Drivers of Flammability of Eucalyptus globulus Labill Leaves: Terpenes, Essential Oils, and Moisture Content
Abstract
:1. Introduction
2. Materials and Methods
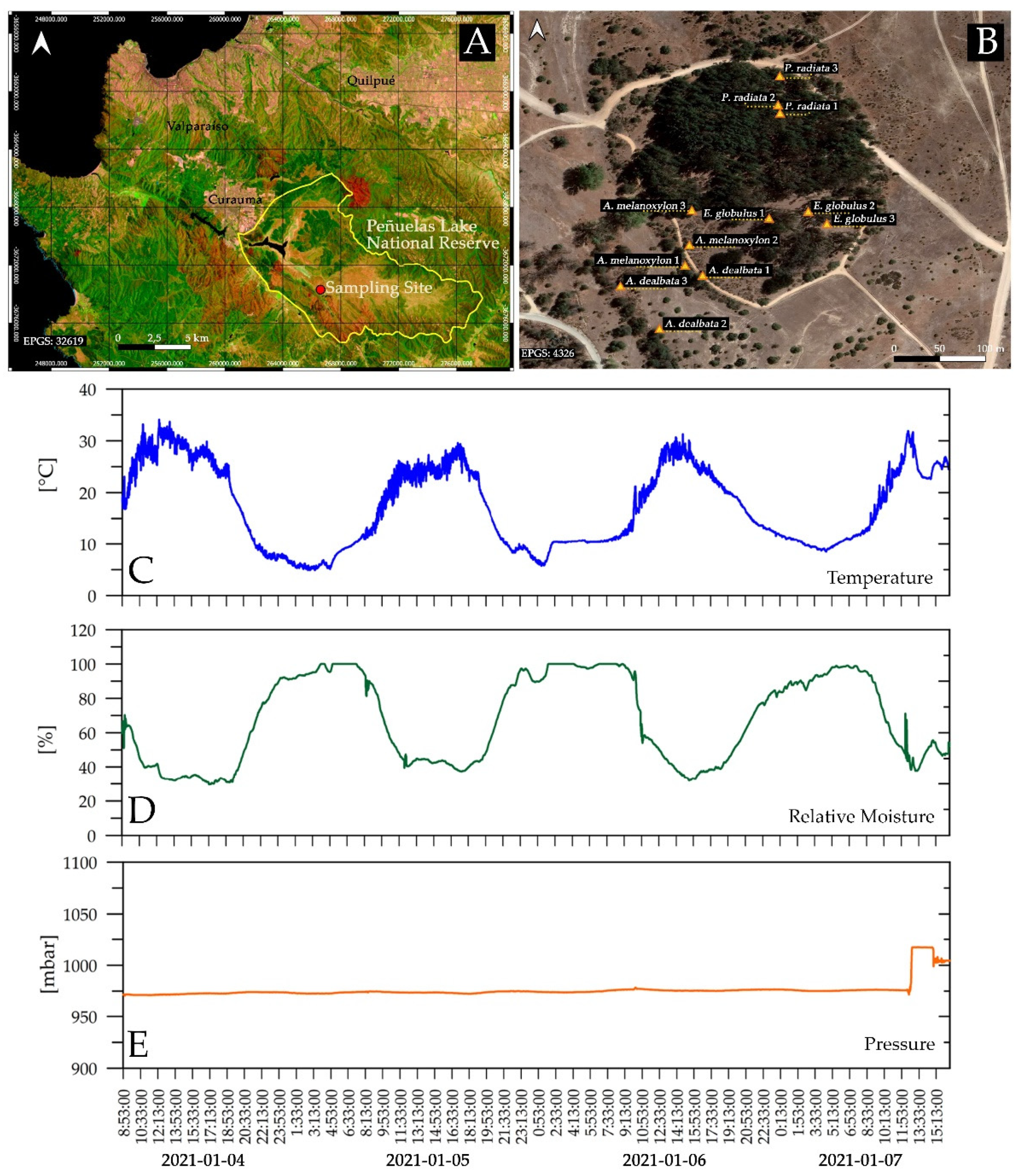
2.1. Study Area and Leaf Sampling
2.2. Flammability Measurements
2.3. Heat of Combustion
2.4. Flashpoint
2.5. Essential Oils
2.6. Chemical Extraction and Analysis
2.7. Moisture Content
2.8. Data Analysis
3. Results
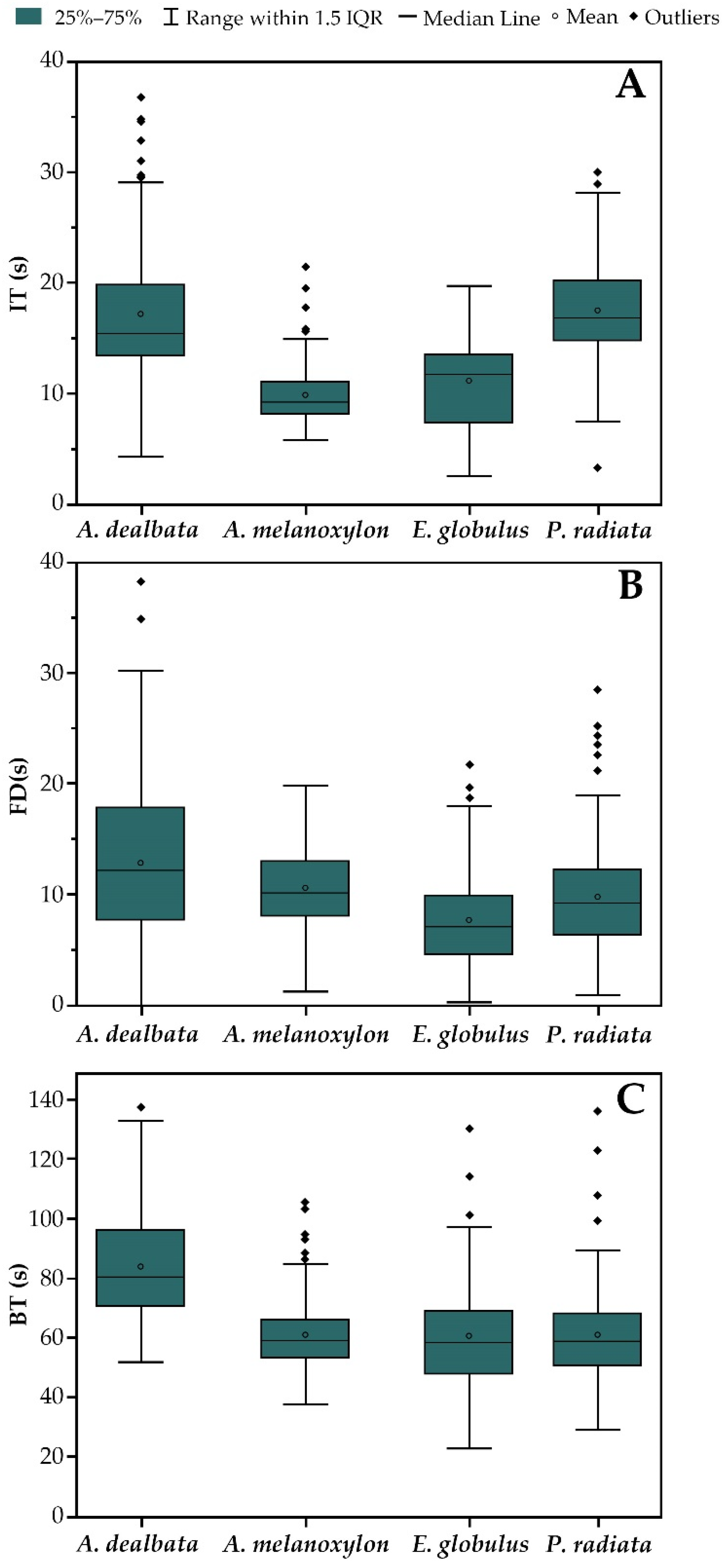
3.1. Leaf Flammability: Ignition Time, Flame Duration, and Burning Time
3.2. Flammability Index
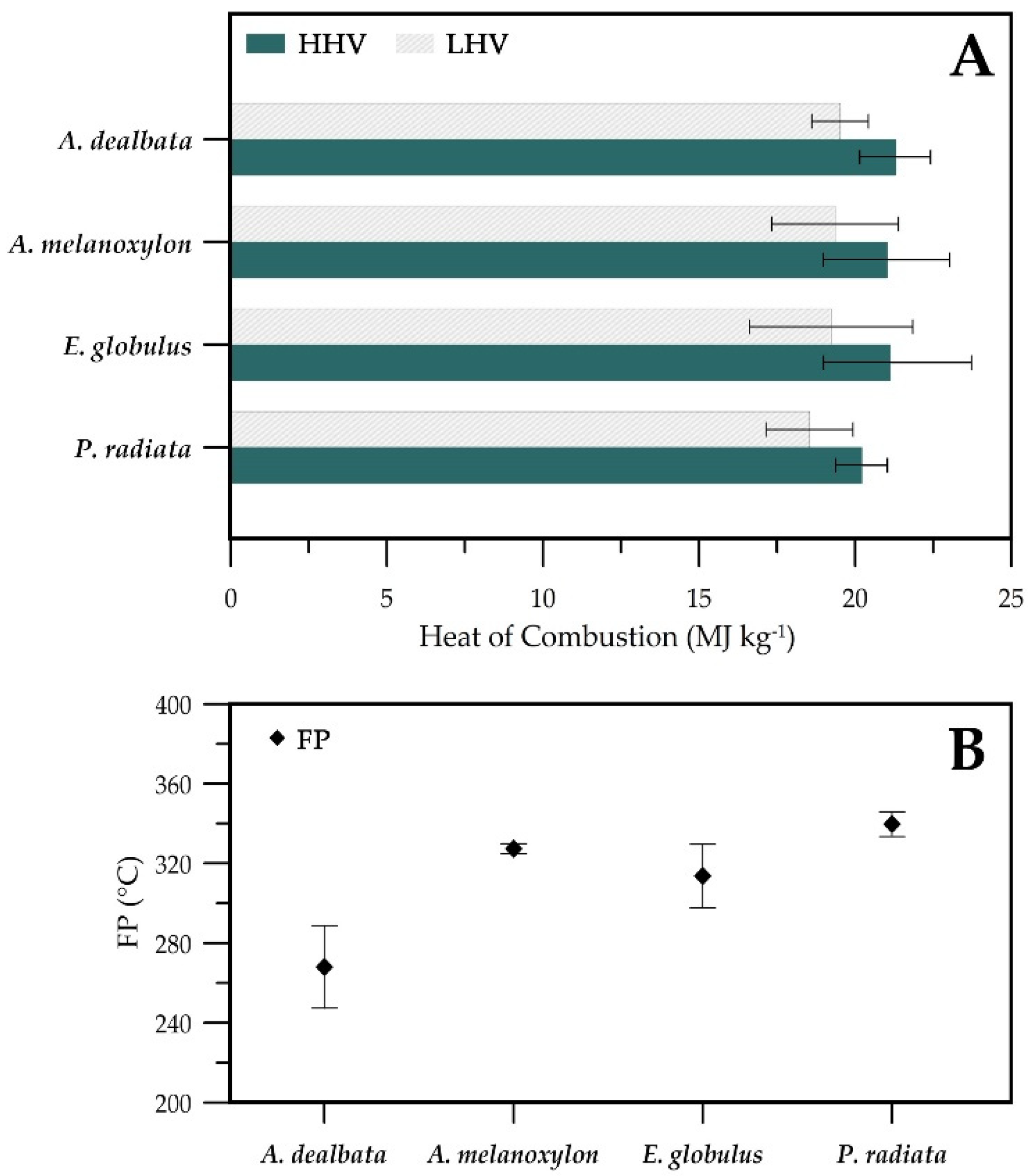
3.3. Heat of Combustion and Flashpoint
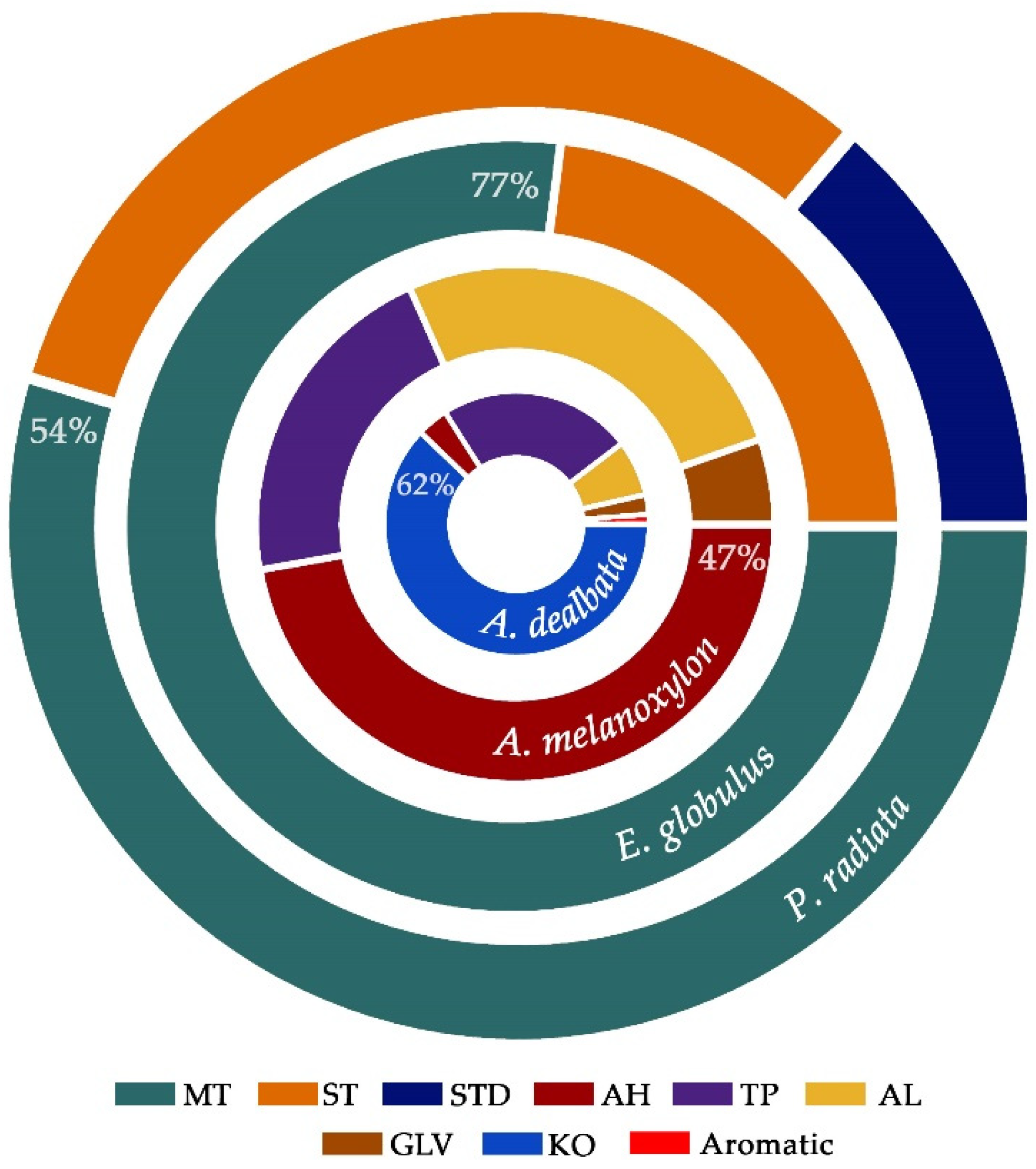
3.4. Identification and Quantification of Chemical Compounds and Essential Oils in Fresh Leaves
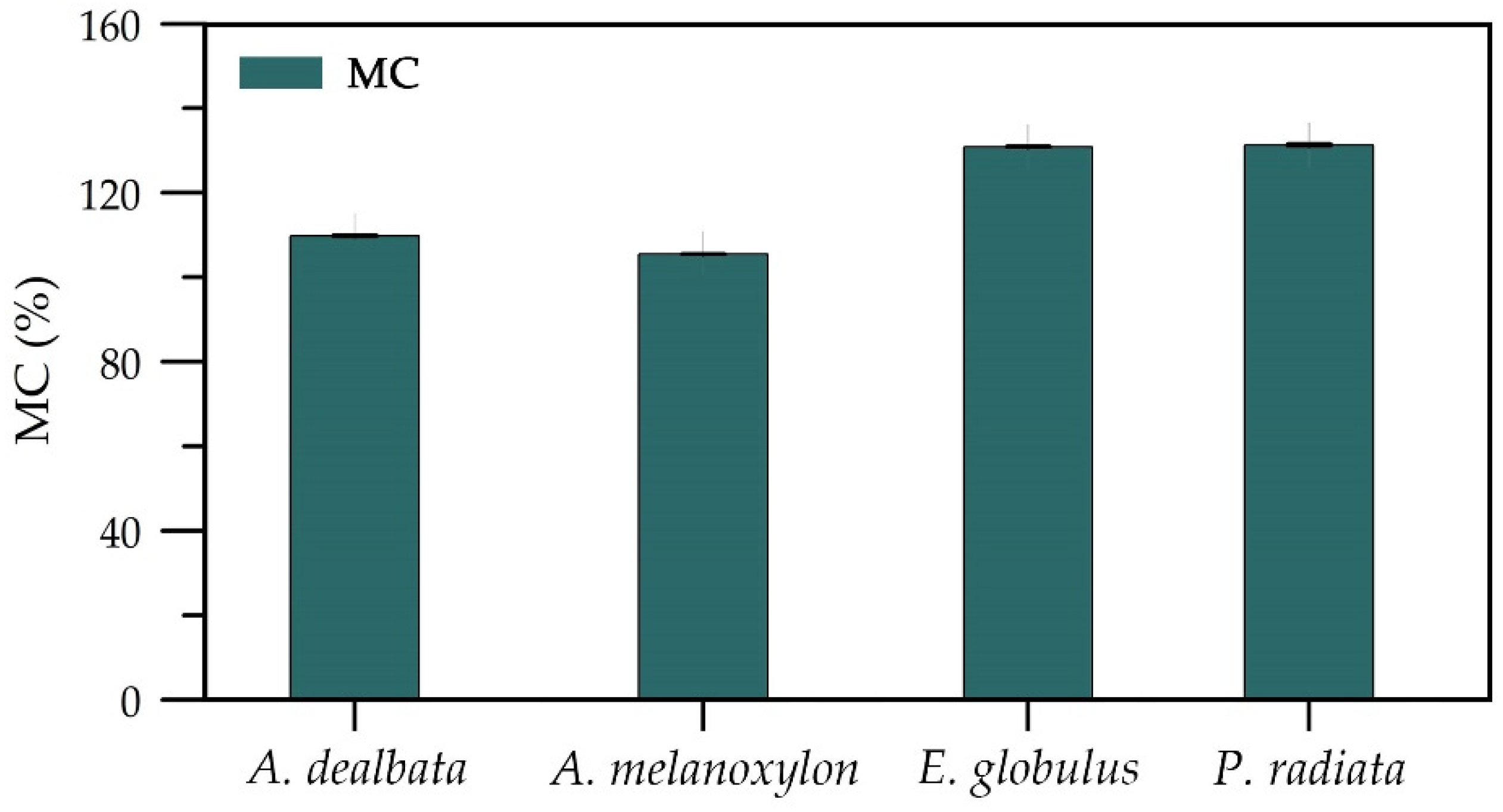
3.5. Moisture Content
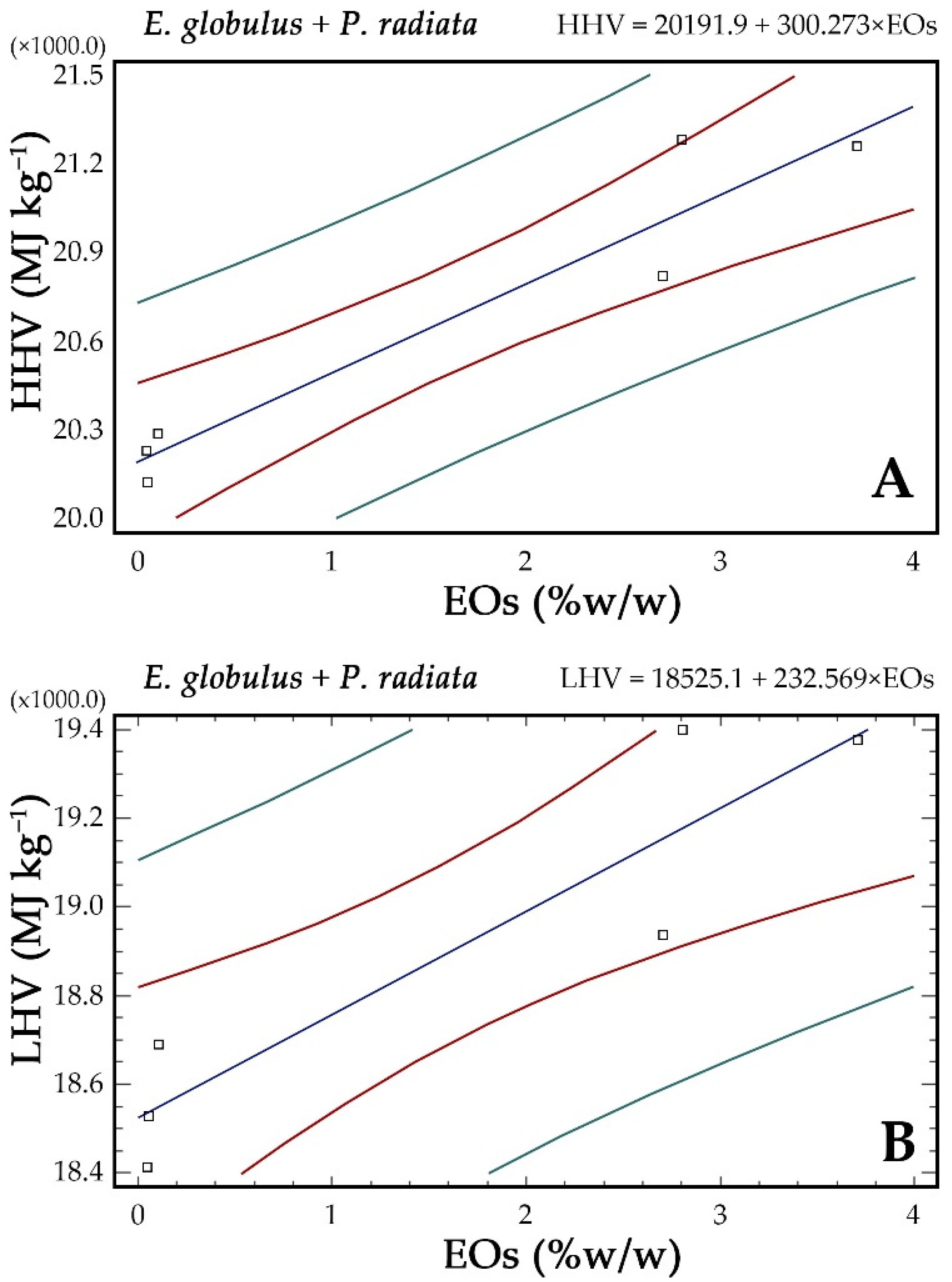
3.6. Relationships between Flammability, Thermochemical Properties, and Chemical Compounds
4. Discussion
4.1. Variation in the Flammability of Exotic Species
4.2. Variations of Heating Values and Flashpoints
4.3. Variations in Terpene Content
4.4. Effects of Drivers on Leaf Flammability
4.5. The Ecological Role of Terpenes: Promoting E. globulus Flammability
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lindenmayer, D.; Messier, C.; Paquette, A.; Hobbs, R.J. Managing tree plantations as novel socioecological systems: Australian and North American perspectives. Can. J. For. Res. 2015, 45, 1427–1433. [Google Scholar] [CrossRef] [Green Version]
- Wolf, K.M.; DiTomaso, J.M. Management of blue gum eucalyptus in California requires region-specific consideration. Calif. Agric. 2016, 70, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Proença, V.; Pereira, H.M. Ecosystem Changes, Biodiversity Loss and Human Well-Being. Ref. Modul. Earth Syst. Environ. Sci. 2015, 1–10. [Google Scholar] [CrossRef]
