Abstract
The soil microbiota is vulnerable to burning; however, it shows some resilience. No indices have yet been developed to assess fire damage related to soil biota. We evaluated the biological soil indices recorded by a Biolog EcoPlate System in a Mediterranean ecosystem. The experiment was carried out in an outdoor forest lysimeter facility (MedForECOtron), where we simulated burns with different burn severities. Burning increased the metabolic diversity of bacteria and most C-substrate utilization groups. Soil organic matter, phosphorus, electric conductivity, and calcium increased with increasing burn severity. Microbial richness and activity, as well as the integrated capacity of soil microbes to use a C source, lowered by burning, but recovered 6 months later. The functional diversity and amount of the C source used by microbes immediately increased after fire, and values remained higher than for unburned soils. We evaluated the changes in the vulnerability and resilience of fire-adapted ecosystems to improve their adaptive forest management. We found that the high burn severity reduced microbial richness, functional diversity, and the C source utilization of soil microbes (marked vulnerability to high temperatures), which recovered in the short term (high resilience). These results help to understand the main mechanisms of the effects of wildfire on semi-arid Mediterranean ecosystems, whose field validation will be helpful for fire prevention planning and restoration of burned areas.
1. Introduction
The European Biodiversity Strategy defines soil biodiversity protection as a main priority [1]. To achieve it, measurement tools to evaluate it are needed, i.e., to measure microbial diversity and functional value [2]. Soil quality has been defined as the capacity of soil to function effectively at present and in the future, or as the capacity of soil to function within ecosystem boundaries to sustain biological productivity, maintain environmental quality, and promote plant and animal health [3,4]. However, the biological soil quality is a more complex term, focused on the contribution of soil organisms to soil functions and providing the capacity to mitigate the effects of disturbances on soil ecosystem services due to their resistance, resilience, and/or functional redundancy [5]. We used the term vulnerability according to [6], defined as the inverse to the resistance, understood as the difficulty to perturb a state variable by an external force, and the term resilience according to [7] as disturbance withstood by an ecosystem with no changes in self-organized processes and structures. The biological soil quality approach defines soil health according to its stability, resilience to disturbance or stress, biological diversity, and level of internal nutrient cycling [8]. The fire regime, e.g., the pattern, frequency, and intensity of wildfires, is changing due to human-induced modifications, which have promoted the risk of extinction of a wide taxa and habitat range, including soil-dwelling organisms that play fundamental and irreplaceable roles in ecosystem functioning [9]. In addition, fire regime modulates some main variables of ecosystems, such as nutrient availability, water content or plant–soil interactions, which are related to effects of fire damage on soil microbial diversity [10].
Soil microbiota has been considered the main agent responsible for soil structure, but showed marked vulnerability to burning despite its buffer capacities [11,12]. After a disturbance, soil microorganisms play a key role in the reclaim and regeneration of ecosystems [13], and even soil biodiversity recovery promotes resilience in Mediterranean Basin ecosystems, which can be used to restore essential ecosystem functions [14]. Fire damage to microbes is marked because of their virtual immobility [10]. Burn severity [15] affects ecosystem services and functions [16,17,18], and it deeply modulates the response of soil microbial communities [19]. The plant–soil interphase and its relations are complex, and the role of vegetation recovery is highlighted as a source of C and nutrients for soil microbial communities [20]. The increases in both nutrient availability and SOM after low burn severity promote microbial groups in soil [21,22,23]. However, decreases in C substrate utilization were detected for medium-to-high burn severity, which was related to unfavorable post-fire environmental conditions [24]. In addition, the C use patterns were modified, except for amines, because of the changes in the microbial community that derived from removing vulnerable populations to disturbances, such as fire activity [25] or biological interactions [26]. The impact of high burn severity almost invariably leads to lower bacterial diversity because vulnerable populations are altered, even in the short term, after a fire [27]. In short, independent of burn severity, fire passage causes direct microbiota community restructuring, which does not come from previous findings that showed that low burn severity does not kill microorganisms due to low burn severity [12,16].
In this way, soil biota can be considered the most sensitive indicator of soil quality [28]. The interest of soil managers is increasing in measuring biological next to chemical and physical properties, and in making use of soil biological functions [29]. Knowledge of soil biology for restoring ecosystems is a research priority to evaluate fire damage to soil biota to prioritize and optimize mitigation and restoration [30]. To predict fire damage and ecosystem resilience, some studies have highlighted the importance of assessing the recovery of the microbial community structure and ecosystem functions after fire [10].
The Biolog EcoPlate System is a standard, rapid, simple, and replicable method, widely used and suitable for comparing the functional diversity of the soil microbe community under different treatments or impacts [31,32], which has been used to characterize the abundance and diversity of soil microorganisms after fire [21,23]. The use of enclosed simplified ecosystems in controlled environmental facilities helps to understand complex interactions in real ecosystem disturbances [33,34].
The fire regime affects the soil microbial diversity and structure [35]. The microbial diversity in burned pine forests increases, mainly in low-fire-severity areas [21,23], but fire damage is more marked in severely burned soils because it reduces nitrogen availability and alters soil bacterial composition [36,37]. Even in Mediterranean fire-prone ecosystems, the structure of functional groups of soil microorganisms is negatively impacted by high burn severity from a single fire [38,39].
Our aim was to assess changes due to fire damage in Mediterranean soils [40]. We previously proposed two variables of biological soil quality, the abundance and diversity of soil microorganisms recorded with a Biolog EcoPlate System, to evaluate the response pattern of an ecosystem to fire damage for landscape management [21]. In the current study, we tried to relate fire damage of soil to burn severity in the short-term period, including the novelty of relating burn severity and temporal dynamics of physico-chemical-biological soil indices. Our experiment was performed in an outdoor forest lysimeter facility (MedForECOtron), where we checked that high burn severity induced changes in microbial biomass and phylogenetic composition [37]. In this way, we hypothesized that fire damage could be directly measured by evaluating changes in the bacterial community composition, because high burn severity reduces microbial richness, functional diversity, and the C source utilization of soil microbes.
2. Materials and Methods
2.1. Study Site
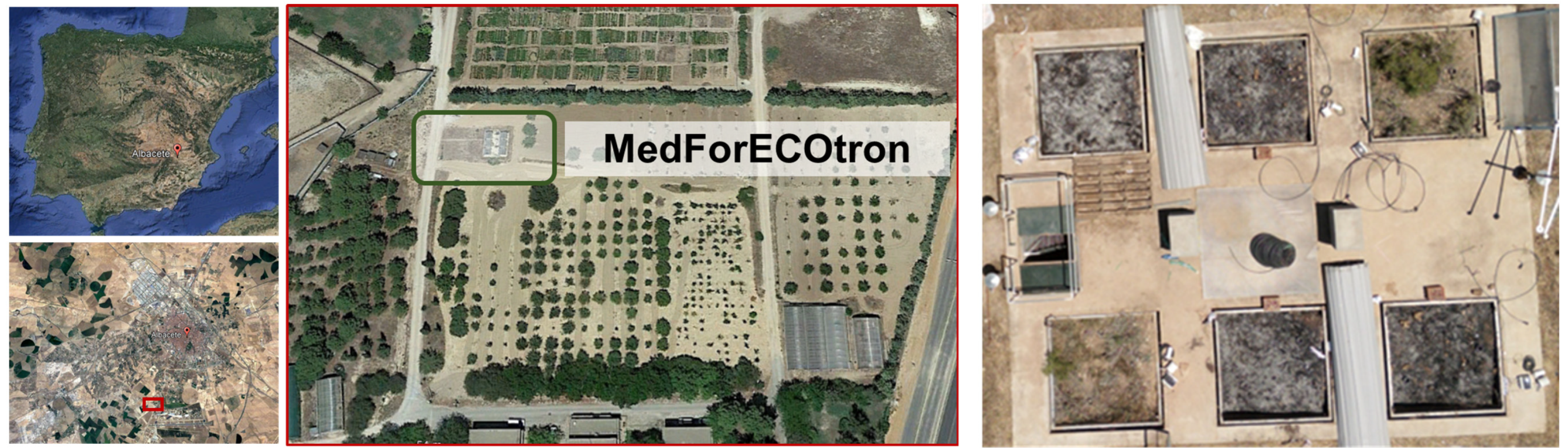
The Mediterranean Forest Ecotron (MedForECOtron) is a controlled environmental facility that lies in the Professional Forestry Practice Areas of Escuela Técnica Superior Ingenieros Agrónomos y Montes, Albacete in Spain (38°59′00″ N, 01°51′00″ W). Based on conclusions from [33], we built a mesocosm of a 4 m2 surface (2 × 2) and 1 m depth to retain some replication. Six intact lysimeters were dug in natural Aleppo pine (Pinus halepensis Mill.) forests (originally located 38°33′12″ N, 2°4′15″ W) from the SE Iberian Peninsula (Figure 1) (more details in [37]). Monoliths showed over 60% vegetation coverage on average, mostly consisting of P. halepensis Mill., Juniperus oxycedrus L., and J. phoenicea L. Other representative companion species were Genista scorpius L. DC., Rosmarinus officinalis L., or Brachypodium phoenicoides L. Roem. & Schult. Monoliths’ soil developed on limestone and was classified as Haplocalcids [41]. The soil texture was sandy loam (averaged as 66% sand, 20% silt, and 14% clay).

