Oak Decline Syndrome in Korean Forests: History, Biology, and Prospects for Korean Oak Wilt
Abstract
:1. Introduction
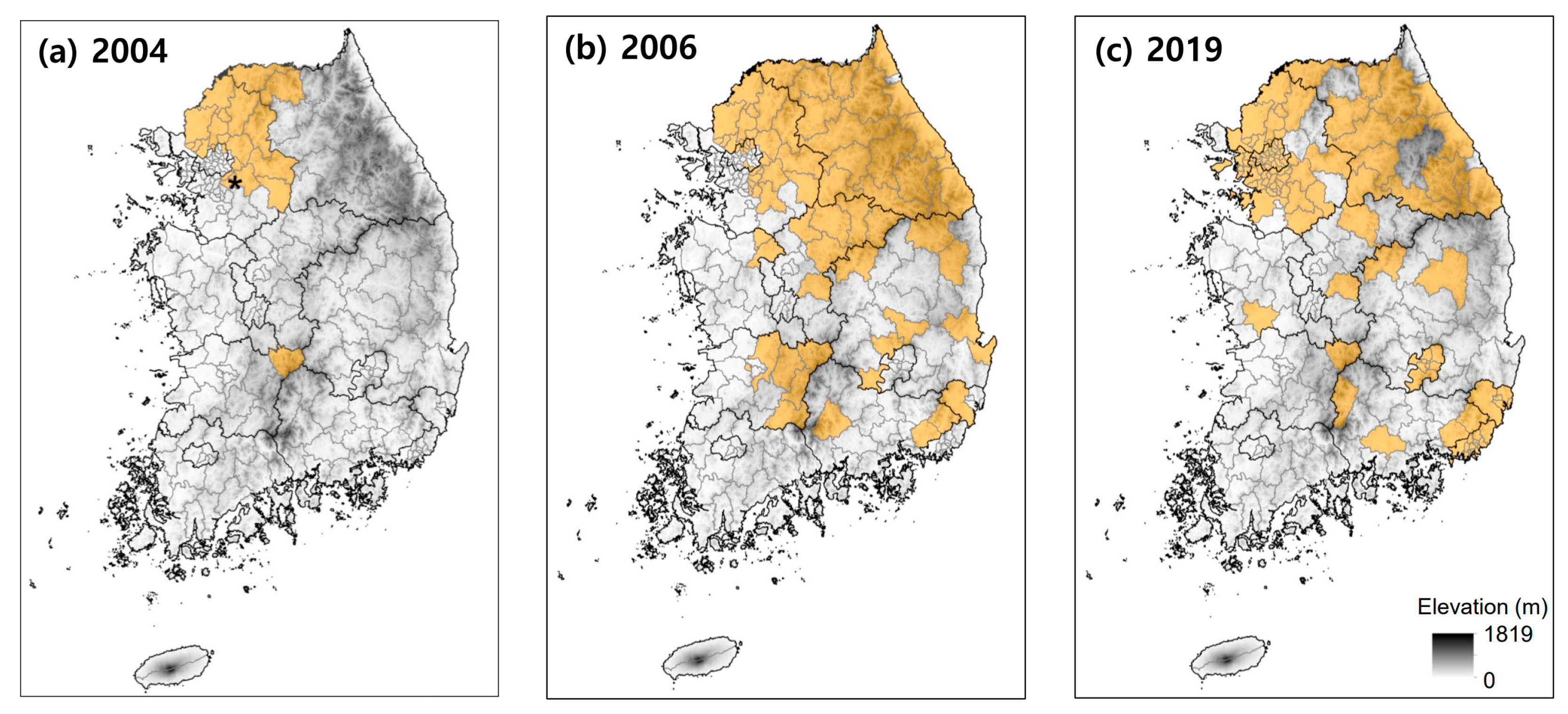
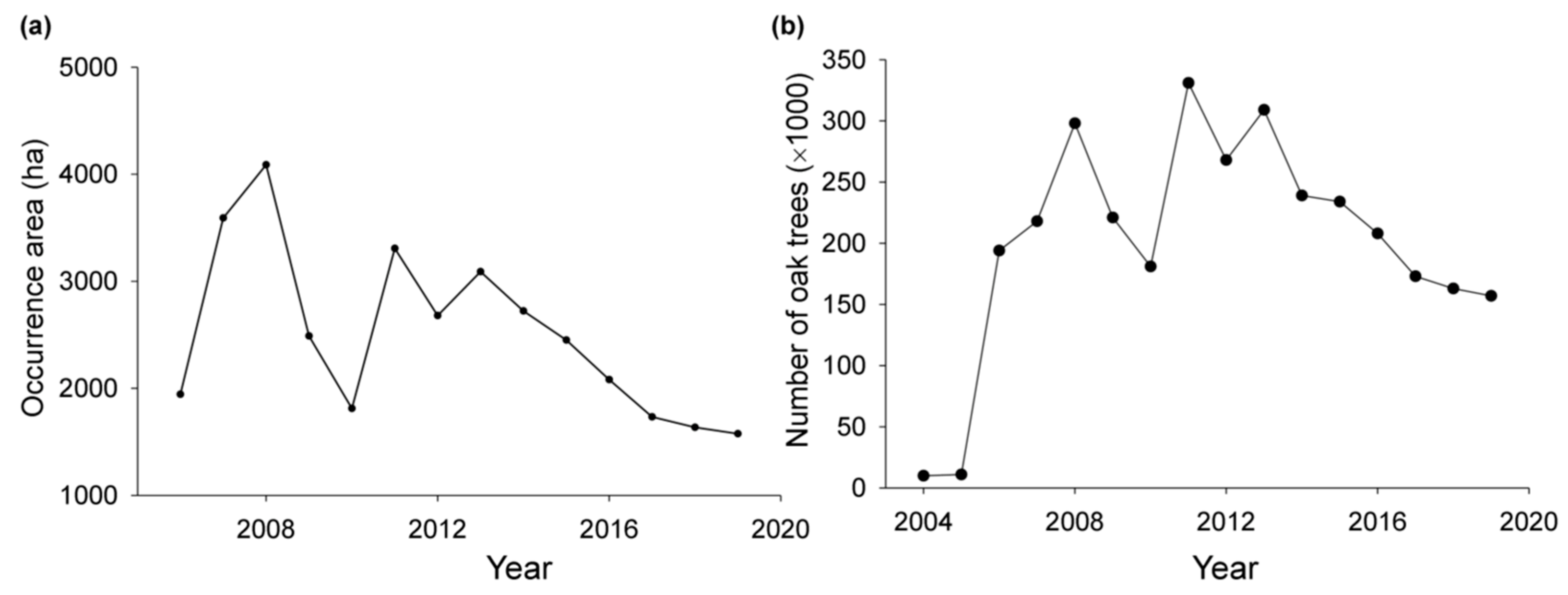
2. History of Korean Oak Wilt (KOW)
3. Disease Cycle of KOW
3.1. Host and Symptom
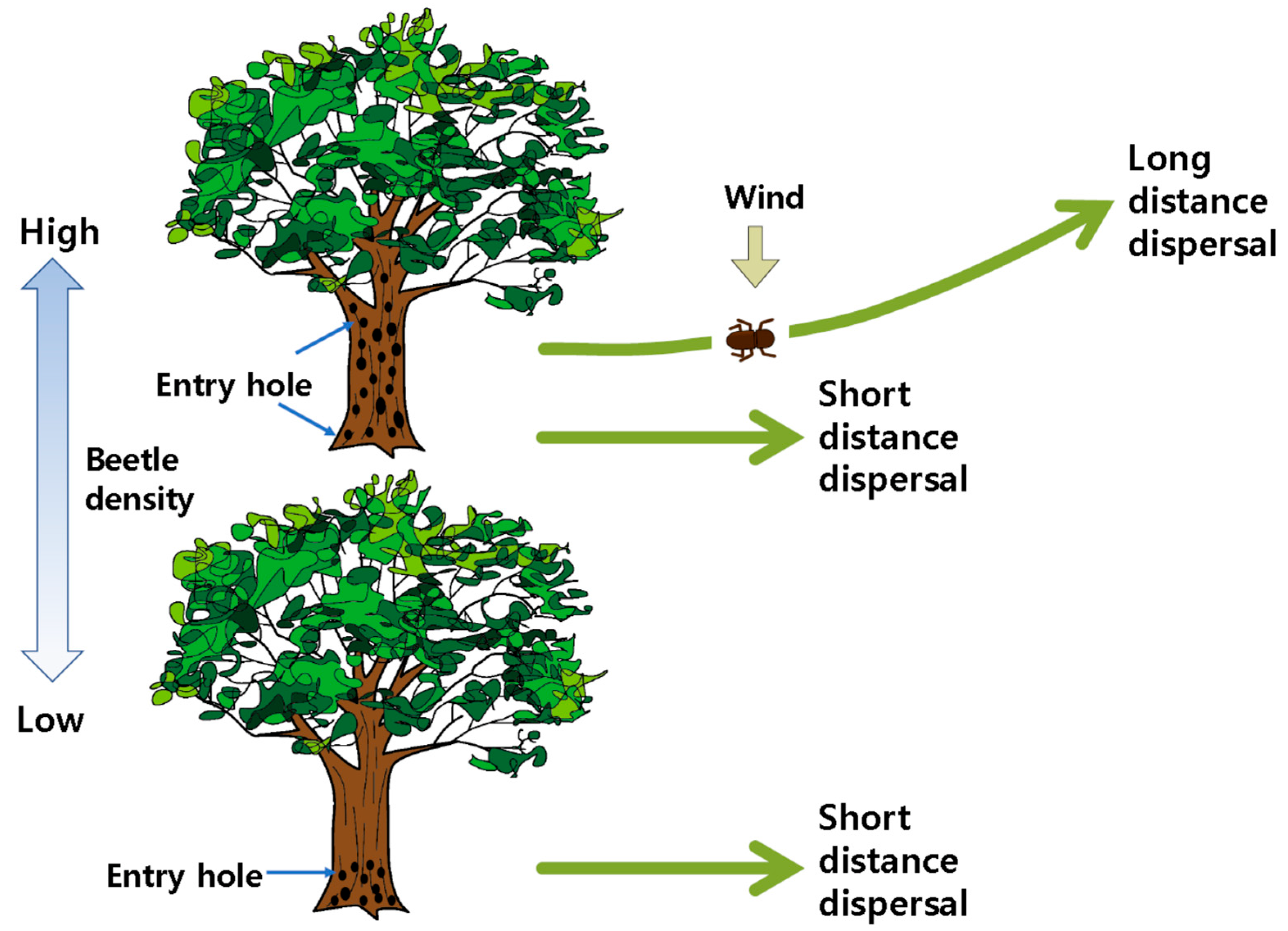
3.2. Vector Beetle
3.3. Fungus as the Causal Agent of KOW
3.4. Yeast
4. Management of KOW
5. Causes of KOW
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haavik, L.J.; Billings, S.A.; Guldin, J.M.; Stephen, F.M. Emergent insects, pathogens and drought shape changing patterns in oak decline in North America and Europe. For. Ecol. Manag. 2015, 354, 190–205. [Google Scholar] [CrossRef]
- Gottschalk, K.W.; Wargo, P.M. Oak decline around the world. In Proceedings, US Department of Agriculture Interagency Gypsy Moth Research Forum 1996, 16–19 January 1996; Annapolis, MD. Gen. Tech. Rep. NE-230; Fosbroke, S.L.C., Gottschalk, K.W., Eds.; US Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Radnor, PA, USA, 1997; pp. 3–13. [Google Scholar]
- Burlacu, E.; Nisca, A.; Tanase, C. A comprehensive review of phytochemistry and biological activities of Quercus species. Forests 2020, 11, 904. [Google Scholar] [CrossRef]
- Thomas, F.M.; Blank, R.; Hartmann, G. Abiotic and biotic factors and their interactions as causes of oak decline in Central Europe. For. Pathol. 2002, 32, 277–307. [Google Scholar] [CrossRef]
- Pinna, C.; Linaldeddu, B.T.; Deiana, V.; Maddau, L.; Montecchio, L.; Lentini, A. Plant pathogenic fungi associated with Coraebus florentinus (Coleoptera: Buprestidae) attacks in declining oak forests. Forests 2019, 10, 488. [Google Scholar] [CrossRef] [Green Version]
- da Clara, M.I.E.; de Almeida Ribeiro, N.M.C. Decline of Mediterranean oak trees and its association with Phytophthora cinnamomi: A review. Eur. J. For. Res. 2013, 132, 411–432. [Google Scholar]
- Moradi-Amirabad, Y.; Rahimian, H.; Babaeizad, V.; Denman, S. Brenneria spp. and Rahnella victoriana associated with acute oak decline symptoms on oak and hornbeam in Iran. For. Path. 2019, 49, e12535. [Google Scholar] [CrossRef]
- Choi, W.I. Influence of global warming on forest coleopteran communities with special reference to ambrosia and bark beetles. J. Asia-Pac. Entomol. 2011, 14, 227–231. [Google Scholar] [CrossRef]
- Biedermann, P.H.W.; Vega, F.E. Ecology and evolution of insect–fungus mutualisms. Annu. Rev. Entomol. 2020, 65, 431–455. [Google Scholar] [CrossRef] [Green Version]
