Spring and Autumn Phenology in Sessile Oak (Quercus petraea) Near the Eastern Limit of Its Distribution Range
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Sampling
2.2. Phenological Observations
3. Results
3.1. Budburst and Leaf Development
3.2. Flowering
3.3. Leaf Senescence
3.4. Correlations
4. Discussion
4.1. Budburst Phenology
4.2. Flowering Phenology
4.3. Leaf Senescence
4.4. Correlations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lieth, H. Purposes of a phenological book. In Phenology and Seasonality Modeling; Lieth, H., Ed.; Springer: Berlin, Germany, 1974; pp. 3–23. [Google Scholar]
- Davi, H.; Gillmann, M.; Ibanez, T.; Cailleret, M.; Bontemps, A.; Fady, B.; Lefèvre, F. Diversity of leaf unfolding dynamics among tree species: New insights from a study along an altitudinal gradient. Agric. For. Meteorol. 2011, 151, 1504–1513. [Google Scholar] [CrossRef]
- Zhao, M.; Peng, C.; Xiang, W.; Deng, X.; Tian, D.; Zhou, X.; He, H.; Zhao, Z. Plant phenological modeling and its application in global climate change research: Overview and future challenges. Environ. Rev. 2013, 21, 1–14. [Google Scholar] [CrossRef]
- Tang, J.; Körner, C.; Muraoka, H.; Piao, S.; Shen, M.; Thackeray, S.J.; Yang, X. Emerging opportunities and challenges in phenology: A review. Ecosphere 2016, 7, e01436. [Google Scholar] [CrossRef] [Green Version]
- Workie, T.G.; Debella, H.J. Climate change and its effects on vegetation phenology across ecoregions of Ethiopia. Glob. Ecol. Conserv. 2018, 13, e00366. [Google Scholar] [CrossRef]
- Khare, S.; Drolet, G.; Sylvain, J.D.; Paré, M.C.; Rossi, S. Assessment of Spatio-Temporal Patterns of Black Spruce Bud Phenology across Quebec Based on MODIS-NDVI Time Series and Field Observations. Remote Sens. 2019, 11, 2745. [Google Scholar] [CrossRef] [Green Version]
- Bertin, R.I. Plant phenology and distribution in relation to recent climate change. J. Torrey Bot. Soc. 2008, 135, 126–146. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Friedl, M.A.; Schaaf, C.B. Sensitivity of vegetation phenology detection to the temporal resolution of satellite data. Int. J. Remote Sens. 2009, 30, 2061–2074. [Google Scholar] [CrossRef]
- Chen, X.; Xu, L. Phenological responses of Ulmus pumila (Siberian Elm) to climate change in the temperate zone of China. Int. J. Biometeorol. 2012, 56, 695–706. [Google Scholar] [CrossRef]
- Richardson, A.D.; Keenan, T.F.; Migliavacca, M.; Ryu, Y.; Sonnentag, O.; Toomey, M. Climate change, phenology, and phenological control of vegetation feedbacks to the climate system. Agric. For. Meteorol. 2013, 169, 156–173. [Google Scholar] [CrossRef]
- Campbell, R.K.; Sugano, A.I. Phenology of Bud Burst in Douglas-Fir Related to Provenance, Photoperiod, Chilling, and Flushing Temperature. Bot. Gaz. 1975, 136, 290–298. [Google Scholar] [CrossRef]
- White, M.A.; Thornton, P.E.; Running, S.W. A continental phenology model for monitoring vegetation responses to interannual climatic variability. Glob. Biogeochem. Cycles 1997, 11, 217–234. [Google Scholar] [CrossRef]
