Dendroclimatological Analysis of Fir (A. borisii-regis) in Greece in the frame of Climate Change Investigation
Abstract
:1. Introduction
2. Materials and Methods
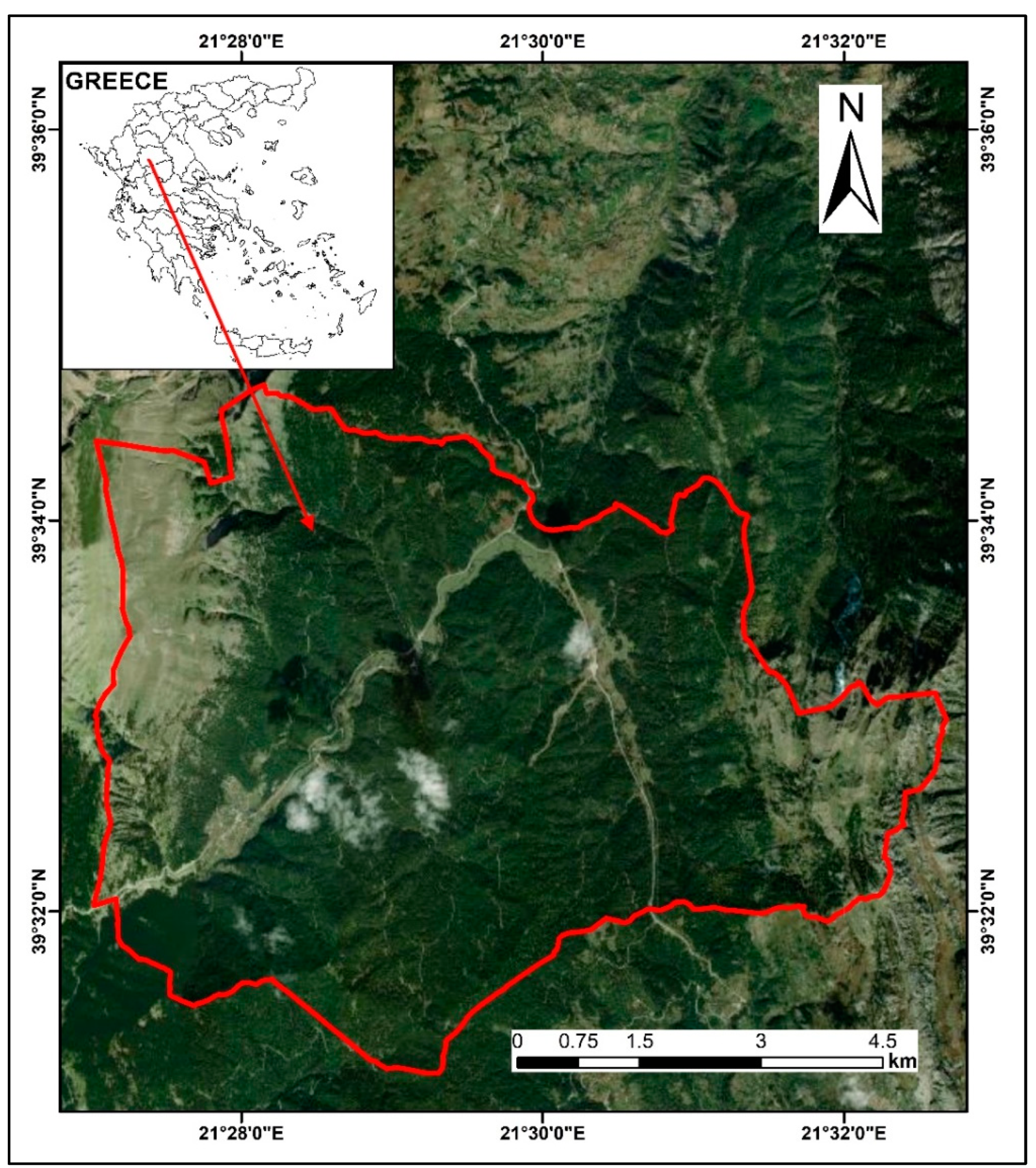
2.1. Study Area
2.2. Sampling and Tree-Ring Analysis
2.3. Climate Data and Trend Analysis
2.4. Climatic Conditions and Tree-Ring Width Correlation Analysis
3. Results and Discussion
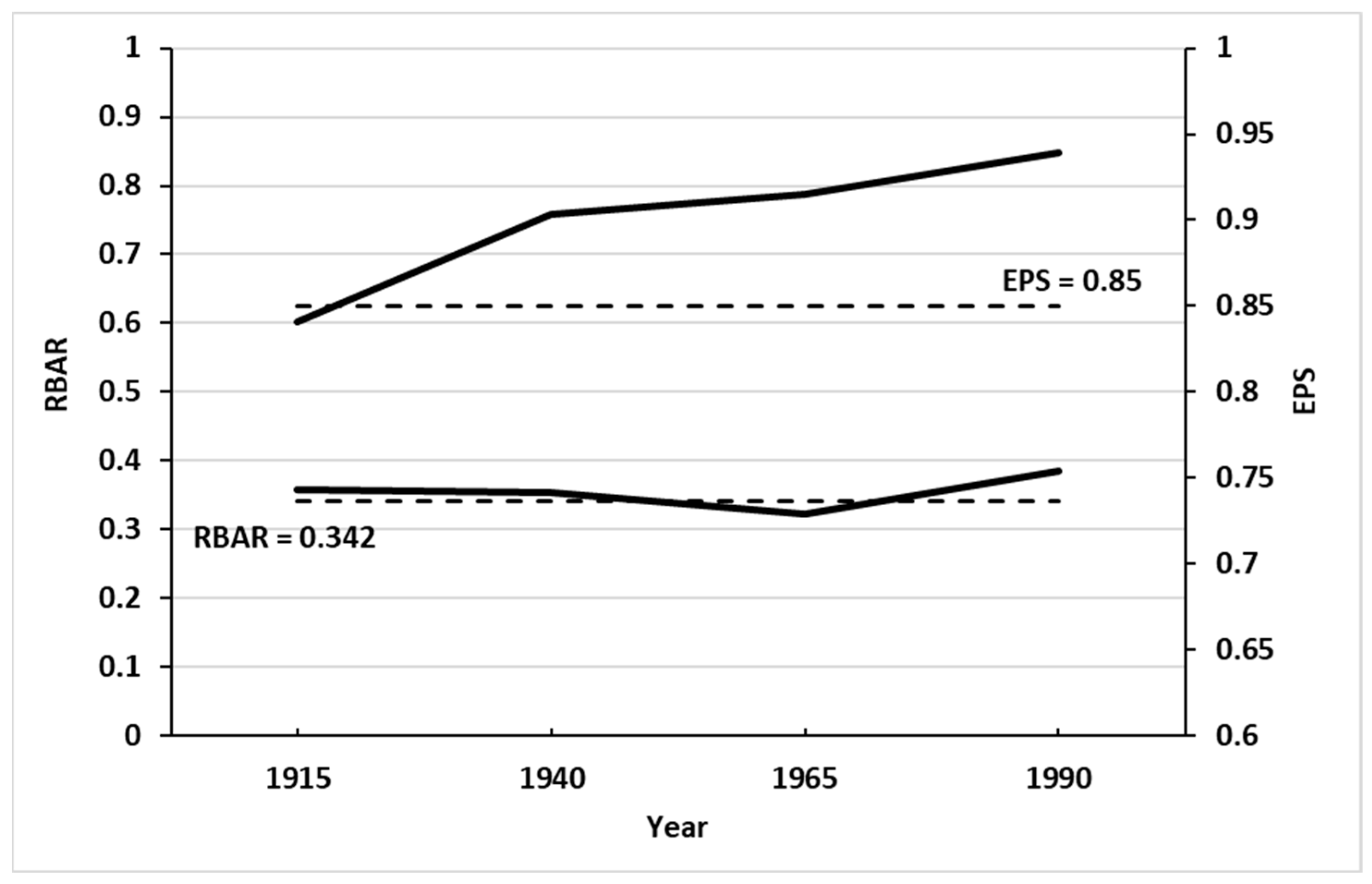
3.1. Tree-Ring Analysis
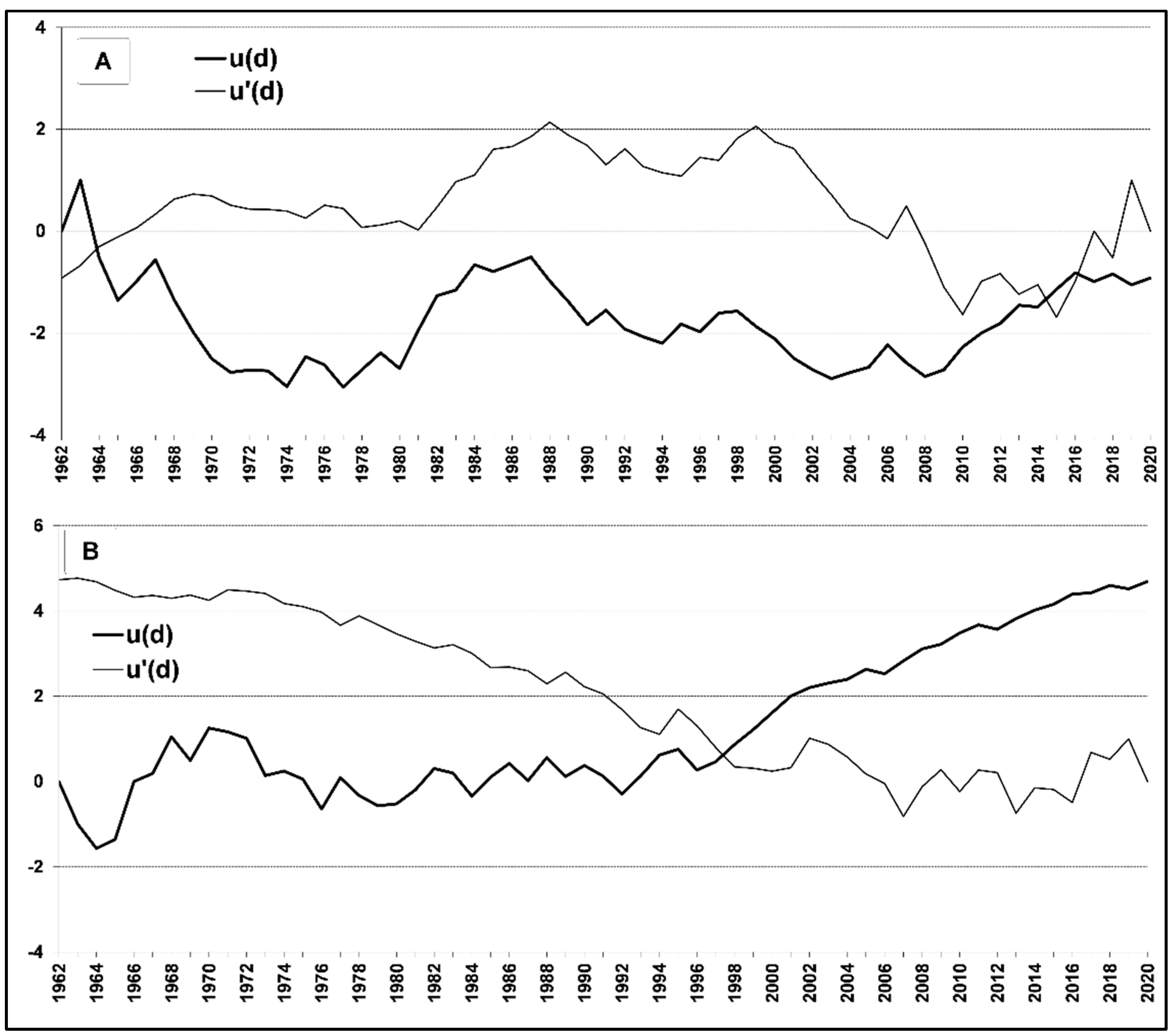
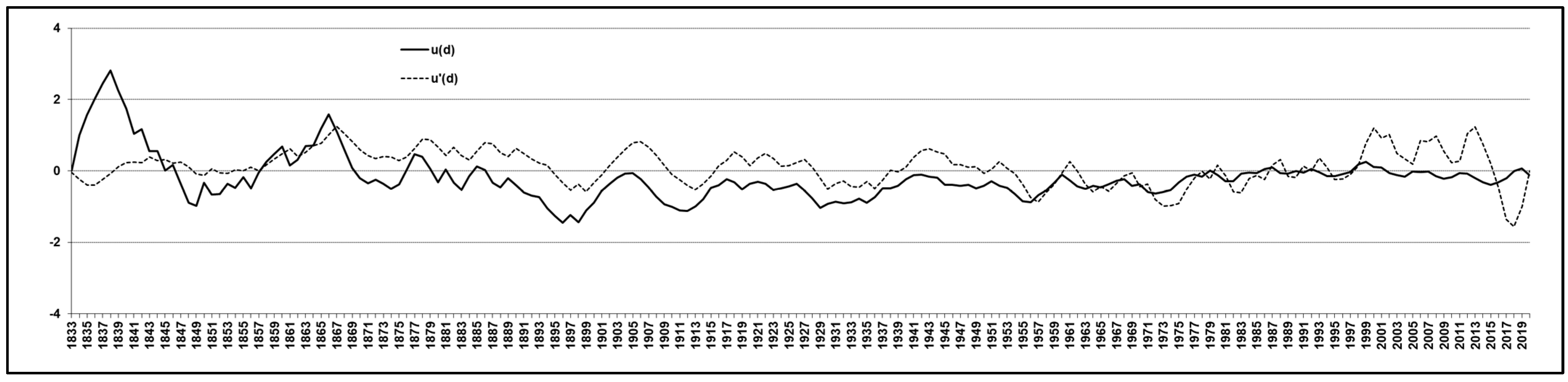
3.2. Climate Trend Analysis
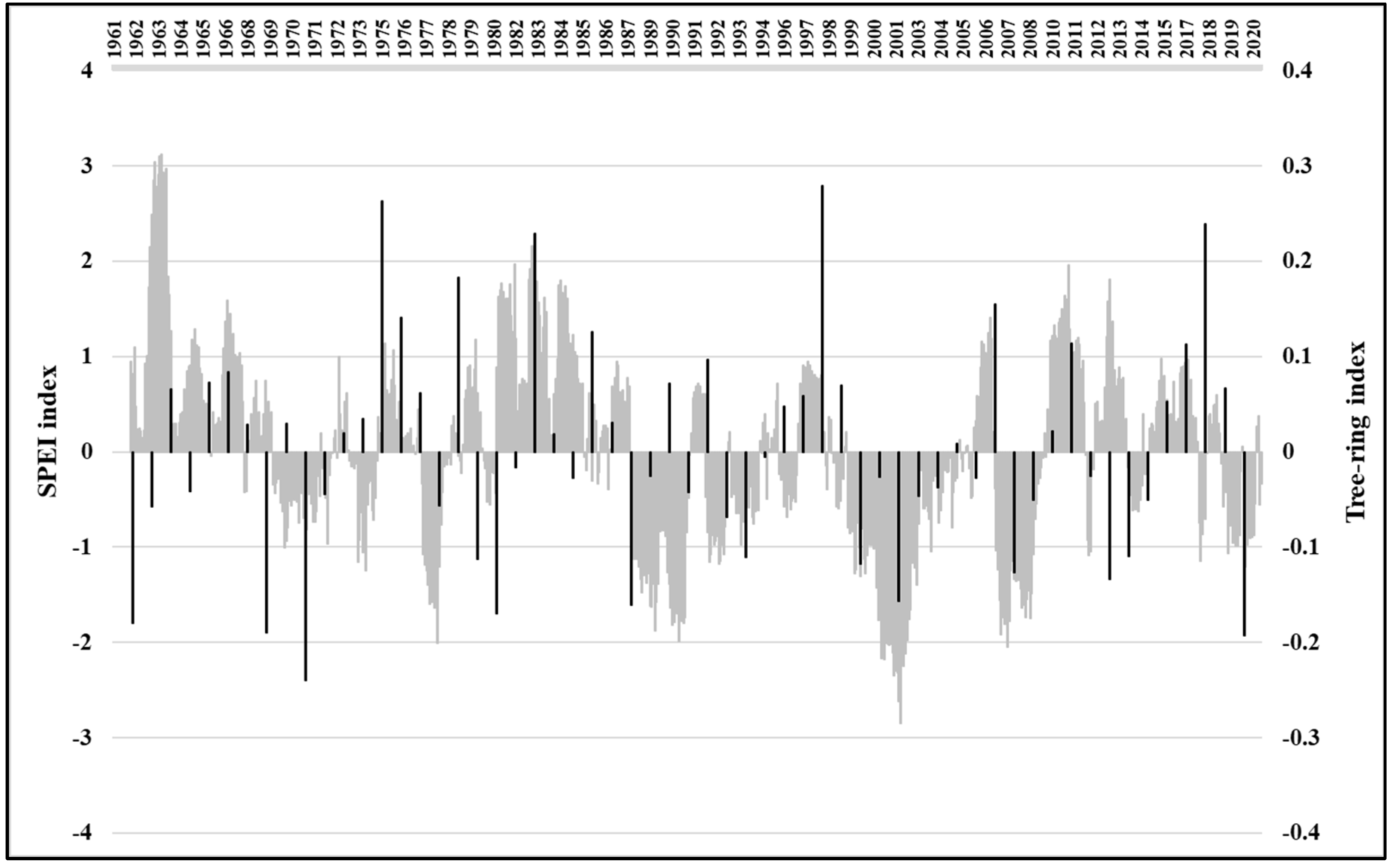
3.3. Relations between Tree-Ring Growth and Climatic Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Novak, K.; de Luis, M.; Saz, M.A.; Longares, L.A.; Serrano-Notivoli, R.; Raventós, J.; Cufar, K.; Gricar, J.; Di Filippo, A.; Piovesan, G.; et al. Missing Rings in Pinus halepensis–The Missing Link to Relate the Tree-Ring Record to Extreme Climatic Events. Front. Plant Sci. 2016, 7, 727. [Google Scholar] [CrossRef] [PubMed]
- Fritts, H.C. Tree Rings and Climate; The Black Burn Press: Caldwell, NJ, USA, 2001. [Google Scholar]
- Papadopoulos, A.; Raftoyannis, Y.; Pantera, A. Fir decline in Greece: A dendroclimatological approach. In Proceedings of the 10th International Conference on Environmental Science and Technology, Kos, Greece, 5–7 September 2007; pp. 571–578. [Google Scholar]
- Roibu, C.C.; Sfeclă, V.; Mursa, A.; Ionita, M.; Nagavciuc, V.; Chiriloaei, F.; Leșan, I.; Popa, I. The Climatic Response of Tree Ring Width Components of Ash (Fraxinus excelsior L.) and Common Oak (Quercus robur L.) from Eastern Europe. Forests 2020, 11, 600. [Google Scholar] [CrossRef]
- Sapountzis, M.; Kastridis, A.; Kazamias, A.-P.; Karagiannidis, A.; Nikopoulos, P.; Lagouvardos, K. Utilization and uncertainties of satellite precipitation data in flash flood hydrological analysis in ungauged watersheds. Glob. NEST J. 2021, 23, 388–399. [Google Scholar] [CrossRef]
