Habitat Significantly Affect CWD Decomposition but No Home-Field Advantage of the Decomposition Found in a Subtropical Forest, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling Design
2.3. Sample Collection and Processing
2.4. Measurements of RCWD
2.5. Determination of Sample C, N, P, Cellulose, Lignin Content, and PLFA
2.6. Data Analysis
3. Results
3.1. RCWD, Substrate Quality of CWD, and Soil Properties
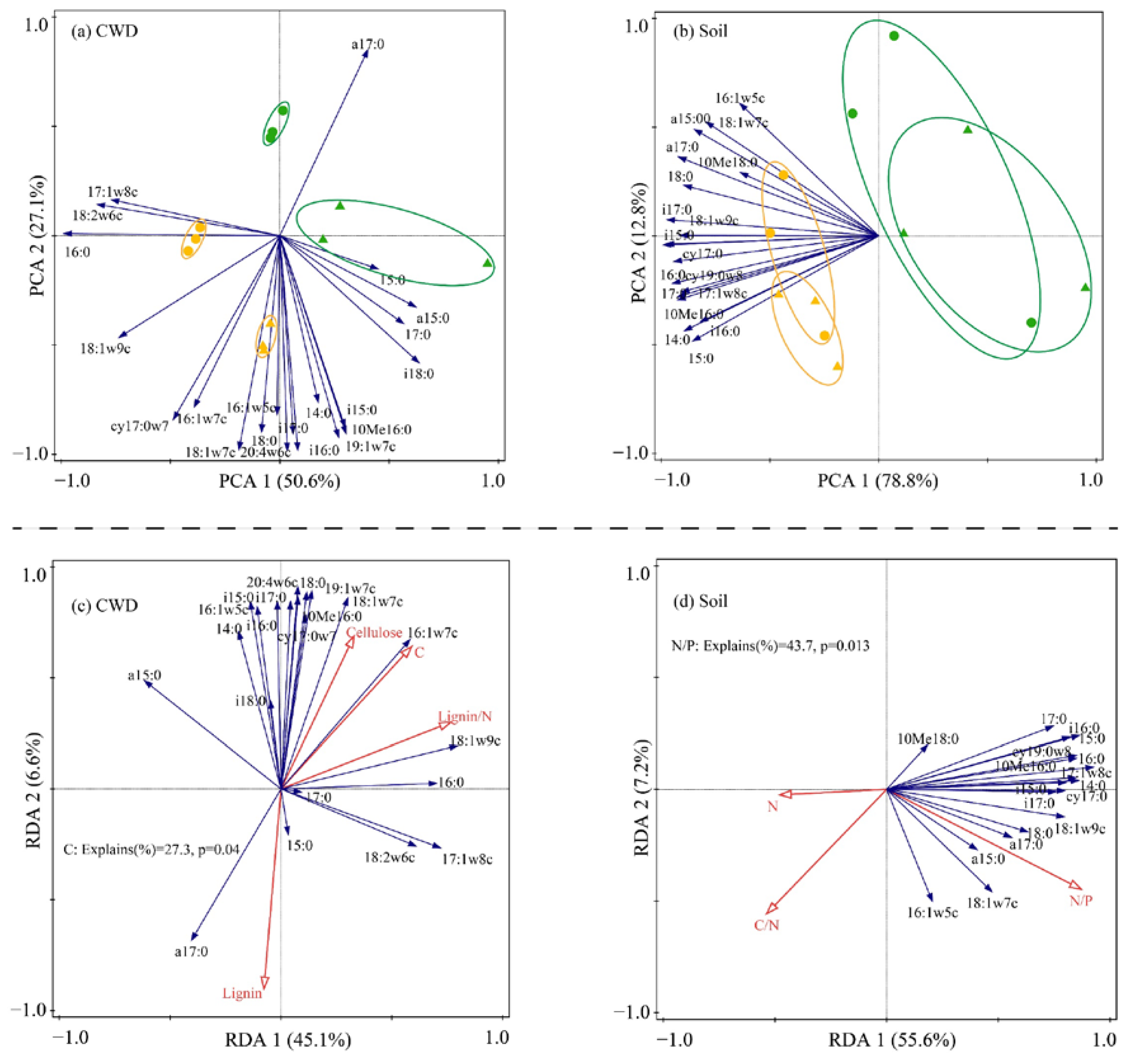
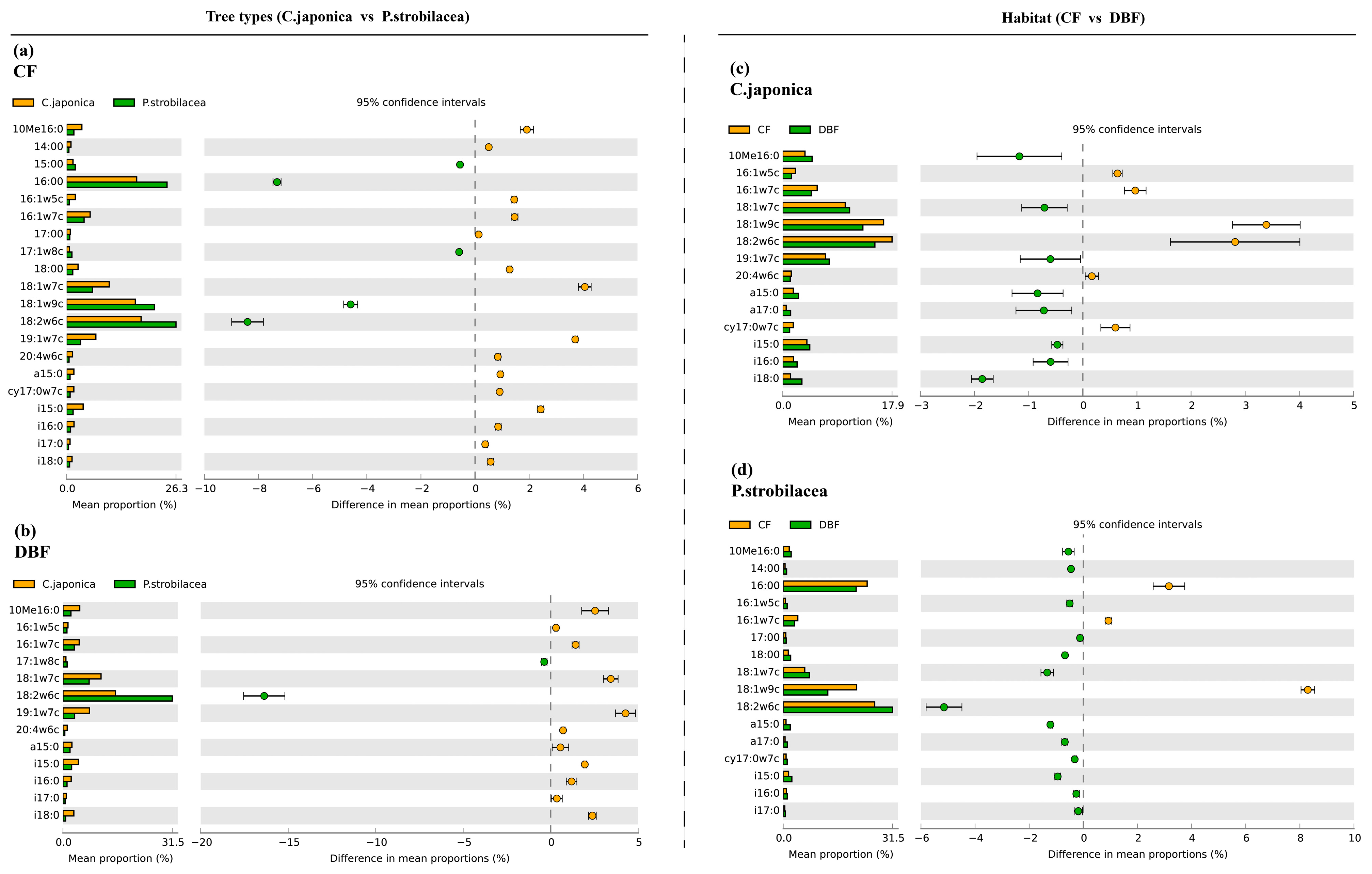
3.2. PLFA Analysis
3.3. Relationships between Respiration of CWD and Influencing Factors
4. Discussion
4.1. Home-Field Advantage of CWD Decomposition
4.2. Decomposer Community Composition
4.3. Fungi Drive CWD Decomposition
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eaton, J.M.; Lawrence, D. Woody debris stocks and fluxes during succession in a dry tropical forest. For. Ecol. Manag. 2006, 232, 46–55. [Google Scholar] [CrossRef]
- Harmon, M.E.; Franklin, J.F.; Swanson, F.J.; Sollins, P.; Gregory, S.; Lattin, J.; Anderson, N.; Cline, S.; Aumen, N.; Sedell, J.R. Ecology of coarse woody debris in temperate ecosystems. Adv. Ecol. Res. 1986, 15, 133–302. [Google Scholar]
- Hicks, W.T.; Harmon, M.E.; Myrold, D.D. Substrate controls on nitrogen fixation and respiration in woody debris from the Pacific Northwest, USA. For. Ecol. Manag. 2003, 176, 25–35. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [Green Version]
- Pregitzer, K.S.; Euskirchen, E.S. Carbon cycling and storage in world forests: Biome patterns related to forest age. Glob. Chang. Biol. 2004, 10, 2052–2077. [Google Scholar] [CrossRef]
- Chambers, J.Q.; Schimel, J.P.; Nobre, A.D. Respiration from coarse wood litter in central Amazon forests. Biogeochemistry 2001, 52, 115–131. [Google Scholar] [CrossRef]
- Purahong, W.; Kahl, T.; Kruger, D.; Buscot, F.; Hoppe, B. Home-Field Advantage in Wood Decomposition Is Mainly Mediated by Fungal Community Shifts at “Home” Versus “Away”. Microb. Ecol. 2019, 78, 725–736. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, Z.; Wang, H.; Huang, G.; Shu, C.; Kong, F.; Zhang, Y.; Geoff Wang, G.; Liu, Y. Home-field advantage of CWD decomposition in subtropical forests varied by field sites. For. Ecol. Manag. 2019, 444, 127–137. [Google Scholar] [CrossRef]