- Hulcr, J.; Stelinski, L.L. The ambrosia symbiosis: From evolutionary ecology to practical management. Ann. Rev. Entomol. 2017, 62, 285–303. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-S.; Haack, R.A.; Choi, W.I. Attack pattern of Platypus koryoensis (Coleoptera: Platypodidae) in relation to crown dieback of Mongolian oak in Korea. Environ. Entomol. 2011, 40, 1363–1369. [Google Scholar] [CrossRef]
- Nakajima, H.; Ishida, M. Decline of Quercus crispula in abandoned coppice forests caused by secondary succession and Japanese oak wilt disease: Stand dynamics over twenty years. For. Ecol. Manag. 2014, 334, 18–27. [Google Scholar] [CrossRef]
- Kim, K.-H.; Choi, Y.-J.; Seo, S.-T.; Shin, H.-D. Raffaelea quercus-mongolicae sp. nov. associated with Platypus koryoensis on oak in Korea. Mycotaxon 2009, 110, 189–197. [Google Scholar] [CrossRef]
- Shoda-Kagaya, E.; Saito, S.; Okada, M.; Nozaki, A.; Nunokawa, K.; Tsuda, Y. Genetic structure of the oak wilt vector beetle Platypus quercivorus: Inferences toward the process of damaged area expansion. MBC Ecol. 2010, 10, 21. [Google Scholar] [CrossRef] [Green Version]
- Choi, W.I.; Nam, Y.; Lee, C.Y.; Choi, B.K.; Shin, Y.J.; Lim, J.-H.; Koh, S.-H.; Park, Y.-S. Changes in major insect pests of pine forests in Korea over the last 50 years. Forests 2019, 10, 692. [Google Scholar] [CrossRef] [Green Version]
- Um, T.-W.; Chun, J.-H.; Kim, K.H. Stand structure characteristics of oak wilt infected forest, Korea. Korean J. Environ. Ecol. 2009, 23, 220–232. [Google Scholar]
- Park, S.; Lyu, D. Checklist of the family Platypodidae (Coleoptera) in Korea. J. Asia-Pac. Entomol. 2007, 10, 275–280. [Google Scholar] [CrossRef]
- Korea Forest Research Institute. Annual Report of Monitoring for Forest Insect Pests and Diseases in Korea; SeongMunSa: Seoul, Korea, 2020. [Google Scholar]
- Murayama, J. Révisions des Familiiies des Ipides et des Platypides de Corée. J. Chosen Nat. Hist. Soc. 1930, 11, 3–38. [Google Scholar]
- Korea Forest Research Institute. Researches on Disease Cycle and Management of Korean Oak Wilt; Usgo Munhwasa: Seoul, Korea, 2010. [Google Scholar]
- Park, J.H.; Nam, Y.; Lee, S.G.; Jung, J.K.; Lee, D.J.; Jung, B.N.; Jang, Y.; Eom, C.-D.; Lee, H.M.; Kim, G.H.; et al. Development of Customized Management Methods for Korean Oak Wilt (KOW) and Research on Utilization of Woods Damaged by KOW; National Institute of Forest Science: Seoul, Korea, 2021. [Google Scholar]
- Cha, S.; Lee, W.-K.; Kim, M.; Lee, S.G.; Jo, H.-W.; Choi, W.I. Characterizing the spatial distribution of oak wilt disease using remote sensing data. J. Korean For. Soc. 2017, 106, 310–319. [Google Scholar]