Figure 1.
Location of the study area in the Iberian Peninsula (red dot in the top left image) and location in Albacete (red rectangle in the bottom left image). The pilot plant facility was installed at the Higher Technical School of Agricultural and Forestry Engineers in Albacete (green rectangle in the right-hand image). Zenital perspective (bird’s eye view) of the burned monoliths at MedForECOtron (right image): two unburned plots (green vegetation), two plots simulating low burn severity (dark black-grayish ash cover), and two simulating high burn severity (light gray-whitish ash cover).
On 14 July 2016, we randomly burned paired monoliths to simulate different fire severities. We increased fuel load by adding the light fuel load collected from the original Aleppo pine forest (small stems, branches, needles, and cones) to promote high burn severity (9 cm thick layer) and low burn severity (3 cm thick layer) to mimic natural stands that remained unburned in the long term [42] (Figure 1). In the burned monoliths, we recorded the mean residence time (time in which the recorded temperature was higher than 100 °C) (timeRESIDENCE) and characterized the fire intensity in the monoliths by recording temperatures (averaged maximum temperature (Tmax) every minute on the soil surface and at a shallow depth (2 cm below soil). For more details, see [37].
2.2. Soil Sampling and Analysis
Following [21,43], three composite soil samples were collected in each monolith on three different dates (18 samples per date, 90 g each): prefire conditions (7 July 2016, before burning (PRE)), immediately after burning (15 July 2016, 1 day after (1Dafter)), and the short-term post burning dynamics 4 January 2017 (6 months after burning (180Dafter)). Each one was made up of three subsamples, randomly gathered according to a split-plot design (18 soil samples, 6 per collection date). After surface litter removal, we used steel cylinders to dig the upper 2 cm of the mineral soil layer where most of the microbial activity occurs [44,45]. We mixed all the subsamples in a bag to be analyzed. They were dried, passed through a 2 mm sieve and sent to the laboratory for the physico-chemical and biological analyses (they were left at 4 °C in a fridge for the latter).
2.2.1. Physico-Chemical Analysis
The indicators of soil characteristics related to the physico-chemical soil approach were: texture (% w/w); total soil organic matter (SOM, %); pH; total nitrogen (N, %); available phosphorus (P, ppm); sodium (Na+, meq 100 g−1); calcium (Ca2+, meq 100 g−1); potassium (K+, meq 100 g−1); magnesium (Mg2+, meq 100 g−1); total soil organic carbon (Corg, %); cation exchange capacity (CEC, meq 100 g−1); electrical conductivity (EC, dS m−1).
We followed the international Robinson pipette method to calculate the percentage distribution of the individual soil particles according to size [46], and we calculated texture with the Soil Texture Calculator [41]. We also determined SOM and Corg by the potassium dichromate oxidation method [47]. We measured pH and EC in deionized water (1:2.5 and 1:5 w:w, respectively) at 20 °C and CEC following the method by [48].
Mg2+, Na+, Ca2+, and K+ were extracted with 1 M ammonium acetate [49] and further analyzed by atomic absorption spectrometry (Analyst 200, PerkinElmer, Inc., Waltham, MA, USA). N was established by the Kjeldahl method [50]. P and N were determined following [50,51], respectively.
2.2.2. Biological Analysis
We used BiologTM EcoPlates (Biolog Inc., Hayward, CA, USA) to analyze soil bacterial functional diversity, an approach followed to describe the microbial community-level physiological profiles [52,53]. We characterized the community-level physiological profiles (CLPPs) after 96 h of incubation [21,22,54].
To evaluate microbial activity and integrated capacity of soil microbes in soil [55], we calculated the average well-color development rate (AWCD). We also evaluated microbial richness (MR, number of spent substrates) and the Shannon diversity index (H’) [56] to characterize the level of microbial functional diversity and metabolic diversity [57]. The CLPPs of the soil bacterial community analysis were divided into six substrate categories [56]: carboxylic acids (CA); amines or amides (AMN); amino acids (AAC), carbohydrates (CH); phenolic compounds (PHE); polymers (POL).
2.3. Statistical Analysis
We selected analysis of variance with the values obtained from the variables obtained in the laboratory after checking the influence of the interaction of the studied factors, BURNSEV and TIME, on the bacterial community metabolic profiles and the physico-chemical variables. Whenever the F statistics were significant, we ran Tukey’s minimum significant difference test to compare the mean values with a low type-I error (p < 0.05).
The Spearman’s rank-order correlation coefficient (RHO) is nonparametric and does not rely upon an assumption of normality (a Shapiro–Wilk test validated that assumption for our data). We used it to evaluate the association between pairs of variables. In addition, a principal component analysis (PCA) was carried out for the main indices related to the soil physico-chemical characteristics and the CLPPs. A varimax normalization (varimax-normalized PCA), an orthogonal rotation, transformed the initial factors in a particular simpler subspace, which were easier to interpret.
The variables were reduced to a combination constrained in two or three significant axes. Statistical analyses were performed with RStudio v. 1.3.1073 [58] and IBM SPSS Statistics v. 24.0 [59].
3. Results
3.1. Validating Burn Severity Levels
The unburned monoliths were considered as the control (UNB), and the expected burn severity was validated in line with [60]. According to the temperatures reached, we classified the burn severity for each group of monoliths as HIGHsev (Tmax was 595 °C on the surface, 289 °C at a 2 cm depth; timeRESIDENCE 6.5 and 7.0 h on the surface and at a 2 cm depth, respectively) and LOWsev (Tmax was 547 °C on the surface, 132 °C at a 2 cm depth; timeRESIDENCE 10 and 17 min on the surface and at a 2 cm depth, respectively).
3.2. Changes in the Physico-Chemical Soil Properties
The physico-chemical soil characteristics were analyzed to check for any significant differences in the increases in values before and after a fire (Table 1). We did not find any significant effects of either BURNsev or TIME on the pH, Corg, or CEC values of the other studied nutrients (N, K+, Na+, and Mg2+). Furthermore, the soil texture did not change: the reported results showed loam soils with negligible variations in clay, silt, and sand contents. We detected fire damage related to SOM, P, EC, and Ca2+.

Table 1.
The main soil physico-chemical properties of the burned plots before (PRE), immediately after burning (1Dafter), and 6 months after (180Dafter) (mean ± standard error) in the MedForECOtron plots: unburned (UNB), low burn severity (LOWsev), and low burn severity (HIGHsev). Capital letters indicate significant differences between the means of the different groups (Fisher’s least significant difference test); MSE: mean squared error.
SOM increased in the burned monoliths, specifically in HIGHsev 180Dafter burning. The value was not significantly affected by fire passage (3.59% ± 0.24% to 4.87% ± 1.01% and 5.76% ± 0.74% to 5.58% ± 0.97% in prefire and 1Dafter conditions for LOWsev and HIGHsev, respectively), and did not vary in LOWsev after TIME (4.87 ± 1.01% to 4.68 ± 0.28%). However, the SOM in HIGHsev significantly increased from 5.58% ± 0.97% to 6.85% ± 0.88% six months after fire (180Dafter). Soil P increased after burning (from 5.03 ± 0.11 ppm to 9.58 ± 0.38 ppm in LOWsev and 5.79 ± 1.43 ppm to 11.43 ± 0.43 ppm after fire passage), and significantly did so in HIGHsev 180Dafter (reaching 18.00 ± 3.02 ppm). EC and Ca2+ rose according to TIME in all the monoliths, but the higher BURNsev promoted more marked increases in their values.
