Can Mechanical Strain and Aspect Ratio Be Used to Determine Codominant Unions in Red Maple without Included Bark
Abstract
:1. Introduction
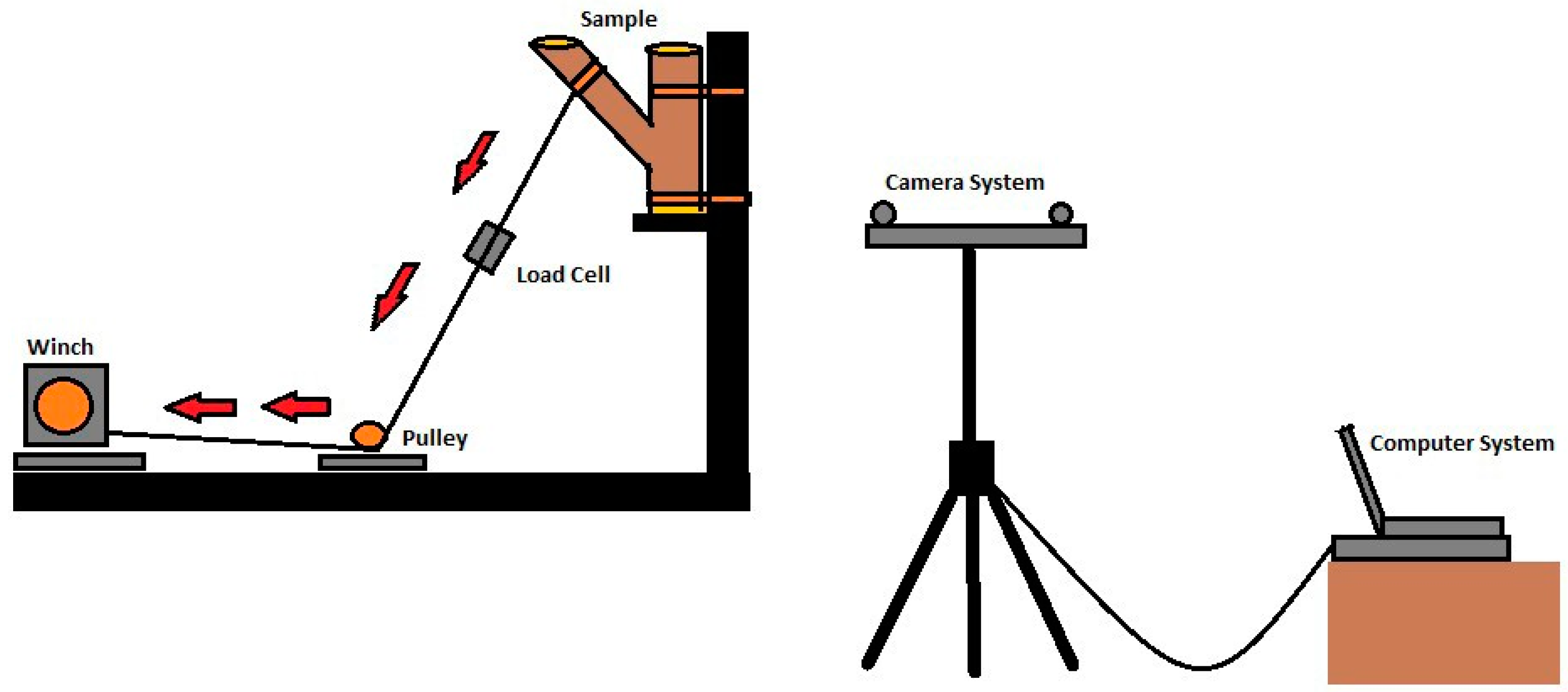
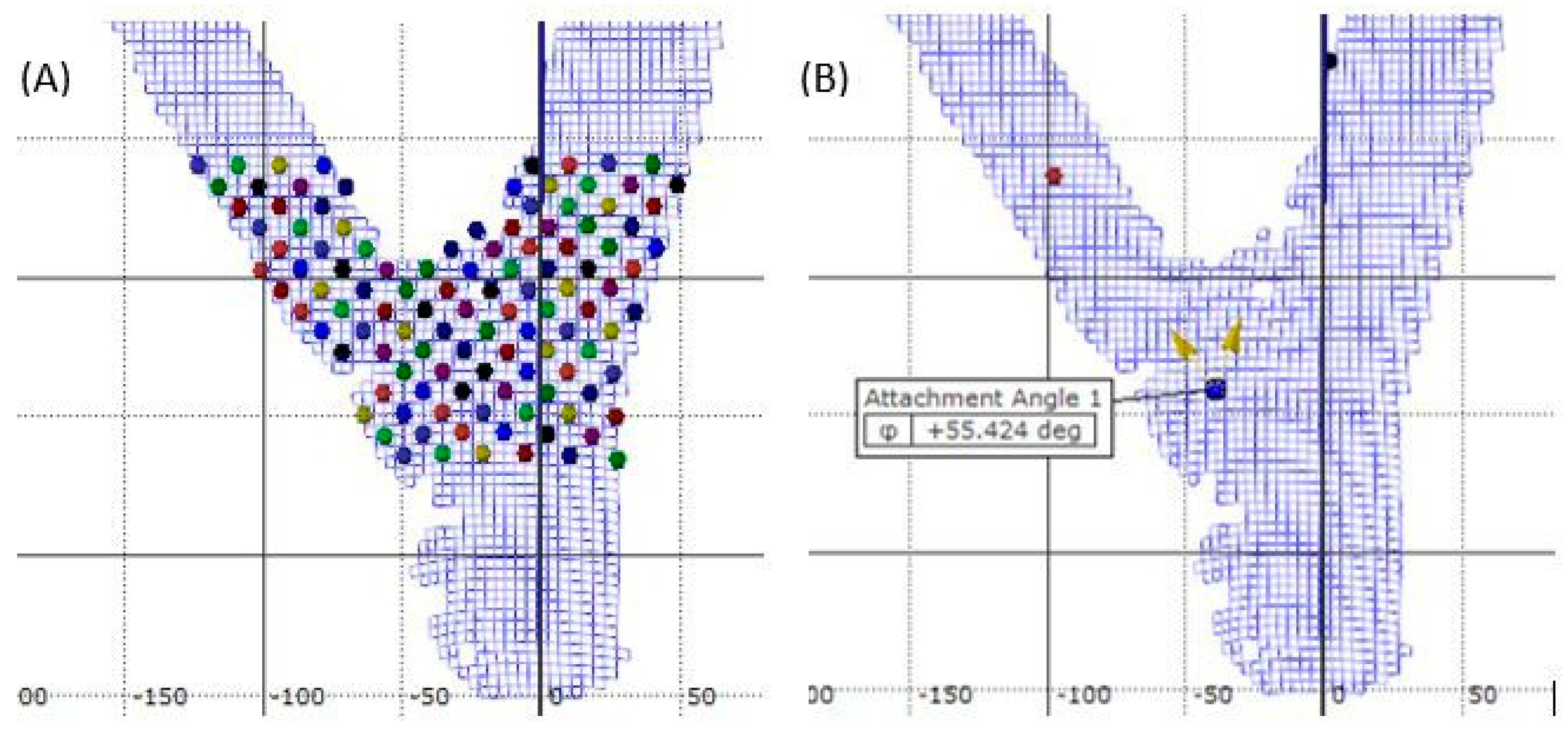
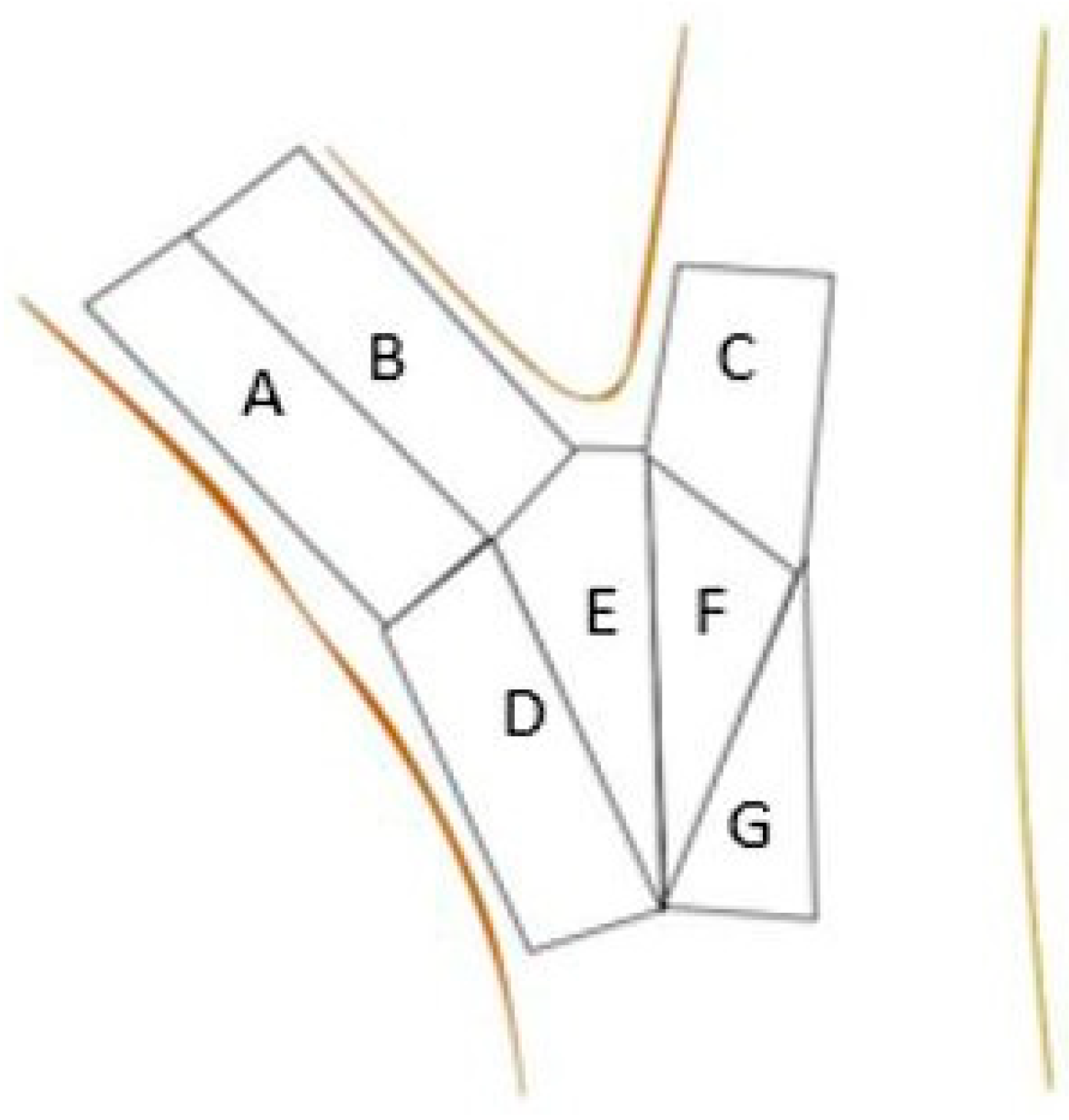
2. Materials and Methods
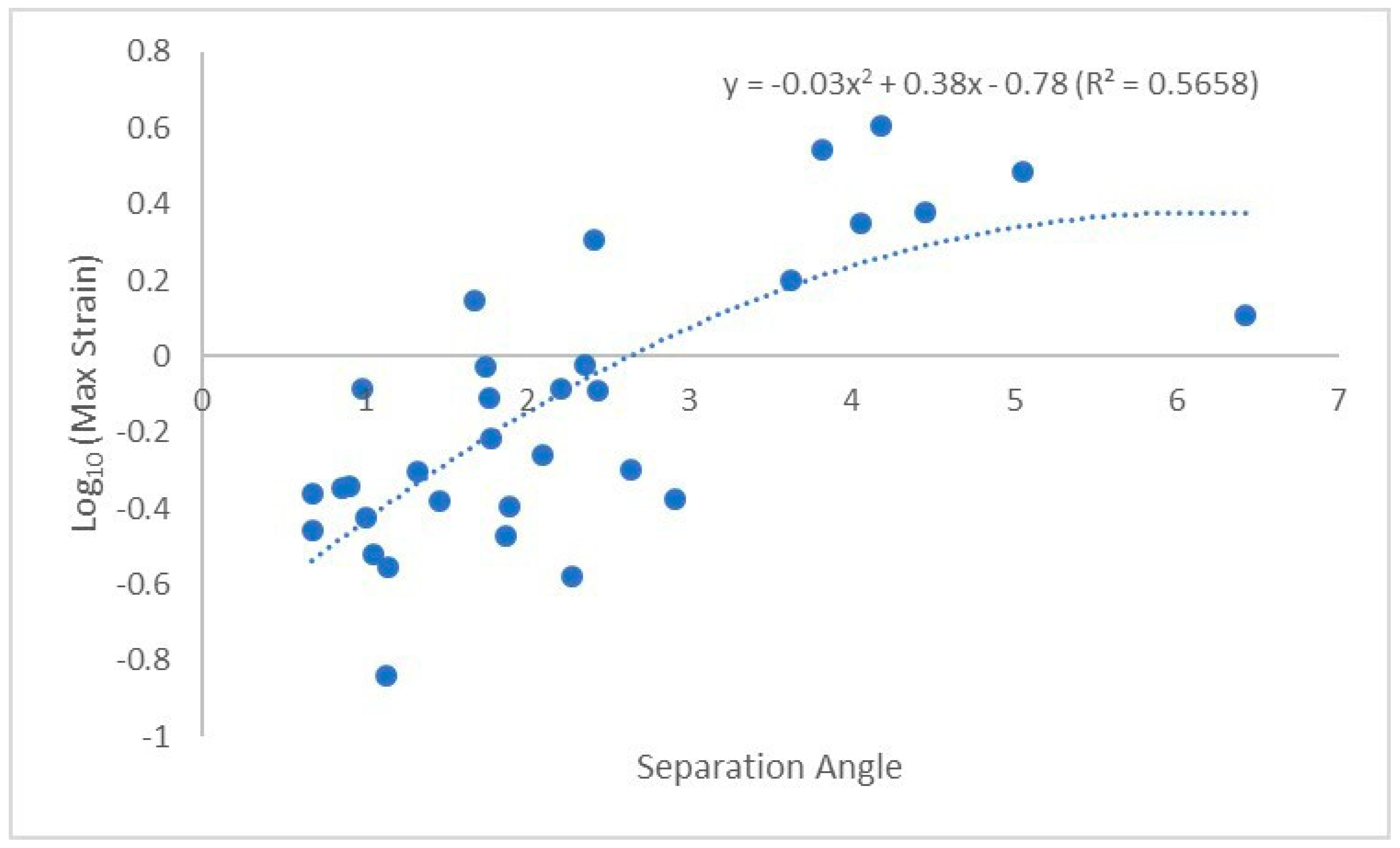
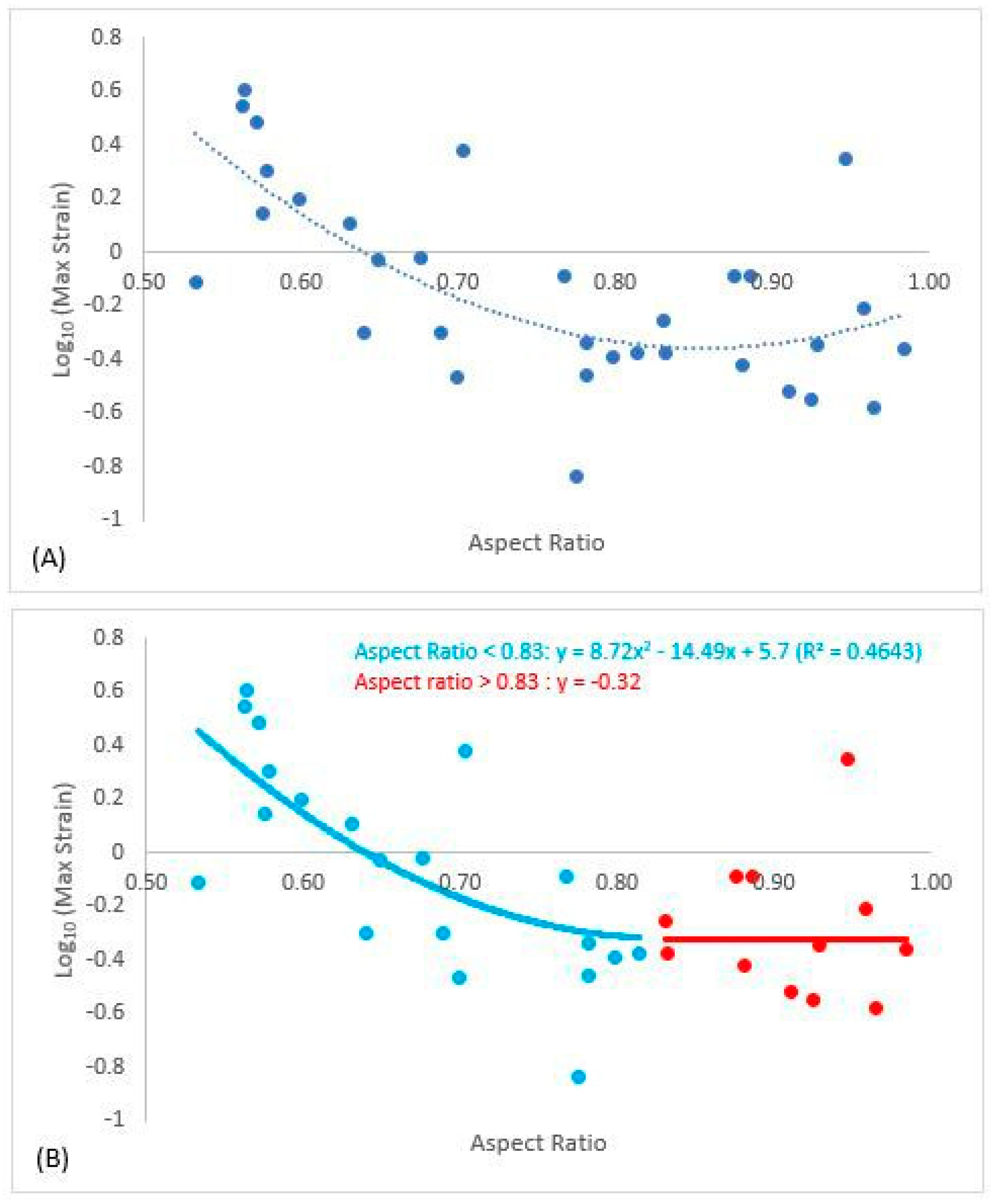
3. Results
4. Discussion
5. Implications
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dahle, G.A.; Grabosky, J.; Kane, B.; Miesbauer, J.; Peterson, W.; Telewski, F.W.; Koeser, A.; Watson, G.W. Tree biomechanics: A white paper from the 2021 international meeting and research summit at the Morton Arboretum (Lisle, Illinois, U.S.). Arboric. Urban For. 2014, 40, 309–318. [Google Scholar]
- Goodfellow, J.W. Utility Tree Risk Assessment—Best Management Practices; International Society of Arboriculture: Champaign, IL, USA, 2020. [Google Scholar]
- Smiley, E.T.; Matheny, N.; Lilly, S. Best Management Practices: Tree Risk Assessment; International Society of Arboriculture: Champaign, IL, USA, 2017. [Google Scholar]
- James, K.R.; Dahle, G.A.; Grabosky, J.; Kane, B.; Detter, A. Tree biomechanics literature review: Dynamics. J. Arboric. Urban For. 2014, 40, 1–15. [Google Scholar] [CrossRef]
- Dahle, G.A.; James, K.R.; Kane, B.; Grabosky, J.; Detter, A. A review of factors that affect the load-bearing capacity of urban trees. Arboric. Urban For. 2017, 43, 89–106. [Google Scholar] [CrossRef]
- Skea, J.; Shukla, P.; Kilkiş, Ş. Climate Change 2022 Mitigation of Climate Change. Working Group III Contribution of the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. 2022. Available online: https://report.ipcc.ch/ar6wg3/pdf/IPCC_AR6_WGIII_FinalDraft_F (accessed on 17 May 2022).
