Species Diversity, Biomass and Carbon Stock Assessment of Kanhlyashay Natural Mangrove Forest
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of Study Site
2.2. Data Collection
2.3. Species Composition and Diversity
- H′ = the value of the Shannon–Wiener diversity index
- Pi = the proportion of ith species individuals to total species individuals
- ln = the natural logarithm of Pi
Aboveground and Belowground Biomass Estimation and Carbon Stocks
- AGB (kg) = aboveground biomass estimates in kg per tree
- BGB (kg) = belowground biomass estimate in kg per tree
- D = diameter at breast height (dbh) in cm
- ρ = wood density in g cm−3
2.4. Statistical Analyses and Modelling Work
3. Results and Discussion
3.1. Species Composition
3.2. Structural Analysis
| Species | Mean Stem Density (No. of Trees ha−1) | Mean BA (m2 ha−1) | RD (%) | RF (%) | RBA (%) | IVI (%) |
|---|---|---|---|---|---|---|
| Avicennia officinalis | 868 ± 463 | 26.155 ± 16.940 | 78.77 | 52.27 | 87.66 | 218.70 |
| Sonneratia apetala | 154 ± 162 | 2.633 ± 4.503 | 13.97 | 22.73 | 8.83 | 45.53 |
| Sonneratia caseolaris | 46 ± 116 | 0.807 ± 1.994 | 4.17 | 9.09 | 2.71 | 15.97 |
| Aegiceras corniculutum | 32 ± 84 | 0.238 ± 0.646 | 2.90 | 11.36 | 0.80 | 15.06 |
| Avicennia alba | 25 ± 0.0 | 0.0525 ± 0.000 | 0.09 | 2.27 | 0.01 | 2.37 |
| Bruguiera sexangula | 25 ± 0.0 | 0.050 ± 0.000 | 0.09 | 2.27 | 0.01 | 2.37 |
3.3. Biomass and Carbon Stock of Mangrove
3.4. The Relationships between Carbon Density and Structural Variables
3.5. Influence of Structural Variables on Aboveground Carbon-Stock
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Myint, S.W.; Giri, C.P.; Wang, L.; Zhu, Z.; Gillette, S.C. Identifying Mangrove Species and Their Surrounding Land Use and Land Cover Classes Using an Object-Oriented Approach with a Lacunarity Spatial Measure. GISci. Remote Sens. 2008, 45, 188–208. [Google Scholar] [CrossRef]
- Naylor, L.A.; Viles, H.; Carter, N.E.A. Biogeomorphology revisited: Looking towards the future. Geomorphology 2002, 47, 3–14. [Google Scholar] [CrossRef]
- Abino, A.C.; Castillo, J.A.A.; Lee, Y.J. Assessment of species diversity, biomass and carbon sequestration potential of a natural mangrove stand in Samar, the Philippines. For. Sci. Technol. 2014, 10, 2–8. [Google Scholar] [CrossRef]
- Alongi, D.M. Present state and future of the world’s mangrove forests. Environ. Conserv. 2002, 29, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Donato, D.C.; Kauffman, J.B.; Murdiyarso, D.; Kurnianto, S.; Stidham, M.; Kanninen, M. Mangroves among the most carbon-rich forests in the tropics. Nat. Geosci. 2011, 4, 293–297. [Google Scholar] [CrossRef]
- Lovelock, C.E. Soil Respiration and Belowground Carbon Allocation in Mangrove Forests. Ecosystems 2008, 11, 342–354. [Google Scholar] [CrossRef]
- Alongi, D.M. Mangrove forests: Resilience, protection from tsunamis, and responses to global climate change. Estuar. Coast. Shelf Sci. 2008, 76, 1–13. [Google Scholar] [CrossRef]
- Rasquinha, D.N.; Mishra, D.R. Tropical cyclones shape mangrove productivity gradients in the Indian subcontinent. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon Cycling and Storage in Mangrove Forests. Annu. Rev. Mar. Sci. 2014, 6, 195–219. [Google Scholar] [CrossRef]
- Bhomia, R.K.; Kauffman, J.B.; McFadden, T.N. Ecosystem carbon stocks of mangrove forests along the Pacific and Caribbean coasts of Honduras. Wetl. Ecol. Manag. 2016, 24, 187–201. [Google Scholar] [CrossRef]
- Alongi, D.M. Global Significance of Mangrove Blue Carbon in Climate Change Mitigation. Science 2020, 2, 67. [Google Scholar] [CrossRef]
- Kankare, V.; Vastaranta, M.; Holopainen, M.; Räty, M.; Yu, X.; Hyyppä, J.; Hyyppä, H.; Alho, P.; Viitala, R. Retrieval of Forest Aboveground Biomass and Stem Volume with Airborne Scanning LiDAR. Remote Sens. 2013, 5, 2257–2274. [Google Scholar] [CrossRef] [Green Version]
- Kosoy, N.; Corbera, E. Payments for ecosystem services as commodity fetishism. Ecol. Econ. 2010, 69, 1228–1236. [Google Scholar] [CrossRef]