3.3. Changes in the Microbiological Soil Properties
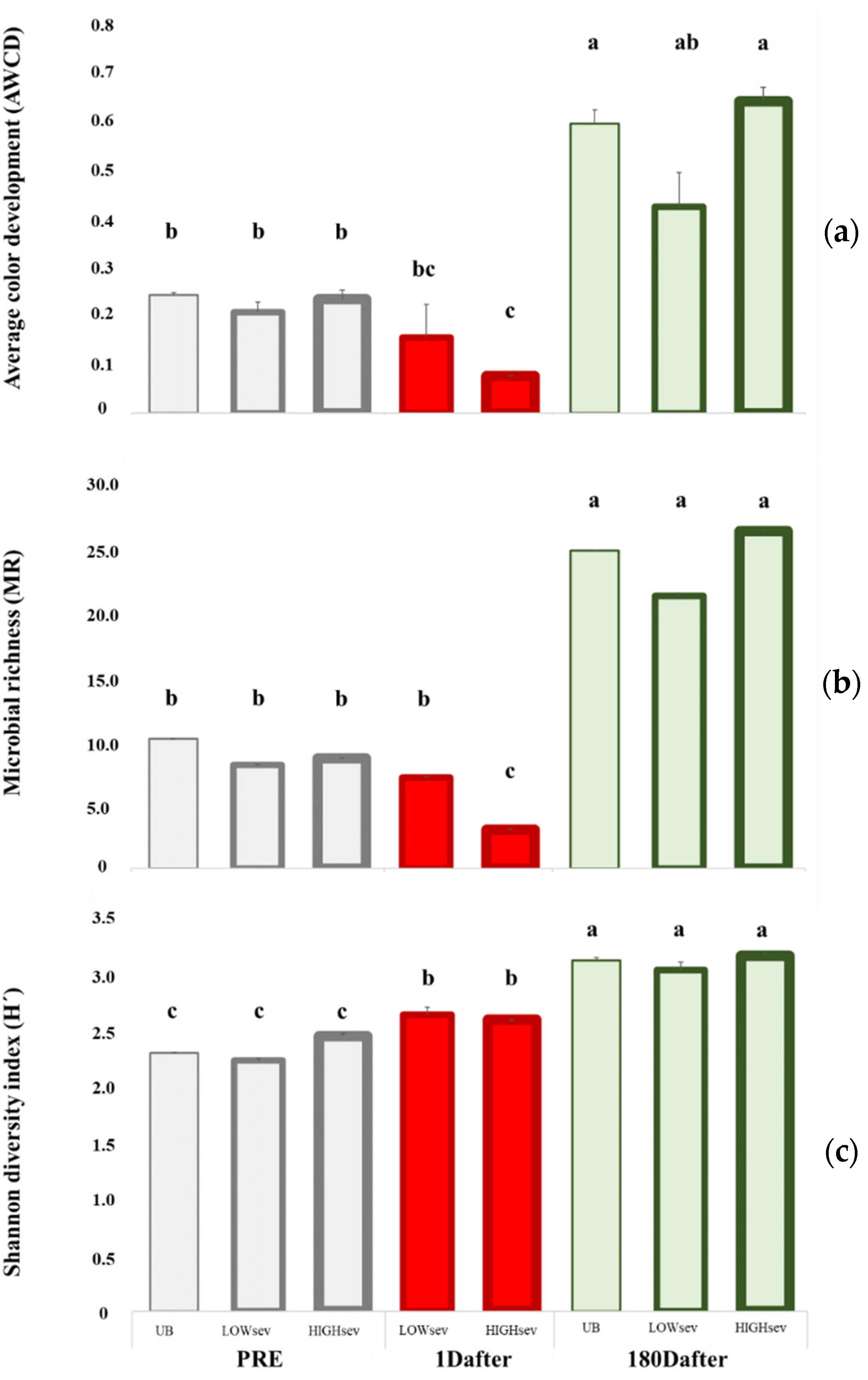
The midexponential growth phase (96 h of incubation) was analyzed to characterize microbial activity, metabolic diversity, and the integrated capacity of soil microbes to use different C sources (Figure 2). We found no differences in the UB plots 1Dafter, but did find an increase six months later. The prefire AWCD, MR, and H’ values indicated no significant differences in the paired monoliths, although the AWCD and MR values decreased in the HIGHsev monoliths. Only 1 day after fire, H’ increased in both burn severities. 180Dafter, their values were similar to those of the UB treatment, and no differences were found for BURNsev. The AWCD and MR indices showed more marked changes related to HIGHsev, which disappeared 180Dafter, when all three values increased according to TIME. The H’ index increased according to TIME, but was not related to BURNsev.
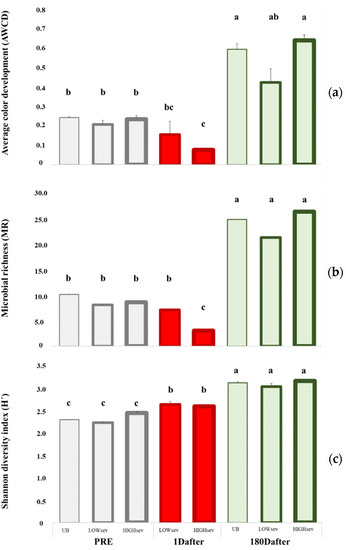
Figure 2.
Community-level physiological profiles (CLPPs) were characterized after 96 h of incubation in the midexponential growth phase in the soil samples of the burned lysimeters at MedForECOtron (14 July 2016) collected prefire (7 days before, PRE), 1 day after (1Dafter) and 6 months after (180Dafter): (a) average well-color development (AWCD); (b) microbial richness (MR); (c) the Shannon diversity index (H’). Burn severity levels: unburned (UB), low burn severity (LOWsev), and high burn severity (HIGHsev). Vertical bars represent the standard error of the mean, and lowercase letters indicate significant differences between the means of the different groups (Fisher’s least significant difference test).
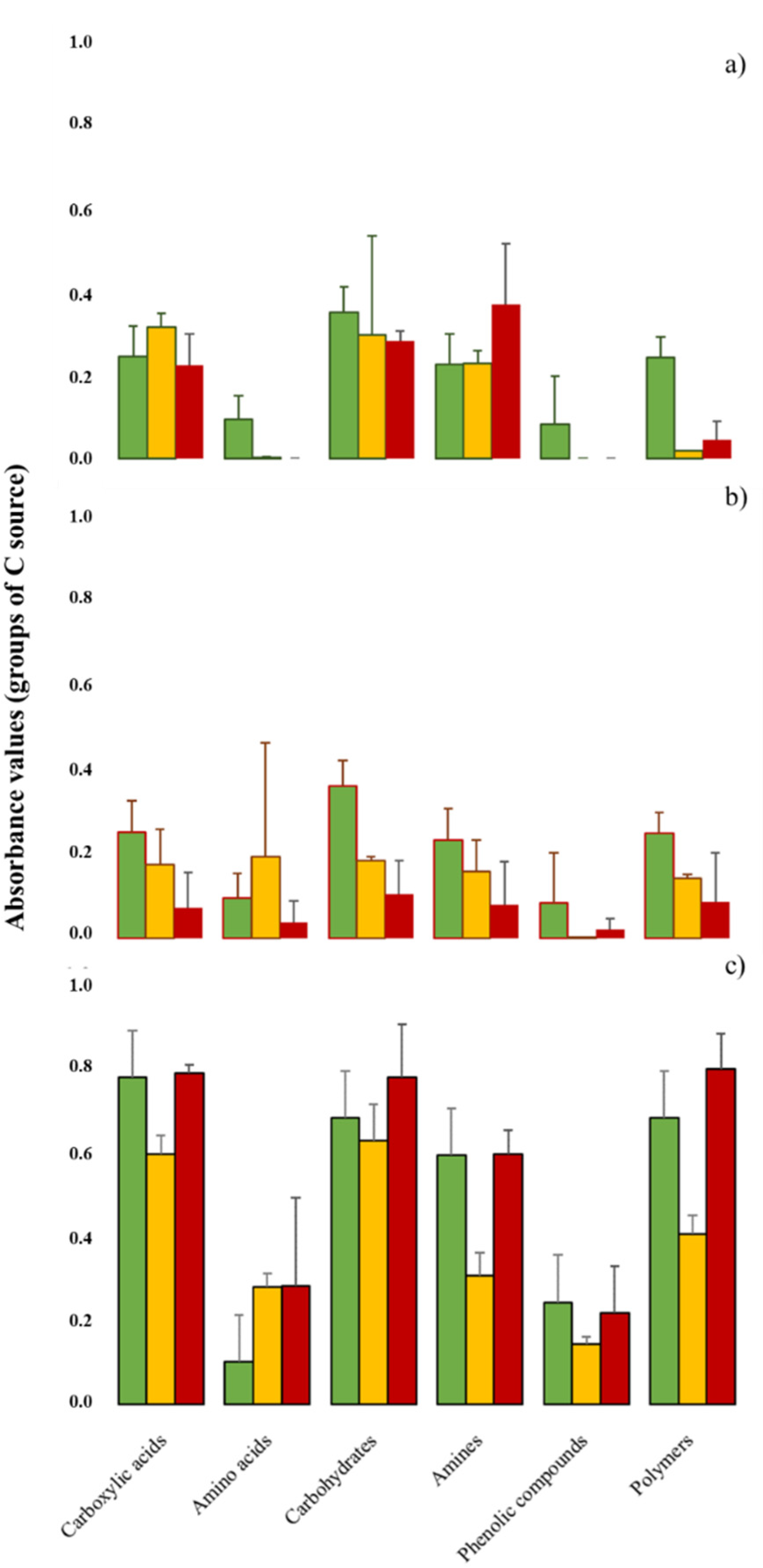
When focusing on the midexponential growth phase, BURNsev and TIME significantly correlated with the use of substrate groups (Figure 3). Although the initial averaged use of CA, AMN, CH, AAC, PHE, and POL differed, we observed trends in the time dynamics, as all the values rose according to TIME, except for AMN. After fire passage, the CA and CH values lowered, while AMN increased. The heating effect differed depending on BURNsev for the AAC and PHE values, which lowered in LOWsev, but increased for HIGHsev. Conversely, the POL values increased in LOWsev, but lowered for HIGHsev. The carbon sources increased 180Dafter in all the monoliths, but the effect of TIME was especially intense for the increase recorded in CA, CH, AAC, and POL, mainly for HIGHsev.