- Ayres, E.; Steltzer, H.; Simmons, B.L.; Simpson, R.T.; Steinweg, J.M.; Wallenstein, M.D.; Mellor, N.; Parton, W.J.; Moore, J.C.; Wall, D.H. Home-field advantage accelerates leaf litter decomposition in forests. Soil Biol. Biochem. 2009, 41, 606–610. [Google Scholar] [CrossRef]
- Gholz, H.L.; Wedin, D.A.; Smitherman, S.M.; Harmon, M.E.; Parton, W.J. Long-term dynamics of pine and hardwood litter in contrasting environments: Toward a global model of decomposition. Glob. Chang. Biol. 2000, 6, 751–765. [Google Scholar] [CrossRef]
- Asplund, J.; Kauserud, H.; Bokhorst, S.; Lie, M.H.; Ohlson, M.; Nybakken, L. Fungal communities influence decomposition rates of plant litter from two dominant tree species. Fungal Ecol. 2018, 32, 1–8. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, S. Leaf litter decomposition in urban forests: Test of the home-field advantage hypothesis. Ann. For. Sci. 2016, 73, 1063–1072. [Google Scholar] [CrossRef]
- Hoppe, B.; Purahong, W.; Wubet, T.; Kahl, T.; Bauhus, J.; Arnstadt, T.; Hofrichter, M.; Buscot, F.; Kruger, D. Linking molecular deadwood-inhabiting fungal diversity and community dynamics to ecosystem functions and processes in Central European forests. Fungal Divers. 2016, 77, 367–379. [Google Scholar] [CrossRef]
- Binkley, D.; Giardina, C. Why do Tree Species Affect Soils? The Warp and Woof of Tree-soil Interactions. Biogeochemistry 1998, 42, 89–106. [Google Scholar] [CrossRef]
- Ushio, M.; Kitayama, K.; Balser, T.C. Tree species effects on soil enzyme activities through effects on soil physicochemical and microbial properties in a tropical montane forest on Mt. Kinabalu, Borneo. Pedobiologia 2010, 53, 227–233. [Google Scholar] [CrossRef]
- Lamit, L.J.; Busby, P.E.; Lau, M.K.; Compson, Z.G.; Wojtowicz, T.; Keith, A.R.; Zinkgraf, M.S.; Schweitzer, J.A.; Shuster, S.M.; Gehring, C.A.; et al. Tree genotype mediates covariance among communities from microbes to lichens and arthropods. J. Ecol. 2015, 103, 840–850. [Google Scholar] [CrossRef] [Green Version]
- Purahong, W.; Arnstadt, T.; Kahl, T.; Bauhus, J.; Kellner, H.; Hofrichter, M.; Krüger, D.; Buscot, F.; Hoppe, B. Are correlations between deadwood fungal community structure, wood physico-chemical properties and lignin-modifying enzymes stable across different geographical regions? Fungal Ecol. 2016, 22, 98–105. [Google Scholar] [CrossRef] [Green Version]
- Van der Wal, A.; Ottosson, E.; De Boer, W. Neglected role of fungal community composition in explaining variation in wood decay rates. Ecology 2015, 96, 124–133. [Google Scholar] [CrossRef] [Green Version]