- Korea Forest Research Institute. Annual Report of Monitoring for Forest Insect Pests and Diseases in Korea; SeongMunSa: Seoul, Korea, 2014. [Google Scholar]
- Yeum, J.H.; Han, B.H.; Choi, J.-W.; Jeong, H.-U. Mapping of the damaged forest by oak wilt disease in Bukhansan National Park. Korean J. Environ. Ecol. 2013, 27, 704–717. [Google Scholar]
- Choi, W.I.; Lee, J.-S.; Choi, K.-S.; Kim, J.-K.; Shin, S.-C. Tree trunk level distribution of entry hole by Platypus koryoensis (Coleoptera: Platypodidae) and its implication to tree damage. Korean J. Appl. Entomol. 2008, 47, 127–131. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, K. Responses of Quercus sapwood to infection with the pathogenic fungus of a new wilt disease vectored by the ambrosia beetle Platypus quercivorus. J. Wood Sci. 2001, 47, 425–429. [Google Scholar] [CrossRef]
- Lee, Y.G.; Sung, J.H.; Chun, J.H.; Shin, M.Y. Effect of climate changes on the distribution of productive areas for Quercus mongolica in Korea. J. Korean For. Soc. 2014, 103, 605–612. [Google Scholar] [CrossRef]
- Nakajima, H. Region-wide mass mortality of Japanese oak due to ambrosia beetle infestation: Mortality factors and change in oak abundance. For. Ecol. Manag. 2019, 449, 117468. [Google Scholar] [CrossRef]
- Farrell, B.D.; Sequeira, A.S.; O’Meara, B.C.; Normark, B.B.; Chung, J.H.; Jordal, B.H. The evolution of agriculture in beetles (Curculionidae: Scolytinae and Platypodinae). Evolution 2001, 55, 2011–2027. [Google Scholar] [CrossRef]
- Peer, K.; Taborsky, M. Delayed dispersal as a potential route to cooperative breeding in ambrosia beetles. Behav. Ecol. Sociobiol. 2007, 61, 729–739. [Google Scholar] [CrossRef] [Green Version]
- Kubono, T.; Ito, S.I. Raffaelea quercivora sp. nov. associated with mass mortality of Japanese oak, and the ambrosia beetle (Platypus quercivorus). Mycoscience 2002, 43, 0255–0260. [Google Scholar] [CrossRef]
- Yamasaki, M.; Sakimoto, M. Predicting oak tree mortality caused by the ambrosia beetle Platypus quercivorus in a cool-temperate forest. J. Appl. Entomol. 2009, 133, 673–681. [Google Scholar] [CrossRef]
- Hong, K.J.; Kwon, Y.D.; Park, S.W.; Lyu, D.P. Platypus koryoensis (Murayama) (Platypodidae: Coleoptera), the vector of oak wilt disease. Korean J. Appl. Entomol. 2006, 45, 113–117. [Google Scholar]
- Baker, J.M. Ambrosia beetles and their fungi, with particular reference to Platypus cylindrus FAB. Symp. Soc. Gen. Microbiol. 1963, 13, 232–265. [Google Scholar]
