Predicting Climate Change Impacts on the Rare and Endangered Horsfieldia tetratepala in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Current Species Data
2.2. Environmental Variables
2.3. Habitat Suitability Modeling
2.4. Predicting the Suitable Areas of Plants under Global Climate Change
3. Results
3.1. Model Performance and Contributions of the Variables
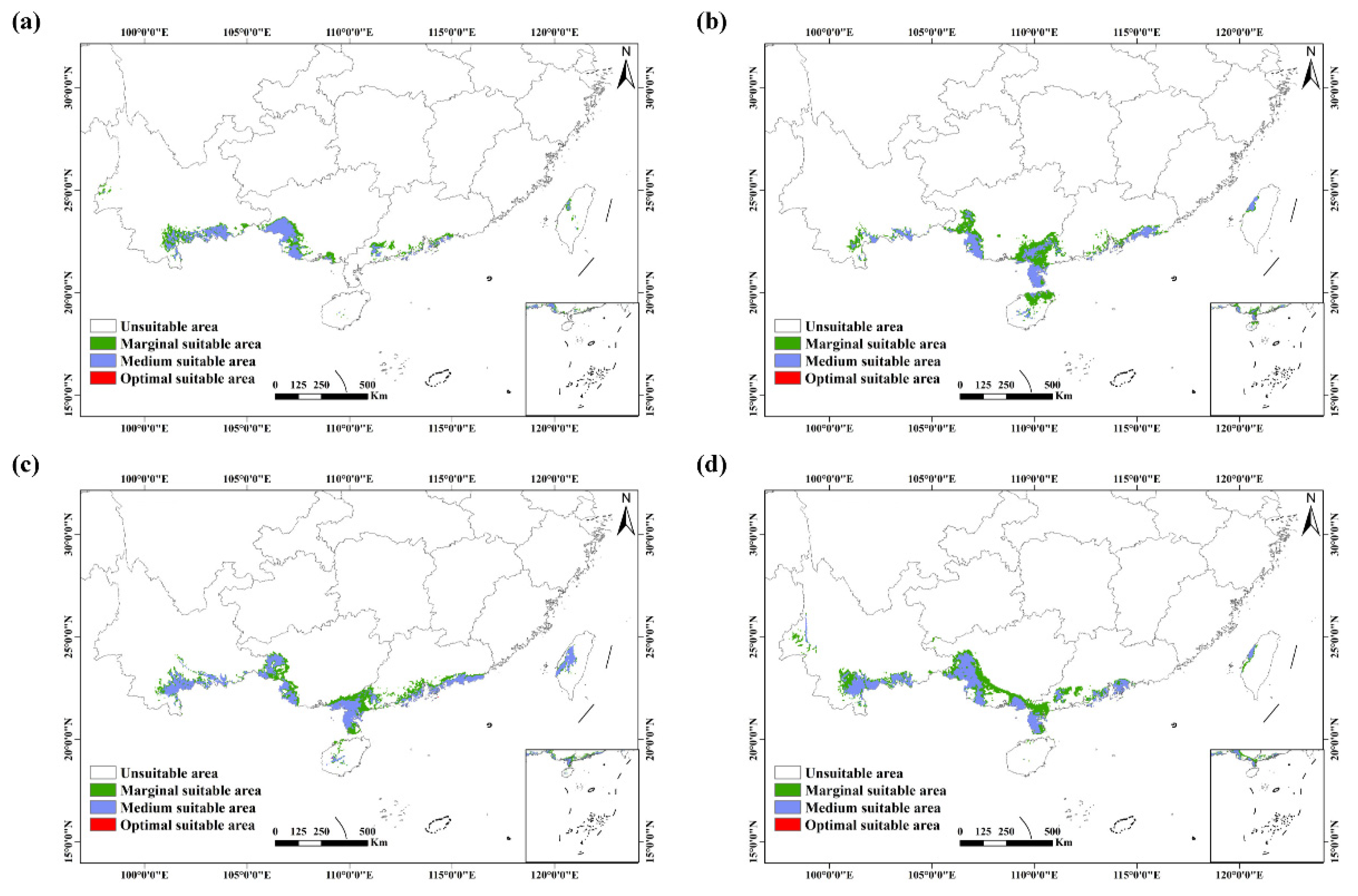
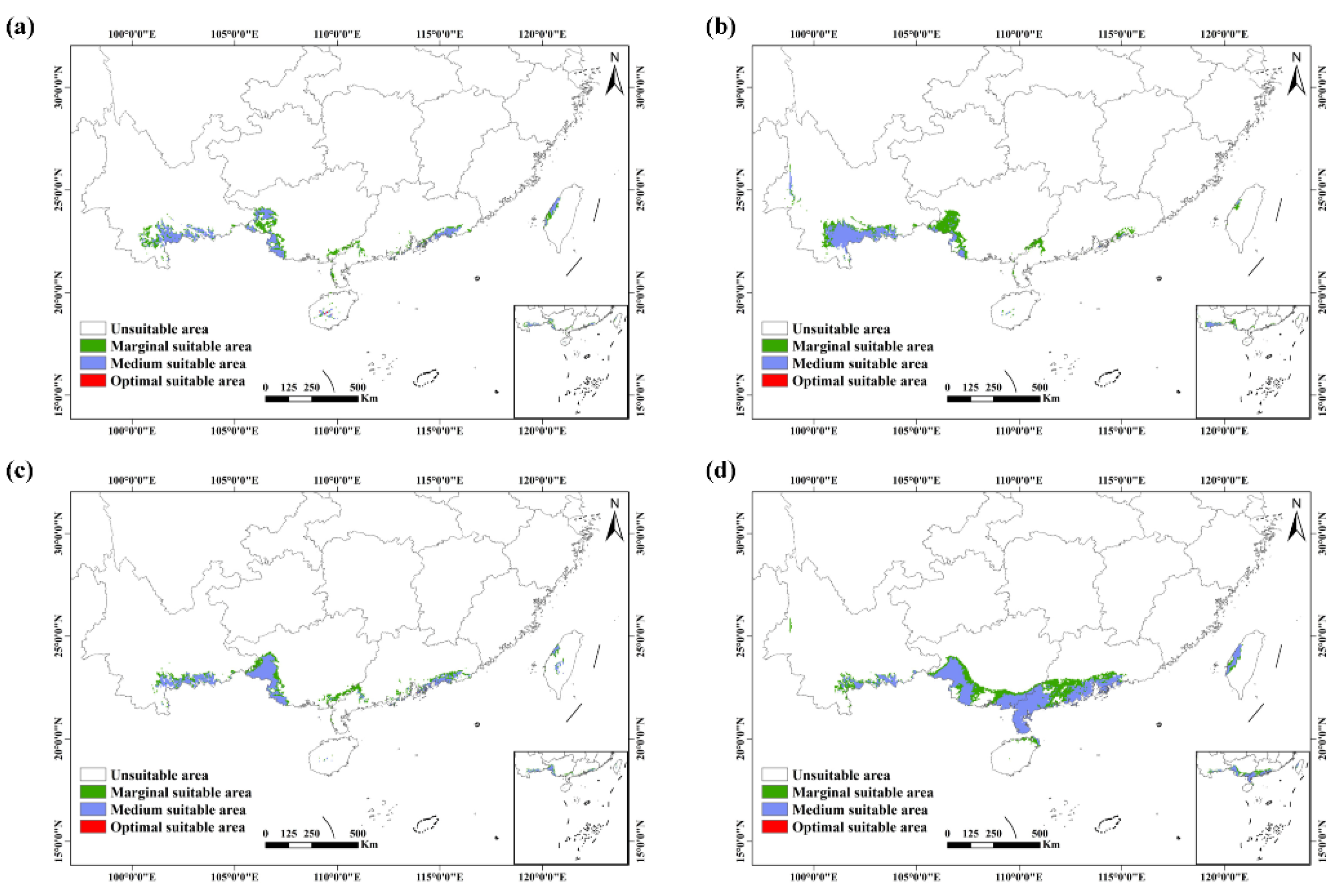
3.2. Potential Distribution under Future Climate Conditions
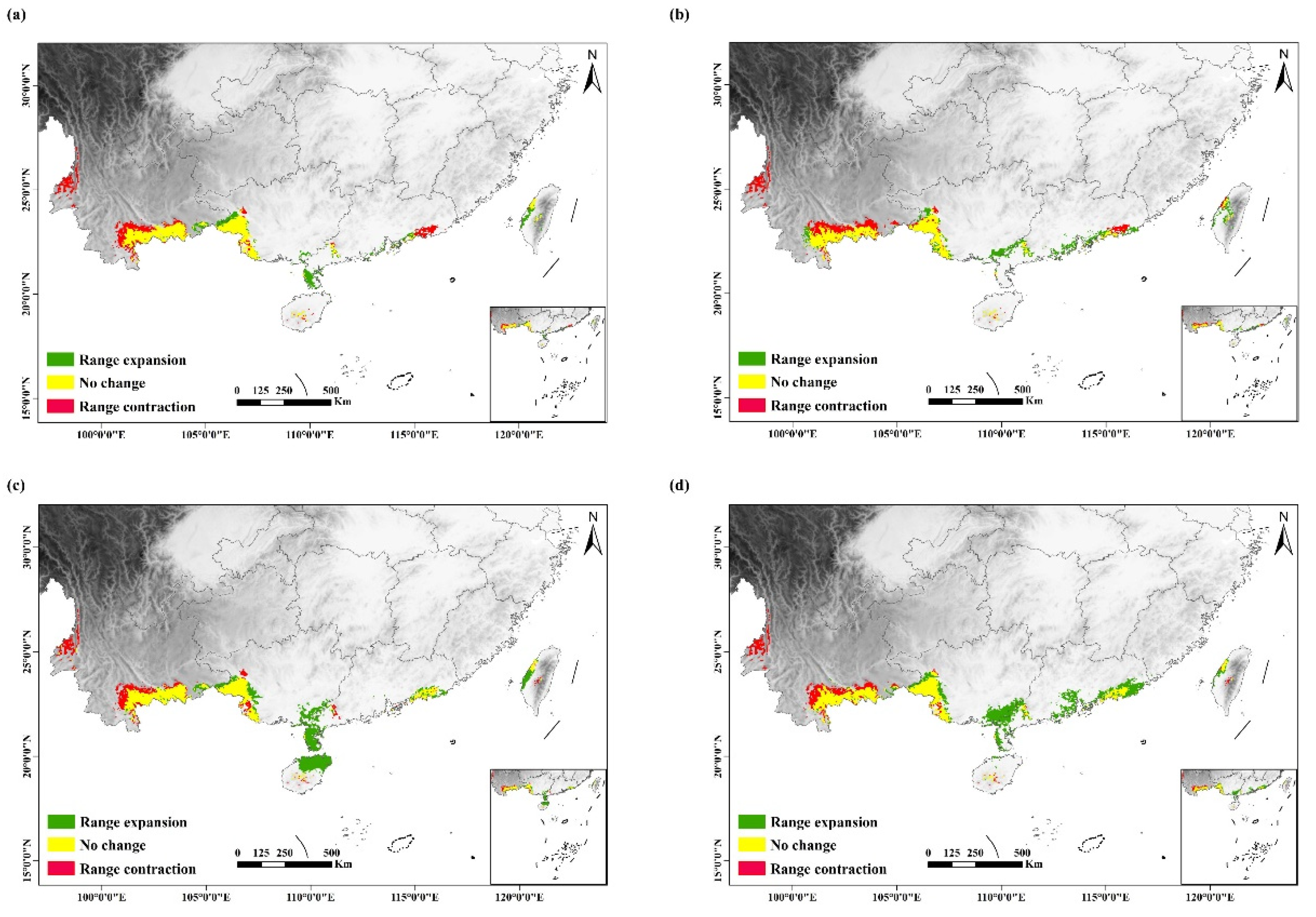
3.3. Future Changes in Suitable Habitats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meng, H.H.; Zhou, S.S.; Li, L.; Tan, Y.H.; Li, J.W.; Li, J. Conflict between biodiversity conservation and economic growth: Insight into rare plants in tropical China. Biodivers. Conserv. 2019, 28, 523–537. [Google Scholar] [CrossRef]
- Isbel, F.; Gonzalez, A.; Loreau, M.; Cowles, J.; Diaz, S.; Hector, A.; Mace, M.M.; Wardle, D.A.; O’Connor, M.I.; Duffy, J.M.; et al. Linking the influence and dependence of people on biodiversity across scales. Nature 2017, 546, 65–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, C.N.; Balmford, A.; Brook, B.W.; Buettel, J.C.; Galetti, M.; Lei, G.; Wilmshurst, J.M. Biodiversity losses and conservation responses in the Anthropocene. Science 2017, 356, 270–275. [Google Scholar] [CrossRef]
- Meng, H.H.; Gao, X.Y.; Song, Y.G.; Cao, G.L.; Li, J. Biodiversity arks in the Anthropocene. Reg. Sustain. 2021, 2, 109–115. [Google Scholar] [CrossRef]
- Lawton, J.H.; May, R.M. Extinction Rates; Oxford University Press: Oxford, UK, 1995. [Google Scholar]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.P.; Shen, X.L.; Grumbine, R.; Corlett, R. China’s biodiversity conservation research in progress. Biol. Conserv. 2017, 210, 1–2. [Google Scholar] [CrossRef]
- Parmesan, C. Climate and species’ range. Nature 1996, 382, 765–766. [Google Scholar] [CrossRef]
- Leach, K.; Zalat, S.; Gilbert, F. Egypt’s protected area network under future climate change. Biol. Conserv. 2013, 159, 490–500. [Google Scholar] [CrossRef]
- Li, B.; Wilson, T.K. Myristicaceae. In Flora of China; Wu, Z.Y., Raven, P.H., Eds.; Science Press: Beijing, China; Missouri Botanical Garden Press: St Louis, MO, USA, 2008; Volume 7, pp. 99–101. [Google Scholar]