- Haavik, L.J.; Billings, S.A.; Guldin, J.M.; Stephen, F.M. Emergent insects, pathogens and drought shape changing patterns in oak decline in North America and Europe. For. Ecol. Manag. 2015, 354, 190–205. [Google Scholar] [CrossRef]
- Robson, J.R.M.; Conciatori, F.; Tardif, J.C.; Knowles, K. Tree-ring response of jack pine and scots pine to budworm defoliation in central Canada. For. Ecol. Manag. 2015, 347, 83–95. [Google Scholar] [CrossRef]
- Kamperidou, V. The biological durability of thermally- and chemically-modified black pine and poplar wood against basidiomycetes and mold action. Forests 2019, 10, 1111. [Google Scholar] [CrossRef] [Green Version]
- Barmpoutis, P.; Stathaki, T.; Kamperidou, V. Estimation of extent of trees and biomass infestation of the suburban forest of Thessaloniki (Seich Sou) using UAV imagery and combining R-CNNs and multichannel texture analysis. In Proceedings of the SPIE-The International Society for Optical Engineering This Link Is Disabled, Amsterdam, The Netherlands, 31 January 2020; Volume 11433, p. 114333C. [Google Scholar]
- Camarero, J.J.; Gazol, A.; Sangüesa-Barreda, G.; Oliva, J.; Vicente-Serrano, M. To die or not to die: Early warnings of tree die back in response to a severe drought. J. Ecol. 2015, 103, 44–57. [Google Scholar] [CrossRef] [Green Version]
- Panayotov, M.P.; Zafirov, N.; Cherubini, P. Fingerprints of extreme climate events in Pinus sylvestris tree rings from Bulgaria. Trees 2013, 27, 211–227. [Google Scholar] [CrossRef] [Green Version]
- Lebourgeois, F. Climatic signals in early wood, latewood and total ring width of Corsican pine from western France. Ann. For. Sci. 2000, 57, 155–164. [Google Scholar] [CrossRef] [Green Version]
- Fonti, P.; VonArx, G.; García-González, I.; Eilmann, B.; Sass-Klaassen, U.; Gärtner, H.; Eckstein, D. Studying global change through investigation of the plastic responses of xylem anatomy intreerings. New Phytol. 2010, 185, 42–53. [Google Scholar] [CrossRef]