- INFOR El Sector Forestal Chileno 2020. Available online: https://wef.infor.cl/sector_forestal/sectorforestal.php (accessed on 8 June 2022).
- CONAF Catastros de uso de suelo y Vegetación. Available online: https://www.ide.cl/index.php/flora-y-fauna/item/1513-catastros-de-uso-de-suelo-y-vegetacion (accessed on 8 June 2022).
- Piñones Aguilera, G. Plan Maestro para la Gestión del Riesgo de Incendios Forestales—Urbanos Valparaíso (Memoria Técnica) 2018. Available online: http://munivalpo.cl/repositorio/archivos/2019/PM/Memoria_PLAN_MAESTRO_INCENDIO_2018.pdf (accessed on 8 June 2022).
- Figueroa, J.A.; Castro, S.A.; Marquet, P.A.; Jaksic, F.M. Exotic plant invasions to the mediterranean region of Chile: Causes, history and impacts Invasión de plantas exóticas en la región mediterránea de Chile: Causas, historia e impactos. Rev. Chil. Hist. Nat. 2004, 77, 465–483. [Google Scholar] [CrossRef] [Green Version]
- Huber, A.; Iroumé, A.; Mohr, C.; Frêne, C. Efecto de plantaciones de Pinus radiata y Eucalyptus globulus sobre el recurso agua en la Cordillera de la Costa de la región del Biobío, Chile. Bosque 2010, 31, 219–230. [Google Scholar] [CrossRef]
- Banfield, C.C.; Braun, A.C.; Barra, R.; Castillo, A.; Vogt, J. Erosion proxies in an exotic tree plantation question the appropriate land use in Central Chile. Catena 2018, 161, 77–84. [Google Scholar] [CrossRef]
- Drake, P.L.; Mendham, D.S.; White, D.A.; Ogden, G.N.; Dell, B. Water use and water-use efficiency of coppice and seedling Eucalyptus globulus Labill.: A comparison of stand-scale water balance components. Plant Soil 2012, 350, 221–235. [Google Scholar] [CrossRef]
- Forrester, D.I.; Theiveyanathan, S.; Collopy, J.J.; Marcar, N.E. Enhanced water use efficiency in a mixed Eucalyptus globulus and Acacia mearnsii plantation. For. Ecol. Manag. 2010, 259, 1761–1770. [Google Scholar] [CrossRef]