- Bosso, L.; Luchi, N.; Maresi, G.; Cristinzio, G.; Smeraldo, S.; Russo, D. Predicting current and future disease outbreaks of Diplodia sapinea shoot blight in Italy: Species distribution models as a tool for forest management planning. For. Ecol. Manag. 2017, 400, 655–664. [Google Scholar] [CrossRef]
- Zhang, K.; Yao, L.; Meng, J.; Tao, J. Maxent modeling for predicting the potential geographical distribution of two peony species under climate change. Sci. Total Environ. 2018, 634, 1326–1334. [Google Scholar] [CrossRef]
- Wang, D.; Cui, B.C.; Duan, S.S.; Chen, J.J.; Fan, H.; Lu, B.B.; Zheng, J.H. Moving north in China: The habitat of Pedicularis kansuensis in the context of climate change. Sci. Total Environ. 2019, 697, 133979. [Google Scholar] [CrossRef]
- Ye, P.C.; Zhang, G.F.; Zhao, X.; Chen, H.; Si, Q.; Wu, J.Y. Potential geographical distribution and environmental explanations of rare and endangered plant species through combined modeling: A case study of Northwest Yunnan, China. Ecol. Evol. 2021, 11, 13052–13067. [Google Scholar] [CrossRef]
- Tang, S.L.; Song, Y.B.; Zeng, B.; Dong, M. Potential distribution of the extremely endangered species Ostrya rehderiana (Betulaceae) in China under future climate change. Environ. Sci. Pollut. Res. 2022, 29, 7782–7792. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Sun, J.J.; El-Kassaby, Y.A.; Yang, X.Y.; Tian, X.N.; Wang, T.L. Predicting potential habitat of a plant species with small populations under climate change: Ostrya rehderiana. Forests 2022, 13, 129. [Google Scholar] [CrossRef]
- Sala, O.E.; Chapin, F.S.; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef] [Green Version]
- Vegas-Vilarrúbia, T.; Nogué, S.; Rull, V. Global warming, habitat shifts and potential refugia for biodiversity conservation in the neotropical Guayana Highlands. Biol. Conserv. 2012, 152, 159–168. [Google Scholar] [CrossRef]
- Fung, E.; Imbach, P.; Corrales, L.; Vilchez, S.; Zamora, N.; Argotty, F.; Hannah, L.; Ramos, A. Mapping conservation priorities and connectivity pathways under climate change for tropical ecosystems. Clim. Change 2017, 141, 77–92. [Google Scholar] [CrossRef]
- Weiskopf, S.R.; Rubenstein, M.A.; Crozier, L.G.; Gaichas, S.; Griffis, R.; Halofsky, J.E.; Hyde, K.J.W.; Morelli, T.L.; Morisette, J.T.; Muñoz, R.C.; et al. Climate Change Effects on Biodiversity, Ecosystems, Ecosystem Services, and Natural Resource Management in the United States. Sci. Total Environ. 2020, 733, 137782. [Google Scholar] [CrossRef]