- Lambert, C.; Landry, S.; Andreu, M.G.; Koeser, A.; Starr, G.; Staudhammer, D. Impact of model choice in predicting urban forest storm damage when data is uncertain. Landsc. Urban Plan. 2022, 226, 104467. [Google Scholar] [CrossRef]
- Walker, M.; Dahle, G.A. Likelihood of failure of trees along electrical utility rights-of-ways: A literature review. Arboric. Urban For. 2022; accepted, in press. [Google Scholar]
- MacDaniels, L.H. The apple-tree crotch. In Bulletin 419 of the Cornell University Agricultural Experiment Station; Cornell University Agricultural Experiment Station: Ithaca, NY, USA, 1923. [Google Scholar]
- MacDaniels, L.H. Factors affecting the breaking strength of apple tree crotches. J. Am. Soc. Hortic. Sci. 1932, 29, 44. [Google Scholar]
- Miller, V.J. Crotch influence on strength and breaking point of apple tree branches. J. Am. Soc. Hortic. Sci. 1958, 73, 27–32. [Google Scholar]
- Smiley, E.T. Does included bark reduce the strength of codominant stems? J. Arboric. 2003, 29, 104–106. [Google Scholar] [CrossRef]
- Gilman, E.F. Branch-to-stem diameter ratio affects strength of attachment. J. Arboric. 2003, 29, 291–294. [Google Scholar] [CrossRef]
- Gilman, E.F. An Illustrated Guide to Pruning, 2nd ed.; Delmar Publications: Independence, KY, USA, 2002. [Google Scholar]
- Fraedrich, F.R. Structural pruning of young trees. In Bartlett Tree Research Laboratories Technical Report; Bartlett Tree Experts: Charlotte, NC, USA, 2014; pp. 1–6. [Google Scholar]
- Slater, D.; Harbinson, C. Towards a new model of branch attachment. Arboric. J. 2010, 33, 95–105. [Google Scholar] [CrossRef]