- Farley, K.A.; Jobbágy, E.G.; Jackson, R.B. Effects of afforestation on water yield: A global synthesis with implications for policy. Glob. Chang. Biol. 2005, 11, 1565–1576. [Google Scholar] [CrossRef]
- Úbeda, X.; Sarricolea, P. Wildfires in Chile: A review. Glob. Planet. Chang. 2016, 146, 152–161. [Google Scholar] [CrossRef]
- Rubio, M.A.; Lissi, E.; Gramsch, E.; Garreaud, R.D. Effect of nearby forest fires on ground level ozone concentrations in Santiago, Chile. Atmosphere 2015, 6, 1926–1938. [Google Scholar] [CrossRef] [Green Version]
- McWethy, D.B.; Pauchard, A.; García, R.A.; Holz, A.; González, M.E.; Veblen, T.T.; Stahl, J.; Currey, B. Correction: Landscape drivers of recent fire activity (2001-2017) in south-central Chile. PLoS ONE 2018, 13, e0205287. [Google Scholar] [CrossRef] [PubMed]
- Sarricolea, P.; Serrano-Notivoli, R.; Fuentealba, M.; Hernández-Mora, M.; de la Barrera, F.; Smith, P.; Meseguer-Ruiz, Ó. Recent wildfires in Central Chile: Detecting links between burned areas and population exposure in the wildland urban interface. Sci. Total Environ. 2020, 706, 135894. [Google Scholar] [CrossRef] [PubMed]
- Villacrés, J.; Arevalo-Ramirez, T.; Fuentes, A.; Reszka, P.; Cheein, F.A. Foliar moisture content from the spectral signature for wildfire risk assessments in Valparaíso-Chile. Sensors 2019, 19, 5475. [Google Scholar] [CrossRef] [Green Version]
- Marris, E. Conservation: Biodiversity as a bonus prize. Nature 2010, 468, 895. [Google Scholar] [CrossRef] [Green Version]
- Urrutia-Jalabert, R.; González, M.E.; González-Reyes, Á.; Lara, A.; Garreaud, R. Climate variability and forest fires in central and south-central Chile. Ecosphere 2018, 9, e02171. [Google Scholar] [CrossRef]
- Díaz-Hormazábal, I.; González, M.E. Spatio-temporal analyses of wildfires in the region of Maule, Chile. Bosque 2016, 37, 147–158. [Google Scholar] [CrossRef] [Green Version]