- Henriques, J.; de Lurdes Inácio, M.; Sousa, E. Ambrosia fungi in the insect-fungi symbiosis in relation to cork oak decline. Rev. Iberoam. Micol. 2006, 23, 185–188. [Google Scholar] [CrossRef]
- Akbulut, S.; Keten, A.; Yüksel, B. Wood destroying insects in Düzce province. Turk. J. Zool. 2008, 32, 343–350. [Google Scholar]
- Ponel, P.; Orgeas, J.; Samways, M.J.; Andrieu-Ponel, V.; de Beaulieu, J.-L.; Reille, M.; Roche, P.; Tatoni, T. 110,000 years of quaternary beetle diversity change. Biodivers. Conserv. 2003, 12, 2077–2089. [Google Scholar] [CrossRef]
- Alfaro, R.I.; Humble, L.M.; Gonzalez, P.; Villaverde, R.; Allegro, G. The threat of the ambrosia beetle Megaplatypus mutatus (Chapuis) (=Platypus mutatus Chapuis) to world poplar resources. Forestry 2007, 80, 471–479. [Google Scholar] [CrossRef]
- Choo, H.Y.; Woo, K.S. Supplementary notes on the bark and Ambrosia beetles of Korea. Korean J. Appl. Entomol. 1989, 28, 4–9. [Google Scholar]
- Lee, B.Y.; Chung, Y.J. Tree Insect Pests in Korea; Sungandang: Seoul, Korea, 1997. [Google Scholar]
- Miranda, I.; Sousa, V.; Ferreira, J.; Pereira, H. Chemical characterization and extractives composition of heartwood and sapwood from Quercus faginea. PLoS ONE 2017, 12, e0179268. [Google Scholar] [CrossRef] [Green Version]
- Imai, K.; Yamauchi, K.; Mitsunaga, T. Extractives of Quercus crispula sapwood infected by the pathogenic fungus Raffaelea quercivora II: Isolation and identification of phenolic compounds from infected sapwood. J. Wood Sci. 2013, 59, 517–521. [Google Scholar] [CrossRef]
- Nam, Y.; Choi, W.I.; Won, D.-S.; Kim, J.-K. Density related plasticity in stand-level spatial distribution of the ambrosia beetle, Platypus koryoensis (Coleoptera: Curculionidae). Popul. Ecol. 2013, 55, 3–10. [Google Scholar] [CrossRef]
- Kobayashi, M.; Ueda, A. Preliminary study of mate choice in Platypus quercivorus (Murayama) (Coleoptera: Platypodidae). Appl. Entomol. Zool. 2002, 37, 451–457. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.K.; Seo, J.W.; Kang, W.J.; Lee, J.S.; Cho, W.S.; Seo, S.T.; Kwon, Y.-D.; Kwon, G.H.; Kim, G.H. Attractant effect of citral on Platypus koryoensis (Coleoptera: Curculionidae). Entomol. Res. 2018, 48, 27–31. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Nakashima, T.; Tebayashi, S.-i.; Kim, C.-S. Determination of the Absolute Configuration of Quercivorol, (1S,4R)-p-Menth-2-en-1-ol, an Aggregation Pheromone of the Ambrosia Beetle Platypus quercivorus (Coleoptera: Platypodidae). Biosci. Biotechnol. Biochem. 2006, 70, 2544–2546. [Google Scholar] [CrossRef] [Green Version]