- Fanin, N.; Fromin, N.; Bertrand, I. Functional breadth and home-field advantage generate functional differences among soil microbial decomposers. Ecology 2016, 97, 1023–1037. [Google Scholar] [CrossRef]
- de Graaff, M.A.; Classen, A.T.; Castro, H.F.; Schadt, C.W. Labile soil carbon inputs mediate the soil microbial community composition and plant residue decomposition rates. New Phytol. 2010, 188, 1055–1064. [Google Scholar] [CrossRef] [Green Version]
- Hunt, H.; Coleman, D.; Ingham, E.; Ingham, R.; Elliott, E.; Moore, J.; Rose, S.; Reid, C.; Morley, C.J.B.; Soils, F.O. The detrital food web in a shortgrass prairie. Biol. Fertil. Soils 1987, 3, 57–68. [Google Scholar] [CrossRef]
- Paterson, E.; Osler, G.; Dawson, L.A.; Gebbing, T.; Sim, A.; Ord, B. Labile and recalcitrant plant fractions are utilised by distinct microbial communities in soil: Independent of the presence of roots and mycorrhizal fungi. Soil Biol. Biochem. 2008, 40, 1103–1113. [Google Scholar] [CrossRef]
- Liu, X.Z.; Wang, L. Scientific Survey and Study of Biodiversity on the Lushan Nature Reserve in Jiangxi Province; Science Press: Beijing, China, 2010. [Google Scholar]
- Bao, S.D. Agriculture Chemistry Analysis of Soil; China Agriculture Press: Beijing, China, 1999. [Google Scholar]
- Bossio, D.A.; Scow, K.M. Impacts of Carbon and Flooding on Soil Microbial Communities: Phospholipid Fatty Acid Profiles and Substrate Utilization Patterns. Microb. Ecol. 1998, 35, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Pietri, J.C.A.; Brookes, P.C. Substrate inputs and pH as factors controlling microbial biomass, activity and community structure in an arable soil. Soil Biol. Biochem. 2009, 41, 1396–1405. [Google Scholar] [CrossRef]
- Zelles, L.J. Fatty acid patterns of phospholipids and lipopolysaccharides in the characterisation of microbial communities in soil: A review. Biol. Fertil. Soils 1999, 29, 111–129. [Google Scholar] [CrossRef]
- Frostegård, A.; Bååth, E. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol. Fertil. Soils 1996, 22, 59–65. [Google Scholar] [CrossRef]
- Frostegård, Å.; Bååth, E.; Tunlio, A. Shifts in the structure of soil microbial communities in limed forests as revealed by phospholipid fatty acid analysis. Soil Biol. Biochem. 1993, 25, 723–730. [Google Scholar] [CrossRef]