- Bowman, D.M.J.S.; Moreira-Muñoz, A.; Kolden, C.A.; Chávez, R.O.; Muñoz, A.A.; Salinas, F.; González-Reyes, Á.; Rocco, R.; de la Barrera, F.; Williamson, G.J.; et al. Human-environmental drivers and impacts of the globally extreme 2017 Chilean fires. Ambio 2019, 48, 350–362. [Google Scholar] [CrossRef]
- Garreaud, R.D.; Boisier, J.P.; Rondanelli, R.; Montecinos, A.; Sepúlveda, H.H.; Veloso-Aguila, D. The Central Chile Mega Drought (2010–2018): A climate dynamics perspective. Int. J. Climatol. 2020, 40, 421–439. [Google Scholar] [CrossRef]
- Garreaud, R.D.; Alvarez-Garreton, C.; Barichivich, J.; Pablo Boisier, J.; Christie, D.; Galleguillos, M.; LeQuesne, C.; McPhee, J.; Zambrano-Bigiarini, M. The 2010–2015 megadrought in central Chile: Impacts on regional hydroclimate and vegetation. Hydrol. Earth Syst. Sci. 2017, 21, 6307–6327. [Google Scholar] [CrossRef] [Green Version]
- Nolan, R.H.; Blackman, C.J.; de Dios, V.R.; Choat, B.; Medlyn, B.E.; Li, X.; Bradstock, R.A.; Boer, M.M. Linking forest flammability and plant vulnerability to drought. Forests 2020, 11, 779. [Google Scholar] [CrossRef]
- Ormeño, E.; Ruffault, J.; Gutigny, C.; Madrigal, J.; Guijarro, M.; Hernando, C.; Ballini, C. Increasing cuticular wax concentrations in a drier climate promote litter flammability. For. Ecol. Manag. 2020, 473, 118242. [Google Scholar] [CrossRef]
- Pickett, B.M.; Isackson, C.; Wunder, R.; Fletcher, T.H.; Butler, B.W.; Weise, D.R. Flame interactions and burning characteristics of two live leaf samples. Int. J. Wildl. Fire 2009, 18, 865–874. [Google Scholar] [CrossRef]
- Mitsopoulos, I.D.; Dimitrakopoulos, A.P. Canopy fuel characteristics and potential crown fire behavior in Aleppo pine (Pinus halepensis Mill.) forests. Ann. For. Sci. 2007, 64, 287–299. [Google Scholar] [CrossRef] [Green Version]
- Cornwell, W.K.; Elvira, A.; van Kempen, L.; van Logtestijn, R.S.P.; Aptroot, A.; Cornelissen, J.H.C. Flammability across the gymnosperm phylogeny: The importance of litter particle size. New Phytol. 2015, 206, 672–681. [Google Scholar] [CrossRef]