- de Luis, M.; Cufar, K.; Di Filippo, A.; Novak, K.; Papadopoulos, A.; Piovesan, G.; Rathgeber, C.B.K.; Raventós, J.; Saz, M.A.; Smith, K.T. Plasticity in dendroclimatic response across the distribution range of Aleppo pine (Pinus halepensis). PLoS ONE 2013, 8, e83550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liphschitz, N.; Lev-Yadun, S. Cambial activity of evergreen and seasonal dimorphics around the Mediterranean. IAWA Bull. 1986, 7, 145–153. [Google Scholar] [CrossRef]
- Waszak, N.; Robertson, I.; Puchałka, R.; Przybylak, R.; Pospieszynska, A.; Koprowski, M. Investigating the Climate-Growth Response of Scots Pine (Pinus sylvestris L.) in Northern Poland. Atmosphere 2021, 12, 1690. [Google Scholar] [CrossRef]
- Camarero, J.J.; Rubio-Cuadrado, A. Relating Climate, Drought and Radial Growth in Broadleaf Mediterranean Tree and Shrub Species: A New Approach to Quantify Climate-Growth Relationships. Forests 2020, 11, 1250. [Google Scholar] [CrossRef]
- Subotić, J.; Dukić, V.; Popov, T.; Trbić, G.; Maunaga, Z.; Petrović, D. Relationships Between Climatic Variables and Tree-Ring Width of Silver Fir (Abies alba Mill.) in Kozara National Park (Bosnia and Herzegovina). SEEFOR 2005, 11, 17–27. [Google Scholar] [CrossRef]
- Linares, J.C.; Camarero, J.J.; Carreira, J.A. Plastic responses of Abies pinsapo xylogenesis to drought and competition. Tree Physiol. 2009, 29, 1525–1536. [Google Scholar] [CrossRef]
- Carrer, M.; Nola, P.; Motta, R.; Urbinati, C. Contrasting tree-ring growth to climate responses of Abies alba toward the southern limit of its distribution area. Oikos 2010, 119, 1515–1525. [Google Scholar] [CrossRef]
- Walder, D.; Krebs, P.; Bugmann, H.; Chiara Manetti, M.; Pollastrini, M.; Anzillotti, S.; Conedera, M. Silver fir (Abies alba Mill.) is able to thrive and prosper under meso-Mediterranean conditions. For. Ecol. Manag. 2021, 498, 119537. [Google Scholar] [CrossRef]