- Olsson, P. A Signature fatty acids provide tools for determination of the distribution and interactions of mycorrhizal fungi in soil. FEMS Microbiol. Ecol. 1999, 29, 303–310. [Google Scholar] [CrossRef]
- Wang, C.; Lu, X.K.; Mori, T.; Mao, Q.G.; Zhou, K.J.; Zhou, G.Y.; Nie, Y.X.; Mo, J.M. Responses of soil microbial community to continuous experimental nitrogen additions for 13 years in a nitrogen-rich tropical forest. Soil Biol. Biochem. 2018, 121, 103–112. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Wang, C.; Gower, S.T. Annual carbon flux from woody debris for a boreal black spruce fire chronosequence. J. Geophys. Res. 2002, 108, WFX-1. [Google Scholar] [CrossRef]
- Freschet, G.T.; Aerts, R.; Cornelissen, J.H.C. Multiple mechanisms for trait effects on litter decomposition: Moving beyond home-field advantage with a new hypothesis. J. Ecol. 2012, 100, 619–630. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Cornelissen, J.H.; Amatangelo, K.; Dorrepaal, E.; Eviner, V.T.; Godoy, O.; Hobbie, S.E.; Hoorens, B.; Kurokawa, H.; Perez-Harguindeguy, N.; et al. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol. Lett. 2008, 11, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Santiago, L.S. Extending the leaf economics spectrum to decomposition: Evidence from a tropical forest. Ecology 2007, 88, 1126–1131. [Google Scholar] [CrossRef] [PubMed]
- Weedon, J.T.; Cornwell, W.K.; Cornelissen, J.H.; Zanne, A.E.; Wirth, C.; Coomes, D.A.J.E.L. Global meta-analysis of wood decomposition rates: A role for trait variation among tree species? Ecol. Lett. 2009, 12, 45–56. [Google Scholar] [CrossRef] [PubMed]
- John, M.G.S.; Orwin, K.H.; Dickie, I.A. No ‘home’ versus ‘away’ effects of decomposition found in a grassland–forest reciprocal litter transplant study. Soil Biol. Biochem. 2011, 43, 1482–1489. [Google Scholar] [CrossRef]
- Luo, D.; Cheng, R.M.; Shi, Z.M.; Wang, W.X. Decomposition of Leaves and Fine Roots in Three Subtropical Plantations in China Affected by Litter Substrate Quality and Soil Microbial Community. Forests 2017, 8, 412. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Gundersen, P.; Zhang, T.; Mo, J. Effects of phosphorus addition on soil microbial biomass and community composition in three forest types in tropical China. Soil Biol. Biochem. 2012, 44, 31–38. [Google Scholar] [CrossRef]
- Yu, L.; Wang, Y.; Wang, Y.; Sun, S.; Liu, L. Quantifying components of soil respiration and their response to abiotic factors in two typical subtropical forest stands, southwest China. PLoS ONE 2015, 10, e0117490. [Google Scholar] [CrossRef] [Green Version]