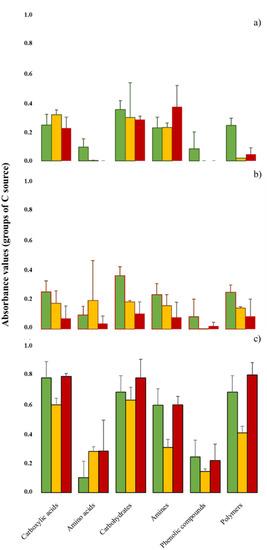
Figure 3.
The six C source groups (carboxylic acids, amino acids, carbohydrates, amines, polymers, and phenolic compounds) were characterized after 96 h of incubation (midexponential growth phase) in soils of burned lysimeters at MedForECOtron (14 July 2016) collected: (a) prefire (7 days before, PRE); (b) one day after (1Dafter); (c) 6 months after (180Dafter). Burn severity levels: unburned (UB, green), low burn severity (LOWsev, orange), and high burn severity (HIGHsev, red).
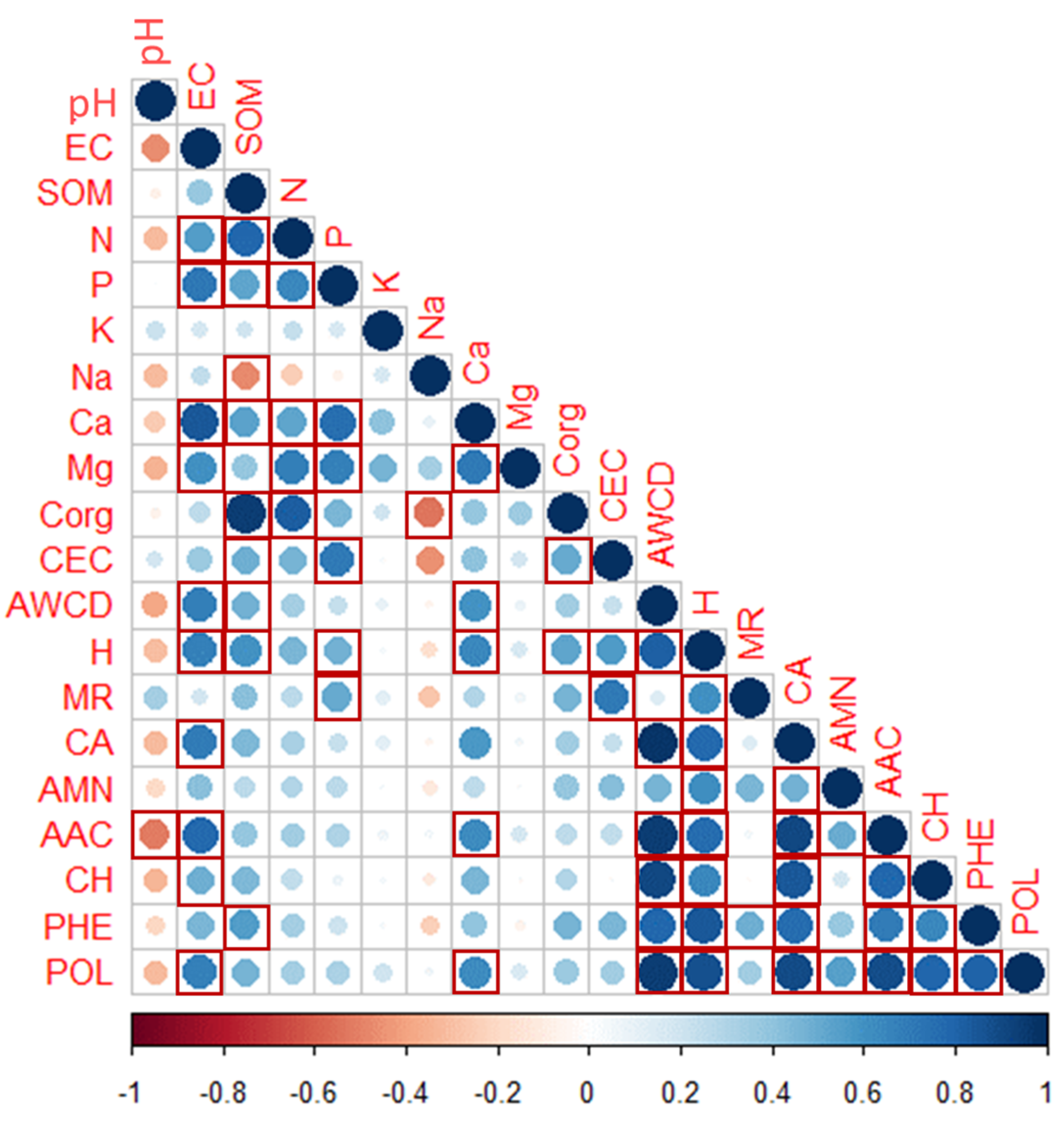
Spearman’s linear correlation analysis (Figure 4) showed that after 96 h of incubation, the average use of the CLPP substrates and the six C source groups were positively and significantly correlated and with other physico-chemical parameters. The highest correlation related AWCD to CA (+0.97), AAC (+0.94), CH (+0.91), and POL (+0.95). H’ strongly correlated with POL (+0.85) and PHE (+0.88). Other correlations appeared of SOM with Corg (+0.94), while the most important negative ones were AAC and pH (−0.51) and Corg with Na (−0.54).
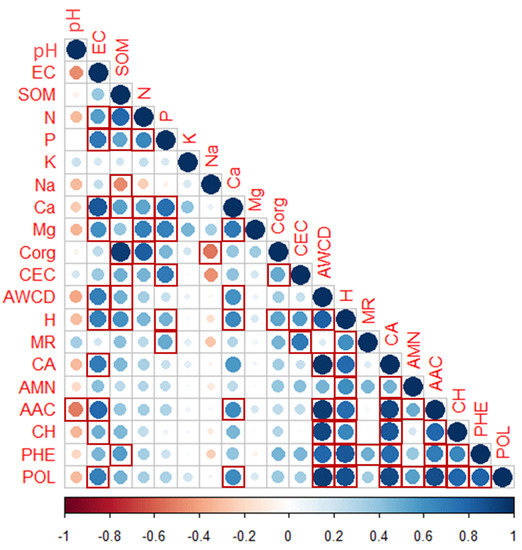
Figure 4.
Spearman’s rank correlations (RHO) of the soil physico-chemical properties and community-level physiological profiles (characterized after 96 h of incubation) of the burned plots in the MedForECOtron plots at three different times (PRE, 1Dafter, and 180Dafter) at three burn severity levels (UB, LOWsev, and HIGHsev). Dark tones mean strong correlation (either positive (in blue) or negative (in red)), whereas light tones or white color mean weak (or lack of) correlation.