- Zylstra, P.; Bradstock, R.A.; Bedward, M.; Penman, T.D.; Doherty, M.D.; Weber, R.O.; Gill, A.M.; Cary, G.J. Biophysical mechanistic modelling quantifies the effects of plant traits on fire severity: Species, not surface fuel loads, determine flame dimensions in eucalypt forests. PLoS ONE 2016, 11, e0160715. [Google Scholar] [CrossRef] [Green Version]
- Michelaki, C.; Fyllas, N.M.; Galanidis, A.; Aloupi, M.; Evangelou, E.; Arianoutsou, M.; Dimitrakopoulos, P.G. Adaptive flammability syndromes in thermo-Mediterranean vegetation, captured by alternative resource-use strategies. Sci. Total Environ. 2020, 718, 137437. [Google Scholar] [CrossRef]
- Krix, D.W.; Phillips, M.L.; Murray, B.R. Relationships among leaf flammability attributes and identifying low-leaf-flammability species at the wildland-urban interface. Int. J. Wildl. Fire 2019, 28, 295–297. [Google Scholar] [CrossRef]
- Krix, D.W.; Murray, B.R. Landscape variation in plant leaf flammability is driven by leaf traits responding to environmental gradients. Ecosphere 2018, 9, e02093. [Google Scholar] [CrossRef]
- Guerrero, F.; Toledo, M.; Ripoll, N.; Espinoza, L.; Morales, R.; Muñoz, A.; Taborga, L.; Carrasco, Y. Thermo-and physicochemical properties of native and exotic forest species of Valparaíso, Chile, as essential information for fire risk management. Int. J. Wildl. Fire 2020, 29, 675–685. [Google Scholar] [CrossRef]
- Guerrero, F.; Hernández, C.; Toledo, M.; Espinoza, L.; Carrasco, Y.; Arriagada, A.; Muñoz, A.; Taborga, L.; Bergmann, J.; Carmona, C. Leaf Thermal and Chemical Properties as Natural Drivers of Plant Flammability of Native and Exotic Tree Species of the Valpara í so Region, Chile. Int. J. Environ. Res. Public Health 2021, 18, 7191. [Google Scholar] [CrossRef] [PubMed]
- CONAF Reserva Nacional Lago Peñuelas. Available online: https://www.conaf.cl/parques/reserva-nacional-lago-penuelas/ (accessed on 8 June 2022).