- Garfi, G. Climatic signal in tree-rings of Quercus pubescens s.l. and Celtis australis L. in South-Eastern Sicily. Dendrochronologia 2000, 18, 41–51. [Google Scholar]
- Cherubini, P.; Gartner, B.; Tognetti, R.; Brakker, O.; Schoch, W.; Innes, J. Identification, measurement and interpretation of tree rings in woody species from mediterranean climates. Biol. Rev. 2003, 78, 119–148. [Google Scholar] [CrossRef] [Green Version]
- Barmpoutis, P.; Stathaki, T.; Kamperidou, V. Monitoring of Trees’ Health Condition Using a UAV Equipped with Low-cost Digital Camera. In Proceedings of the ICASSP, IEEE International Conference on Acoustics, Speech and Signal Processing, Brighton, UK, 12–17 May 2019; pp. 8291–8295. [Google Scholar]
- Kastridis, A.; Theodosiou, G.; Fotiadis, G. Investigation of Flood Management and Mitigation Measures in Ungauged NATURA Protected Watersheds. Hydrology 2021, 8, 170. [Google Scholar] [CrossRef]
- Athanasiadis, N. Forest Botany (Trees and Shrubs of the Greek Forests), PartII, Giahoudis-Giapoudis. Thessaloniki 1986, 309. (In Greek) [Google Scholar]
- Linares, J.C. Biogeography and evolution of Abies (Pinaceae) in the Mediterranean Basin: The roles of long-term climatic change and glacialrefugia. J. Biogeogr. 2011, 38, 619–630. [Google Scholar] [CrossRef]
- Papadopoulos, A. Tree-ring patterns and climate response of Mediterranean fir populations in Central Greece. Dendrochronologia 2016, 40, 17–25. [Google Scholar] [CrossRef]
- Rathgeber, C.; Nicault, A.; Kaplan, J.O.; Guiot, J. Using a biogeochemistry model in simulating forest productivity responses to climatic change and (CO2) increase: Example of Pinus halepensis in Provence (south-east France). Ecol. Model. 2003, 166, 239–255. [Google Scholar] [CrossRef]
- Papadopoulos, A.; Serre-Bachet, F.; Tessier, L. Tree ring to climate relationships of Aleppo pine (Pinus halepensis Mill.) in Greece. Ecol. Mediterr. 2001, 27, 89–98. [Google Scholar] [CrossRef]