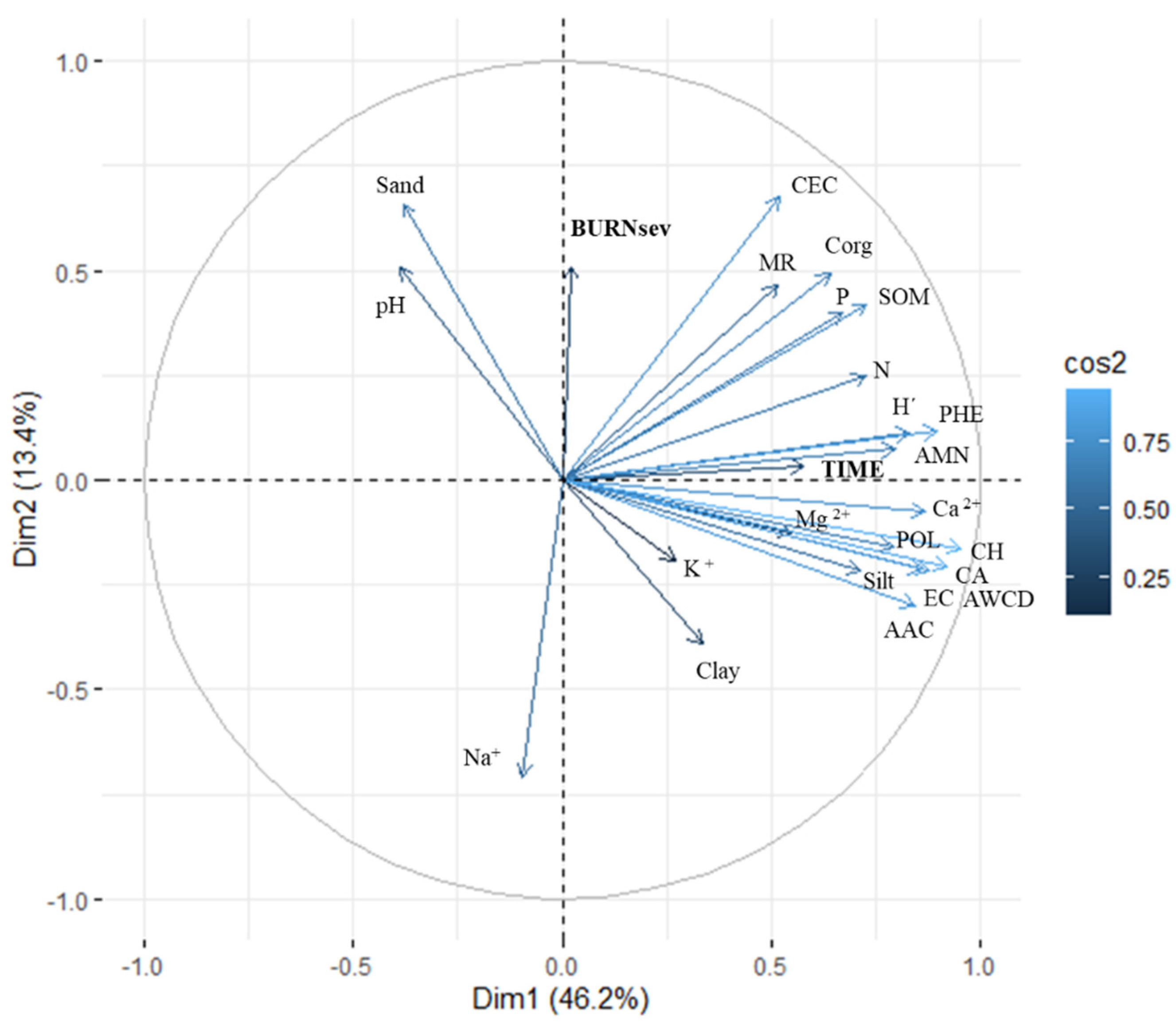
The varimax-normalized PCA was performed with the values of the six substrate groups, CLPPs, and physico-chemical values recorded from PRE (1Dafter and 180Dafter) (Figure 5). The ordination was spaced to two dimensions (eigenvalues > 1) to maximize the explained variability (59.60%) in the simplest way: 46.20% for dimension 1 (DIM1) and 13.40% for dimension 2 (DIM2). The burned soil samples clustered in relation to TIME to the CLPPs (positive values in DIM1), and mainly indices POL, ACWD, H’, and CA, which each contributed almost 10% of the total explained variability. DIM2 related BURNsev to the physico-chemical properties, mainly Na, CEC, and Sand, which, respectively, contributed 15.1%, 13.0%, and 12.7% to the total explained variability.
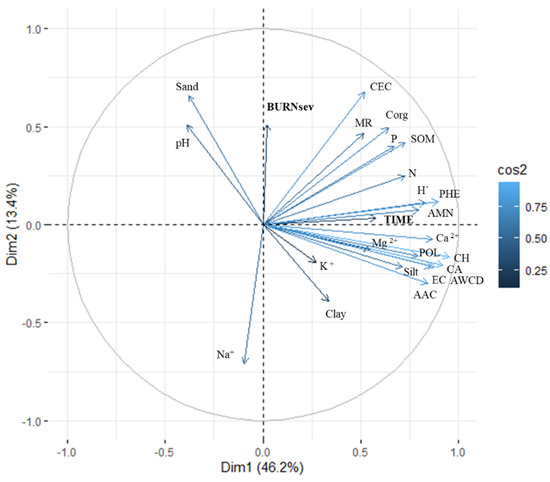
Figure 5.
The diagram of principal component analysis (PCA) after varimax-normalization for the main soil physico-chemical properties and community-level physiological profiles (characterized after 96 h of incubation) of the burned plots before (PRE), immediately after burning (1Dafter), and 6 months after (180Dafter) in the MedForECOtron plots: unburned (UNB), low burn severity (LOWsev), and low burn severity (HIGHsev).
4. Discussion
Some studies have showed how biological soil communities are vulnerable to burning using BiologTM EcoPlates [21,61], even at the mesocosm scale [37]. Our results confirmed that microbial communities were resilient (recovered in the short term) under low and moderately severe fires [23,62]. However, microbial activity depends on nutrient cycles, which are modulated by the severity of disturbances and postfire recovery, mainly in dry and semiarid Mediterranean forest ecosystems [63]. Additionally, the response of the microbial community differs with vegetation coverage and burn severity. Even soil bacterial functional diversity can be improved after low-severity fire and natural regeneration of plants [21].
We assumed that for predicting natural ecosystem recovery, it is important to define the threshold for natural recovery in which interactions related to burn severity and time after fire should be clarified. At MedForECOtron, showing initial homogeneous conditions, some physico-chemical soil indices (SOM, EC, pooled P, and Ca2+) increased according to burn severity and time after fire, as in former studies [64]. Fire triggered microbes and benefited the microbial community structure [14,19]. In the monoliths with high burn severity, AWCD and MR reduced but H’ increased, recovering to their initial levels 6 months after burning.
The changes that took place after fire in the C substrate utilization profiles were almost negligible and ascribed to changes in SOM [54], but they returned to the prefire state in the short term. Both low vulnerability and high resilience have been related to the recovery of vegetation cover and plant composition [65]. The changes detected in the CLPPs were related to time after fire due to the increases in charcoal and ash that promoted soil functionality, changes in phenolic compounds and polymers, and plant recovery [18,66,67], affected by seasonal dynamics [21,68]. The changes in microbial diversity progressively confirmed seasonal variations [69]. However, changes in phylogeny could increase some C-substrate utilization due to the over-representation of fire-resistant lineages [14], but a decrease in bacterial diversity implies a reduction in ecosystem functions [14,70].
We found that burning immediately decreased microbial richness, microbial activity, and the integrated capacity of using a C source; however, these increased 6 months later. This provided information about how fire damage (burn severity) changed the CLPPs of the microbial community, as was previously found in a laboratory test [71]. The AWCD, MR, and the C-substrate utilization groups (except amines) displayed ephemeral changes, even with high burn severity, which corroborates the resilience of Mediterranean pine forest soil ecosystems [72]. However, fire damage may be underestimated with the CLLP technique because microbial communities are functionally redundant, and different microbial communities can utilize the same carbon source [73].
To achieve the effective restoration and mitigation of fire damage treatments [74], fire damage assessments should include indicators for biological soil properties: microbial functional diversity (the higher the burn severity, the lower the value), microbial activity (the higher the burn severity, the higher the value), and recalcitrant soil organic matter (negative correlation of SOM and burn severity). At MedForECOtron, burning immediately increased SOM, P, Ca, and EC after the fire, and large amounts remained 6 months later, which influenced the richness and functional diversity of the microbial community.
5. Conclusions
We used soil-quality indices to assess changes related to both burn severity (vulnerability) and natural recovery (resilience) to provide essential information on the dynamics of soil–plant interactions after disturbance because it is essential to better integrate soil microbial ecology with macroecology and forest and landscape management practices (see [36]). In our study, carried out at MedForECOtron, a correlation appeared between soil biological responses and time since fire, which will help to evaluate changes in vulnerability and/or resilience of fire-adapted ecosystems, and can be directly applied to adaptive forest management.
