Abstract
Biochar, a soil conditioner, has the potential to improve soil properties and plant productivity. However, in forestry planting, especially in subtropical moso bamboo forests, the response of seedling growth to biochar addition is still not well known. We conducted a comprehensive factorial experiment with biochar and nitrogen (N) addition as factors (no biochar and no N addition; 0.64% biochar + 0% NH4NO3; 1.28% biochar + 0% NH4NO3; T3: 0% biochar + 1.28% NH4NO3; T4: 0.64% biochar + 1.28% NH4NO3; T5: 1.28% biochar + 1.28% NH4NO3) to study their effects on moso bamboo seedling growth, rhizosphere soil nutrient contents, and enzymatic activity. Our results indicate that applying biochar without N did not promote the growth of moso bamboo seedlings (biomass of leaves and branches) but increased soil nutrient content and affected soil-enzyme activity. The combined application of biochar and N significantly increased the leaf and branch biomass of moso bamboo seedlings and soil nutrient content and affected soil-enzyme activity. In conclusion, biochar should be mixed with an adequate amount of N for its application in subtropical moso bamboo forests to promote seedling growth and improve economic benefits.
1. Introduction
Biochar is a highly aromatic substance with 60%–85% carbon (C) content, which is produced by the pyrolysis of plant biomass under complete or partial hypoxia conditions [1]. Soil amendments using biochar have been shown to affect soil physical properties, such as texture, porosity structure, pore size distribution, and available water capacity [2,3,4]. A recent meta-analysis showed that biochar treatment significantly improved soil physical properties by reducing soil bulk density by an average of 7.6% and increasing porosity by an average of 8.4%, aggregate stability by 8.2%, saturated water conductivity by 25.2%, and effective water-holding capacity (AWC) by 15.1% [5]. In addition, recent studies have highlighted the important role of biochar in mitigating heavy-metal pollution in soils [6,7].
In addition to the physical properties of soil, biochar can also contribute to soil fertility improvement and affect plant growth [8]. A stable-nitrogen (N) isotopic-labeling experiment showed that 29%–45% of N from biochar was incorporated into the soil [9]. A recent study showed that biochar can also control the N cycle and retain soil nutrients by reducing NO2 emissions [10]. Clough and Condron (2010) [11] showed that adding biochar to soil could change the process of N migration, including soil N transformation, loss, and leaching, providing plants with higher nutrient utilization efficiency. In addition, biochar can also help plants cope with drought and other stresses; for example, Abbas et al. found that the addition of biochar (3.0% and 5.0% (w/w)) could significantly reduce the level of drought stress in 45-day-old wheat plants [12]. Ding et al. suggested that cellulose in biochar could potentially control sodium and potassium uptake under salt stress, thereby promoting plant growth [13]. Moreover, biochar can also change soil enzyme activity and enhance plant development [7]. For example, Turan found that there was a potential for a pistachio shell biochar and dicalcium phosphate combination to improve soil enzymatic activities, plant nutritional quality, and antioxidant defense system [14].
Although many studies have shown that biochar can lead to soil improvement, its mechanism of increasing yield is still in the stage of conjecture and exploration [7,14]. A recent study showed that biochar reduced nitrate leaching and increased soil water content but did not increase yield [15]. Furthermore, the addition of biochar without additional fertilizer can reduce crop yields [16]. Biochar modification in soil–plant systems has been observed with different results in different subjects; therefore, more research is needed to predict the effects and possibilities of biochar use on a global scale, and to provide insights into the impact of biochar on changing soil properties or plant growth processes [12]. However, most studies on biochar focus on agricultural systems, and only a few reports have focused on forestry, especially in subtropical economic forests, which seriously restricts the promotion and utilization of biochar [17].
Moso bamboo (Phyllostachys pubescens J.Houz.) is one of the most important non-wood forest products worldwide and an excellent alternative for wood production [15]. In China, moso bamboo forests are among the most important economic plantations in subtropical areas. These plantations comprise 4.56 million ha accounting for 74% of all bamboo forests in the country [18]. Compared to most other forest types, moso bamboo shows higher biomass productivity and a shorter rotation period (4–5 years) [19]. Nutrient consumption from budding to bamboo is very fast, resulting in a decline in soil fertility [20]. The lack of fertile soil may restrict the growth of moso bamboo seedlings, resulting in a reduction in the economic profit from the moso bamboo forest. Therefore, the application of N fertilizer has become an increasingly common phenomenon in moso bamboo forests in China [21].
Given the shortage of research into biochar and its application in forestry and the frequent application of N fertilizer in moso bamboo producing areas in southeast China, it is necessary to evaluate the effects of biochar on bamboo seedling growth with or without N fertilizer application. In this study, a comprehensive factorial experiment with biochar and N addition as factors was carried out. Specifically, our objectives were to: (1) verify how rhizosphere soil properties and enzymatic activity respond to biochar application, (2) determine whether biochar application can promote moso bamboo seedling growth, and (3) determine whether the effect of biochar was altered by N application. The results of this study will reveal the relationship between biochar, soil, and plants and may provide new insights into biochar fertilization in moso bamboo plantations to improve productivity.
2. Materials and Methods
2.1. Sample and Biochar Preparation
The experiment was conducted in the moso bamboo forest of Miaoshanwu Nature Reserve, Fuyang District, Zhejiang Province (119.95° E, 29.48° N) (Figure 1). The altitude of this area is 130 m, and it has a typical subtropical monsoon climate, with an average annual temperature of 16.1 °C and an average annual rainfall of 1441.9 mm, which occurs mainly from April to September.
Figure 1.
Location of the experimental area, Miaoshanwu Nature Reserve, Zhejiang province, China.
The soil was collected from the 0–15 cm soil layer of the Miaoshanwu Nature Reserve. After air drying, the soil was sieved through a 2 mm mesh screen to remove visible stones and roots. Initial chemical properties were analyzed according to previously described methods [14], as follows: soil organic C (SOC) 18.70 ± 2.63 g kg−1, soil total N (TN) 0.91 ± 0.12 g kg−1, soil total potassium (TK) 11.2 ± 0.08 g kg−1, soil total phosphorus (TP) 0.27 ± 0.02 g kg−1, and pH 4.80 ± 0.23. Moso bamboo seedlings with relatively uniform height (38.35 ± 1.02 cm) and basal diameter (2.77 ± 0.58 mm) were used as experimental materials.
The properties of biochar largely depend on the raw material. In the present study, moso bamboo branches were used as raw material to prepare biochar. Moso bamboo branches are typically considered waste, being directly burned and causing environmental and social problems such as air pollution and fires. In the experiment, biochar in the experiment was obtained by carbonizing moso bamboo branches at 500 °C for 3 h at the Yaoshi Biochar Industry Corporation (Lin’an, Zhejiang, China), with initial pH of 9.02, TN of 7.9 g kg−1, TP of 1.2 g kg−1, TK of 7.8 g kg−1, SOC of 785.2 g kg−1, and ash content of 17.4%.