- Mäkipää, R.; Rajala, T.; Schigel, D.; Rinne, K.T.; Pennanen, T.; Abrego, N.; Ovaskainen, O. Interactions between soil- and dead wood-inhabiting fungal communities during the decay of Norway spruce logs. ISME J. 2017, 11, 1964–1974. [Google Scholar] [CrossRef] [Green Version]
- Güsewell, S.; Gessner, M.O. N:P ratios influence litter decomposition and colonization by fungi and bacteria in microcosms. Funct. Ecol. 2009, 23, 211–219. [Google Scholar] [CrossRef]
- Johnston, E.R.; Kim, M.; Hatt, J.K.; Phillips, J.R.; Yao, Q.; Song, Y.; Hazen, T.C.; Mayes, M.A.; Konstantinidis, K.T. Phosphate addition increases tropical forest soil respiration primarily by deconstraining microbial population growth. Soil Biol. Biochem. 2019, 130, 43–54. [Google Scholar] [CrossRef]
- Fukasawa, Y.; Hyodo, F.; Kawakami, S. Foraging association between myxomycetes and fungal communities on coarse woody debris. Soil Biol. Biochem. 2018, 121, 95–102. [Google Scholar] [CrossRef]
- Lin, G.G.; Chen, Z.X.; Zeng, D.H. Presence of Mycorrhizal Fungal Hyphae Rather than Living Roots Retards Root Litter Decomposition. Forests 2019, 10, 502. [Google Scholar] [CrossRef] [Green Version]
- Arnstadt, T.; Hoppe, B.; Kahl, T.; Kellner, H.; Kruger, D.; Bassler, C.; Bauhus, J.; Hofrichter, M. Patterns of laccase and peroxidases in coarse woody debris of Fagus sylvatica, Picea abies and Pinus sylvestris and their relation to different wood parameters. Eur. J. For. Res. 2016, 135, 109–124. [Google Scholar] [CrossRef] [Green Version]
- Greaves, H. The bacterial factor in wood decay. Wood Sci. Technol. 1971, 5, 6–16. [Google Scholar] [CrossRef]
- Kielak, A.M.; Scheublin, T.R.; Mendes, L.W.; van Veen, J.A.; Kuramae, E.E. Bacterial Community Succession in Pine-Wood Decomposition. Front. Microbiol. 2016, 7, 231. [Google Scholar] [CrossRef] [Green Version]
- Hoppe, B.; Kahl, T.; Karasch, P.; Wubet, T.; Bauhus, J.; Buscot, F.; Kruger, D. Network Analysis Reveals Ecological Links between N-Fixing Bacteria and Wood-Decaying Fungi. PLoS ONE 2014, 9, e88141. [Google Scholar] [CrossRef] [Green Version]
- Robie, J.; White, D. Lipid analysis in microbial ecology: Quantitative approaches to the study of microbial communities. Bioscience 1989, 39, 535–541. [Google Scholar] [CrossRef]