- Friess, D.A.; Rogers, K.; Lovelock, C.E.; Krauss, K.W.; Hamilton, S.E.; Lee, S.Y.; Lucas, R.; Primavera, J.; Rajkaran, A.; Shi, S. The State of the World’s Mangrove Forests: Past, Present, and Future. Annu. Rev. Environ. Resour. 2019, 44, 89–115. [Google Scholar] [CrossRef] [Green Version]
- Veettil, B.K.; Pereira, S.F.R.; Quang, N.X. Rapidly diminishing mangrove forests in Myanmar (Burma): A review. Hydrobiologia 2018, 822, 19–35. [Google Scholar] [CrossRef]
- Spalding, M. World Atlas of Mangroves; Routledge: London, UK, 2010; ISBN 9781849776608. [Google Scholar]
- Oo, N. Present state and problems of mangrove management in Myanmar. Trees 2002, 16, 218–223. [Google Scholar] [CrossRef]
- De Alban, J.D.T.; Jamaludin, J.; de Wen, D.W.; Than, M.M.; Webb, E.L. Improved estimates of mangrove cover and change reveal catastrophic deforestation in Myanmar. Environ. Res. Lett. 2020, 15, 034034. [Google Scholar] [CrossRef]
- GGGI MM03 Mangrove Restoration Program in Myanmar—Global Green Growth Institute. Available online: https://gggi.org/project/project-reference-profiles-myanmarmm03-mangrove-restoration-program-in-myanmar/ (accessed on 29 November 2021).
- Aye, W.N.; Wen, Y.; Marin, K.; Thapa, S.; Tun, A.W. Contribution of Mangrove Forest to the Livelihood of Local Communities in Ayeyarwaddy Region, Myanmar. Forests 2019, 10, 414. [Google Scholar] [CrossRef] [Green Version]
- Galidaki, G.; Zianis, D.; Gitas, I.; Radoglou, K.; Karathanassi, V.; Tsakiri–Strati, M.; Woodhouse, I.; Mallinis, G. Vegetation biomass estimation with remote sensing: Focus on forest and other wooded land over the Mediterranean ecosystem. Int. J. Remote Sens. 2017, 38, 1940–1966. [Google Scholar] [CrossRef] [Green Version]
- Hashim, T.; Suratman, M.N. Mangrove Biomass: Review on Estimation Methods and the Applications of Remote Sensing Approach; Mangroves; FRIM: Kuala Lumpr, Malaysia, 2020. [Google Scholar]
- DMH. Department of Meteorology and Hydrology. Available online: https://www.moezala.gov.mm/ (accessed on 15 November 2021).
- Asadi, M.A.; Yona, D.; Saputro, S.E. Species Diversity, Biomass, and Carbon Stock Assessments of Mangrove Forest in Labuhan, Indonesia. In Proceedings of the IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018; Volume 151. [Google Scholar]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1963; ISBN 9780252725487. [Google Scholar]
- Gevaña, D.; Pampolina, N.M. Plant Diversity and Carbon Storage of a Rhizopora Stand in Verde Passage, San Juan, Batangas, Philippines. J. Environ. Sci. Manag. 2010, 12, 1–10. [Google Scholar]
- Sharma, C.M.; Baduni, N.P.; Gairola, S.; Ghildiyal, S.K.; Suyal, S. Tree diversity and carbon stocks of some major forest types of Garhwal Himalaya, India. For. Ecol. Manag. 2010, 260, 2170–2179. [Google Scholar] [CrossRef]
- Lumbres, R.I.C.; Palaganas, J.A.; Micosa, S.C.; Laruan, K.A.; Besic, E.D.; Yun, C.-W.; Lee, Y.-J. Floral diversity assessment in Alno communal mixed forest in Benguet, Philippines. Landsc. Ecol. Eng. 2014, 10, 361–368. [Google Scholar] [CrossRef]
- Wim, G.; Stephan, W.; Max, Z.; Liesbeth, S. Mangrove Guidebook Forsoutheastasia; FAO Regional Office for Asia and the Pacific: Bangkok, Thailand, 2006; ISBN 9747946858. [Google Scholar]
- Komiyama, A.; Poungparn, S.; Kato, S. Common allometric equations for estimating the tree weight of mangroves. J. Trop. Ecol. 2005, 21, 471–477. [Google Scholar] [CrossRef]
- Chave, J.; Coomes, D.; Jansen, S.; Lewis, S.L.; Swenson, N.G.; Zanne, A.E. Towards a worldwide wood economics spectrum. Ecol. Lett. 2009, 12, 351–366. [Google Scholar] [CrossRef]
- FAO. State of the World’s Forests 2011; Food and Agriculture Organization of the United Nations: Rome, Italy, 2011. [Google Scholar]
- IPCC 2006 IPCC Guidelines for National Greenhouse Gas Inventories—IPCC. Available online: https://www.ipcc.ch/report/2006-ipcc-guidelines-for-national-greenhouse-gas-inventories/ (accessed on 17 September 2021).