2.2. Experimental Design
Based on Tammeorg et al. [16] and local moso bamboo fertilization levels, two N addition ratio treatments (0% NH4NO3 and 1.28% NH4NO3 (w/w)) and three biochar addition ratio treatments (0% biochar, 0.64% biochar, and 1.28% biochar (w/w)) were used. Overall, there were six treatments with six replicates (stands) each in this study: (1) no biochar and no N addition (CK); (2) low biochar and no N addition (T1, 0.64% biochar + 0% NH4NO3); (3) high biochar and no N addition (T2, 1.28% biochar + 0% NH4NO3); (4) no biochar and N addition (T3, 0% biochar + 1.28% NH4NO3); (5) low biochar and N addition (T4, 0.64% biochar + 1.28% NH4NO3); (6) high biochar and N addition (T5, 1.28% biochar + 1.28% NH4NO3). Each pot was filled with 6 kg of prepared soil or soil mixture in the proportions mentioned in the above six treatments. Each plot was managed at 65% of the maximum water-holding capacity of the soil. The plants were placed in 25 cm × 30 cm plastic containers (approximately 10 L capacity) in March 2018. To avoid the influence of rainfall on the soil water content, the experiment was performed in a greenhouse with sunlight and ventilation, but no contact with rain. All other physical conditions were maintained as consistently as possible to ensure the comparability of samples.
2.3. Measurement
In November 2018, four healthy moso bamboo seedlings were harvested from each treatment. The leaves and branches of all moso bamboo seedlings were separated, bagged, marked, and then transported to the laboratory for drying at 70 °C before weighing.
The rhizosphere soil of each pot was collected by the shaking method. Specifically, plants were removed with small shovels, and the roots were vigorously shaken to separate the attached soil. Soil samples were then passed through a 2 mm mesh and stored at 4 °C for soil analysis.
TN content in the leaves and branches of moso bamboo seedlings were measured directly using an elemental analyzer (CHN-O-RAPID, Heraeus, Germany). Soil pH was determined using a soil:deionized water paste (1:2.5, w/v). Determination of SOC was performed using the potassium dichromate (K2Cr2O7) heating oxidation method, soil TP was analyzed using the ammonium molybdate method with colorimetry, and TN was measured with an elemental analyzer (CHN-O-RAPID, Heraeus, Germany). The weight difference between fresh (20 g) and dried soil at 105 °C for 24 h was used to measure the soil water content [17].
Soil enzyme activities related to soil C and N turnover were also determined. Invertase was determined using 3,5-dinitrosalicylic acid colorimetric method, and the activity was expressed as the mass (mg) of glucose produced by 1 g of soil after 24 h. Cellulase activity was determined using the nitrosalicylic acid colorimetric method, and the activity was expressed as the mass of glucose (mg) produced by 10 g of soil after 72 h. Urease was determined using the sodium phenol–sodium hypochlorite colorimetric method, and the activity was expressed as the mass (mg) of NH3–N in 1 g of soil after 24 h. Peroxidase and polyphenol oxidase were determined using the colorimetric method, and the activity was expressed as the mass (mg) of purple gallic acid in 1 g of soil after 2 h [22].
2.4. Data Analysis
One-way analysis of variance (ANOVA) was used to compare the effects of different treatments on organ biomass, nutrient content, and soil characteristics of moso bamboo seedlings. Analyses were performed in SPSS 23.0 (IBM, Armonk, NY, USA), and the significance level of all statistical tests was p ≤ 0.05. Correlation analysis was used to evaluate the relationship between the soil properties and biomass. AMOS 25.0 (SPSS Inc., Armonk, NY, USA) was used for path analysis of soil properties and the leaf and branch biomass of moso bamboo.
3. Results
3.1. Leaf and Branch Biomass under Different Treatments
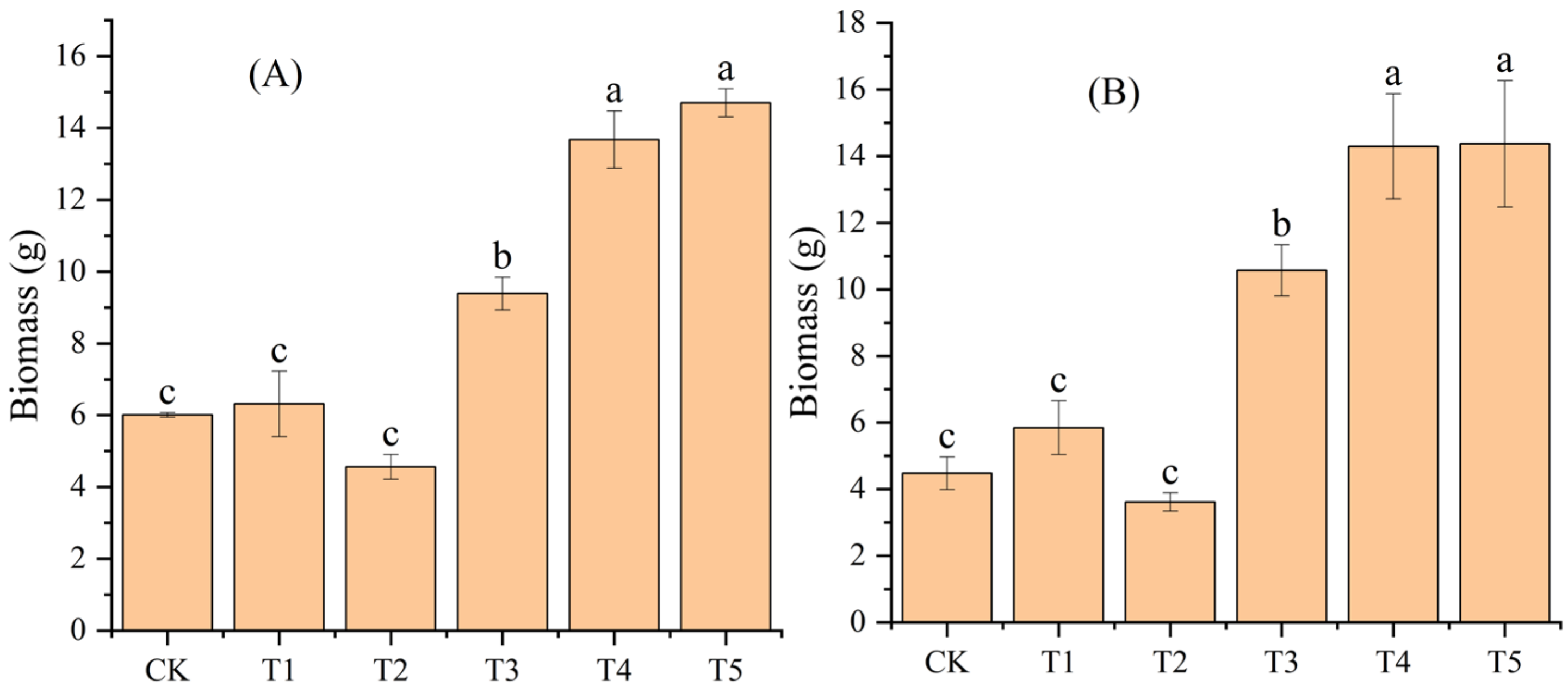
Compared to CK, the addition of biochar (T1 and T2) had no significant effect on the leaf and branch biomass of moso bamboo seedlings (p > 0.05), while adding N (T3, T4, and T5) significantly increased them (p < 0.05) (Figure 2). Compared to the addition of N (T3), the addition of biochar and N (T4 and T5) increased the leaf and branch biomass of moso bamboo seedlings in both groups (Figure 2)
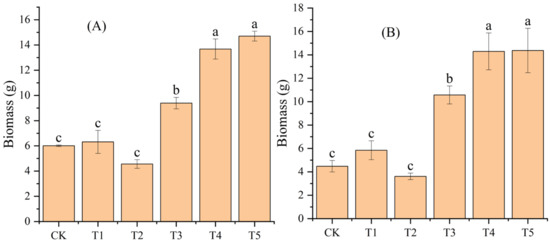
Figure 2.