- Hauenstein, E.; Muñoz-Pedreros, A.; Yánez, J.; Sánchez, P.; Möller, P.; Guiñez, B.; Gil, C. Flora y vegetación de la Reserva Nacional Lago Peñuelas, Reserva de la Biósfera, Región de Valparaíso, Chile. Bosque 2009, 30, 159–179. [Google Scholar] [CrossRef] [Green Version]
- CONAF Controlan Extenso Incendio Forestal Que Afectó a Valparaíso y Quilpué. Available online: https://www.conaf.cl/controlan-extenso-incendio-forestal-que-afecto-a-valparaiso-y-quilpue/ (accessed on 8 June 2022).
- Wolf, K.D.; Higuera, P.E.; Davis, K.T.; Dobrowski, S.Z. Wildfire impacts on forest microclimate vary with biophysical context. Ecosphere 2021, 12, e03467. [Google Scholar] [CrossRef]
- White, R.H.; Zipperer, W.C. Testing and classification of individual plants for fire behaviour: Plant selection for the wildlandurban interface. Int. J. Wildl. Fire 2010, 19, 213–227. [Google Scholar] [CrossRef]
- Blackhall, M.; Raffaele, E. Flammability of Patagonian invaders and natives: When exotic plant species affect live fine fuel ignitability in wildland-urban interfaces. Landsc. Urban Plan. 2019, 189, 1–10. [Google Scholar] [CrossRef]
- Ganteaume, A.; Jappiot, M.; Lampin, C.; Guijarro, M.; Hernando, C. Flammability of some ornamental species in wildland-urban interfaces in southeastern France: Laboratory assessment at particle level. Environ. Manag. 2013, 52, 467–480. [Google Scholar] [CrossRef] [Green Version]
- Valette, J.-C. Inflammabilités des espèces forestières méditerranéennes: Conséquences sur la combustibilité des formations forestières. Rev. For. Française 1990, 42, 76–92. [Google Scholar] [CrossRef] [Green Version]
- Núñez-Regueira, L.; Rodríguez-Añón, J.; Proupín, J.; Vilanova Diz, A. Calorimetry as a tool to design campaigns to prevent and fight forest fires originating from shrub species. Thermochim. Acta 2002, 394, 279–289. [Google Scholar] [CrossRef]
- Nuňez-Regueira, L.; Rodríguez-Aňón, J.A.; Proupín, J.; Mouriňo, B.; Artiaga-Diaz, R. Energetic study of residual forest biomass using calorimetry and thermal analysis. J. Therm. Anal. Calorim. 2005, 80, 457–464. [Google Scholar] [CrossRef]
- ASTM D240-00; ASTM Standard Test Method for Heat of Combustion of Liquid Hydrocarbon Fuels by Bomb Calorimeter. ASTM: West Conshohoken, PA, USA, 2000.
- Brandes, E.; Möller, W.; Bundesanstalt, B.P.-T. Safety Characteristic Data, Volume 1: Flammable Liquids and Gases; Wirtschaftsverl. NW, Verlag f. Neue Wiss.: Vienna, Austria, 2008. [Google Scholar]
- ASTM D-92-72; Standard Test Method for Flash and Fire Points by Cleveland Open Cup. ASTM: West Conshohoken, PA, USA, 2000.
- ISO 6571:1984; Spices, Condiments and Herbs—Determination of Volatile Oil Content (Hydrodistillation Method). ISO: Geneva, Switzerland, 1984; Volume 2.
- Sánchez, Y.; Correa, T.M.; Abreu, Y.; Pino, O. Efecto del aceite esencial de Piper auritum Kunth y sus componentes sobre Xanthomonas albilineans (Ashby) Dowson y Xanthomonas campestris pv. campestris (Pammel) Dowson. Rev. Prot. Veg. 2012, 27, 39–44. [Google Scholar]
- Infante, D.; Martínez, B.; Sánchez, Y.; Pino Pérez, O. Efecto de aceites esenciales sobre cuatro cepas de Trichoderma asperellum Samuels. Rev. Protección Veg. 2013, 28, 153–157. [Google Scholar]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas—liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Peter, G. Waterman Identification of essential oil components by gas chromatography. Biochem. Syst. Ecol. 1996, 24, 594. [Google Scholar]
- Ormeño, E.; Céspedes, B.; Sánchez, I.A.; Velasco-García, A.; Moreno, J.M.; Fernandez, C.; Baldy, V. The relationship between terpenes and flammability of leaf litter. For. Ecol. Manag. 2009, 257, 471–482. [Google Scholar] [CrossRef]
- Rodríguez, Y.C.; Rodríguez, M.P.R.; Mesa, F.J.; Hernández, Y.C.; Becerra, L.W.M. Inflamabilidad de especies vegetales del ecosistema de pinares. Rev. Cuba. Cienc. For. 2016, 4, 36–47. [Google Scholar]
- Ganteaume, A. Does plant flammability differ between leaf and litter bed scale? Role of fuel characteristics and consequences for flammability assessment. Int. J. Wildl. Fire 2018, 27, 342–352. [Google Scholar] [CrossRef] [Green Version]
- Behm, A.L.; Duryea, M.L.; Long, A.J.; Zipperer, W.C. Flammability of native understory species in pine flatwood and hardwood hammock ecosystems and implications for the wildland-urban interface. Int. J. Wildl. Fire 2004, 13, 355–365. [Google Scholar] [CrossRef] [Green Version]
- Bianchi, L.O.; Oddi, F.J.; Muñoz, M.; Defossé, G.E. Comparison of leaf moisture content and ignition characteristics among native species and exotic conifers in Northwestern Patagonia, Argentina. For. Sci. 2019, 65, 375–386. [Google Scholar] [CrossRef]
- Popović, Z.; Bojović, S.; Marković, M.; Cerdà, A. Tree species flammability based on plant traits: A synthesis. Sci. Total Environ. 2021, 800, 149625. [Google Scholar] [CrossRef]
- Núñez-Regueira, L.; Rodríguez Añón, J.A.; Proupín Castiñeiras, J. Calorific values and flammability of forest species in Galicia. Coastal and hillside zones. Bioresour. Technol. 1996, 57, 283–289. [Google Scholar] [CrossRef]
- Ganteaume, A.; Marielle, J.; Corinne, L.M.; Thomas, C.; Laurent, B. Effects of vegetation type and fire regime on flammability of undisturbed litter in Southeastern France. For. Ecol. Manag. 2011, 261, 2223–2231. [Google Scholar] [CrossRef]
- Merino, A.; Chávez-Vergara, B.; Salgado, J.; Fonturbel, M.T.; García-Oliva, F.; Vega, J.A. Variability in the composition of charred litter generated by wildfire in different ecosystems. Catena 2015, 133, 52–63. [Google Scholar] [CrossRef]