- Chuine, I.; Cour, P.; Rousseau, D. Selecting models to predict the timing of flowering of temperate trees: Implications for tree phenology modelling. Plant Cell Environ. 1999, 22, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Chuine, I.; de Cortazar-Atauri, I.G.; Kramer, K.; Hänninen, H. Plant development models. In Phenology: An Integrative Environmental Science; Springer: Dordrecht, The Netherlands, 2013; pp. 275–293. [Google Scholar]
- Menzel, A.; Estrella, N.; Fabian, P. Spatial and temporal variability of the phenological seasons in Germany from 1951 to 1996. Glob. Chang. Biol. 2001, 7, 657–666. [Google Scholar] [CrossRef]
- Chmielewski, F.M.; Rötzer, T. Response of tree phenology to climate change across Europe. Agric. For. Meteorol. 2001, 108, 101–112. [Google Scholar] [CrossRef]
- Schieber, B. Spring phenology of European beech (Fagus sylvatica L.) in a submountain beech stand with different stocking in 1995–2004. J. For. Sci. 2006, 52, 208–216. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Yan, Z.; Zhang, S. Forest Phenology Shifts in Response to Climate Change over China–Mongolia–Russia International Economic Corridor. Forests 2020, 11, 757. [Google Scholar] [CrossRef]
- Chuine, I.; Beaubien, E.G. Phenology is a major determinant of tree species range. Ecol. Lett. 2001, 4, 500–510. [Google Scholar] [CrossRef] [Green Version]
- Chuine, I. Why does phenology drive species distribution? Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 3149–3160. [Google Scholar] [CrossRef] [Green Version]
- Soularue, J.P.; Kremer, A. Evolutionary responses of tree phenology to the combined effects of assortative mating, gene flow and divergent selection. Heredity 2014, 113, 485–494. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Jiang, J.M.; Chen, Y.T. Genetic diversity of central and peripheral populations of Toona ciliata var. pubescens, an endangered tree species endemic to China. Genet. Mol. Res. 2014, 13, 4579–4590. [Google Scholar] [CrossRef]
- Kelleher, C.T.; de Vries, S.M.G.; Baliuckas, V.; Bozzano, M.; Frýdl, J.; Gonzalez Goicoechea, P.; Ivankovic, M.; Kandemir, G.; Koskela, J.; Kozioł, C.; et al. Approaches to the Conservation of Forest Genetic Resources in Europe in the Context of Climate Change; European Forest Genetic Resources Programme (EUFORGEN), Bioversity International: Rome, Italy, 2015; p. XIV 3. [Google Scholar]
- Fady, B.; Aravanopoulos, F.A.; Alizoti, P.; Mátyás, C.; von Wühlisch, G.; Westergren, M.; Belletti, P.; Cvjetkovic, B.; Ducci, F.; Huber, G.; et al. Evolution-based approach needed for the conservation and silviculture of peripheral forest tree populations. For. Ecol. Manag. 2016, 375, 66–75. [Google Scholar] [CrossRef] [Green Version]
- Ducci, F.; Donnelly, K. Forest tree marginal populations in Europe-report on the state of knowledge on forest tree marginal and peripheral populations in Europe. Ann. Silvic. Res. 2017, 41, 1–12. Available online: https://journals-crea.4science.it/index.php/asr (accessed on 13 March 2022).