Effects of different treatments on leaf (A) and branch (B) biomass of moso bamboo seedlings. CK: no biochar and no N addition; T1: 0.64% biochar + 0% NH4NO3; T2: 1.28% biochar + 0% NH4NO3; T3: 0% biochar + 1.28% NH4NO3; T4: 0.64% biochar + 1.28% NH4NO3; T5: 1.28% biochar + 1.28% NH4NO3. Bars (standard deviation) with different lowercase letters are significantly different at p < 0.05.
3.2. TN and TC of Leaves and Branches under Different Treatments
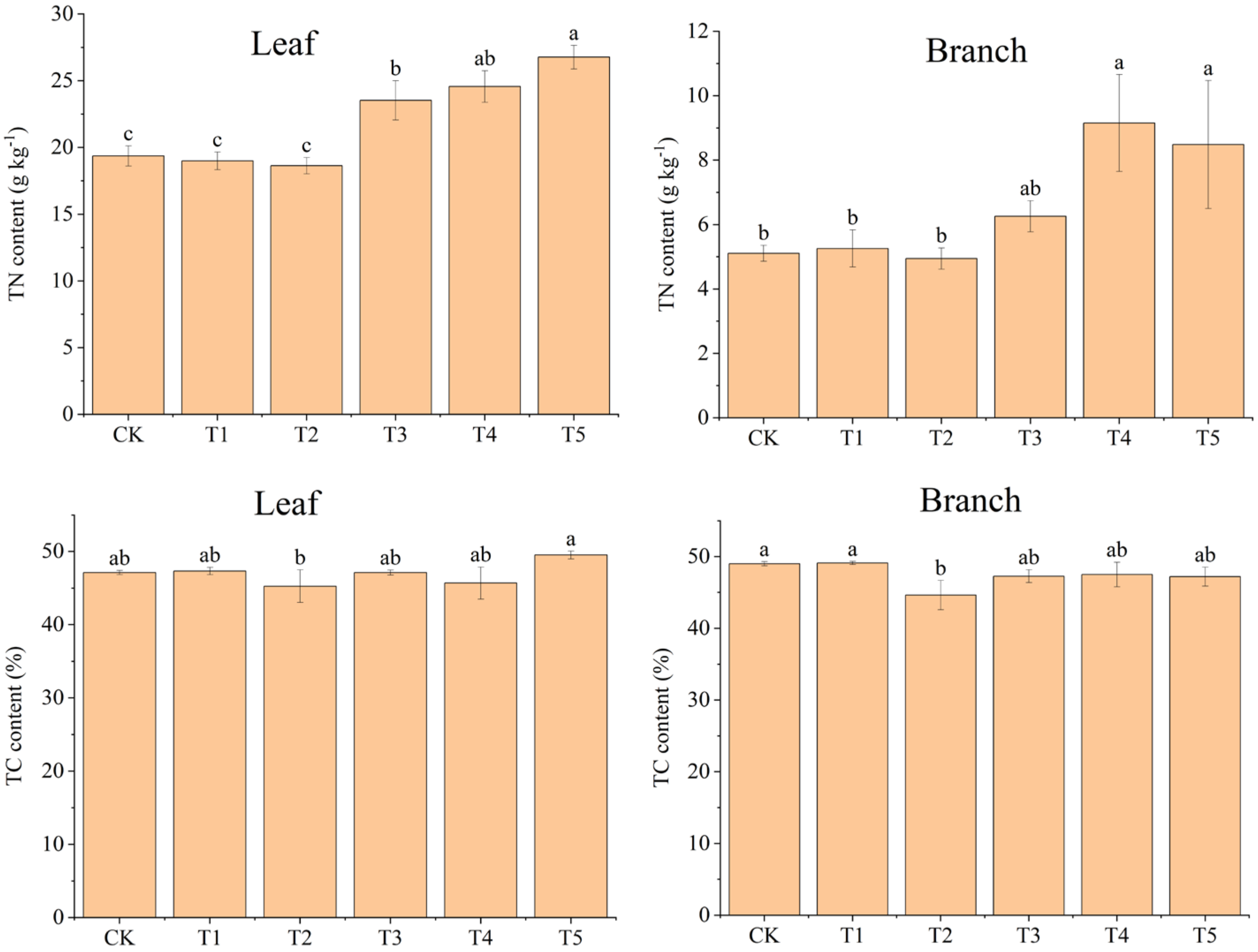
Compared to CK, the addition of biochar (T1 and T2) had no significant effect on the TN content of moso bamboo seedling leaves and branches (p > 0.05), while adding N (T3, T4, and T5) significantly increased it in both organs (p < 0.05) (Figure 3). Compared to solely N addition (T3), adding biochar and N (T4 and T5) increased TN content in the leaves and branches of moso bamboo seedlings (Figure 3).
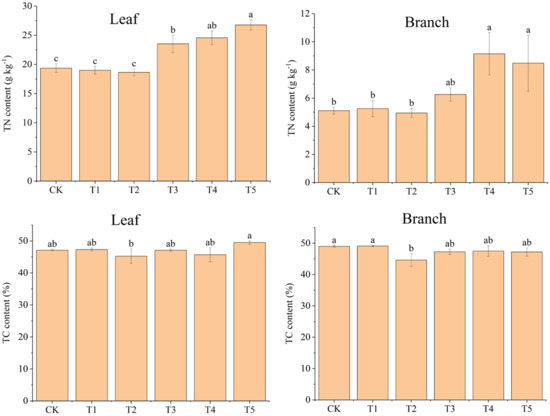
Figure 3.
Effects of different treatments on total nitrogen (TN) and total carbon (TC) contents of moso bamboo (leaf and branch). CK: no biochar and no N addition; T1: 0.64% biochar + 0% NH4NO3; T2: 1.28% biochar + 0% NH4NO3; T3: 0% biochar + 1.28% NH4NO3; T4: 0.64% biochar + 1.28% NH4NO3; T5: 1.28% biochar + 1.28% NH4NO3. Bars (standard deviation) followed by different lowercase letters indicate significant difference at p < 0.05.