- Walther, G.R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef]
- Root, T.L.; Price, J.T.; Hall, K.R.; Schneider, S.H.; Rosenzweig, C.; Pounds, J.A. Fingerprints of global warming on wild animals and plants. Nature 2003, 421, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Mawdsley, J.R.; O’Malley, R.; Ojima, D.S. A review of climate-change adaptation strategies for wildlife management and biodiversity conservation. Conserv. Biol. 2009, 23, 1080–1089. [Google Scholar] [CrossRef]
- Chen, I.C.; Hill, J.K.; Ohlemuller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- Feeley, K.J.; Hurtado, J.; Saatchi, S.; Silman, M.R.; Clark, D.B. Compositional shifts in Costa Rican forests due to climate-driven species migrations. Glob. Change Biol. 2013, 19, 3472–3480. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Torres, D.A.; Navarro-Siguenza, A.G.; Santiago-Alarcon, D.; Rojas-Soto, O.R. Response of the endangered tropical dry forests to climate change and the role of Mexican Protected Areas for their conservation. Glob. Change Biol. 2016, 22, 364–379. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.G.; Petitpierre, B.; Deng, M.; Wu, J.P.; Kozlowski, G. Predicting climate change impacts on the threatened Quercus arbutifolia in montane cloud forests in southern China and Vietnam: Conservation implications. For. Ecol. Manag. 2019, 444, 269–279. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.X.; Wang, Y.; Zang, Z.H.; Deng, S.Y.; Lan, T.Y.; Xie, Z.Q.; Xiong, G.M.; Li, J.Q.; Shen, G.Z. Climate warming has changed phenology and compressed the climatically suitable habitat of Metasequoia glyptostroboides over the last half century. Glob. Ecol. Conserv. 2020, 23, e01140. [Google Scholar] [CrossRef]
- Lawler, J.J.; Shafer, S.L.; White, D.; Kareiva, P.; Maurer, E.P.; Blaustein, A.R.; Bartlein, P.J. Projected climate-induced faunal change in the Western Hemisphere. Ecology 2009, 90, 588–597. [Google Scholar] [CrossRef] [Green Version]
- Guillera-Arroita, G.; Lahoz-Monfort, J.J.; Elith, J.; Gordon, A.; Kujala, H.; Lentini, P.E.; McCarthy, M.A.; Tingley, R.; Wintle, B.A. Is my species distribution model fit for purpose? Matching data and models to applications. Glob. Ecol. Biogeogr. 2015, 24, 276–292. [Google Scholar] [CrossRef]