- Papadopoulos, A.M. Investigations dendroclimatologiques du Sapin de Céphalonie en Grèce Centrale. Geogr. Tech. 2009, 2, 34–38. [Google Scholar]
- Manetti, M.C.; Cutini, A. Tree-ring growth of silver fir (Abies alba Mill.) in twostands under different silvicultural systems in central Italy. Dendrochronologia 2006, 23, 145–150. [Google Scholar] [CrossRef]
- Akkemik, Ü.; D’Arrigo, R.; Cherubini, P.; Köse, N.; Jacoby, G.C. Tree-ringreconstructions of precipitation and streamflow for north-western Turkey. Int. J. Climatol. 2008, 28, 173–183. [Google Scholar] [CrossRef]
- Touchan, R.; Anchukaitis, K.J.; Shishov, V.V.; Sivrikaya, F.; Attieh, J.; Ketmen, M.; Stephan, J.; Mitsopoulos, I.; Christou, A.; Meko, D.M. Spatial patterns of Eastern Mediterranean climate influence on tree growth. Holocene 2004, 24, 381–392. [Google Scholar] [CrossRef]
- Rolland, C. Tree-ring and climate relationships for Abies alba in the internal Alps. Tree-Ring Bull. 1993, 53, 1–11. [Google Scholar]
- Fund for the Administration and Management of University Forests. Pertouli University Forest Management Plan of the Decade 2009–2018 (2018). Available online: http://uniforest.auth.gr/wp-content/uploads/2016/04/4_ΜΕΡOΣ-ΠΡΩΤO.pdf (accessed on 22 September 2020).
- Grissino-Mayer, H.D. Evaluating crossdating accuracy: A manual and tutorial for the computer program COFECHA. Tree Ring Res. 2001, 57, 205–221. [Google Scholar]
- Cook, E.R.; Holmes, R.L. User’s Manual for Program ARSTAN; Laboratory of Tree-rings Research: Tucson, AZ, USA; University of Arizona: Tucson, AZ, USA, 1999. [Google Scholar]
- Cook, E.R.; Peters, K. The smoothing spline: A new approach to standardizing forest interior tree-ring width series for dendroclimatic studies. Tree Ring Bull. 1981, 41, 45–53. [Google Scholar]