- Weißhaupt, P.; Pritzkow, W.; Noll, M. Nitrogen metabolism of wood decomposing basidiomycetes and their interaction with diazotrophs as revealed by IRMS. Int. J. Mass Spectrom. 2011, 307, 225–231. [Google Scholar] [CrossRef]
- Purahong, W.; Stempfhuber, B.; Lentendu, G.; Francioli, D.; Reitz, T.; Buscot, F.; Schloter, M.; Kruger, D. Influence of Commonly Used Primer Systems on Automated Ribosomal Intergenic Spacer Analysis of Bacterial Communities in Environmental Samples. PLoS ONE 2015, 10, e0118967. [Google Scholar] [CrossRef] [Green Version]


| Study Areas | Longitude | Latitude | Average Altitude | Slope in Degrees | Slope Position | Tree Species (n) | Average Diameter | Average Length | Average Density |
|---|---|---|---|---|---|---|---|---|---|
| CF | 115°57′ E | 29°30′ N | 843 m | 37° | Northwest | C. japonica (3) | 16.5 cm | 148.2 cm | 0.4 g cm−3 |
| P. strobilacea (3) | 15.4 cm | 152.2 cm | 0.5 g cm−3 | ||||||
| DBF | 115°59′ E | 29°30′ N | 845 m | 36° | Northwest | C. japonica (3) | 16.1 cm | 152.7 cm | 0.4 g cm−3 |
| P. strobilacea (3) | 15.3 cm | 146.7 cm | 0.5 g cm−3 |
| Factor | The Substrate Quality of CWD | ||||||
|---|---|---|---|---|---|---|---|
| C (g kg−1) | N (g kg−1) | Cel (%) | Lig (%) | C/N | Lig/N | ||
| C. japonica | Initial values | 383.60 b | 4.48 b | 37.98 a | 18.09 b | 85.58 a | 40.38 b |
| P. strobilacea | Initial values | 421.80 a | 4.73 b | 35.30 b | 21.24 a | 89.27 b | 44.80 a |
| After decomposition | |||||||
| C. japonica | CF | 360.6 (10.4) a | 4.12 (0.3) b | 30.2 (1.7) a | 22.3 (1.2) b | 87.5 (11.8) a | 54.4 (5.2) ab |
| DBF | 331.5 (27.4) ab | 4.81 (1.4) b | 25.8 (1.5) ab | 24.5 (2.1) ab | 68.9 (10.4) a | 54.7 (16.8) ab | |
| P. strobilacea | CF | 353.4 (31.2) a | 3.64 (0.4) b | 27.2 (2.5) ab | 24.8 (0.4) ab | 97.1 (16.4) a | 67.9 (10.5) a |
| DBF | 309.2 (13.5) b | 7.69 (2.6) a | 22.0 (6.3) b | 27.5 (2.1) a | 40.2 (7.32) b | 37.8 (9.1) b | |
| Factor | The Soil Properties | ||||||
|---|---|---|---|---|---|---|---|
| C (g kg−1) | N (g kg−1) | P (g kg−1) | C/N | C/P | N/P | ||
| Initial values | CF | 42.33 b | 1.79 b | 0.27 b | 23.65 b | 156.78 b | 6.63 b |
| Initial values | DBF | 51.21 a | 2.02 b | 0.31 b | 25.35 a | 165.19 b | 6.52 b |
| After decomposition | |||||||
| C. japonica | CF | 53.71 (3.97) b | 3.37 (0.65) a | 0.58 (0.04) bc | 16.35 (3.31) b | 93.28 (13.4) b | 5.85 (1.23) ab |
| DBF | 89.76 (3.27) a | 3.96 (0.33) a | 0.87 (0.01) a | 22.7 (1.30) ab | 103.6 (4.76) b | 4.57 (0.39) b | |