- Nam, Y.; Koh, S.-H.; Won, D.-S.; Kim, J.-K.; Choi, W.I. An empirical predictive model of the flight period of the ambrosia beetle Platypus koryoensis (Coleoptera: Platypodinae). Appl. Entomol. Zool. 2013, 48, 515–524. [Google Scholar] [CrossRef]
- Park, G.H. Ecological Characteristics and Chemical Control of Platypus kortoensis (Coleoptera: Platypodidae); Chungbuk National University: Cheong-Ju, Korea, 2008. [Google Scholar]
- Lee, C.Y.; Nam, Y.; Seo, Y.O.; Bae, Y.J.; Choi, W.I. Characterization dispersal and flight behavior of Platypus koryoensis (Coleoptera: Curculionidae) by mark-release-recapture. J. Econ. Entomol. 2019, 112, 720–728. [Google Scholar] [CrossRef]
- Nam, Y.; Choi, W.I. An empirical predictive model for the spring emergence of Thecodiplosis japonensis (Diptera: Cecidomyiidae): Model construction and validation on the basis of 25 years field observations data. J. Econ. Entomol. 2014, 107, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Igeta, Y.; Esaki, K.; Kato, K.; Kamata, N. Spatial distribution of a flying ambrosia beetle Platypus quercivorus (Coleoptera: Platypodidae) at the stand level. Appl. Entomol. Zool. 2004, 39, 583–589. [Google Scholar] [CrossRef] [Green Version]
- Okada, R.; Pham, D.L.; Ito, Y.; Yamasaki, M.; Ikeno, H. Measuring the flight ability of the ambrosia beetle, Platypus quercivorus (Murayama), using a low-cost, small, and easily constructed flight mill. J. Vis. Exp. 2018, 138, e57468. [Google Scholar] [CrossRef] [Green Version]
- Inácio, M.L.; Marcelino, J.; Lima, A.; Sousa, E.; Nóbrega, F. Raffaelea quercina sp. nov. associated with cork oak (Quercus suber L.) decline in Portugal. Forests 2021, 12, 513. [Google Scholar] [CrossRef]
- Saragih, S.A.; Kusumoto, D.; Takemoto, S.; Torii, M.; Kamata, N. Virulence of fungi isolated from ambrosia beetles to Acer amoenum branches. Plant Dis. 2021, 105, 3087–3091. [Google Scholar] [CrossRef]
- Trollip, C.; Carnegie, A.J.; Dinh, Q.; Kaur, J.; Smith, D.; Mann, R.; Rodoni, B.; Edwards, J. Ophiostomatoid fungi associated with pine bark beetles and infested pines in south-eastern Australia, including Graphilbum ipis-grandicollis sp. nov. IMA Fungus 2021, 12, 24. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Y.T.; Kasson, M.T.; Macias, A.M.; Skelton, J.; Carlson, P.S.; Yin, M.; Hulcr, J. Specific and promiscuous ophiostomatalean fungi associated with Platypodinae ambrosia beetles in the southeastern United States. Fungal Ecol. 2018, 35, 42–50. [Google Scholar] [CrossRef]