- Cook, E.R. A Time Series Analysis Approach to Tree-Ring Standardization. Ph.D. Thesis, University of Arizona, Tucson, AZ, USA, 1985. Available online: http://hdl.handle.net/10150/188110 (accessed on 13 April 2020).
- Wigley, T.M.L.; Briffa, K.R.; Jones, P.D. On the average value of correlated time-series, with applications in dendroclimatology and hydrometeorology. J. Appl. Meteorol. Climatol. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Cook, E.R.; Palmer, J.G.; Cook, B.I.; Hogg, A.; D’Arrigo, R.D. A multi-millennial palaeoclimatic resource from lagarostrobos colensoi tree-rings at oroko swamp, new zealand. Glob. Planet. Change 2002, 33, 209–220. [Google Scholar] [CrossRef]
- Buras, A. A comment on the expressed population signal. Dendrochronologia 2017, 44, 130–132. [Google Scholar] [CrossRef]
- Mann, H.B. Nonparametric tests against trend. Econometrica 1945, 13, 245–259. [Google Scholar] [CrossRef]
- Kendall, M.G. Rank Correlation Methods; Oxford University Press: New York, NY, USA, 1975. [Google Scholar]
- Sen, P.K. Estimates of the regression coefficient based on Kendall’s tau. J. Am. Stat. Assoc. 1968, 63, 1379–1389. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Beguerıa, S.; Lopez-Moreno, J.I. A multi-scalar drought index sensitive to global warming: The standardized precipitation evapotranspiration index–SPEI. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef] [Green Version]
- Tirivarombo, S.; Osupile, D.; Eliasson, P. Drought monitoring and analysis: Standardised Precipitation Evapotranspiration Index (SPEI) and Standardised Precipitation Index (SPI). Phys. Chem. Earth 2018, 106, 1–10. [Google Scholar] [CrossRef]
- Heikkinen, O. Relationships between tree growth and climate in the subalpine Cascade Range of Washington, USA. Ann. Bot. Fenn. 1985, 22, 1–14. [Google Scholar]
- Koprowski, M.; Duncker, P. Tree ring width and wood density as the indicators of climatic factors and insect outbreaks affecting spruce growth. Ecol. Indic. 2012, 23, 332–337. [Google Scholar] [CrossRef]