The TC content of moso bamboo seedling leaves in T5 (49.55%) was the highest among all treatments, and the other treatments presented TC content between 45.27% and 47.33% (Figure 3). The TC content of the moso bamboo seedling branches in T2 (44.63%) was the lowest among all treatments, and the other treatments presented a range of 47.27%–49.1% (Figure 3).
3.3. Soil Properties under Different Treatments
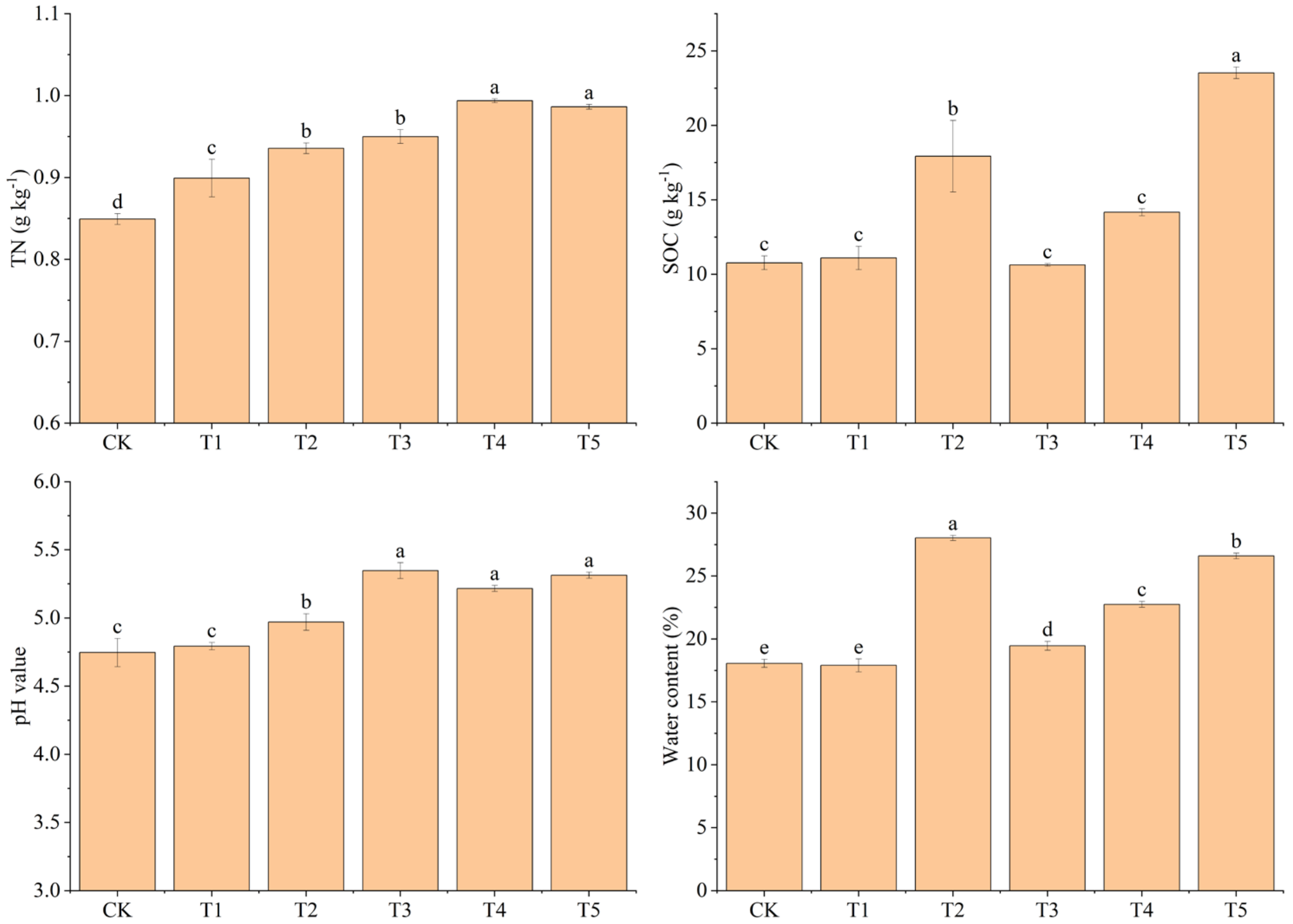
The addition of biochar increased the SOC content, with T5 presenting the highest value (23.53 g kg−1), followed by T2 (17.93 g kg−1) (Figure 4). Soil TN contents in T4 and T5, both of which were 0.99 g kg−1, were significantly higher than in other treatments (p < 0.05), and those in T2 (0.94 g kg−1) and T1 (0.90 g kg−1) were significantly higher than that in CK (0.85 g kg−1) (p < 0.05) (Figure 4). The soil pH value of T2 (4.97) was significantly higher than that of CK (4.75) (p < 0.05), while there was no significant difference between T1 (4.79) and CK (p > 0.05) (Figure 4). There was no significant difference in soil pH values of T3, T4, and T5 (p > 0.05), but they were all significantly higher than that of CK (p < 0.05). Soil water content in T2 (28.03%) was significantly higher than that in CK (18.07%) (p < 0.05), while there was no significant difference between T1 (17.90%) and CK (p > 0.05). The soil water contents of T3 (19.46%), T4 (22.75%), and T5 (26.59%) were significantly different (p < 0.05) (Figure 4).
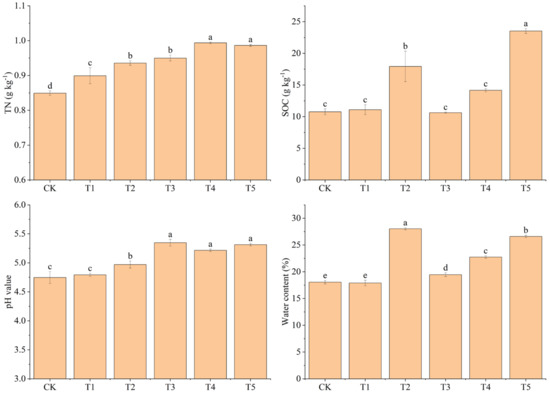
Figure 4.
Effects of different treatments on soil organic carbon (SOC), soil total nitrogen (TN), soil pH values, and soil water content. CK: no biochar and no N addition; T1: 0.64% biochar + 0% NH4NO3; T2: 1.28% biochar + 0% NH4NO3; T3: 0% biochar + 1.28% NH4NO3; T4: 0.64% biochar + 1.28% NH4NO3; T5: 1.28% biochar + 1.28% NH4NO3. Bars (standard deviation) followed by different lowercase letters indicate significant difference at p < 0.05.