- Mátyás, C.; Berki, I.; Bidló, A.; Csóka, G.; Czimber, K.; Führer, E.; Gálos, B.; Gribovszki, Z.; Illés, G.; Hirka, A.; et al. Sustainability of Forest Cover under Climate Change on the Temperate-Continental Xeric Limits. Forests 2018, 9, 489. [Google Scholar] [CrossRef] [Green Version]
- Popescu, R.; Șofletea, N. Spring and autumn phenology in sub-mesothermal beech stands from the southwestern extremity of the Carpathians. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 1057–1069. [Google Scholar] [CrossRef]
- Gapare, W.J.; Aitken, S.N.; Ritland, C.E. Genetic diversity of core and peripheral Sitka spruce (Picea sitchensis (Bong.) Carr) populations: Implications for conservation of widespread species. Biol. Conserv. 2005, 123, 113–123. [Google Scholar] [CrossRef]
- Lesica, P.; Allendorf, F.W. When are peripheral populations valuable for conservation? Conserv. Biol. 1995, 9, 753–760. [Google Scholar] [CrossRef]
- Nielsen, J.L.; Scott, J.M.; Aycrigg, J.L. Endangered species and peripheral populations: Cause for conservation. Endanger. Species Update 2001, 18, 194–197. [Google Scholar]
- Ursenbacher, S.; Guillon, M.; Cubizolle, H.; Dupoué, A.; Blouin-Demers, G.; Lourdais, O. Postglacial recolonization in a cold climate specialist in western Europe: Patterns of genetic diversity in the adder (Vipera berus) support the central–marginal hypothesis. Mol. Ecol. 2015, 24, 3639–3651. [Google Scholar] [CrossRef]
- Eckert, C.G.; Samis, K.E.; Lougheed, S.C. Genetic variation across species’ geographical ranges: The central–marginal hypothesis and beyond. Mol. Ecol. 2008, 17, 1170–1188. [Google Scholar] [CrossRef]
- Lázaro-Nogal, A.; Matesanz, S.; García-Fernández, A.; Traveset, A.; Valladares, F. Population size, center–periphery, and seed dispersers’ effects on the genetic diversity and population structure of the Mediterranean relict shrub Cneorum tricoccon. Ecol. Evol. 2017, 7, 7231–7242. [Google Scholar] [CrossRef]
- Macdonald, S.L.; Llewelyn, J.; Moritz, C.; Phillips, B.L. Peripheral isolates as sources of adaptive diversity under climate change. Front. Ecol. Evol. 2017, 5, 88. [Google Scholar] [CrossRef] [Green Version]
- Vucetich, J.A.; Waite, T.A. Spatial patterns of demography and genetic processes across the species’ range: Null hypotheses for landscape conservation genetics. Conserv. Genet. 2003, 4, 639–645. [Google Scholar] [CrossRef]
- Hardie, D.C.; Hutchings, J.A. Evolutionary ecology at the extremes of species’ ranges. Environ. Rev. 2010, 18, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Eliades, N.G.H.; Papageorgiou, A.C.; Fady, B.; Gailing, O.; Leinemann, L.; Finkeldey, R. An approach to genetic resources conservation of peripheral isolated plant populations: The case of an island narrow endemic species. Biodivers. Conserv. 2019, 28, 3005–3035. [Google Scholar] [CrossRef]
- Mátyás, C.; Vendramin, G.G.; Fady, B. Forests at the limit: Evolutionary–genetic consequencesof environmental changes at the receding (xeric) edge of distribution. Report from a research workshop. Ann. For. Sci. 2009, 66, 800. [Google Scholar] [CrossRef]
- Mátyás, C. Forecasts needed for retreating forests. Nature 2010, 464, 1271. [Google Scholar] [CrossRef]
- Borovics, A.; Mátyás, C. Decline of genetic diversity of sessile oak at the retracting (xeric) limits. Ann. For. Sci. 2013, 70, 835–844. [Google Scholar] [CrossRef] [Green Version]