- Slater, D.; Ennos, A.R. Determining the mechanical properties of hazel forks by testing their component parts. Trees 2013, 27, 1515–1524. [Google Scholar] [CrossRef]
- Slater, D.; Bradley, R.S.; Withers, P.J.; Ennos, A.R. The anatomy and grain pattern in forks of hazel (Corylus avellana L.) and other tree species. Trees 2014, 28, 1437–1448. [Google Scholar] [CrossRef]
- Kane, B. Branch strength of Bradford pear (Pyrus calleryana var. ‘Bradford’). Arboric. Urban For. 2007, 33, 283–291. [Google Scholar] [CrossRef]
- Kane, B.; Clouston, P. Tree pulling tests of large shade trees in the genus Acer. Arboric. Urban For. 2008, 34, 101–109. [Google Scholar] [CrossRef]
- Kane, B.; Farrell, R.; Zedaker, S.M.; Lofersky, J.R.; Smith, D.W. Failure mode and prediction of the strength of branch attachments. Arboric. Urban For. 2008, 34, 308–316. [Google Scholar] [CrossRef]
- Eisner, N.J.; Gilman, E.F.; Grabosky, J.C.; Beeson, R.C., Jr. Branch junction characteristics affect hydraulic segmentation in red maple. J. Arboric. 2002, 28, 245–251. [Google Scholar] [CrossRef]
- Eckenrode, R.T.; Dahle, G.A.; Smiley, E.T.; DeVallance, D. Examining Strain Propagation in the Lateral Branch Attachment of White Oak (Quercus alba L.) and Chestnut Oak (Quercus prinus L.). Urban For. Urban Green. 2022, 73, 127591. [Google Scholar] [CrossRef]
- Beer, F.P.; Johnston, E.R., Jr.; DeWolf, J.T. Stress and strain–Axial loading. In Mechanics of Materials, 3rd ed.; Plant, J., Ed.; McGraw-Hill: New York, NY, USA, 2001; pp. 48–57. [Google Scholar]
- Burgert, I. Exploring the micromechanical design of plant cell walls. Am. J. Bot. 2006, 93, 1391–1401. [Google Scholar] [CrossRef]