- Feldpausch, T.R.; Rondon, M.A.; Fernandes, E.C.M.; Riha, S.J.; Wandelli, E. Carbon and Nutrient Accumulation in Secondary Forests Regenerating on Pastures in Central Amazonia. Ecol. Appl. 2004, 14, 164–176. [Google Scholar] [CrossRef] [Green Version]
- Kauffman, J.B.; Donato, D.C. Protocols for the Measurement, Monitoring and Reporting of Structure, Biomass and Carbon Stocks in Mangrove Forests; CFIOR: Bogor, Indonesia, 2012. [Google Scholar] [CrossRef]
- Kauffman, J.B.; Arifanti, V.B.; Basuki, I.; Kurnianto, S.; Novita, N.; Murdiyarso, D.; Donato, D.C.; Warren, M.W. Protocols for the Measurement, Monitoring, and Reporting of Structure, Biomass, Carbon Stocks and Greenhouse Gas Emissions in Tropical Peat Swamp Forests; Center for International Forestry Research: Bogor, Indonesia, 2016. [Google Scholar] [CrossRef]
- Baskerville, G.L. Use of Logarithmic Regression in the Estimation of Plant Biomass. Can. J. For. Res. 1972, 2, 49–53. [Google Scholar] [CrossRef]
- Altanzagas, B.; Luo, Y.; Altansukh, B.; Dorjsuren, C.; Fang, J.; Hu, H. Allometric Equations for Estimating the Above-Ground Biomass of Five Forest Tree Species in Khangai, Mongolia. Forests 2019, 10, 661. [Google Scholar] [CrossRef] [Green Version]
- Sprugel, D.G. Correcting for Bias in Log-Transformed Allometric Equations. Ecology 1983, 64, 209–210. [Google Scholar] [CrossRef]
- Edwards, P.J.; Tomlinson, P.B. The Botany of Mangroves. Kew Bull. 1986, 42. [Google Scholar] [CrossRef]
- Zöckler, C.; Aung, C. The Mangroves of Myanmar. In Sabkha Ecosystems; Springer: Berlin/Heidelberg, Germany, 2019; pp. 253–268. [Google Scholar] [CrossRef]
- Faridah-Hanum, I.; Kudus, K.A.; Saari, N.S. Plant Diversity and Biomass of Marudu Bay Mangroves in Malaysia. Pakistan J. Bot. 2012, 44, 151–156. [Google Scholar]
- Thatoi, H.; Samantaray, D.; Das, S.K. The genus Avicennia, a pioneer group of dominant mangrove plant species with potential medicinal values: A review. Front. Life Sci. 2016, 9, 267–291. [Google Scholar] [CrossRef] [Green Version]
- Duke, N. Phenologies and Litter Fall of Two Mangrove Trees, Sonneratia alba Sm. and S. caseolaris (L.) Engl., And Their Putative Hybrid, S. × Gulngai N.C. Duke. Aust. J. Bot. 1988, 36, 473–482. [Google Scholar] [CrossRef]
- Nasrin, S.; Hossain, M.; Alam, R.M. A Monograph on Sonneratia Apetala Buch.-Ham.; Lap Lambert Academic Publishing: Saarbrücken, Germany, 2017; ISBN 3330052511. [Google Scholar]
- Schaefer-Novelli, C.; Cintron, G.; Novelli, Y.S. Methods for Studying Mangrove Structure. In The Mangrove Ecosystem: Research Methods; Snedaker, S.C., Snedaker, J.G., Eds.; United Nations Educational, Scientific and Cultural Organization: Paris, France, 1984; pp. 91–113. Available online: http://www.sciepub.com/reference/14979 (accessed on 2 December 2021).