- Ciocîrlan, E.; Sofletea, N.; Ducci, F.; Curtu, A.L. Patterns of genetic diversity in European beech (Fagus sylvatica L.) at the eastern margins of its distribution range. Iforest-Biogeosci. For. 2017, 10, 916. [Google Scholar] [CrossRef] [Green Version]
- Liepe, K. Growth-chamber trial on frost hardiness and field trial on flushing of sessile oak (Quercus petraea Liebl). In Annales des Sciences Forestières; EDP Sciences: Les Ulis, France, 1993; Volume 50, pp. 208s–214s. [Google Scholar] [CrossRef]
- Deans, J.; Harvey, F. Phenologies of sixteen European provenances of sessile oak growing in Scotland. For. Int. J. For. Res. 1995, 68, 265–274. [Google Scholar] [CrossRef]
- Ducousso, A.; Guyon, J.; Kremer, A. Latitudinal and altitudinal variation of bud burst in western populations of sessile oak (Quercus petraea (Matt) Liebl). Ann. Des Sci. For. 1996, 53, 775–782. [Google Scholar] [CrossRef]
- Vitasse, Y.; François, C.; Delpierre, N.; Dufrêne, E.; Kremer, A.; Chuine, I.; Delzon, S. Assessing the effects of climate change on the phenology of European temperate trees. Agric. For. Meteorol. 2011, 151, 969–980. [Google Scholar] [CrossRef]
- Alberto, F.; Bouffier, L.; Louvet, J.M.; Lamy, J.B.; Delzon, S.; Kremer, A. Adaptive responses for seed and leaf phenology in natural populations of sessile oak along an altitudinal gradient. J. Evol. Biol. 2011, 24, 1442–1454. [Google Scholar] [CrossRef] [PubMed]
- Alberto, F.J.; Derory, J.; Boury, C.; Frigerio, J.-M.; Zimmermann, N.E.; Kremer, A. Imprints of natural selection along environmental gradients in phenology-related genes of Quercus petraea. Genetics 2013, 195, 495–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vander Mijnsbrugge, K.; Turcsán, A.; Maes, J.; Duchêne, N.; Meeus, S.; Steppe, K.; Steenackers, M. Repeated summer drought and re-watering during the first growing year of oak (Quercus petraea) delay autumn senescence and bud burst in the following spring. Front. Plant Sci. 2016, 7, 419. [Google Scholar] [CrossRef]
- Delpierre, N.; Guillemot, J.; Dufrêne, E.; Cecchini, S.; Nicolas, M. Tree phenological ranks repeat from year to year and correlate with growth in temperate deciduous forests. Agric. For. Meteorol. 2017, 234, 1–10. [Google Scholar] [CrossRef]
- Firmat, C.; Delzon, S.; Louvet, J.M.; Parmentier, J.; Kremer, A. Evolutionary dynamics of the leaf phenological cycle in an oak metapopulation along an elevation gradient. J. Evol. Biol. 2017, 30, 2116–2131. [Google Scholar] [CrossRef] [Green Version]
- Sáenz-Romero, C.; Lamy, J.B.; Ducousso, A.; Musch, B.; Ehrenmann, F.; Delzon, S.; Cavers, S.; Chałupka, W.; Dağdaş, S.; Hansen, J.K.; et al. Adaptive and plastic responses of Quercus petraea populations to climate across Europe. Glob. Chang. Biol. 2017, 23, 2831–2847. [Google Scholar] [CrossRef] [Green Version]
- Denéchère, R.; Delpierre, N.; Apostol, E.N.; Berveiller, D.; Bonne, F.; Cole, E.; Delzon, S.; Dufrêne, E.; Gressler, E.; Jean, F.; et al. The within-population variability of leaf spring and autumn phenology is influenced by temperature in temperate deciduous trees. Int. J. Biometeorol. 2019, 65, 369–379. [Google Scholar] [CrossRef]
- Czúcz, B.; Gálhidy, L.; Mátyás, C. Present and forecasted xeric climatic limits of beech and sessile oak distribution at low altitudes in Central Europe. Ann. For. Sci. 2011, 68, 99–108. [Google Scholar] [CrossRef] [Green Version]
- NFI (National Forest Inventory). 2019. Available online: http://roifn.ro/site/rezultate-ifn-2/ (accessed on 5 December 2020).