Biochar addition did not change the activities of polyphenol oxidase and peroxidase in the soil. The addition of biochar significantly increased cellulase activity; cellulase activity in T5 was significantly higher than that in T4 and T3 (Table 1). It was observed that biochar increased the activity of invertase in both no N addition (T1 and T2) and N addition (T4 and T5) groups (Table 1). Biochar did not increase urease activity in either no N addition or N addition groups, while N addition significantly increased urease activity (p < 0.05) (Table 1).

Table 1.
Effects of different treatments on enzyme activities. The values are means ± SE (n = 3). CK: no biochar and no N addition; T1: 0.64% biochar + 0% NH4NO3; T2: 1.28% biochar + 0% NH4NO3; T3: 0% biochar + 1.28% NH4NO3; T4: 0.64% biochar + 1.28% NH4NO3; T5: 1.28% biochar + 1.28% NH4NO3. Different letters indicate statistical significance at p < 0.05 in the different treatments.
3.4. Relationship between Soil Properties and Biomass
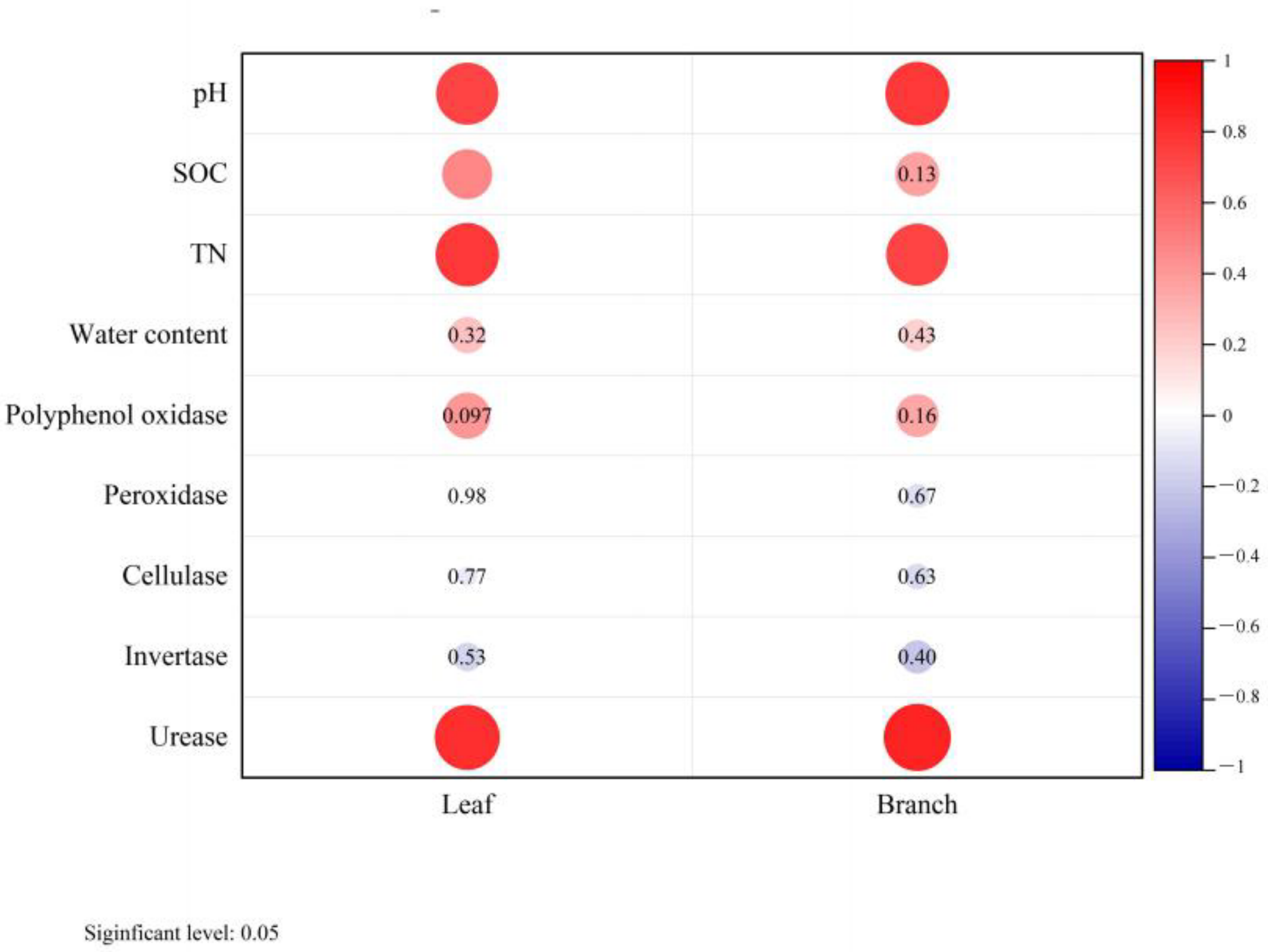
Correlation analysis reflected the relationship between soil factors and biomass. Leaf and branch biomass were both positively correlated with soil TN content and pH value, while urease activity was significantly correlated with the biomass of leaves, branches, and roots (Figure 5).
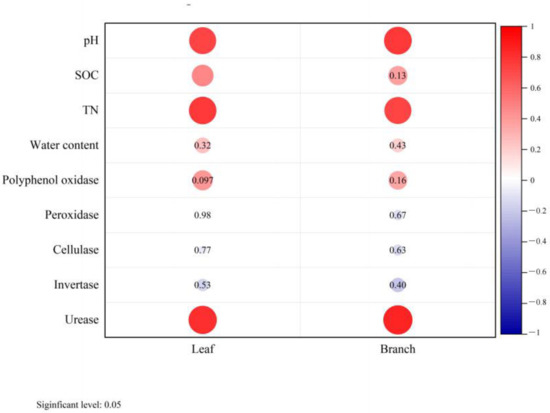
Figure 5.
Relationship between soil properties and biomass of moso bamboo leaf and branch. SOC: soil organic carbon, TN: soil total nitrogen. Red color indicates significant difference at p < 0.05.