- Hauck, M.; Zimmermann, J.; Jacob, M.; Dulamsuren, C.; Bade, C.; Ahrends, B.; Leuschner, C. Rapid recovery of stem increment in Norway spruce at reduced SO2 levels in the Harz Mountains, Germany. Environ. Pollut. 2012, 164, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Serre-Bachet, F. La dendrochronology dans le bassin méditerranéen. Dendrochronologia 1985, 3, 77–92. [Google Scholar]
- Rigling, A.; Waldner, P.; Forster, T.; Bräker, O.; Pouttu, A. Ecological interpretation of tree-ring width and intraannual density fluctuations in Pinus sylvestris on dry sites in the central Alps and Siberia. Can. J. For. Res. 2001, 31, 18–31. [Google Scholar] [CrossRef]
- Wilson, R.; Topham, J. Violins and climate. Theor. Appl. Climatol. 2004, 77, 9–24. [Google Scholar] [CrossRef]
- Pasho, E.; Toromani, E.; Alla, A.Q. Climate impact on tree-ring widths in Abies borissi-regis forest from South-East Albania. Dendrochronologia 2014, 32, 237–244. [Google Scholar] [CrossRef]
- Bhuyan, U.; Zang, C.; Menzel, A. Different responses of multispecies tree ring growth to various drought indices across Europe. Dendrochronologia 2017, 44, 1–8. [Google Scholar] [CrossRef]
- Koutavas, A. CO2 fertilization and enhanced drought resistance in Greek firs from Cephalonia Island, Greece. Glob. Change Biol. 2013, 19, 529–539. [Google Scholar] [CrossRef]
- Hughes, M.K.; Kuniholm, P.I.; Garfin, G.M.; Griggs, C.; Latini, C. Aegean tree-ring signature years explained. Tree Ring Res. 2001, 57, 67–73. [Google Scholar]
- Csilléry, K.; Buchmann, N.; Fady, B. Adaptation to Drought is Coupled with Slow Growth, but Independent from Phenology in Marginal Silver Fir (Abies Alba Mill.) Populations. Evol. Appl. 2020, 13, 2357–2376. [Google Scholar] [CrossRef] [PubMed]
- Mihai, G.; Alexandru, A.M.; Stoica, E.; Birsan, M.V. Intraspecific Growth Response to Drought of Abies alba in the Southeastern Carpathians. Forests 2021, 12, 387. [Google Scholar] [CrossRef]