We confirmed that AWCD can be used as an indicator of fire damage in the biological soil section as burning reduces the oxidative capacity of soil microorganisms and inhibits microbial populations due to reduced soil organic matter [61,75].
Further soil studies are required to assess how different fire regime parameters influence the long-term dynamics of the richness and functional diversity of microbial communities. This should be complemented by including long-term temporal dynamics in relation to the mechanisms linking plant–soil and soil–soil processes. All this knowledge can be applied in biophysical processes to develop or improve models by considering soil biology and fire. This will help with evaluations of the changes in the vulnerability and resilience of fire-adapted ecosystems by including the soil–plant interphase as a key factor in postfire restoration to mitigate fire damage [23]. Habitat type has to be included as a useful index for adaptive forest management, especially in future scenarios with predicted extreme wildfire events [76]. All this can be supported and validated with information from remote sensors, as they already provide good results for monitoring natural regeneration and the efficiency of restoration treatments on the landscape scale [77].
Author Contributions
D.M.: conceptualization, methodology, investigation, writing—original draft preparation, and writing—reviewing and editing, T.F.: data curation, writing—original draft preparation, and formal analysis, E.P.: investigation, formal analysis, and writing—reviewing and editing, R.A.-S.: investigation, formal analysis, and writing—reviewing and editing, P.A.P.-Á.: investigation, formal analysis, and writing—reviewing and editing, J.G.-R.: investigation, formal analysis, and writing—reviewing and editing, M.E.L.-B.: visualization, investigation, and writing—reviewing and editing, J.d.L.H.: conceptualization, writing—reviewing and editing, supervision, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by funds from the Spanish Institute for Agricultural Research and Technology CSIC-INIA (VIS4FIRE, RTA2017-00042-C05-00), MCIN/AEI/10.13039/501100011033 “FEDER una manera de hacer Europa” (ENFIRES: PID2020-116494RR-C43) and regional funds from Junta de Comunidades Castilla-La Mancha (PRESFIRE, SBPLY/19/180501/000130). Pedro Antonio Plaza Álvarez was supported by a predoctoral fellowship (FPU16/03296). J. González-Romero holds a postdoctoral position from Universidad Castilla-La Mancha (from the Regional Government), and Esther Peña was granted as predoctoral research staff (2020-PREDUCLM-16032).
Acknowledgments
The authors thank the support and field sampling performed by the personnel of the Castilla-La Mancha Regional Forest Service and GEACAM. We also thank Helen Warburton for her professional English editing and suggestions.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| CLPP | community-level physiological profiles |
| AWCD | average well-color development |
| MR | microbial richness |
| H’ | Shannon diversity index |
| CA | carboxylic acids; AMN: amines/amides |
| AAC | amino acids |
| CH | carbohydrates |
| PHE | phenolic compounds |
| POL | polymers |
References
- European Commission Our Life Insurance. Our Natural Capital: An EU Biodiversity Strategy to 2020; European Commission: Brussels, Belgium, 2011; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52011DC0244&from=EN (accessed on 21 June 2022).
- Orgiazzi, A.; Panagos, P.; Yigini, Y.; Dunbar, M.B.; Gardi, C.; Montanarella, L.; Ballabio, C. A Knowledge-Based Approach to Estimating the Magnitude and Spatial Patterns of Potential Threats to Soil Biodiversity. Sci. Total Environ. 2016, 545–546, 11–20. [Google Scholar] [CrossRef]
- Doran, J.W.; Parkin, T.B. Defining and Assessing Soil Quality. In Defining Soil Quality for a Sustainable Environment; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1994; pp. 1–21. ISBN 978-0-89118-930-5. [Google Scholar]
- Fließbach, A.; Oberholzer, H.-R.; Gunst, L.; Mäder, P. Soil Organic Matter and Biological Soil Quality Indicators after 21 Years of Organic and Conventional Farming. Agric. Ecosyst. Environ. 2007, 118, 273–284. [Google Scholar] [CrossRef]
- Allison, S.D.; Martiny, J.B.H. Resistance, Resilience, and Redundancy in Microbial Communities. Proc. Natl. Acad. Sci. USA 2008, 105, 11512–11519. [Google Scholar] [CrossRef] [Green Version]
- Carpenter, S.; Walker, B.; Anderies, J.M.; Abel, N. From Metaphor to Measurement: Resilience of What to What? Ecosystems 2001, 4, 765–781. [Google Scholar] [CrossRef]
- Gunderson, L.H. Ecological Resilience—In Theory and Application. Annu. Rev. Ecol. Syst. 2000, 31, 425–439. [Google Scholar] [CrossRef] [Green Version]
- Rüdisser, J.; Tasser, E.; Peham, T.; Meyer, E.; Tappeiner, U. The Dark Side of Biodiversity: Spatial Application of the Biological Soil Quality Indicator (BSQ). Ecol. Indic. 2015, 53, 240–246. [Google Scholar] [CrossRef]
- Kelly, L.T.; Giljohann, K.M.; Duane, A.; Aquilué, N.; Archibald, S.; Batllori, E.; Bennett, A.F.; Buckland, S.T.; Canelles, Q.; Clarke, M.F.; et al. Fire and Biodiversity in the Anthropocene. Science 2020, 370, eabb0355. [Google Scholar] [CrossRef]
- Certini, G.; Moya, D.; Lucas-Borja, M.E.; Mastrolonardo, G. The Impact of Fire on Soil-Dwelling Biota: A Review. For. Ecol. Manag. 2021, 488, 118989. [Google Scholar] [CrossRef]
- Acea, M.J.; Carballas, T. Microbial Fluctuations after Soil Heating and Organic Amendment. Bioresour. Technol. 1999, 67, 65–71. [Google Scholar] [CrossRef]
- Mataix-Solera, J.; Navarro-Pedreño, J.; Guerrero, C.; Gómez Lucas, I.; Marco, B.; Mataix, J. Effects of an Experimental Fire on Soil Microbial Populations in a Mediterranean Environment. In Man and Soil at the Third Millenium; Geoforma Ediciones: Logroño, Spain, 2002; pp. 1607–1614. ISBN 978-84-87779-47-3. [Google Scholar]
- Lladó, S.; López-Mondéjar, R.; Baldrian, P. Forest Soil Bacteria: Diversity, Involvement in Ecosystem Processes, and Response to Global Change. Microbiol. Mol. Biol. Rev. 2017, 81, e00063-16. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Valera, E.; Verdú, M.; Navarro-Cano, J.A.; Goberna, M. Soil Microbiome Drives the Recovery of Ecosystem Functions after Fire. Soil Biol. Biochem. 2020, 149, 107948. [Google Scholar] [CrossRef]
- Moya, D.; Madrigal, J.; Fonturbel, T.; Marino, E.; Hernando, C.; Guijarro, M.; Fernandez, C.; Jimenez, E.; Lucas-Borja, M.; Vega, J.; et al. Fire Severity Assessments in Both the Laboratory and the Field. In Fire Effects on Soil Properties; Pereira, P., Mataix-Solera, J., Ubeda, X., Rein, G., Cerda, A., Eds.; CSIRO Publishing: Clayton, Australia, 2019; pp. 241–266. [Google Scholar]