- Dahle, G.A. Influence of bark on the measurement of mechanical strain using digital image correlation. Wood Sci. Technol. 2017, 51, 1469–1477. [Google Scholar] [CrossRef]
- Müller, U.; Gindl, W.; Jeronimidis, G. Biomechanics of a branch–stem junction in softwood. Trees 2006, 20, 643–648. [Google Scholar] [CrossRef]
- Sebera, V.; Kunecký, J.; Praus, L.; Tippner, J.; Horáček, P. Strain transfer from xylem to bark surface analyzed by digital image correlation. Wood Sci. Technol. 2016, 50, 773–787. [Google Scholar] [CrossRef]
- Sebera, V.; Praus, L.; Tippner, J.; Kunecký, J.; Čepela, J.; Wimmer, R. Using optical full-field measurement based on digital image correlation to measure strain on a tree subjected to mechanical load. Trees 2014, 28, 1173–1184. [Google Scholar] [CrossRef]
- Löchteken, H.; Rust, S. Analyse optischer Verfahren zur Messung der Randfaser für die Verwendung bei baumstatischen Untersuchungen. In Jahrbuch der Baumpflege; Dujesiefken, D., Ed.; Haymarket Media: Cologne, Germany, 2015; pp. 267–292. [Google Scholar]
- Tippner, J.; Praus, L.; Brabec, M.; Sebera, V.; Vojáčková, B.; Milch, J. Using 3D digital image correlation in an identification of defects of trees subjected to bending. Urban For. Urban Green. 2019, 46, 126513. [Google Scholar] [CrossRef]
- Beezley, K.E.; Dahle, G.A.; Miesbauer, J.; DeVallance, D. Strain Patterns Across the Root-Stem Transition Zone in Urban Trees. Arboric. Urban For. 2020, 6, 321–332. [Google Scholar] [CrossRef]
- Vojáčková, B.; Tippner, J.; Horáček, P.; Sebera, V.; Praus, L.; Mařík, R.; Brabec, M. The effect of the stem and root-plate defects to the tree response during static loading—numerical analysis. Urban For. Urban Green. 2021, 59, 127002. [Google Scholar] [CrossRef]