- Eyre, A.C.; Briscoe, N.J.; Harley, D.K.P.; Lumsden, L.F.; McComb, L.B.; Lentini, P.E. Using species distribution models and decision tools to direct surveys and identify potential translocation sites for a critically endangered species. Divers. Distrib. 2022, 28, 700–711. [Google Scholar] [CrossRef]
- Franklin, J. Species distribution models in conservation biogeography: Developments and challenges. Divers. Distrib. 2013, 19, 1217–1223. [Google Scholar] [CrossRef]
- Sun, J.J.; Feng, L.; Wang, T.L.; Tian, X.N.; He, X.; Xia, H.; Wang, W.F. Predicting the potential habitat of three endangered species of Carpinus genus under climate change and human activity. Forests 2021, 12, 1216. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W. Predicting species distribution: Offering more than simple habitat models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef] [PubMed]
- Tulloch, A.I.T.; Sutcliffe, P.; Naujokaitis-Lewis, I.; Tingley, R.; Brotons, L.; Ferraz, K.; Possingham, H.; Guisan, A.; Rhodes, J.R. Conservation planners tend to ignore improved accuracy of modelled species distributions to focus on multiple threats and ecological processes. Biol. Conserv. 2016, 199, 157–171. [Google Scholar] [CrossRef] [Green Version]
- Li, J.Y.; Chang, H.; Liu, T.; Zhang, C. The potential geographical distribution of Haloxylon across Central Asia under climate change in the 21st century. Agric. For. Meteorol. 2019, 275, 243–254. [Google Scholar] [CrossRef]
- Ramasamy, M.; Das, B.; Ramesh, R. Predicting climate change impacts on potential worldwide distribution of fall armyworm based on CMIP6 projections. J. Pest Sci. 2022, 95, 841–854. [Google Scholar] [CrossRef]
- Evans, J.S.; Murphy, M.A.; Holden, Z.A.; Cushman, S.A. Modeling Species distribution and change using random forest. In Predictive Species and Habitat Modeling in Landscape Ecology; Drew, C., Wiersma, Y., Huettmann, F., Eds.; Springer: New York, NY, USA, 2011. [Google Scholar]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Guisan, A.; Edwards, T.C.; Hastie, T. Generalized linear and generalized additive models in studies of species distributions: Setting the scene. Ecol. Model. 2002, 157, 89–100. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Jayasinghe, S.L.; Kumar, L. Modeling the climate suitability of tea [Camellia sinensis (L.) O. Kuntze] in Sri Lanka in response to current and future climate change scenarios. Agric. For. Meteorol. 2019, 272, 102–117. [Google Scholar] [CrossRef]