- Şofletea, N.; Curtu, L. Dendrologie; Editura Universităţii Transilvania: Brasov, Romania, 2007; pp. 156–164. [Google Scholar]
- Paşcovschi, S.; Doniță, N. Vegetaţia Lemnoasă din Silvostepa României; Editura Academiei Republicii Socialiste România: Bucharest, Romania, 1967. [Google Scholar]
- Sanda, V.; Barabaş, N.; Ştefănuţ, S. Atlas Florae Romaniae. III- Genul Quercus; Editura “Ion Borcea”: Bacau, Romania, 2004; p. 173. [Google Scholar]
- Chesnoiu, E.N.; Șofletea, N.; Curtu, A.L.; Toader, A.; Radu, R.; Enescu, M. Bud burst and flowering phenology in a mixed oak forest from Eastern Romania. Ann. For. Res. 2009, 52, 199–206. [Google Scholar] [CrossRef]
- Crăciunesc, I. Evaluation of Natural Hybridization in Native Species of Oaks: A Case Study in Bejan-Deva Natural Reserve. Ph.D. Thesis, Transilvania University of Brasov Degree-Granting University, Brasov, Romania, 2013; p. 175. [Google Scholar]
- Ducousso, A.; Bordacs, S. EUFORGEN Technical Guidelines for Genetic Conservation and use for Pedunculate and Sessile Oaks (Quercus robur and Q. petraea); International Plant Genetic Resources Institute: Rome, Italy, 2004; 6 pages, Available online: www.euforgen.org (accessed on 13 March 2022).
- Pârnuța, G.; Lorent, A.; Tudoroiu, M.; Petrila, M. Regiuni de Provenienta Pentru Materiale de Baza din Care se Obtin Materiale Forestiere de Reproducere în România; Editura Silvica: Bucharest, Romania, 2010. [Google Scholar]
- Pâques, L.E. TREES4FUTURE—Designing Trees for the Future. Alternative Methodologies for Phenology Assessment in Forest Trees. 2016, pp. 4–6. Available online: http://www.trees4future.eu/uploads/t4f-training-activities/Annex%20I%201%20Alternative%20methodologies%20for%20phenology%20assessment%20in%20forest%20trees.pdf (accessed on 13 March 2022).
- Bacilieri, R.; Ducousso, A.; Kremer, A. Genetic, morphological, ecological and phenological differentiation between Quercus petraea (Matt.) Liebl. and Quercus robur L. in a mixed stand of northwest of France. Silvae Genet. 1995, 44, 1–9. [Google Scholar]
- Preushler, T. Manual on methods and criteria for harmonized sampling, assessment, monitoring and analysis of the effects of air pollution on forest. In Part IX, Phenological Observatio; UNECE: Geneva, Switzerland, 1999. [Google Scholar]
- STATISTICA, Version 8.0; StatSoft Inc.: Tulsa, OK, USA, 2007.
- Wiersma, J.H. Enkete kwantitatieve aspecten van het exotenvraagstuk. Ned. Bosb. Tijtschrift 1962, 34, 175–184. [Google Scholar]
- Tomescu, A. Fazele Periodice de Vegetație la Speciile Forestiere—Sinteza Pentru Perioada 1946–1955; Editura Agro-Silvică de Stat: Bucharest, Romania, 1957; p. 123. [Google Scholar]
- Chesnoiu, E.N. Taxonomic Discrimination through Leaf Descriptors and Analysis of Spring Phenology in Pedunculate Oak (Quercus robur L.) and Grayish Oak (Quercus pedunculiflora K. Koch). Ph.D. Thesis, Transilvania University of Brasov Degree-Granting University, Brasov, Romania, 2017. Summary. p. 67. [Google Scholar]
- Perot, T.; Balandier, P.; Couteau, C.; Delpierre, N.; Jean, F.; Perret, S.; Korboulewsky, N. Budburst date of Quercus petraea is delayed in mixed stands with Pinus sylvestris. Agric. For. Meteorol. 2021, 300, 108326. [Google Scholar] [CrossRef]