- Lilly, S.; Sydnor, T.D. Comparison of branch failure during static loading of silver and norway maples. J. Arboric. 1995, 21, 302–305. [Google Scholar] [CrossRef]
- Dahle, G.A. Strength Loss and Decay Patterns in Trees Converted from Round-Overs to V-trims During Electrical Line Clearance Operations. Master’s Thesis, Purdue University, West Lafayette, IN, USA, 2004. [Google Scholar]
- Miesbauer, J.W.; Gilman, E.F.; Masters, F.; Nitesh, S. Impact of branch reorientation on breaking stress in Liriodendron tulipifera L. Urban For. Urban Green. 2014, 13, 526–533. [Google Scholar] [CrossRef]
- Rathjen, R.; Sydnor, D.; Grabosky, J.; Dahle, G.A. Structural Pruning in Callery Pear Does Not Change Apparent Branch Union Strength in 7th Year Static Load Field Testing. Arboric. Urban For. 2021, 47, 123–130. [Google Scholar] [CrossRef]
- Lavers, G.M. The strength properties of timber. In Building Research Establishment Report; HMSO: London, UK, 1983. [Google Scholar]
- Kretschmann, D.E. Mechanical properties of wood. In Wood Handbook: Wood as an Engineering Material; USDA: Madison, WI, USA, 2010. [Google Scholar]
- Spatz, H.C.; Pfisterer, J. Mechanical properties of green wood and their relevance for tree risk assessment. Arboric. Urban For. 2013, 39, 218–225. [Google Scholar]
- Ennos, A.R.; van Casteren, A. Transverse stresses and modes of failure in tree branches and other beams. Proc. R. Soc. B 2010, 277, 1253–1258. [Google Scholar] [CrossRef] [Green Version]