| P. strobilacea | CF | 62.53 (11.7) b | 2.93 (0.29) a | 0.44 (0.05) c | 21.2 (1.82) ab | 139.8 (10.3) a | 6.59 (0.31) a |
| DBF | 87.06 (5.88) a | 3.59 (0.78) a | 0.70 (0.17) ab | 25.23 (6.94) a | 131.5 (16.8) a | 5.18 (0.76) ab | |
| Factor | The Microbial Community of CWD | The Microbial Community of Soil | ||||||
|---|---|---|---|---|---|---|---|---|
| F | df | p | % | F | df | p | % | |
| Habitat | 42.8 | 1 | 0.002 | 43.4 | 1.42 | 1 | 0.007 | 56.4 |
| Tree types | 35.1 | 1 | 0.003 | 35.7 | 12.6 | 1 | 0.262 | 6.37 |
| Habitat*types | 12.6 | 1 | 0.005 | 12.8 | 0.33 | 1 | 0.686 | 1.47 |
| Factor | The Microbial Community Structure of CWD | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Bacteria | Fungi | GP | GN | Actino | B/F | GP/GN | ||
| PLFAs (nmol g−1) | |||||||||
| C. japonica | CF | 110.3 (5.2) b | 61.7 (2.8) a | 34.6 (1.5) b | 11.3 (0.6) a | 27.5 (1.3) a | 5.1 (0.3) a | 1.8 (0.03) b | 0.4 (0.01) a |
| DBF | 81.5 (5.1) c | 47.5 (2.8) b | 17.9 (1.9) c | 11.0 (0.6) a | 20.0 (1.2) b | 4.5 (0.1) b | 2.7 (0.1) a | 0.5 (0.02) a | |
| P. strobilacea | CF | 152.4 (10.5) a | 63.7 (3.1) a | 57.3 (3.2) a | 7.4 (0.3) b | 20.5 (0.9) b | 3.1 (0.1) d | 1.1 (0.01) d | 0.4 (0.01) a |
| DBF | 89.9 (4.4) c | 42.5 (2.8) b | 30.8 (2.0) b | 7.9 (0.4) b | 14.8 (1.0) c | 2.4 (0.3) c | 1.4 (0.02) c | 0.5 (0.01) a | |
| Relative abundance (%) | |||||||||
| C. japonica | CF | 55.9 (0.2) b | 29.6 (0.5) c | 10.3 (0.1) b | 24.8 (0.1) a | 4.6 (0.1) b | |||
| DBF | 58.3 (0.5) a | 20.9 (1.0) d | 13.5 (0.5) a | 24.5 (0.1) a | 5.5 (0.4) a | ||||
| P. strobilacea | CF | 41.8 (0.9) d | 37.1 (0.5) a | 4.9 (0.2) d | 13.5 (0.5) c | 2.0 (0.1) c | |||
| DBF | 47.2 (0.8) c | 33.3 (0.6) b | 8.8 (0.1) c | 16.4 (0.3) b | 2.7 (0.2) c | ||||
| Factor | The Microbial Community Structure of Soil | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Bacteria | Fungi | GP | GN | Actino | B/F | GP/GN | ||
| PLFAs (nmol g−1) | |||||||||
| C. japonica | CF | 31.2 (2.4) a | 22.2 (1.8) ab | 2.8 (0.2) a | 6.8 (0.5) a | 9.7 (1.2) ab | 2.9 (0.2) a | 8.1 (0.4) a | 0.7 (0.03) a |
| DBF | 19.8 (4.4) b | 13.7 (3.3) c | 1.9 (0.4) b | 4.2 (1.3) b | 5.9 (1.5) c | 2.1 (0.4) b | 7.2 (0.9) ab | 0.7 (0.08) a | |
| P. strobilacea | CF | 32.7 (2.6) a | 23.4 (2.2) a | 2.9 (0.1) a | 7.0 (0.6) a | 10.7 (1.7) a | 2.9 (0.1) a | 8.0 (0.6) a | 0.7 (0.06) a |
| DBF | 23.4 (5.5) b | 16.3 (4.4) bc | 2.7 (0.6) a | 4.8 (1.5) b | 7.1 (2.5) bc | 2.0 (0.4) b | 6.0 (0.8) b | 0.7 (0.05) a | |