3.5. Path Analysis
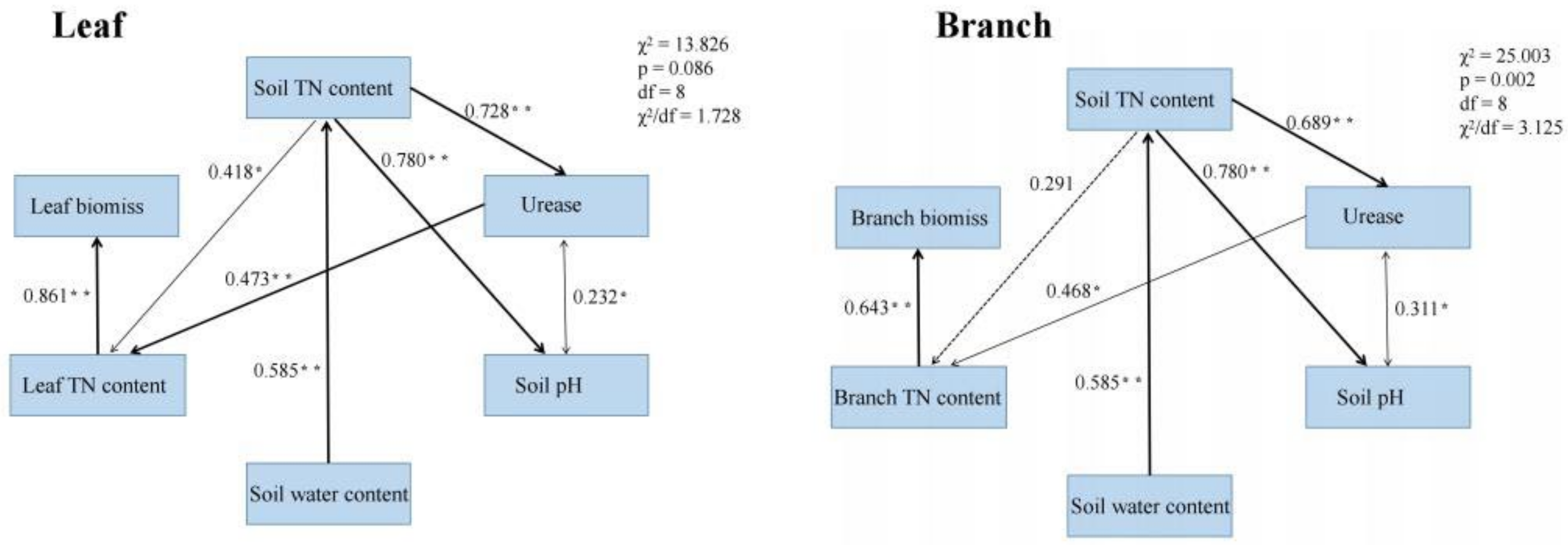
Path analysis showed that the TN content of leaves and branches positively affected the biomass of leaves and branches, respectively (path coefficients were 0.521 and 0.643, respectively; Figure 6). Soil water content positively affected soil TN content (path coefficient = 0.585, Figure 6), whereas soil TN content positively affected leaf TN content (path coefficient = 0.418, Figure 6). Soil pH was correlated with urease content; increased urease activity positively affected leaf and branch TN contents (path coefficients 0.473 and 0.468, Figure 6). Therefore, soil TN content was the key factor promoting the increase in leaf and branch biomass, while the change in soil properties caused by biochar addition indirectly affected the leaf and branch biomass by enhancing the soil TN content.
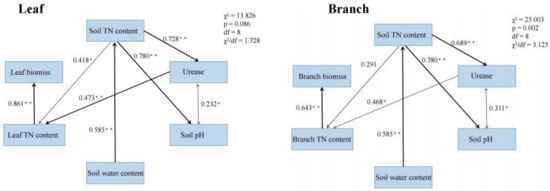
Figure 6.
Path analysis of leaf and branch biomass. Standardized path coefficients are shown next to the arrows. Asterisks (* and **) indicate significance at p ≤ 0.05 and ≤ 0.01, respectively. The goodness of fit statistics are displayed next to the modeling framework.
4. Discussion
4.1. Effects of Biochar on Rhizosphere Soil Characteristics
The results of our research confirmed the effectiveness of biochar in increasing SOC, TN, and soil water contents, which was consistent with findings of previous studies in which biochar significantly improved soil nutrient indicators [23]. Biochar is a carbon-rich material produced by the pyrolysis of biomass at high temperatures in the absence of oxygen, and when added to soil, biochar can increase the amount of SOC by releasing dissolved organic matter derived from it [24]. The ability of biochar to improve soil water content has been reported in many studies [25,26]. Owing to its developed pore structure and large surface energy, biochar increases the original pore structure and drainage capacity of the soil, enabling it to absorb more water and improve its water-holding capacity [26]. A recent study proposed the following important mechanisms for N retention after biochar addition to soil: (a) adsorption of NH4+-N due to the high cation exchange capacity of biochar and (b) reduced leaching of NO3−-N due to increased soil water content [27].
Biochar addition has been shown to increase soil pH values [27]. However, our study found that only a high amount of biochar addition significantly increased soil pH value without N addition; neither high nor low amounts of biochar addition significantly increased soil pH with N addition. Li et al. [28] observed that biochar application (10, 20, 40, and 60 t ha−1) did not affect soil pH in semi-arid regions and Werner et al. [29] found that the pH of sandy loam did not change with biochar addition. Therefore, whether biochar can change soil pH may be related to the original soil properties and the nature of the biochar used.
Biochar can alter soil enzyme activity by creating microhabitats and introducing unstable organic compounds for bacterial growth [30]. Enzymes are important factors of microbial activities in the soil microbial community and provide a driving force for biogeochemical cycles in soil ecosystems, playing an irreplaceable role in soil organic matter transformation, nutrient release, and fertility maintenance [31]. In this study, soil invertase and cellulase showed higher activities as more biochar was added to the soil. It has been reported that biochar contains stable C, and its addition increases the C content in the soil, and improves the ratio of C to N in the soil, and microbial activity, thus affecting sucrase activity [32]. In addition, the activities of polyphenol oxidase and peroxidase also changed with the addition of biochar. The net effect of biochar on soil enzyme activity can be indirect (e.g., microbial synthesis) or direct (e.g., surface adsorption), and the process is complex [33]. Therefore, the final soil enzyme activity depends on the chemical composition of biochar, application amount of biochar, soil type, and hydrothermal conditions.
4.2. Effects of Biochar on Moso Bamboo Seedling Growth
We found that adding of biochar alone did not significantly increase the biomass of moso bamboo seedlings, while the combination of biochar and N significantly increased the biomass of leaves and branches. Biochar has often been shown to improve crop productivity by improving soil water content and nutrient availability [34]. However, a report showed that crop yields did not improve after biochar addition; although applying 5–10 t ha−1 of biochar to fertile sandy clay loam in a boreal climate relieved transient water deficit, it did not improve yield [16]. Our study also observed the improvement in soil properties by biochar addition, such as increased soil water, SOC, and soil TN contents. However, the addition of biochar increases the soil pH value, thereby reducing the availability of certain nutrients (i.e., P, Mn, and Zn), which may offset the benefits of soil property improvement [35]. It should also be noted that (1) N in biochar is essentially unavailable because it is mainly present as heterocyclic aromatic N [36], and (2) the mineralizable portion of organic C in biochar may lead to initial net N immobilization [37], which is likely to neutralize the benefits of biochar in improving soil properties. The addition of rice-husk biochar reduced the available N content by 21% compared to that of the control soil due to the immobilization of N [29]. Lu et al. [28] concluded that biochar application could reduce NH4+-N and NO3–-N contents in soil by 6% and 12%, respectively.