- Olatinwo, R.; Hwang, J.; Johnson, W. First report of laurel wilt disease caused by Raffaelea lauricola on spicebush in Louisiana. Plant Dis. 2021, 105, 2250. [Google Scholar] [CrossRef]
- Procter, M.; Nel, W.J.; Marincowitz, S.; Crous, P.W.; Wingfield, M.J. A new species of Raffaelea from beetle-infested Leucaena leucocephala. Fungal Syst. Evol. 2020, 6, 305–314. [Google Scholar] [CrossRef]
- Ibarra-Juarez, L.A.; Burton, M.A.J.; Biedermann, P.H.W.; Cruz, L.; Desgarennes, D.; Ibarra-Laclette, E.; Latorre, A.; Alonso-Sánchez, A.; Villafan, E.; Hanako-Rosas, G.; et al. Evidence for succession and putative metabolic roles of fungi and bacteria in the farming mutualism of the Ambrosia beetle Xyleborus affinis. mSystems 2020, 5, e00541-20. [Google Scholar] [CrossRef]
- Mayfield, A.E.; Smith, J.A.; Hughes, M.; Dreaden, T.J. First report of laurel wilt disease caused by a Raffaelea sp. on avocado in Florida. Plant Dis. 2008, 92, 976. [Google Scholar] [CrossRef]
- Harrington, T.; Stephen, F.; Aghayeva, D. Raffaelea lauricola, a new ambrosia beetle symbiont and pathogen on the Lauraceae. Mycotaxon. Mycotaxon 2008, 104, 399–404. [Google Scholar]
- Ploetz, R.C.; Hulcr, J.; Wingfield, M.J.; de Beer, Z.W. Destructive tree diseases associated with ambrosia and bark beetles: Black swan events in tree pathology? Plant Dis. 2013, 97, 856–872. [Google Scholar] [CrossRef] [Green Version]
- Torii, M.; Matsuda, Y.; Seo, S.T.; Kim, K.H.; Ito, S.-i.; Moon, M.J.; Kim, S.H.; Yamada, T. The effect of Raffaelea quercus-mongolicae inoculations on the formation of non-conductive sapwood of Quercus mongolica. Mycobiology 2014, 42, 210–214. [Google Scholar] [CrossRef] [Green Version]
- Yi, S.H.; Lee, J.H.; Seo, S.T.; Lee, J.K. In vivo pathogenicity test of oak wilt fungus (Raffaelea quercus-mongolicae) on oriental chestnut oak (Quercus acutissima). J. For. Environ. Sci. 2017, 33, 342–347. [Google Scholar]
- Kinuura, H.; Kobayashi, M. Death of Quercus crispula by inoculation with adult Platypus quercivorus (Coleoptera: Platypodidae). Appl. Entomol. Zool. 2006, 41, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, Y.; Matsushita, N.; Hogetsu, T. Spatial distribution of Raffaelea quercivora in xylem of naturally infested and inoculated oak trees. Phytopathology 2010, 100, 747–755. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.S.; Hohenlohe, P.A.; Kim, K.H.; Seo, S.T.; Klopfenstein, N.B. Genetic diversity and population structure of Raffaelea quercus-mongolicae, a fungus associated with oak mortality in South Korea. For. Pathol. 