- Ab Lah, N.Z.; Yusop, Z.; Hashim, M.; Mohd Salim, J.; Numata, S. Predicting the habitat suitability of Melaleuca cajuputi based on the MaxEnt species distribution model. Forests 2021, 12, 1449. [Google Scholar] [CrossRef]
- State Forestry Administration of China. The Implementation Plan of Rescuing and Conserving China’s PSESP (2011–2015) (Nonpublic Publication); State Forestry Administration of China: Beijing, China, 2012. [Google Scholar]
- Jiang, Y.H. Ecological Characteristics and Endangered Reason Analysis of Horsfeldia hainanensis Merr. as an Extremely Small Population. Ph.D. Thesis, Central South University of Forestry and Technology, Changsha, China, 2018. [Google Scholar]
- Jiang, Y.H.; Liu, X.S.; Xiang, W.H.; Jiang, Y.; He, Y.H. Genetic diversity and structure analysis of the endangered plant species Horsfieldia hainanensis Merr. in China. Biotechnol. Biotechnol. Equip. 2018, 32, 95–101. [Google Scholar] [CrossRef] [Green Version]
- Kitamura, S.; Poonswad, P. Nutmeg-vertebrate interactions in the Asia-Pacific region: Importance of frugivores for seed dispersal in Myristicaceae. Trop Conserv. Sci. 2013, 6, 608–636. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.L.; Cai, N.H.; Wu, Y.; Duan, A.A. Fatty acid composition of several plants of Horsfieldia. China Oils Fats 2012, 37, 80–82. [Google Scholar]
- Jiang, Y.H.; Xiang, W.H.; Jiang, Y.; He, Y.H.; Lin, J.Y. Floristic composition, structure and phytogeographic characteristics of Horsfieldia hainanensis Merr. community in Guangxi. J. Beijing For. Univ. 2016, 381, 74–82. [Google Scholar]
- National Forestry and Grassland Administration. List of National Key Protected Wild Plants in China; National Forestry and Grassland Administration: Beijing, China, 2021. [Google Scholar]
- Cai, C.N.; Xiao, J.H.; Ci, X.Q.; Conran, J.G.; Li, J. Genetic diversity of Horsfieldia tetratepala (Myristicaceae), an endangered Plant Species with Extremely Small Populations to China: Implications for its conservation. Plant Syst. Evol. 2021, 307, 50. [Google Scholar] [CrossRef]
- He, G.Z.; Cai, L.; Liang, G.; Shi, Q.; Chen, L.W. Sowing and breeding technique of Horsfieldia hainanensis Merr. Pract. For. Technol. 2013, 6, 35–37. [Google Scholar]