- Núñez-Regueira, L.; Añón, J.A.R.; Castiñeiras, J.P. Design of risk index maps as a tool to prevent forest fires in the northern coast of Galicia (N.W. Spain). Bioresour. Technol. 1999, 69, 23–33. [Google Scholar] [CrossRef]
- Wyse, S.V.; Perry, G.L.W.; O’Connell, D.M.; Holland, P.S.; Wright, M.J.; Hosted, C.L.; Whitelock, S.L.; Geary, I.J.; Maurin, K.J.L.; Curran, T.J. A quantitative assessment of shoot flammability for 60 tree and shrub species supports rankings based on expert opinion. Int. J. Wildl. Fire 2016, 25, 466–477. [Google Scholar] [CrossRef] [Green Version]
- Wyse, S.V.; Perry, G.L.W.; Curran, T.J. Shoot-Level Flammability of Species Mixtures is Driven by the Most Flammable Species: Implications for Vegetation-Fire Feedbacks Favouring Invasive Species. Ecosystems 2018, 21, 886–900. [Google Scholar] [CrossRef] [Green Version]
- Dewhirst, R.A.; Smirnoff, N.; Belcher, C.M. Pine species that support crown fire regimes have lower leaf-level terpene contents than those native to surface fire regimes. Fire 2020, 3, 17. [Google Scholar] [CrossRef]
- Núñez-Regueira, L.; Proupín-Castiñeiras, J.; Rodríguez-Añón, J.A. Design of risk index maps as a tool to prevent forest fires in the hill-side zone of Galicia (NW Spain). Bioresour. Technol. 2000, 73, 123–131. [Google Scholar] [CrossRef]
- Pausas, J.G.; Alessio, G.A.; Moreira, B.; Segarra-Moragues, J.G. Secondary compounds enhance flammability in a Mediterranean plant. Oecologia 2016, 180, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Palma, A.; Díaz, M.J.; Ruiz-Montoya, M.; Morales, E.; Giráldez, I. Ultrasound extraction optimization for bioactive molecules from Eucalyptus globulus leaves through antioxidant activity. Ultrason. Sonochem. 2021, 76, 105654. [Google Scholar] [CrossRef]
- Luís, Â.; Duarte, A.; Gominho, J.; Domingues, F.; Duarte, A.P. Chemical composition, antioxidant, antibacterial and anti-quorum sensing activities of Eucalyptus globulus and Eucalyptus radiata essential oils. Ind. Crops Prod. 2016, 79, 274–282. [Google Scholar] [CrossRef]
- Ioannou, E.; Koutsaviti, A.; Tzakou, O.; Roussis, V. The genus Pinus: A comparative study on the needle essential oil composition of 46 pine species. Phytochem. Rev. 2014, 13, 741–768. [Google Scholar] [CrossRef]
- Mimoune, N.; Mimoune, D.; Yataghene, A. Chemical composition and antimicrobial activity of the essential oils of Pinus pinaster. J. Coast. Life Med. 2013, 1, 54–58. [Google Scholar] [CrossRef]
- Ganteaume, A.; Romero, B.; Fernandez, C.; Ormeño, E.; Lecareux, C. Volatile and semi-volatile terpenes impact leaf flammability: Differences according to the level of terpene identification. Chemoecology 2021, 31, 259–275. [Google Scholar] [CrossRef]
- O’Reilly-Wapstra, J.M.; Humphreys, J.R.; Potts, B.M. Stability of genetic-based defensive chemistry across life stages in a Eucalyptus species. J. Chem. Ecol. 2007, 33, 1876–1884. [Google Scholar] [CrossRef]
- Silvestre, A.J.D.; Cavaleiro, J.A.S.; Delmond, B.; Filliatre, C.; Bourgeois, G. Analysis of the variation of the essential oil composition of Eucalyptus globulus Labill. from Portugal using multivariate statistical analysis. Ind. Crops Prod. 1997, 6, 27–33. [Google Scholar] [CrossRef]
- Chandorkar, N.; Tambe, S.; Amin, P.; Madankar, C. A systematic and comprehensive review on current understanding of the pharmacological actions, molecular mechanisms, and clinical implications of the genus Eucalyptus. Phytomedicine Plus 2021, 1, 100089. [Google Scholar] [CrossRef]
- Panikar, S.; Shoba, G.; Arun, M.; Sahayarayan, J.J.; Usha Raja Nanthini, A.; Chinnathambi, A.; Alharbi, S.A.; Nasif, O.; Kim, H.J. Essential oils as an effective alternative for the treatment of COVID-19: Molecular interaction analysis of protease (Mpro) with pharmacokinetics and toxicological properties. J. Infect. Public Health 2021, 14, 601–610. [Google Scholar] [CrossRef]
- Asif, M.; Saleem, M.; Saadullah, M.; Yaseen, H.S.; Al Zarzour, R. COVID-19 and therapy with essential oils having antiviral, anti-inflammatory, and immunomodulatory properties. Inflammopharmacology 2020, 28, 1153–1161. [Google Scholar] [CrossRef]
- Cory, J.S.; Hoover, K. Plant-mediated effects in insect-pathogen interactions. Trends Ecol. Evol. 2006, 21, 278–286. [Google Scholar] [CrossRef]
- Owens, M.K.; Lin, C.D.; Taylor, C.A.; Whisenant, S.G. Seasonal patterns of plant flammability and monoterpenoid content in Juniperus ashei. J. Chem. Ecol. 1998, 24, 2115–2129. [Google Scholar] [CrossRef]
- Rocca, G.D.; Madrigal, J.; Marchi, E.; Michelozzi, M.; Moya, B.; Danti, R. Relevance of terpenoids on flammability of Mediterranean species: An experimental approach at a low radiant heat flux. IForest 2017, 10, 766–775. [Google Scholar] [CrossRef] [Green Version]
- Tarvainen, V.; Hakola, H.; Hellén, H.; Bäck, J.; Hari, P.; Kulmala, M. Temperature and light dependence of the VOC emissions of Scots pine. Atmos. Chem. Phys. 2005, 5, 989–998. [Google Scholar] [CrossRef] [Green Version]
- Chetehouna, K.; Barboni, T.; Zarguili, I.; Leoni, E.; Simeoni, A.; Fernandez-Pello, A.C. Investigation on the Emission of Volatile Organic Compounds from Heated Vegetation and Their Potential to Cause an Accelerating Forest Fire. Combust. Sci. Technol. 2009, 181, 1273–1288. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.-J.; Shu, L.-F.; Wang, Q.-H. Terpenoid emissions from heated needles of Pinus sylvestris and their potential influences on forest fires. Acta Ecol. Sin. 2012, 32, 33–37. [Google Scholar] [CrossRef]