- Van Casteren, A.; Sellers, W.I.; Thorpe, S.K.S.; Coward, S.; Crompton, R.H.; Ennos, A.R. Why don’t branches snap? The mechanics of bending failure in three temperate angiosperm trees. Trees 2011, 26, 789–797. [Google Scholar] [CrossRef]
- Panshin, A.J.; de Zeeuw, C. Textbook of Wood Technology; McGraw-Hill: New York, NY, USA, 1980. [Google Scholar]
- Pfiesterer, J.A. Toward a better understating of tree failure: Investigations into bending stresses of branch junctions and reiterated of European filbert (Corylus avellane L.) as a model organism. In Proceedings of the Second International Symposium on Plant Health in Urban Horticulture, Berlin, Germany, 27–29 August 2003; pp. 272–279. [Google Scholar]
- Buckley, G.; Slater, D.; Ennos, R. Angle of inclination affects the morphology and strength of bifurcations in hazel (Corylus avellana L.). Arboric. J. 2015, 37, 99–112. [Google Scholar] [CrossRef]
- Farnsworth, K.D.; Niklas, K.J. Theories of optimization, form and function in branching architecture of plants. Funct. Ecol. 1995, 9, 355–363. [Google Scholar] [CrossRef]
- Dahle, G.A.; Grabosky, J.C. Review of literature on the function and allometric relationships of tree stems and branches. Arboric. Urban For. 2009, 35, 311–320. [Google Scholar] [CrossRef]
- Dahle, G.A.; Grabosky, J.C. Variation in modulus of elasticity (E) along Acer platanoides L. (Aceraceae) branches. Urban For. Urban Green. 2010, 9, 227–233. [Google Scholar] [CrossRef]
- Woodrum, C.L.; Ewers, F.W.; Telewski, F.W. Hydraulic, biomechanical, and anatomical interactions of xylem from five species of Acer (Aceraceae). Am. J. Bot. 2003, 90, 693–699. [Google Scholar] [CrossRef]
- Read, J.; Stokes, A. Plant biomechanics in an ecological context. Am. J. Bot. 2006, 93, 1546–1565. [Google Scholar] [CrossRef]