- Curtis, J.T.; McIntosh, R.P. The Interrelations of Certain Analytic and Synthetic Phytosociological Characters. Ecology 1950, 31, 434–455. [Google Scholar] [CrossRef]
- Sahu, S.C.; Kumar, M.; Ravindranath, N.H. Carbon Stocks in Natural and Planted Mangrove forests of Mahanadi Mangrove Wetland, East Coast of India. Curr. Sci. 2016, 110, 2253–2260. [Google Scholar] [CrossRef]
- Kridiborworn, P.; Chidthaisong, A.; Yuttitham, M.; Tripetchkul, S. Carbon Sequestration by Mangrove Forest Planted Specifically for Charcoal Production in Yeesarn, Samut Songkram. J. Sustain. Energy Environ. 2012, 3, 87–92. [Google Scholar]
- Kairo, J.; Mbatha, A.; Murithi, M.M.; Mungai, F. Total Ecosystem Carbon Stocks of Mangroves in Lamu, Kenya; and Their Potential Contributions to the Climate Change Agenda in the Country. Front. For. Glob. Chang. 2021, 4, 151. [Google Scholar] [CrossRef]
- Salum, R.B.; Souza-Filho, P.W.M.; Simard, M.; Silva, C.A.; Fernandes, M.E.B.; Cougo, M.F.; Do Nascimento, W.; Rogers, K. Improving mangrove above-ground biomass estimates using LiDAR. Estuar. Coast. Shelf Sci. 2020, 236, 106585. [Google Scholar] [CrossRef]
- Harishma, K.M.; Sandeep, S.; Sreekumar, V.B. Biomass and carbon stocks in mangrove ecosystems of Kerala, southwest coast of India. Ecol. Process. 2020, 9, 1–9. [Google Scholar] [CrossRef]
- Komiyama, A.; Ong, J.E.; Poungparn, S. Allometry, biomass, and productivity of mangrove forests: A review. Aquat. Bot. 2008, 89, 128–137. [Google Scholar] [CrossRef]
- Cairns, M.A.; Brown, S.; Helmer, E.H.; Baumgardner, G.A. Root biomass allocation in the world’s upland forests. Oecologia 1997, 111, 1–11. [Google Scholar] [CrossRef]
- O’Connor, E. ENERGY CROPS | Biomass Production. In Encyclopedia of Applied Plant Sciences; Elsevier: Amsterdam, The Netherlands, 2003; pp. 266–273. [Google Scholar]