2016, 46, 164–167. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.H.; Jung, J.M.; Seo, S.T. Population genetic structure of Raffaelea quercus-mongolicae indicates a recent fungal introduction event to Jeju Island from inland areas of South Korea. Plant Pathol. 2021, 70, 1871–1882. [Google Scholar] [CrossRef]
- Seo, S.T.; Kim, K.H.; Lee, S.H.; Kwon, Y.N.; Shin, C.H.; Kim, H.J.; Lee, S.Y. Genotypic characterization of oak wilt pathogen Raffaelea quercus-mongolicae and R. quercivora strains. Res. Plant Dis. 2010, 16, 219–223. [Google Scholar] [CrossRef] [Green Version]
- Son, S.Y. Analysis of Pathogenicity, Genetic Diversity of Raffaelea quercus-mongolicae and Control of Oak Wilt Disease. Ph.D. Thesis, Chonbuk National University, Jeonju, Korea, 2017. [Google Scholar]
- Lee, D.-H.; Son, S.-Y.; Seo, S.-T.; Lee, J.K. Investigation of the mating-type distribution of Raffaelea quercus-mongolicae in South Korea. For. Pathol. 2020, 50, e12590. [Google Scholar] [CrossRef]
- Yun, Y.H.; Suh, D.Y.; Yoo, H.D.; Oh, M.H.; Kim, S.H. Yeast associated with the ambrosia beetle, Platypus koryoensis, the pest of oak trees in Korea. Mycobiology 2015, 43, 458–466. [Google Scholar] [CrossRef] [Green Version]
- Endoh, R.; Suzuki, M.; Okada, G.; Takeuchi, Y.; Futai, K. Fungus symbionts colonizing the galleries of the ambrosia beetle Platypus quercivorus. Microb. Ecol. 2011, 62, 106–120. [Google Scholar] [CrossRef]
- Madden, A.A.; Epps, M.J.; Fukami, T.; Irwin, R.E.; Sheppard, J.; Sorger, D.M.; Dunn, R.R. The ecology of insect-yeast relationships and its relevance to human industry. Proc. R. Soc. B Biol. Sci. 2018, 285, 20172733. [Google Scholar] [CrossRef] [Green Version]
- Korea Forest Service. Guideline for the Control of Forest Disease and Insect Pests; Korea Forest Service: Daejeon, Korea, 2015. [Google Scholar]
- Park, I.-K.; Nam, Y.; Seo, S.-T.; Kim, S.-W.; Jung, C.-S.; Han, H.-R. Development of a mass trapping device for the ambrosia beetle, Platypus koryoensis, an insect vector of oak wilt disease in Korea. J. Asia Pac. Entomol. 2016, 19, 39–43. [Google Scholar] [CrossRef]
- Son, S.Y.; Seo, S.T.; Park, J.H. Control efficacy of fungicide injection on oak wilt in the field. Res. Plant Dis. 2014, 20, 295–298. [Google Scholar] [CrossRef] [Green Version]
- Korea Forest Service. Statistical Yearbook of Forestry; Korea Forest Service: Daejeon, Korea, 2016. [Google Scholar]