- Ma, Q.; Zhan, R.; Chen, Y.G. Chemical constituents and biological activity of Horsfieldia plants. Guangzhou Chem. Ind. 2014, 42, 11–13. [Google Scholar]
- Wu, Y.; Mao, C.L.; Zhang, F.L.; Yang, X.L.; Zeng, J.S.; Duan, A.A. Taxonomic position of Horsfieldia pandurifolia Hu. (Myristicaceae). Bull. Bot. Res. 2015, 35, 652–659. [Google Scholar]
- Jiang, Y.H.; Liu, X.S.; Jiang, Y.; He, Y.H.; Xiang, W.H. Anatomical structure characteristics of vegetative organs of endangered plant Horsfieldia hainanensis. Guihaia 2018, 38, 843–850. [Google Scholar]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Wang, C.J.; Wan, J.Z. Assessing the habitat suitability of 10 serious weed species in global croplands. Glob. Ecol. Conserv. 2020, 23, e01142. [Google Scholar] [CrossRef]
- Du, J.J.; Chen, Z.W. Method of path analysis with SPSS linear regression. Bull. Biol. 2010, 45, 4–6. [Google Scholar]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Yi, Y.J.; Cheng, X.; Yang, Z.F.; Zhang, S.H. Maxent modeling for predicting the potential distribution of endangered medicinal plant (H. riparia Lour) in Yunnan, China. Ecol. Eng. 2016, 92, 260–269. [Google Scholar] [CrossRef]
- O’Neill, B.C.; Kriegler, E.; Ebi, K.L.; Kemp-Benedict, E.; Riahi, K.; Rothman, D.S.; van Ruijven, B.J.; van Vuuren, D.P.; Birkmann, J.; Kok, K.; et al. The roads ahead: Narratives for shared socioeconomic pathways describing world futures in the 21st century. Glob. Environ. Change 2017, 42, 169–180. [Google Scholar] [CrossRef] [Green Version]
- Riahi, K.; van Vuuren, D.P.; Kriegler, E.; Edmonds, J.; O’Neill, B.C.; Fujimori, S.; Bauer, N.; Calvin, K.; Dellink, R.; Fricko, O.; et al. The shared socioeconomic pathways and their energy, land use, and greenhouse gas emissions implications: An overview. Global Environ. Change 2017, 42, 153–168. [Google Scholar] [CrossRef] [Green Version]
- Efron, B. Bootstrap method: Another look at the jackknife. Ann. Stat. 1979, 7, 1–26. [Google Scholar] [CrossRef]
- Evangelista, P.H.; Kumar, S.; Stohlgren, T.J.; Young, N.E. Assessing forest vulnerability and the potential distribution of pine beetles under current and future climate scenarios in the interior west of the US. For. Ecol. Manag. 2011, 262, 307–316. [Google Scholar] [CrossRef]