- Camacho, L.D.; Gevaña, D.T.; Carandang, A.P.; Camacho, S.C.; Combalicer, E.A.; Rebugio, L.L.; Youn, Y.-C. Tree biomass and carbon stock of a community?managed mangrove forest in Bohol, Philippines. For. Sci. Technol. 2011, 7, 161–167. [Google Scholar] [CrossRef]
- Abino, A.C.; Lee, Y.J.; Castillo, J.A.A. Species Diversity, Biomass, and Carbon Stock Assessments of a Natural Mangrove Forest in Palawan, Philippines. Pakistan J. Bot. 2014, 46, 1955–1962. [Google Scholar]
- Jachowski, N.R.A.; Quak, M.S.Y.; Friess, D.A.; Duangnamon, D.; Webb, E.L.; Ziegler, A.D. Mangrove biomass estimation in Southwest Thailand using machine learning. Appl. Geogr. 2013, 45, 311–321. [Google Scholar] [CrossRef]
- Brahma, B.; Nath, A.J.; Deb, C.; Sileshi, G.W.; Sahoo, U.K.; Das, A.K. A critical review of forest biomass estimation equations in India. Trees For. People 2021, 5, 100098. [Google Scholar] [CrossRef]
- Ghasemi, A.; Fallah, A.; Joibary, S.S. Allometric equations for estimating standing biomass of Avicennia marina in Bushehr of Iran. Off. J. İstanbul Univ.-Cerrahpaşa Fac. For. 2016, 66, 691–697. [Google Scholar] [CrossRef]
- Kebede, B.; Soromessa, T. Allometric equations for aboveground biomass estimation of Olea europaea L. subsp. cuspidatain Mana Angetu Forest. Ecosyst. Health Sustain. 2018, 4, 1–12. [Google Scholar] [CrossRef] [Green Version]



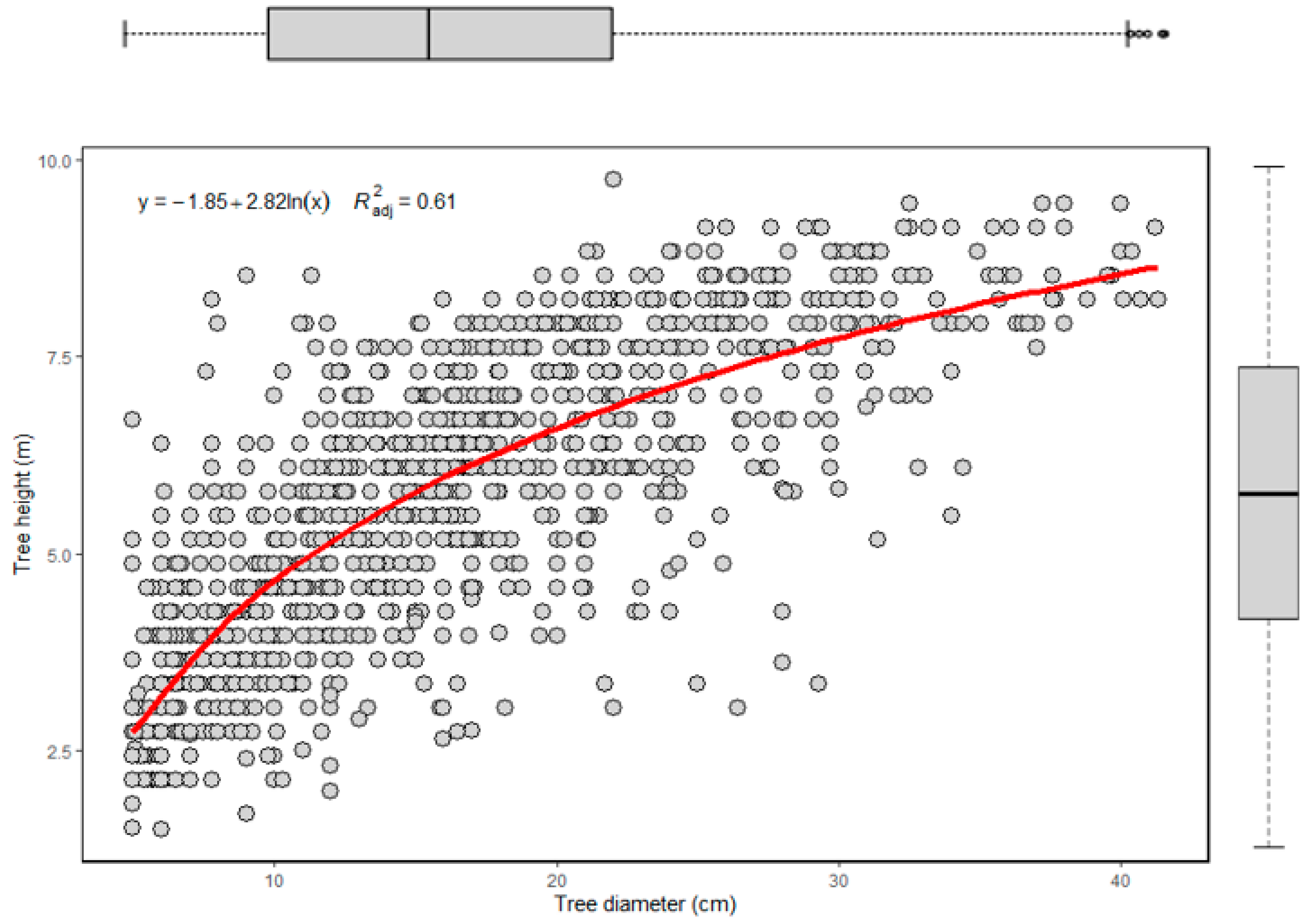
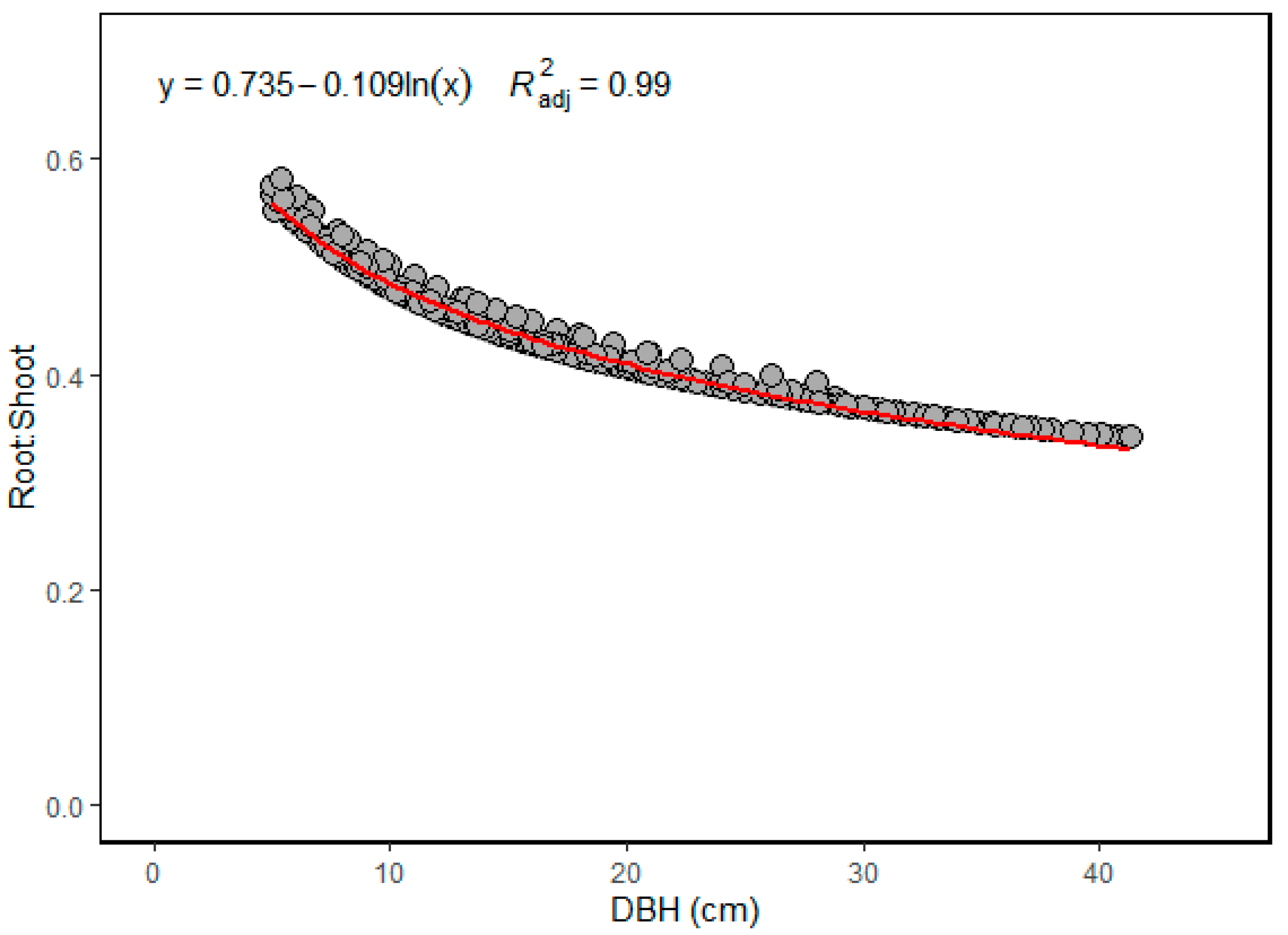
| Plot | Stand Density (Stems ha−1) | Species | DBH Range (cm) | Height Range (m) | Basal Area (m2 ha−1) | Biomass (Mg ha−1) | C-Stock Mg C ha−1 | CO2 Equivalent (MgCO2 eq) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| AGB | BGB | TB | ||||||||
| 1 | 1250 | Ao, Sa | 5.00–29.00 | 3.05–9.75 | 19.950 | 131.077 | 55.307 | 186.38 | 83.18 | 305.26 |