Path analysis showed that soil TN content was the key factor in increasing leaf and branch biomass and had a positive effect. Although biochar addition could increase the total N content in the soil, it did not increase the N content in leaves and branches; the combined application of biochar and N significantly increased the total N content in soil, leaves, and branches. Biochar with a high C content has been suggested to increase the soil C/N ratio and lead to a decrease in available N in the soil, thus reducing plant N absorption [38]. The yield increase of biochar combined with N was related to the complementary or synergistic effect between biochar and N, because biochar extended the nutrient release period of N and reduced nutrient loss, whereas N eliminated the nutrient deficiency of biochar and reduced the soil C/N ratio [39].
In general, the present study has provided important insights regarding the application of biochar. Nevertheless, some specific issues will be better addressed in future research: (1) This study was a short-term greenhouse experiment, and a larger field experiment is needed in the future to evaluate the long-term interaction between soil, plants, and microorganisms for a better understanding of the long-term effects of combined application of biochar and N on the whole ecosystem (including aboveground–underground coupling); (2) For different types of soil, it is necessary to comprehensively compare the raw materials produced at different pyrolysis temperatures, which will help to determine the impact of raw materials on soil physical and chemical properties and enzyme activities; (3) There is a need to know more about how biochar affects soil nutrient transformation, to improve the understanding of the mechanism of biochar action [40].
5. Conclusions
In this study, it was found that the addition of biochar increased soil water, SOC, and TN contents and changed soil enzyme activity but did not promote the growth of moso bamboo seedlings. However, the combined application of biochar and N not only improved soil fertility but also significantly increased leaf and branch biomass of moso bamboo seedlings. Moreover, the path analysis showed that N was the key factor for the effect of biochar. Overall, our study provides useful insights regarding the utilization of biochar in forestry, particularly in moso bamboo. However, there is little literature on the usage of biochar in moso bamboo forests, and long-term field trials are needed to explore the mechanisms and potential applications of these relationships.
Author Contributions
Formal analysis, Y.X.; funding acquisition, X.G.; investigation, B.Z.; project administration, X.G. and B.Z.; software, N.Y.; writing–original draft, Y.X. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Public Projects of Zhejiang Province (LGN21C030002), the National Natural Science Foundation of China (31600492), the Fundamental Research Funds for the Central Non-profit Research Institution (CAFYBB2016SY006), and the National Key R&D Program of China (2021YFD220040205).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lehmann, J. A handful of carbon. Nature 2007, 447, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Amendola, C.; Montagnoli, A.; Terzaghi, M.; Trupiano, D.; Oliva, F.; Baronti, S.; Miglietta, F.; Chiatante, D.; Scippa, G.S. Short-term effects of biochar on grapevine fine root dynamics and arbuscular mycorrhizae production. Agric. Ecosyst. Environ. 2017, 239, 236–245. [Google Scholar] [CrossRef]
- Herath, H.M.S.K.; Camps-Arbestain, M.; Hedley, M. Effect of biochar on soil physical properties in two contrasting soils: An Alfisol and an Andisol. Geoderma 2013, 209–210, 188–197. [Google Scholar] [CrossRef]
- Kinney, T.; Masiello, C.; Dugan, B.; Hockaday, W.; Dean, M.; Zygourakis, K.; Barnes, R. Hydrologic properties of biochars produced at different temperatures. Biomass Bioenerg. 2012, 41, 34–43. [Google Scholar] [CrossRef]
- Oduor, M.O.; Xia, X.; Nahayo, A.; Liu, X.; Korai, P.K.; Pan, G. Quantification of biochar effects on soil hydrological properties using meta-analysis of literature data. Geoderma 2016, 274, 28–34. [Google Scholar] [CrossRef]
- Turan, V. Calcite in combination with olive pulp biochar reduces Ni mobility in soil and its distribution in chili plant. Int. J. Phytoremediat. 2022, 24, 166–176. [Google Scholar] [CrossRef]
- Turan, V. Arbuscular mycorrhizal fungi and pistachio husk biochar combination reduces Ni distribution in mungbean plant and improves plant antioxidants and soil enzymes. Physiol. Plant. 2021, 173, 418–429. [Google Scholar] [CrossRef]
- Cayuela, M.L.; van Zwieten, L.; Singh, B.P.; Jeffery, S.; Roig, A.; Sánchez-Monedero, M.A. Biochar’s role in mitigating soil nitrous oxide emissions: A review and meta-analysis. Agric. Ecosyst. Environ. 2014, 191, 5–16. [Google Scholar] [CrossRef]
- Taghizadeh-Toosi, A.; Clough, T.J.; Sherlock, R.R.; Condron, L.M. Biochar absorbed ammonia is bioavailable. Plant Soil 2012, 350, 57–69. [Google Scholar] [CrossRef]
- Bai, S.H.; Reverchon, F.; Xu, C.Y.; Xu, Z.; Blumfield, T.J.; Zhao, H.; Zwieten, L.V.; Wallace, H.M. Wood biochar increases nitrogen retention in field settings mainly through abiotic processes. Soil Biol. Biochem. 2015, 90, 232–240. [Google Scholar] [CrossRef]
- Clough, T.J.; Condron, L.M. Biochar and the nitrogen cycle: Introduction. J. Environ. Qual. 2010, 39, 1218–1223. [Google Scholar] [CrossRef] [PubMed]
- Abbas, T.; Rizwan, M.; Ali, S.; Adrees, M.; Mahmood, A.; Zia-ur-Rehman, M.; Ibrahim, M.; Arshad, M.; Qayyum, M.F. Biochar application increased the growth and yield and reduced cadmium in drought stressed wheat grown in an aged contaminated soil. Ecotoxicol. Environ. Saf. 2018, 148, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, L.; Zheng, B. Biochar to improve soil fertility. A review. Agron. Sustain. Dev. 2016, 36, 36. [Google Scholar] [CrossRef] [Green Version]
- Turan, V. Potential of pistachio shell biochar and dicalcium phosphate combination to reduce Pb speciation in spinach, improved soil enzymatic activities, plant nutritional quality, and antioxidant defense system. Chemosphere 2020, 245, 125611. [Google Scholar] [CrossRef] [PubMed]
- Haider, G.; Steffens, D.; Moser, G.; Müller, C.; Kammann, C.I. Biochar reduced nitrate leaching and improved soil moisture content without yield improvements in a four-year field study. Agric. Ecosyst. Environ. 2017, 237, 80–94. [Google Scholar] [CrossRef]