- Radosavljevic, A.; Anderson, R.P. Making better Maxent models of species distributions: Complexity, overfitting and evaluation. J. Biogeogr. 2013, 41, 629–643. [Google Scholar] [CrossRef]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.L.; Bennett, J.R.; French, C.M. SDMtoolbox 2.0: The next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ 2017, 5, e4095. [Google Scholar] [CrossRef] [Green Version]
- Hughes, G.; Madden, L.V. Evaluating predictive models with application in regulatory policy for invasive weeds. Agric. Syst. 2003, 76, 755–774. [Google Scholar] [CrossRef]
- Lobo, J.M.; Jiménez-Valverde, A.; Real, R. AUC: A misleading measure of the performance of predictive distribution models. Glob. Ecol. Biogeogr. 2008, 17, 145–151. [Google Scholar] [CrossRef]
- Peterson, A.T.; Papeş, M.; Soberón, J. Rethinking receiver operating characteristic analysis applications in ecological niche modeling. Ecol. Model. 2008, 213, 63–72. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, T.; De Bie, C.; Skidmore, A.; Liu, X.; Song, S.; Zhang, L.; Wang, J.; Shao, X. Satellite-derived vegetation indices contribute significantly to the prediction of epiphyllous liverworts. Ecol. Indic. 2014, 38, 72–80. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Z.; Zhu, B.; Cheng, X.; Yang, L.; Gao, M.; Kong, R. MaxEnt Modeling Based on CMIP6 Models to Project Potential Suitable Zones for Cunninghamia lanceolata in China. Forests 2021, 12, 752. [Google Scholar] [CrossRef]
- Boisvert-Marsh, L.; de Blois, S. Unravelling potential northward migration pathways for tree species under climate change. J. Biogeogr. 2021, 48, 1088–1100. [Google Scholar] [CrossRef]
- Mehta, P.; Sekar, K.C.; Bhatt, D.; Tewari, A.; Bisht, K.; Upadhyay, S.; Negi, V.S.; Soragi, B. Conservation and prioritization of threatened plants in Indian Himalayan Region. Biodivers. Conserv. 2020, 29, 1723–1745. [Google Scholar] [CrossRef]
- Martin, T.G.; Kehoe, L.; Mantyka-Pringle, C.; Chades, I.; Wilson, S.; Bloom, R.G.; Davis, S.K.; Fisher, R.; Keith, J.; Mehl, K. Prioritizing recovery funding to maximize conservation of endangered species. Conserv. Lett. 2018, 11, e12604. [Google Scholar] [CrossRef]