- Alcañiz, M.; Outeiro, L.; Francos, M.; Úbeda, X. Effects of Prescribed Fires on Soil Properties: A Review. Sci. Total Environ. 2018, 613–614, 944–957. [Google Scholar] [CrossRef]
- Cawson, J.G.; Sheridan, G.J.; Smith, H.G.; Lane, P.N.J. Effects of Fire Severity and Burn Patchiness on Hillslope-Scale Surface Runoff, Erosion and Hydrologic Connectivity in a Prescribed Burn. For. Ecol. Manag. 2013, 310, 219–233. [Google Scholar] [CrossRef]
- Lucas-Borja, M.E.; Delgado-Baquerizo, M.; Muñoz-Rojas, M.; Plaza-Álvarez, P.A.; Gómez-Sanchez, M.E.; González-Romero, J.; Peña-Molina, E.; Moya, D.; de las Heras, J. Changes in Ecosystem Properties after Post-Fire Management Strategies in Wildfire-Affected Mediterranean Forests. J. Appl. Ecol. 2021, 58, 836–846. [Google Scholar] [CrossRef]
- Pérez-Valera, E.; Verdú, M.; Navarro-Cano, J.A.; Goberna, M. Resilience to Fire of Phylogenetic Diversity across Biological Domains. Mol. Ecol. 2018, 27, 2896–2908. [Google Scholar] [CrossRef]
- Muñoz-Rojas, M.; Barcenas-Moreno, G. Microbiology. In Fire Effects on Soil Properties; Pereira, P., Ubeda, X., Mataix-Solera, J., Rein, G., Cerda, A., Eds.; CSIRO Publishing: Clayton, Australia, 2019; pp. 157–174. [Google Scholar]
- Moya, D.; Fonturbel, M.T.; Lucas-Borja, M.E.; Peña, E.; Alfaro-Sanchez, R.; Plaza-Álvarez, P.A.; González-Romero, J.; de Las Heras, J. Burning Season and Vegetation Coverage Influenced the Community-Level Physiological Profile of Mediterranean Mixed-Mesogean Pine Forest Soils. J. Environ. Manag. 2021, 277, 111405. [Google Scholar] [CrossRef]
- Wang, Q.; Zhong, M.; Wang, S. A Meta-Analysis on the Response of Microbial Biomass, Dissolved Organic Matter, Respiration, and N Mineralization in Mineral Soil to Fire in Forest Ecosystems. For. Ecol. Manag. 2012, 271, 91–97. [Google Scholar] [CrossRef]
- Fontúrbel, M.T.; Barreiro, A.; Vega, J.A.; Martín, A.; Jiménez, E.; Carballas, T.; Fernández, C.; Díaz-Raviña, M. Effects of an Experimental Fire and Post-Fire Stabilization Treatments on Soil Microbial Communities. Geoderma 2012, 191, 51–60. [Google Scholar] [CrossRef]
- Fontúrbel, M.T.; Fernández, C.; Vega, J.A. Prescribed Burning versus Mechanical Treatments as Shrubland Management Options in NW Spain: Mid-Term Soil Microbial Response. Appl. Soil Ecol. 2016, 107, 334–346. [Google Scholar] [CrossRef]
- Pérez-Valera, E.; Goberna, M.; Verdú, M. Fire modulates ecosystem functioning through the phylogenetic structure of soil bacterial communities. Soil Biol. Biochem. 2019, 129, 80–89. [Google Scholar] [CrossRef] [Green Version]
- Turan, V.; Schröder, P.; Bilen, S.; Insam, H.; Fernández-Delgado Juárez, M. Co-Inoculation Effect of Rhizobium and Achillea millefolium L. Oil Extracts on Growth of Common Bean (Phaseolus vulgaris L.) and Soil Microbial-Chemical Properties. Sci. Rep. 2019, 9, 15178. [Google Scholar] [CrossRef] [Green Version]
- Lucas-Borja, M.E.; Ortega, R.; Miralles, I.; Plaza-Álvarez, P.A.; González-Romero, J.; Peña-Molina, E.; Moya, D.; Zema, D.A.; Wagenbrenner, J.W.; de las Heras, J. Effects of Wildfire and Logging on Soil Functionality in the Short-Term in Pinus Halepensis M. Forests. Eur. J. For. Res. 2020, 139, 935–945. [Google Scholar] [CrossRef]
- Bastida, F.; Zsolnay, A.; Hernández, T.; García, C. Past, Present and Future of Soil Quality Indices: A Biological Perspective. Geoderma 2008, 147, 159–171. [Google Scholar] [CrossRef]
- Barrios, E. Soil Biota, Ecosystem Services and Land Productivity. Ecol. Econ. 2007, 64, 269–285. [Google Scholar] [CrossRef]
- Doblas-Miranda, E.; Martínez-Vilalta, J.; Lloret, F.; Álvarez, A.; Ávila, A.; Bonet, F.J.; Brotons, L.; Castro, J.; Yuste, J.C.; Díaz, M.; et al. Reassessing Global Change Research Priorities in Mediterranean Terrestrial Ecosystems: How Far Have We Come and Where Do We Go from Here? Glob. Ecol. Biogeogr. 2015, 24, 25–43. [Google Scholar] [CrossRef] [Green Version]
- Baraza-Ruiz, E.; Bota, J.; Romero-Munar, A.; Nogales, B. Aplicación de la técnica BiologTM ECO-plate para el estudio del perfil fisiológico de las comunidades microbianas del suelo agrícola. Ecosistemas 2019, 28, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Sofo, A.; Ricciuti, P. A Standardized Method for Estimating the Functional Diversity of Soil Bacterial Community by Biolog® EcoPlatesTM Assay—The Case Study of a Sustainable Olive Orchard. Appl. Sci. 2019, 9, 4035. [Google Scholar] [CrossRef] [Green Version]
- Lawton, J.H.; Naeem, S.; Woodfin, R.M.; Brown, V.K.; Gange, A.; Godfray, H.J.C.; Heads, P.A.; Lawler, S.; Magda, D.; Thomas, C.D.; et al. The Ecotron: A Controlled Environmental Facility for the Investigation of Population and Ecosystem Processes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1993, 341, 181–194. [Google Scholar] [CrossRef]
- Granjou, C.; Walker, J. Promises That Matter: Reconfiguring Ecology in the Ecotrons. Sci. Technol. Stud. 2016, 29, 49–67. [Google Scholar] [CrossRef]
- Alcañiz, M.; Outeiro, L.; Francos, M.; Farguell, J.; Úbeda, X. Long-term dynamics of soil chemical properties after a prescribed fire in a Mediterranean forest (Montgrí Massif, Catalonia, Spain). Sci. Total Environ. 2016, 572, 1329–1335. [Google Scholar] [CrossRef]
- Ammitzboll, H.; Jordan, G.J.; Baker, S.C.; Freeman, J.; Bissett, A. Contrasting Successional Responses of Soil Bacteria and Fungi to Post-Logging Burn Severity. For. Ecol. Manag. 2022, 508, 120059. [Google Scholar] [CrossRef]
- Lucas-Borja, M.E.; Miralles, I.; Ortega, R.; Plaza-Álvarez, P.A.; Gonzalez-Romero, J.; Sagra, J.; Soriano-Rodríguez, M.; Certini, G.; Moya, D.; Heras, J. Immediate Fire-Induced Changes in Soil Microbial Community Composition in an Outdoor Experimental Controlled System. Sci. Total Environ. 2019, 696, 134033. [Google Scholar] [CrossRef]
- López-Poma, R.; Bautista, S. Plant Regeneration Functional Groups Modulate the Response to Fire of Soil Enzyme Activities in a Mediterranean Shrubland. Soil Biol. Biochem. 2014, 79, 5–13. [Google Scholar] [CrossRef]
- Moya, D.; González-De Vega, S.; Lozano, E.; García-Orenes, F.; Mataix-Solera, J.; Lucas-Borja, M.E.; de las Heras, J. The Burn Severity and Plant Recovery Relationship Affect the Biological and Chemical Soil Properties of Pinus halepensis Mill. Stands in the Short and Mid-Terms after Wildfire. J. Environ. Manag. 2019, 235, 250–256. [Google Scholar] [CrossRef]
- Martinez-Salgado, M.M.; Gutiérrez-Romero, V.; Jannsens, M.; Ortega-Blu, R. Biological soil quality indicators: A review. Curr. Res. Technol. Educ. Top. Appl. Microbiol. Microb. Biotechnol. 2010, 1, 319–328. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2014.