- Dantec, C.F.; Vitasse, Y.; Bonhomme, M.; Louvet, J.M.; Kremer, A.; Delzon, S. Chilling and heat requirements for leaf unfolding in European beech and sessile oak populations at the southern limit of their distribution range. Int. J. Biometeorol. 2014, 58, 1853–1864. [Google Scholar] [CrossRef]
- de Sauvage, J.C.; Vitasse, Y.; Meier, M.; Delzon, S.; Bigler, C. Temperature rather than individual growing period length determines radial growth of sessile oak in the Pyrenees. Agric. For. Meteorol. 2022, 317, 108885. [Google Scholar] [CrossRef]
- Jensen, J.S. Provenance variation in phenotypic traits in Quercus robur and Quercus petraea in Danish provenance trials. Scand. J. For. Res. 2000, 15, 297–308. [Google Scholar] [CrossRef]
- Sampaio, T.; Branco, M.; Guichoux, E.; Petit, R.J.; Pereira, J.S.; Varela, M.C.; Almeida, M.H. Does the geography of cork oak origin influence budburst and leaf pest damage? For. Ecol. Manag. 2016, 373, 33–43. [Google Scholar] [CrossRef]
- Chmura, D.J.; Rozkowski, R. Variability of beech provenances in spring and autumn phenology. Silvae Genet. 2002, 51, 123–127. [Google Scholar]
- Anandhi, A.; Perumal, S.; Gowda, P.H.; Knapp, M.; Hutchinson, S.; Harrington, J.; Murray, L.; Kirkham, M.B.; Rice, C.W. Long-term spatial and temporal trends in frost indices in Kansas, USA. Clim. Change 2013, 120, 169–181. [Google Scholar] [CrossRef] [Green Version]
- Vanoni, M.; Bugmann, H.; Nötzli, M.; Bigler, C. Drought and frost contribute to abrupt growth decreases before tree mortality in nine temperate tree species. For. Ecol. Manag. 2016, 382, 51–63. [Google Scholar] [CrossRef] [Green Version]
- Gazol, A.; Camarero, J.J.; Colangelo, M.; de Luis, M.; del Castillo, E.M.; Serra-Maluquer, X. Summer drought and spring frost, but not their interaction, constrain European beech and Silver fir growth in their southern distribution limits. Agric. For. Meteorol. 2019, 278, 107695. [Google Scholar] [CrossRef]
- Sakai, A.; Larcher, W. Frost Survival of Plants: Responses and Adaptation to Freezing Stress; Springer Science & Business Media: Berlin, Germany, 2012; Volume 62. [Google Scholar]
- Schermer, É.; Bel-Venner, M.C.; Gaillard, J.M.; Dray, S.; Boulanger, V.; Le Roncé, I.; Oliver, G.; Chuine, I.; Delzon, S.; Venner, S. Flower phenology as a disruptor of the fruiting dynamics in temperate oak species. N. Phytol. 2020, 225, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Garca-Mozo, H.; Pablo, J.H.; Carmen, G.; Gomez-casero, M.T.; Dominguez, E. Catkin frost damage in Mediterranean cork-oak (Quercus suber L.). Isr. J. Plant Sci. 2001, 49, 42–47. [Google Scholar] [CrossRef]
- Chaar, H.; Colin, F. Impact of late frost on height growth in young sessile oak regenerations. Ann. For. Sci. 1999, 56, 417–429. [Google Scholar] [CrossRef] [Green Version]
- Grundström, M.; Adams-Groom, B.; Pashley, C.H.; Dahl, Å.; Rasmussen, K.; de Weger, L.A.; Thibaudon, M.; Fernández-Rodríguez, S.; Silva-Palacios, I.; Skjøth, C.A. Oak pollen seasonality and severity across Europe and modelling the season start using a generalized phenological model. Sci. Total Environ. 2019, 663, 527–536. [Google Scholar] [CrossRef]
- Gerst, K.L.; Rossington, N.L.; Mazer, S.J. Phenological responsiveness to climate differs among four species of Quercus in North America. J. Ecol. 2017, 105, 1610–1622. [Google Scholar] [CrossRef] [Green Version]
- Tomescu, A. Cercetări Fenologice la Principalele Specii Forestiere Autohtone din RSR-Sinteza Pentru Perioada 1956–1965; Centrul de Documentare Tehnică Pentru Economia Forestieră: Bucharest, Romania, 1967; p. 100. [Google Scholar]