- Hughes, M.K.; Swetnam, T.W.; Diaz, H.F. Dendroclimatology. In Developments in Paleoenvironmental Research; Springer: Dordrecht, The Netherlands, 2011; p. 54. [Google Scholar] [CrossRef]
- Vitali, V.; Büntgen, U.; Bauhus, J. Seasonality matters—The effects of past and projected seasonal climate change on the growth of native and exotic conifer species in Central Europe. Dendrochronologia 2018, 48, 1–9. [Google Scholar] [CrossRef]



| Study Area | Latitude | Longitude | Altitude (m) | Exposition | Mean Annual Temperature (°C) | Mean Annual Precipitation (mm) | Mean DBH (cm) |
|---|---|---|---|---|---|---|---|
| Pertouli University Forest | 39.542484° | 21.464476° | 1200–1500 | SE-E | 9.1 | 1471.4 | 67.5 |
| Predictors | R2 | f Value | df | p Value |
|---|---|---|---|---|
| Precipitation (May, June, July) | 0.312 | 8.3 | 58 | 0.00012 |
| Mean temperature (June) | 0.15 | 8.9 | 52 | 0.004 |
| Max temperature (April, June) | 0.22 | 7.1 | 52 | 0.002 |
| Precipitation (May, June, July) with mean temperature (June) | 0.364 | 6.8 | 52 | 0.00018 |
| Precipitation (May, June, July) with Max temperature (April, June) | 0.385 | 5.6 | 52 | 0.00038 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kastridis, A.; Kamperidou, V.; Stathis, D. Dendroclimatological Analysis of Fir (A. borisii-regis) in Greece in the frame of Climate Change Investigation. Forests 2022, 13, 879. https://doi.org/10.3390/f13060879
Kastridis A, Kamperidou V, Stathis D. Dendroclimatological Analysis of Fir (A. borisii-regis) in Greece in the frame of Climate Change Investigation. Forests. 2022; 13(6):879. https://doi.org/10.3390/f13060879
Chicago/Turabian StyleKastridis, Aristeidis, Vasiliki Kamperidou, and Dimitrios Stathis. 2022. "Dendroclimatological Analysis of Fir (A. borisii-regis) in Greece in the frame of Climate Change Investigation" Forests 13, no. 6: 879. https://doi.org/10.3390/f13060879