- Andrews, P.L.; Bevins, C.D.; Seli, R.C. BehavePlus Fire Modeling System, Version 4.0: User’s Guide; General Technical Report; Department of Agriculture, Forest Service, Rocky Mountain Research Station: Ogden, UT, USA, 2008. [Google Scholar]
- Jiménez-Morillo, N.T.; Almendros, G.; De la Rosa, J.M.; Jordán, A.; Zavala, L.M.; Granged, A.J.P.; González-Pérez, J.A. Effect of a Wildfire and of Post-Fire Restoration Actions in the Organic Matter Structure in Soil Fractions. Sci. Total Environ. 2020, 728, 138715. [Google Scholar] [CrossRef]
- Lange, M.; Eisenhauer, N.; Sierra, C.A.; Bessler, H.; Engels, C.; Griffiths, R.I.; Mellado-Vázquez, P.G.; Malik, A.A.; Roy, J.; Scheu, S.; et al. Plant Diversity Increases Soil Microbial Activity and Soil Carbon Storage. Nat. Commun. 2015, 6, 6707. [Google Scholar] [CrossRef]
- Badía, D.; López-García, S.; Martí, C.; Ortíz-Perpiñá, O.; Girona-García, A.; Casanova-Gascón, J. Burn Effects on Soil Properties Associated to Heat Transfer under Contrasting Moisture Content. Sci. Total Environ. 2017, 601–602, 1119–1128. [Google Scholar] [CrossRef]
- Gee, W.G.; Or, D. Particle-Size Analysis. In Methods of Soil Analysis. Book Series: 5. Part 4; Dane, J., Topp, G.C., Eds.; Soil Science Society of America; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2002; pp. 255–293. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1996; pp. 961–1010. ISBN 978-0-89118-866-7. [Google Scholar]
- Lax, A.; Roig, A.; Costa, F. A Method for Determining the Cation-Exchange Capacity of Organic Materials. Plant Soil 1986, 94, 349–355. [Google Scholar] [CrossRef]
- Knudsen, D.; Peterson, G.A.; Pratt, P.F. Lithium, Sodium, and Potassium. In Methods of Soil Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1983; pp. 225–246. ISBN 978-0-89118-977-0. [Google Scholar]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen—Total. In Methods of Soil Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1983; pp. 595–624. ISBN 978-0-89118-977-0. [Google Scholar]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1983; pp. 403–430. ISBN 978-0-89118-977-0. [Google Scholar]
- Rutgers, M.; Wouterse, M.; Drost, S.M.; Breure, A.M.; Mulder, C.; Stone, D.; Creamer, R.E.; Winding, A.; Bloem, J. Monitoring Soil Bacteria with Community-Level Physiological Profiles Using BiologTM ECO-Plates in the Netherlands and Europe. Appl. Soil Ecol. 2016, 97, 23–35. [Google Scholar] [CrossRef]
- Garland, J.L.; Mills, A.L. Classification and Characterization of Heterotrophic Microbial Communities on the Basis of Patterns of Community-Level Sole-Carbon-Source Utilization. Appl. Environ. Microbiol. 1991, 57, 2351–2359. [Google Scholar] [CrossRef] [Green Version]
- Guénon, R.; Gros, R. Frequent-Wildfires with Shortened Time-since-Fire Affect Soil Microbial Functional Stability to Drying and Rewetting Events. Soil Biol. Biochem. 2013, 57, 663–674. [Google Scholar] [CrossRef]
- Zabinski, C.A.; Gannon, J.E. Effects of Recreational Impacts on Soil Microbial Communities. Environ. Manag. 1997, 21, 233–238. [Google Scholar] [CrossRef]
- Zak, J.C.; Willig, M.R.; Moorhead, D.L.; Wildman, H.G. Functional Diversity of Microbial Communities: A Quantitative Approach. Soil Biol. Biochem. 1994, 26, 1101–1108. [Google Scholar] [CrossRef]
- Bending, G.D.; Putland, C.; Rayns, F. Changes in Microbial Community Metabolism and Labile Organic Matter Fractions as Early Indicators of the Impact of Management on Soil Biological Quality. Biol. Fertil. Soils 2000, 31, 78–84. [Google Scholar] [CrossRef]
- RStudio Team RStudio: Integrated Development for R. RStudio. Available online: http://www.rstudio.com (accessed on 2 February 2021).
- IBM Corp IBM SPSS Statistics for Windows, Version 24.0. Available online: www.ibm.com/mysupport/s/question/0d50z00006pfp7zcad/how-do-you-cite-spss-version-24-in-a-publication (accessed on 2 February 2021).
- Vega, J.A.; Fontúrbel, T.; Merino, A.; Fernández, C.; Ferreiro, A.; Jiménez, E. Testing the Ability of Visual Indicators of Soil Burn Severity to Reflect Changes in Soil Chemical and Microbial Properties in Pine Forests and Shrubland. Plant Soil 2013, 369, 73–91. [Google Scholar] [CrossRef]
- Guénon, R.; Vennetier, M.; Dupuy, N.; Ziarelli, F.; Gros, R. Soil Organic Matter Quality and Microbial Catabolic Functions along a Gradient of Wildfire History in a Mediterranean Ecosystem. Appl. Soil Ecol. 2011, 48, 81–93. [Google Scholar] [CrossRef] [Green Version]
- Fontúrbel, M.T.; Vega, J.A.; Bara, S.; Bernardez, I. Influence of Prescribed Burning of Pine Stands in NW Spain on Soil Microorganisms. Eur. J. Soil Biol. 1995, 31, 13–20. [Google Scholar]
- Doblas-Miranda, E.; Alonso, R.; Arnan, X.; Bermejo, V.; Brotons, L.; de las Heras, J.; Estiarte, M.; Hódar, J.A.; Llorens, P.; Lloret, F.; et al. A Review of the Combination among Global Change Factors in Forests, Shrublands and Pastures of the Mediterranean Region: Beyond Drought Effects. Glob. Planet. Chang. 2017, 148, 42–54. [Google Scholar] [CrossRef] [Green Version]
- Certini, G. Effects of Fire on Properties of Forest Soils: A Review. Oecologia 2005, 143, 1–10. [Google Scholar] [CrossRef]
- Brunel, C.; Da Silva, A.-M.F.; Gros, R. Environmental Drivers of Microbial Functioning in Mediterranean Forest Soils. Microb. Ecol. 2020, 80, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Knicker, H. How Does Fire Affect the Nature and Stability of Soil Organic Nitrogen and Carbon? A Review. Biogeochemistry 2007, 85, 91–118. [Google Scholar] [CrossRef]
- Merino, A.; Omil, B.; Fonturbel, M.T.; Vega, J.A.; Balboa, M.A. Reclamation of Intensively Managed Soils in Temperate Regions by Addition of Wood Bottom Ash Containing Charcoal: SOM Composition and Microbial Functional Diversity. Appl. Soil Ecol. 2016, 100, 195–206. [Google Scholar] [CrossRef]
- Hamman, S.T.; Burke, I.C.; Knapp, E.E. Soil Nutrients and Microbial Activity after Early and Late Season Prescribed Burns in a Sierra Nevada Mixed Conifer Forest. For. Ecol. Manag. 2008, 256, 367–374. [Google Scholar] [CrossRef]
- Aponte, C.; Marañón, T.; García, L.V. Microbial C, N and P in Soils of Mediterranean Oak Forests: Influence of Season, Canopy Cover and Soil Depth. Biogeochemistry 2010, 101, 77–92. [Google Scholar] [CrossRef] [Green Version]
- Lucas-Borja, M.E.; Plaza-Álvarez, P.A.; Ortega, R.; Miralles, I.; Gonzalez-Romero, J.; Sagra, J.; Moya, D.; Zema, D.A.; de las Heras, J. Short-term changes in soil functionality after wildfire and straw mulching in a Pinus halepensis M. forest. For. Ecol. Manag. 2020, 457, 117700. [Google Scholar] [CrossRef]
- Lombao, A.; Barreiro, A.; Fontúrbel, M.T.; Martín, A.; Carballas, T.; Díaz-Raviña, M. Key Factors Controlling Microbial Community Responses after a Fire: Importance of Severity and Recurrence. Sci. Total Environ. 2020, 741, 140363. [Google Scholar] [CrossRef]
- D’Ascoli, R.; Rutigliano, F.A.; Pascale, R.A.D.; Gentile, A.; Santo, A.V.D. Functional Diversity of the Microbial Community in Mediterranean Maquis Soils as Affected by Fires. Int. J. Wildland Fire 2005, 14, 355–363. [Google Scholar] [CrossRef]
- Staddon, W.J.; Duchesne, L.C.; Trevors, J.T. Microbial Diversity and Community Structure of Postdisturbance Forest Soils as Determined by Sole-Carbon-Source Utilization Patterns. Microb. Ecol. 1997, 34, 125–130. [Google Scholar] [CrossRef]
- Giai, C.; Boerner, R.E.J. Effects of Ecological Restoration on Microbial Activity, Microbial Functional Diversity, and Soil Organic Matter in Mixed-Oak Forests of Southern Ohio, USA. Appl. Soil Ecol. 2007, 35, 281–290. [Google Scholar] [CrossRef]
- Gomez, E.; Ferreras, L.; Toresani, S. Soil Bacterial Functional Diversity as Influenced by Organic Amendment Application. Bioresour. Technol. 2006, 97, 1484–1489. [Google Scholar] [CrossRef] [PubMed]
- Tedim, F.; Leone, V.; Amraoui, M.; Bouillon, C.; Coughlan, M.R.; Delogu, G.M.; Fernandes, P.M.; Ferreira, C.; McCaffrey, S.; McGee, T.K.; et al. Defining Extreme Wildfire Events: Difficulties, Challenges, and Impacts. Fire 2018, 1, 9. [Google Scholar] [CrossRef] [Green Version]
- Chuvieco, E.; Aguado, I.; Salas, J.; García, M.; Yebra, M.; Oliva, P. Satellite Remote Sensing Contributions to Wildland Fire Science and Management. Curr. For. Rep. 2020, 6, 81–96. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).