- Gartner, B.L. Stem hydraulic properties of vines vs. shrubs of western poison oak, Toxicodendron diversilobum. Oecologia 1991, 87, 180–189. [Google Scholar] [CrossRef]
- Chiu, S.T.; Ewers, F.W. Xylem structure and water transport in a twiner, a scrambler, and a shrub of Lonicera (Caprifoliaceae). Trees 1992, 6, 216–224. [Google Scholar] [CrossRef]
- Niklas, K.J. Size-dependent allometry of tree height, diameter and trunk-taper. Ann. Bot. 1995, 75, 217–227. [Google Scholar] [CrossRef]
- Dahle, G.A.; Grabosky, J.C. Allometric patterns in Acer platanoides (Aceraceae) branches. Trees 2010, 24, 321–326. [Google Scholar] [CrossRef]
- Hibbeler, R.C. Mechanics of Material, 6th ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2005. [Google Scholar]
- James, K.R.; Moore, J.R.; Slater, D.; Dahle, G.A. Tree Biomechanics Review. CAB Rev. 2017, 12, 1–11. [Google Scholar] [CrossRef]


| Failure Mode | Branch Diameter | Branch Length | Aspect Ratio | Displacement | N |
|---|---|---|---|---|---|
| (cm) | (cm) | Angle | |||
| Limb | 1.59 ± 0.09 b | 344.00 ± 14.98 a | 0.59 ± 0.02 a | 4.81 ± 0.82 b | 3 |
| Ball and Socket | 1.59 ± 0.12 b | 323.67 ± 11.68 a | 0.64 ± 0.03 a | 2.24 ± 0.42 ab | 9 |
| Imbedded | 1.87 ± 0.07 ab | 389.70 ± 23.95 a | 0.78 ± 0.03 b | 2.08 ± 0.32 a | 10 |
| Flat p-Value | 2.20 ± 0.15 a 0.0043 | 465.50 ± 49.02 a 0.0300 | 0.90 ± 0.02 c <0.0001 | 1.57 ± 0.33 a 0.0063 | 10 |
| Failure Mode | Max Strain ± SE | N |
|---|---|---|
| Limb | 3.46 ± 1.18 a | 3 |
| Ball and Socket | 1.38 ± 0.39 b | 9 |
| Imbedded | 1.34 ± 0.21 b | 10 |
| Flat | 1.08 ± 0.15 b | 10 |
| p-value | 0.0284 |