| 2 | 1125 | Ao, Sa | 8.00–34.00 | 3.05–8.23 | 21.725 | 145.769 | 61.048 | 206.82 | 92.32 | 338.82 |
| 3 | 875 | Ao, Sa | 5.50–34.00 | 2.44–7.93 | 26.000 | 214.807 | 83.486 | 298.29 | 135.52 | 490.01 |
| 4 | 875 | Ao | 5.00–35.30 | 2.13–8.53 | 19.375 | 159.381 | 62.136 | 221.52 | 99.14 | 363.14 |
| 5 | 875 | Ao | 5.20–31.80 | 2.74–8.23 | 25.725 | 211.055 | 82.610 | 293.67 | 131.41 | 482.29 |
| 6 | 875 | Ao | 5.30–37.00 | 2.74–8.23 | 33.900 | 294.506 | 111.881 | 406.39 | 182.05 | 668.13 |
| 7 | 625 | Ao | 16.40–40.00 | 5.18–9.45 | 42.950 | 405.162 | 147.636 | 552.80 | 248.00 | 910.18 |
| 8 | 1000 | Ao | 5.50–40.70 | 2.74–9.14 | 47.750 | 425.534 | 159.583 | 585.12 | 262.24 | 962.42 |
| 9 | 1625 | Ao | 7.60–41.20 | 3.05–9.14 | 69.125 | 603.928 | 228.753 | 832.68 | 373.06 | 1369.13 |
| 10 | 1000 | Ao | 6.60–37.20 | 4.27–9.50 | 30.025 | 248.525 | 96.739 | 345.26 | 154.54 | 567.14 |
| 11 | 1125 | Ao | 6.00–40.10 | 3.66–9.45 | 41.025 | 352.219 | 134.529 | 486.75 | 218.01 | 800.09 |
| 12 | 750 | Ao | 6.10–39.50 | 3.35–9.14 | 29.575 | 259.835 | 98.089 | 357.92 | 160.38 | 588.58 |
| 13 | 1375 | Ao | 5.00–41.30 | 3.05–9.14 | 51.650 | 446.873 | 170.086 | 616.96 | 276.36 | 1014.26 |
| 14 | 1525 | Ac, Ao, Sa | 5.00–26.60 | 2.13–8.23 | 17.075 | 113.658 | 49.255 | 162.91 | 72.63 | 266.55 |
| 15 | 1075 | Ao, Sa | 5.90–28.80 | 2.13–9.14 | 24.325 | 187.260 | 75.120 | 262.38 | 117.31 | 430.52 |
| 16 | 1875 | Ao, Sa, Sc | 5.10–35.60 | 2.13–9.50 | 41.225 | 302.509 | 121.188 | 423.70 | 189.44 | 695.26 |
| 17 | 975 | Ao | 5.50–38.80 | 2.13–8.84 | 30.275 | 258.542 | 99.003 | 357.55 | 160.13 | 587.66 |
| 18 | 2025 | Ao | 5.40–37.60 | 2.13–9.14 | 43.800 | 346.801 | 138.028 | 484.83 | 216.83 | 795.76 |
| 19 | 1075 | Ao | 6.10–29.00 | 3.66–8.84 | 22.900 | 171.747 | 70.439 | 242.19 | 108.19 | 397.07 |