- Mccaw, L.; Bannister, T.; Sullivan, A.; Tolhurst, K. Weather and fire behaviour during the Victorian bushfires of 7 February 2009. AGU Fall Meet. Abstr. 2009, 2009, A43E-02. [Google Scholar]
- De Lillis, M.; Bianco, P.M.; Loreto, F. The influence of leaf water content and isoprenoids on flammability of some Mediterranean woody species. Int. J. Wildl. Fire 2009, 18, 203–212. [Google Scholar] [CrossRef]
- Alessio, G.A.; Peñuelas, J.; Llusià, J.; Ogaya, R.; Estiarte, M.; De Lillis, M. Influence of water and terpenes on flammability in some dominant Mediterranean species. Int. J. Wildl. Fire 2008, 17, 274–286. [Google Scholar] [CrossRef]
- Ciccioli, P.; Centritto, M.; Loreto, F. Biogenic volatile organic compound emissions from vegetation fires. Plant Cell Environ. 2014, 37, 1810–1825. [Google Scholar] [CrossRef] [Green Version]
- Grote, R.; Niinemets, Ü. Modeling volatile isoprenoid emissions—A story with split ends. Plant Biol. 2008, 10, 8–28. [Google Scholar] [CrossRef]
- Boukhatem, M.N.; Ferhat, M.A.; Kameli, A.; Mekarnia, M. Eucalyptus globulus (Labill.): Un arbre à essence aux mille vertusEucalyptus globulus (Labill.): A perfume tree with several medicinal purposes. Phytothérapie 2017, S203–S214. [Google Scholar] [CrossRef]
- Ramos, M.P. Manejo Del Fuego; Editorial Félix Varela: Havana, Cuba, 2010; ISBN 978-959-07-1322-4. [Google Scholar]
- O’Reilly-Wapstra, J.; Bailey, J.; Mcarthur, C.; Potts, B. Genetic-and chemical-based resistance to two mammalian herbivores varies across the geographic range of Eucalyptus globulus. Evol. Ecol. Res. 2010, 12, 491–505. [Google Scholar]
- Youngentob, K.N.; Wallis, I.R.; Lindenmayer, D.B.; Wood, J.T.; Pope, M.L.; Foley, W.J. Foliage chemistry influences tree choice and landscape use of a gliding marsupial folivore. J. Chem. Ecol. 2011, 37, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Horner, J.D.; Gosz, J.R.; Cates, R.G. The Role of Carbon-Based Plant Secondary Metabolites in Decomposition in Terrestrial Ecosystems. Am. Nat. 1988, 132, 869–883. [Google Scholar] [CrossRef]
- McKiernan, A.B.; Hovenden, M.J.; Brodribb, T.J.; Potts, B.M.; Davies, N.W.; O’Reilly-Wapstra, J.M. Effect of limited water availability on foliar plant secondary metabolites of two Eucalyptus species. Environ. Exp. Bot. 2014, 105, 55–64. [Google Scholar] [CrossRef]
- Jarvis, B.B. Chapter One the Role of Natural Products in Evolution; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2000; Volume 34. [Google Scholar]
- Kobayashi, K. Factors affecting phytotoxic activity of allelochemicals in soil. Weed Biol. Manag. 2004, 4, 1–7. [Google Scholar] [CrossRef]
- Chaves, N. Variation of flavonoid synthesis induced by ecological factors. Princ. Pract. Plant Ecol. 1999, 267–285. [Google Scholar]
- Sandermann, H.; Ernst, D.; Heller, W.; Langebartels, C. Ozone: An abiotic elicitor of plant defence reactions. Trends Plant Sci. 1998, 3, 47–50. [Google Scholar] [CrossRef]
- Zobel, A.M.; Lynch, J.M. Extrusion of UV-absorbing phenolics in Acer spp. in response to UV and freezing temperature. I. UV A-absorbing compounds on the surface of Acer saccharum and Acer platanoides autumn leaves. Allelopath. J. 1997, 4, 269–276. [Google Scholar]
- Cuadra, P.; Harborne, J.B.; Waterman, P.G. Increases in surface flavonols and photosynthetic pigments in Gnaphalium luteo-album in response to UV-B radiation. Phytochemistry 1997, 45, 1377–1383. [Google Scholar] [CrossRef]
- Zobel, A.M.; Clarke, P.A.; Lynch, J.M. Production of phenolics in response to UV irradiation ahd heavy metals in seeding of Acer spp. Recent Adv. Allelopath. Serv. Publicaciones UCA 1999, 231–243. [Google Scholar]


| IT (s) | Fr (%) | ||||
|---|---|---|---|---|---|
| 100–95 | 94–90 | 89–85 | 84–80 | <50 | |
| <12.5 | 5 | 4 | 3 | 3 | 1 |
| 12.5–17.5 | 4 | 3 | 3 | 2 | 1 |
| 17.5–22.5 | 3 | 3 | 2 | 2 | 0 |
| 22.5–27.5 | 3 | 2 | 2 | 1 | 0 |
| 27.5–32.5 | 3 | 2 | 2 | 1 | 0 |
| >32.5 | 3 | 1 | 2 | 0 | 0 |
| Exotic Species | IT (s) | Fr (%) | FI (-) | Classification |
|---|---|---|---|---|
| A. melanoxylon | 9.86 ± 2.48 | 100 | 5 | Extremely flammable |
| E. globulus | 11.19 ± 3.71 | 100 | 5 | Extremely flammable |
| A. dealbata | 17.27 ± 5.92 | 100 | 4 | Very flammable |
| P. radiata | 17.47 ± 4.12 | 100 | 4 | Very flammable |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrero, F.; Carmona, C.; Hernández, C.; Toledo, M.; Arriagada, A.; Espinoza, L.; Bergmann, J.; Taborga, L.; Yañez, K.; Carrasco, Y.; et al. Drivers of Flammability of Eucalyptus globulus Labill Leaves: Terpenes, Essential Oils, and Moisture Content. Forests 2022, 13, 908. https://doi.org/10.3390/f13060908
Guerrero F, Carmona C, Hernández C, Toledo M, Arriagada A, Espinoza L, Bergmann J, Taborga L, Yañez K, Carrasco Y, et al. Drivers of Flammability of Eucalyptus globulus Labill Leaves: Terpenes, Essential Oils, and Moisture Content. Forests. 2022; 13(6):908. https://doi.org/10.3390/f13060908
Chicago/Turabian StyleGuerrero, Fabián, Camilo Carmona, Carla Hernández, Mario Toledo, Andrés Arriagada, Lorena Espinoza, Jan Bergmann, Lautaro Taborga, Karen Yañez, Yulián Carrasco, and et al. 2022. "Drivers of Flammability of Eucalyptus globulus Labill Leaves: Terpenes, Essential Oils, and Moisture Content" Forests 13, no. 6: 908. https://doi.org/10.3390/f13060908
APA StyleGuerrero, F., Carmona, C., Hernández, C., Toledo, M., Arriagada, A., Espinoza, L., Bergmann, J., Taborga, L., Yañez, K., Carrasco, Y., & Muñoz, A. A. (2022). Drivers of Flammability of Eucalyptus globulus Labill Leaves: Terpenes, Essential Oils, and Moisture Content. Forests, 13(6), 908. https://doi.org/10.3390/f13060908