| Zone | Limb | Ball and Socket | Imbedded | Flat |
|---|---|---|---|---|
| (n) | (n) | (n) | (n) | |
| A | 3.39 ± 0.45 a (16) | 1.24 ± 0.24 a (44) | 0.53 ± 0.06 bc (56) | 0.59 ± 0.05 abc (90) |
| B | 2.36 ± 0.49 ab (15) | 0.71 ± 0.12 abc (36) | 0.36 ± 0.03 c (50) | 0.42 ± 0.03 bc (86) |
| C | 0.17 ± 0.03 d (14) | 0.11 ± 0.01 d (35) | 0.19 ± 0.01 d (39) | 0.15 ± 0.01 d (56) |
| D | 1.68 ± 0.25 ab (13) | 0.9 ± 0.15 ab (36) | 0.54 ± 0.04 bc (53) | 0.58 ± 0.06 abc (66) |
| E | 1.45 ± 0.2 b (13) | 0.91 ± 0.13 a (30) | 1.14 ± 0.14 a (41) | 0.66 ± 0.06 ab (72) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahle, G.A.; Eckenrode, R.T., IV; Smiley, E.T.; DeVallance, D.; Holásková, I. Can Mechanical Strain and Aspect Ratio Be Used to Determine Codominant Unions in Red Maple without Included Bark. Forests 2022, 13, 1007. https://doi.org/10.3390/f13071007
Dahle GA, Eckenrode RT IV, Smiley ET, DeVallance D, Holásková I. Can Mechanical Strain and Aspect Ratio Be Used to Determine Codominant Unions in Red Maple without Included Bark. Forests. 2022; 13(7):1007. https://doi.org/10.3390/f13071007
Chicago/Turabian StyleDahle, Gregory A., Robert T. Eckenrode, IV, E. Thomas Smiley, David DeVallance, and Ida Holásková. 2022. "Can Mechanical Strain and Aspect Ratio Be Used to Determine Codominant Unions in Red Maple without Included Bark" Forests 13, no. 7: 1007. https://doi.org/10.3390/f13071007
APA StyleDahle, G. A., Eckenrode, R. T., IV, Smiley, E. T., DeVallance, D., & Holásková, I. (2022). Can Mechanical Strain and Aspect Ratio Be Used to Determine Codominant Unions in Red Maple without Included Bark. Forests, 13(7), 1007. https://doi.org/10.3390/f13071007