| 20 | 925 | Ao | 8.30–23.50 | 3.35–8.23 | 17.750 | 126.148 | 53.263 | 179.41 | 80.06 | 293.83 |
| 21 | 1175 | Ao, Sa, Sc | 6.50–37.00 | 2.13–7.62 | 30.025 | 229.082 | 91.594 | 320.68 | 143.39 | 526.24 |
| 22 | 1075 | Ac, Aa, Ao, Bs, Sc | 5.10–36.70 | 1.70–7.93 | 22.875 | 166.665 | 66.580 | 233.25 | 104.30 | 382.78 |
| 23 | 950 | Ac, Ao, Sa | 5.00–31.00 | 1.50–6.86 | 17.200 | 122.801 | 49.746 | 172.55 | 77.12 | 283.02 |
| 24 | 550 | Ac, Sa | 5.00–17.00 | 2.13–5.49 | 4.925 | 26.691 | 12.363 | 39.05 | 17.37 | 63.73 |
| 25 | 950 | Ac, Sa, Sc | 5.50–25.90 | 2.44–7.62 | 14.800 | 83.713 | 35.868 | 119.58 | 53.33 | 195.73 |
| Mean | 6.22–33.94 | 2.76–8.53 | 29.838 | 241.372 | 94.173 | 335.55 | 150.25 | 551.10 | ||
| Standard deviation | 2.32–6.26 | 0.84–0.96 | 14.032 | 132.731 | 48.728 | 181.41 | 81.35 | 298.64 | ||
| Species | Biomass (Mg ha−1) | C-Stock (Mg C ha−1) | ||
|---|---|---|---|---|
| AGB | BGB | AGC | BGC | |
| Avicennia officinalis | 5484.659 | 2119.947 | 2577.790 | 826.779 |
| Sonneratia apetala | 420.535 | 177.029 | 197.651 | 69.041 |
| Sonneratia caseolaris | 94.672 | 41.148 | 44.496 | 16.048 |
| Aegiceras corniculutum | 33.951 | 15.947 | 15.957 | 6.219 |
| Avicennia alba | 0.213 | 0.120 | 0.100 | 0.047 |
| Bruguiera sexangula | 0.256 | 0.141 | 0.120 | 0.055 |
| Species | Mean DBH | Biomass (Mg ha−1) | Vegetation Carbon Stock (Mg C ha−1) | ||||
|---|---|---|---|---|---|---|---|
| AGB | BGB | TB | AGC | BGC | TVC | ||
| Avicennia officinalis | 17.66 ± 8.47 | 6.319 ± 6.888 | 2.442 ± 2.425 | 8.761 ± 9.312 | 2.970 ± 3.237 | 0.953 ± 0.946 | 3.922 ± 4.183 |
| Sonneratia apetala | 13.43 ± 6.13 | 2.731 ± 2.885 | 1.150 ± 1.105 | 3.880 ± 3.989 | 1.283 ± 1.356 | 0.448 ± 0.431 | 1.732 ± 1.787 |