- Tammeorg, P.; Simojoki, A.; Mäkelä, P.; Stoddard, F.L.; Alakukku, L.; Helenius, J. Biochar application to a fertile sandy clay loam in boreal conditions: Effects on soil properties and yield formation of wheat, turnip rape and faba bean. Plant Soil 2014, 374, 89–107. [Google Scholar] [CrossRef]
- Ge, X.; Cao, Y.; Zhou, B.; Xiao, W.; Tian, X.; Li, M. Combined application of biochar and N increased temperature sensitivity of soil respiration but still decreased the soil CO2 emissions in moso bamboo plantations. Sci. Total Environ. 2020, 730, 139003. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, X.; Ni, H.; Gai, X.; Zhong, Z. Soil carbon and associated bacterial community shifts driven by fine root traits along a chronosequence of moso bamboo (Phyllostachys edulis) plantations in subtropical china. Sci. Total Environ. 2021, 752, 142333. [Google Scholar] [CrossRef]
- Buckingham, K.; Jepson, P.; Wu, L.; Rao, I.V.; Jiang, S.; Liese, W.; Lou, Y.; Fu, M. The potential of bamboo is constrained by outmoded policy frames. Ambio 2011, 40, 544–548. [Google Scholar] [CrossRef]
- Zhou, B.Z.; Gu, L.H.; Ding, Y.H.; Shao, L.; Wu, Z.M.; Yang, X.S.; Li, C.Z.; Li, Z.C.; Wang, X.M.; Cao, Y.H.; et al. The great 2008 Chinese storm its socioeconomic ecological impact and sustainability lessons learned. Bull. Am. Meteorol. Soc. 2011, 92, 47–60. [Google Scholar] [CrossRef] [Green Version]
- Song, X.Z.; Gu, H.H.; Wang, M.; Zhou, G.M.; Li, Q. Management practices regulate the response of moso bamboo foliar stoichiometry to nitrogen deposition. Sci. Rep. 2016, 6, 24107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Song, X.; Yrjälä, K.; Lv, J.; Li, Y.; Wu, J.; Qin, H. Biochar mitigates the effect of nitrogen deposition on soil bacterial community composition and enzyme activities in a Torreya grandis orchard-Science Direct. For. Ecol. Manag. 2020, 457, 117717. [Google Scholar] [CrossRef]
- Ma, H.; Egamberdieva, D.; Wirth, S.; Li, Q.; Omari, R.A.; Hou, M.; Bellingrath-Kimura, S.O. Effect of biochar and irrigation on the interrelationships among soybean growth, root nodulation, plant P uptake, and soil nutrients in a sandy field. Sustainability 2019, 11, 6452. [Google Scholar] [CrossRef] [Green Version]
- Aller, D.; Mazur, R.; Moore, K.; Hintz, R.; Laird, D.; Horton, R. Biochar age and crop rotation impacts on soil quality. Soil Sci. Soc. Am. J. 2017, 81, 1157–1167. [Google Scholar] [CrossRef]
- Zhang, D.; Yan, M.; Niu, Y.; Liu, X.; van Zwieten, L.; Chen, D.; Bian, R.; Cheng, K.; Li, L.; Joseph, S.; et al. Is current biochar research addressing global soil constraints for sustainable agriculturre. Agric. Ecosyst. Environ. 2016, 226, 25–32. [Google Scholar] [CrossRef]
- Jin, Z.; Chen, C.; Chen, X.; Hopkins, I.; Zhang, X.; Han, Z.; Jiang, F.; Billy, G. The crucial factors of soil fertility and rapeseed yield—A five year field trial with biochar addition in upland red soil, China. Sci. Total Environ. 2019, 649, 1467–1480. [Google Scholar] [CrossRef]
- Liu, Z.; He, T.; Cao, T.; Yang, T.; Meng, J.; Chen, W. Effects of biochar application on nitrogen leaching, ammonia volatilization and nitrogen use efficency in two distinct soils. J. Soil Sci. Plant Nut. 2017, 17, 515–528. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Wang, Y.; Liu, Y.; Wang, Y.; He, L.; Zhong, Z.; Yang, S. Effects of water-washed biochar on soil properties, greenhouse gas emissions, and rice yield. Clean Soil Air Water 2018, 46, 1700143. [Google Scholar] [CrossRef]
- Werner, S.; Katzl, K.; Wichern, M.; Buerkert, A.; Steiner, C.; Marschner, B. Agronomic benefits of biochar as a soil amendment after its use as waste water fltration medium. Environ. Pollut. 2018, 233, 561–568. [Google Scholar] [CrossRef]
- Lehmann, J.; Gaunt, J.; Rondon, M. Biochar sequestration in terrestrial ecosystems-a review. Mitig. Adapt. Strateg. Glob. Chang. 2006, 11, 403–427. [Google Scholar] [CrossRef]
- Aponte, H.; Meli, P.; Butler, B.; Paolini, J.; Matus, F.; Merino, C.; Cornejo, P.; Kuzyakov, Y. Meta Analysis of Heavy Metal Effects on Soil Enzyme Activities. Sci. Total Environ. 2020, 737, 139744. [Google Scholar] [CrossRef] [PubMed]
- Case, S.D.C.; McNamara, N.P.; Reay, D.S.; Whitaker, J. Can biochar reduce soil greenhouse gas emissions from a miscanthus bioenergy crop? GCB Bioenergy 2014, 6, 76–89. [Google Scholar] [CrossRef] [Green Version]
- Palansooriya, K.N.; Wong, J.T.F.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Chang, S.X.; Bolan, N.; Wang, H.; Ok, Y.S. Response of microbial communities to biochar amended soils: A critical review. Biochar 2019, 1, 3–22. [Google Scholar] [CrossRef] [Green Version]
- Jeffery, S.; Verheijen, F.G.A.; van der Velde, M.; Bastos, A.C. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Ye, L.; Camps-Arbestain, M.; Shen, Q.; Lehmann, J.; Singh, B.; Sabir, M. Biochar effects on crop yields with and without fertilizer: A meta-analysis of field studies using separate controls. Soil Use Manag. 2020, 36, 2–18. [Google Scholar] [CrossRef]
- Knicker, H. “Black nitrogen”—An important fraction in determining the recalcitrance of charcoal. Org. Geochem. 2010, 41, 947–950. [Google Scholar] [CrossRef]
- Bruun, E.W.; Ambus, P.; Egsgaard, H.; Hauggaard-Nielsen, H. Effects of slow and fast pyrolysis biochar on soil C and N turnover dynamics. Soil Biol. Biochem. 2012, 46, 73–79. [Google Scholar] [CrossRef]
- Asai, H.; Samson, B.K.; Stephan, H.M.; Songyikhangsuthor, K.; Homma, K.; Kiyono, Y.; Inoue, Y.; Shiraiwa, T.; Horie, T. Biochar amendment techniques for upland rice production in Northern Laos 1. Soil physical properties, leaf SPAD and grain yield. Field Crop. Res. 2009, 111, 81–84. [Google Scholar] [CrossRef]
- Laird, D.; Fleming, P.; Wang, B.; Horton, R.; Karlen, D. Biochar impact on nutrient leaching from a Midwestern agricultural soil. Geoderma 2010, 158, 436–442. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Yu, M.; Lu, X.; Tang, C.; Liu, X.; Brookes, P.C.; Xu, J. Combined application of biochar and nitrogen fertilizer benefits nitrogen retention in the rhizosphere of soybean by increasing microbial biomass but not altering microbial community structure. Sci. Total Environ. 2018, 640–641, 1221–1230. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).