| Relative abundance (%) | |||||||||
| C. japonica | CF | 70.9 (0.5) a | 7.1 (0.2) a | 21.7 (0.3) a | 31.1 (1.6) a | 9.2 (0.2) a | |||
| DBF | 69.2 (2.1) a | 7.6 (1.0) a | 21.1 (1.3) a | 29.9 (1.8) a | 10.5 (1.1) a | ||||
| P. strobilacea | CF | 71.3 (1.2) a | 7.0 (0.5) a | 21.4 (0.5) a | 32.6 (2.7) a | 9.1 (0.6) a | |||
| DBF | 69.6 (3.4) a | 8.2 (1.4) a | 20.1 (1.7) a | 30.1 (4.4) a | 8.7 (1.5) a | ||||
| Pearson-Correlations | Microbial PLFAs of CWD (nmol g−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Bacterial | Fungi | GP | GN | Actino | B/F | GP/GN | ||
| Microbial PLFAs of soil (nmol g−1) | Total | 0.68 * | 0.66 * | 0.69 * | −0.28 | 0.40 | 0.06 | −0.81 ** | −0.58 * |
| Bacterial | 0.67 * | 0.64 * | 0.68 * | −0.29 | 0.39 | 0.06 | −0.80 ** | −0.58 | |
| Fungi | 0.51 | 0.32 | 0.62 * | −0.50 | 0.01 | −0.29 | −0.52 | −0.73 ** | |
| GP | 0.63 * | 0.64 * | 0.64 * | −0.23 | 0.43 | 0.13 | −0.78 ** | −0.50 | |
| GN | 0.63 * | 0.57 | 0.66 * | −0.33 | 0.31 | 0.01 | −0.76 ** | −0.56 | |
| Actino | 0.67 * | 0.72 ** | 0.63 * | −0.13 | 0.52 | 0.22 | −0.81 ** | −0.40 | |
| B/F | −0.16 | 0.05 | −0.25 | 0.43 | 0.27 | 0.41 | 0.13 | 0.39 | |
| GP/GN | 0.46 | 0.68 * | 0.35 | 0.19 | 0.66 * | 0.50 | −0.68 * | −0.02 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zhang, L.; Deng, W.; Liu, J.; Wu, C.; Zhang, Y.; Liu, Y. Habitat Significantly Affect CWD Decomposition but No Home-Field Advantage of the Decomposition Found in a Subtropical Forest, China. Forests 2022, 13, 924. https://doi.org/10.3390/f13060924
Wang H, Zhang L, Deng W, Liu J, Wu C, Zhang Y, Liu Y. Habitat Significantly Affect CWD Decomposition but No Home-Field Advantage of the Decomposition Found in a Subtropical Forest, China. Forests. 2022; 13(6):924. https://doi.org/10.3390/f13060924
Chicago/Turabian StyleWang, Hankun, Ling Zhang, Wenping Deng, Junping Liu, Chunsheng Wu, Yi Zhang, and Yuanqiu Liu. 2022. "Habitat Significantly Affect CWD Decomposition but No Home-Field Advantage of the Decomposition Found in a Subtropical Forest, China" Forests 13, no. 6: 924. https://doi.org/10.3390/f13060924
APA StyleWang, H., Zhang, L., Deng, W., Liu, J., Wu, C., Zhang, Y., & Liu, Y. (2022). Habitat Significantly Affect CWD Decomposition but No Home-Field Advantage of the Decomposition Found in a Subtropical Forest, China. Forests, 13(6), 924. https://doi.org/10.3390/f13060924