| Sonneratia caseolaris | 13.81 ± 5.79 | 2.058 ± 2.181 | 0.895 ± 0.853 | 2.953 ± 3.034 | 0.967 ± 1.025 | 0.349 ± 0.333 | 1.316 ± 1.358 |
| Aegiceras corniculutum | 9.24 ± 3.08 | 1.061 ± 0.836 | 0.498 ± 0.357 | 1.559 ± 1.192 | 0.499 ± 0.393 | 0.194 ± 0.139 | 0.693 ± 0.532 |
| Avicennia alba | 5.20 | 0.213 | 0.120 | 0.332 | 0.100 | 0.047 | 0.147 |
| Bruguiera sexangula | 5.10 | 0.256 | 0.141 | 0.397 | 0.120 | 0.055 | 0.175 |
| Total | 16.64 ± 8.23 | 5.476 ± 6.438 | 2.136 ± 2.279 | 7.612 ± 8.716 | 2.574 ± 3.026 | 0.833 ± 0.889 | 3.407 ± 3.914 |
| Structural Variables | Pearson Correlation Coefficient with AGC (Mg C ha−1) | p-Value |
|---|---|---|
| Mean DBH (cm) | 0.8033 | 3.94 × 10−06 |
| Mean H (m) | 0.6838 | 3.21 × 10−04 |
| BA (m2/ha) | 0.9921 | <2.2 × 10−16 |
| Model | Adj. R2 (%) | RMSE | AIC | BIC | bptest | CF | p-Value |
|---|---|---|---|---|---|---|---|
| Model 1 | 67.92 | 0.267 | 10.570 | 13.977 | 0.521 | 1.0398 | 8.14 × 10−07 |
| Model 2 | 46.25 | 0.346 | 22.440 | 25.847 | 0.382 | 1.0677 | 0.000214 |
| Model 3 | 97.21 | 0.079 | −45.586 | −42.180 | 0.230 | 1.0034 | <2.2 × 10−16 |
| Model 4 | 97.28 | 0.076 | −45.350 | −40.808 | 0.187 | 1.0033 | <2.2 × 10−16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aye, W.N.; Tong, X.; Tun, A.W. Species Diversity, Biomass and Carbon Stock Assessment of Kanhlyashay Natural Mangrove Forest. Forests 2022, 13, 1013. https://doi.org/10.3390/f13071013
Aye WN, Tong X, Tun AW. Species Diversity, Biomass and Carbon Stock Assessment of Kanhlyashay Natural Mangrove Forest. Forests. 2022; 13(7):1013. https://doi.org/10.3390/f13071013
Chicago/Turabian StyleAye, Wai Nyein, Xiaojuan Tong, and Aung Wunna Tun. 2022. "Species Diversity, Biomass and Carbon Stock Assessment of Kanhlyashay Natural Mangrove Forest" Forests 13, no. 7: 1013. https://doi.org/10.3390/f13071013