- Fan, Z.; Kabrick, J.M.; Spetich, M.A.; Shifley, S.R.; Jensen, R.G. Oak mortality associated with crown dieback and oak borer attack in the Ozark highlands. For. Ecol. Manag. 2008, 255, 2297–2305. [Google Scholar] [CrossRef]
- Riggins, J.J.; Galligan, L.D.; Stephen, F.M. Rise and fall of red oakborer (Coleoptera: Cerambycidae) in the Ozark mountains of Arkansas, USA. Fla. Entomol. 2009, 92, 426–433, 428. [Google Scholar] [CrossRef]
- Kamata, N.; Esaki, K.; Kato, K.; Igeta, Y.; Wada, K. Potential impact of global warming on deciduous oak dieback caused by ambrosia fungus Raffaelea sp. carried by ambrosia beetle Platypus quercivorus (Coleoptera: Platypodidae) in Japan. Bull. Entomol. Res. 2002, 92, 119–126. [Google Scholar] [CrossRef]
- Kobayashi, M.; Ueda, A. Wilt disease of Fagaceae trees caused by Platypus quercivorus (Murayama) (Coleoptera: Platypodidae) and the associated fungus: Aim is to Clarify the Damage Factor. J. Jpn. For. Soc. 2005, 87, 435–450. [Google Scholar] [CrossRef]
- Okaura, T.; Quang, N.D.; Ubukata, M.; Harada, K. Phylogeographic structure and late Quaternary population history of the Japanese oak Quercus mongolica var. crispula and related species revealed by chloroplast DNA variation. Genes Genet. Syst. 2007, 82, 465–477. [Google Scholar] [CrossRef] [Green Version]
- Reeve, J.D.; Ayres, M.P.; Lorio, P.L., Jr. Host suitability, predation, and bark beetle population dynamics. In Population Dynamics: New Approaches and Synthesis; Cappuccino, N., Price, P.W., Eds.; Academic Press: San Diego, CA, USA, 1995; pp. 339–357. [Google Scholar]
- Six, D.L. Ecological and evolutionary determinants of bark beetle—Fungus symbioses. Insects 2012, 3, 339–366. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, W.I.; Lee, D.-H.; Jung, J.B.; Park, Y.-S. Oak Decline Syndrome in Korean Forests: History, Biology, and Prospects for Korean Oak Wilt. Forests 2022, 13, 964. https://doi.org/10.3390/f13060964
Choi WI, Lee D-H, Jung JB, Park Y-S. Oak Decline Syndrome in Korean Forests: History, Biology, and Prospects for Korean Oak Wilt. Forests. 2022; 13(6):964. https://doi.org/10.3390/f13060964
Chicago/Turabian StyleChoi, Won Il, Dong-Hyeon Lee, Jong Bin Jung, and Young-Seuk Park. 2022. "Oak Decline Syndrome in Korean Forests: History, Biology, and Prospects for Korean Oak Wilt" Forests 13, no. 6: 964. https://doi.org/10.3390/f13060964
APA StyleChoi, W. I., Lee, D. -H., Jung, J. B., & Park, Y. -S. (2022). Oak Decline Syndrome in Korean Forests: History, Biology, and Prospects for Korean Oak Wilt. Forests, 13(6), 964. https://doi.org/10.3390/f13060964