- Fos, S.; Laguna, E.; Jimenez, J.; Angel Gomez-Serrano, M. Plant micro-reserves in Valencia (E. Spain): A model to preserve threatened fora in China? Plant Divers. 2017, 39, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.Z.; Zhang, S.S.; Wang, W.B.; Kang, H.M.; Ma, N. A sophisticated species conservation strategy for Nyssa yunnanensis, a species with extremely small populations in China. Biol. Conserv. 2017, 26, 967–981. [Google Scholar] [CrossRef]
- Yang, J.; Cai, L.; Liu, D.T.; Chen, G.; Gratzfeld, J.; Sun, W.B. China’s conservation program on Plant Species with Extremely Small Populations (PSESP): Progress and perspectives. Biol. Conserv. 2020, 244, 108535. [Google Scholar] [CrossRef]





| Environmental Variables | Code | Percent Contribution |
|---|---|---|
| Mean diurnal range | Bio_2 | 0.3 |
| Temperature seasonality | Bio_4 | 13.4 |
| Mean temperature of coldest quarter | Bio_11 | 3.2 |
| Precipitation seasonality | Bio_15 | 5.9 |
| Precipitation of driest quarter | Bio_17 | 3.7 |
| Precipitation of warmest quarter | Bio_18 | 73.6 |
| Periods | Area of Suitability Changes (km2) | ||||
|---|---|---|---|---|---|
| SSP 2.6 | SSP 4.5 | SSP 7.0 | SSP 8.5 | ||
| Current vs. 2050 | Expansion | 19,110.5 | 13,762.6 | 19,921.56 | 94,031.77 |
| Unchanged | 35,027.47 | 45,773.96 | 38,651.87 | 30,592.01 | |
| Contraction | 28,336.26 | 17,589.77 | 24,711.85 | 32,771.72 | |
| Current vs. 2070 | Expansion | 15,334.02 | 22,532.14 | 40,679.51 | 44,101.15 |
| Unchanged | 40,426.06 | 39,032.05 | 43,061.99 | 42,909.91 | |
| Contraction | 22,937.67 | 24,331.67 | 20,301.74 | 20,453.81 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, C.; Zhang, X.; Zha, J.; Li, J.; Li, J. Predicting Climate Change Impacts on the Rare and Endangered Horsfieldia tetratepala in China. Forests 2022, 13, 1051. https://doi.org/10.3390/f13071051
Cai C, Zhang X, Zha J, Li J, Li J. Predicting Climate Change Impacts on the Rare and Endangered Horsfieldia tetratepala in China. Forests. 2022; 13(7):1051. https://doi.org/10.3390/f13071051
Chicago/Turabian StyleCai, Chaonan, Xiaoyan Zhang, Jianjun Zha, Jie Li, and Junmin Li. 2022. "Predicting Climate Change Impacts on the Rare and Endangered Horsfieldia tetratepala in China" Forests 13, no. 7: 1051. https://doi.org/10.3390/f13071051
APA StyleCai, C., Zhang, X., Zha, J., Li, J., & Li, J. (2022). Predicting Climate Change Impacts on the Rare and Endangered Horsfieldia tetratepala in China. Forests, 13(7), 1051. https://doi.org/10.3390/f13071051