- Liu, G.; Chuine, I.; Denéchère, R.; Jean, F.; Dufrêne, E.; Vincent, G.; Berveiller, D.; Delpierre, N. Higher sample sizes and observer inter-calibration are needed for reliable scoring of leaf phenology in trees. J. Ecol. 2021, 109, 2461–2474. [Google Scholar] [CrossRef]
- Delpierre, N.; Dufrêne, E.; Soudani, K.; Ulrich, E.; Cecchini, S.; Boé, J.; François, C. Modelling interannual and spatial variability of leaf senescence for three deciduous tree species in France. Agric. For. Meteorol. 2009, 149, 938–948. [Google Scholar] [CrossRef]
- Menzel, A.; Fabian, P. Growing season extended in Europe. Nature 1999, 397, 659. [Google Scholar] [CrossRef]
- Schaber, J.; Badeck, F.W. Plant phenology in Germany over the 20th century. Reg. Environ. Change 2005, 5, 37–46. [Google Scholar] [CrossRef]
- Estrella, N.; Menzel, A. Responses of leaf colouring in four deciduous tree species to climate and weather in Germany. Clim. Res. 2006, 32, 253–267. [Google Scholar] [CrossRef] [Green Version]
- Menzel, A.; Sparks, T.H.; Estrella, N.; Koch, E.; Aasa, A.; Ahas, R.; Alm-Kübler, K.; Bissolli, P.; Braslavská, O.; Briede, A.; et al. European phenological response to climate change matches the warming pattern. Glob. Chang. Biol. 2006, 12, 1969–1976. [Google Scholar] [CrossRef]
- Menzel, A.; Estrella, N.; Heitland, W.; Susnik, A.; Schleip, C.; Dose, V. Bayesian analysis of the species-specific lengthening of the growing season in two European countries and the influence of an insect pest. Int. J. Biometeorol. 2008, 52, 209–218. [Google Scholar] [CrossRef] [Green Version]
- Nölte, A.; Yousefpour, R.; Hanewinkel, M. Changes in sessile oak (Quercus petraea) productivity under climate change by improved leaf phenology in the 3-PG model. Ecol. Model. 2020, 438, 109285. [Google Scholar] [CrossRef]
- Thomas, F.M.; Gausling, T. Morphological and physiological responses of oak seedlings (Quercus petraea and Q. robur) to moderate drought. Ann. For. Sci. 2000, 57, 325–333. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.S.; Campioli, M.; Vitasse, Y.; De Boeck, H.J.; Van den Berge, J.; AbdElgawad, H.; Asard, H.; Piao, S.; Deckmyn, G.; Janssens, I.A. Variation in leaf flushing date influences autumnal senescence and next year’s flushing date in two temperate tree species. Proc. Natl. Acad. Sci. USA 2014, 111, 7355–7360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morin, X.; Roy, J.; Sonié, L.; Chuine, I. Changes in leaf phenology of three European oak species in response to experimental climate change. N. Phytol. 2010, 186, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Vitasse, Y.; Bresson, C.C.; Kremer, A.; Michalet, R.; Delzon, S. Quantifying phenological plasticity to temperature in two temperate tree species. Funct. Ecol. 2010, 24, 1211–1218. [Google Scholar] [CrossRef]
- Crabbe, R.A.; Dash, J.; Rodriguez-Galiano, V.F.; Janous, D.; Pavelka, M.; Marek, M.V. Extreme warm temperatures alter forest phenology and productivity in Europe. Sci. Total Environ. 2016, 563, 486–495. [Google Scholar] [CrossRef]
- Vitasse, Y.; Porté, A.J.; Kremer, A.; Michalet, R.; Delzon, S. Responses of canopy duration to temperature changes in four temperate tree species: Relative contributions of spring and autumn leaf phenology. Oecologia 2009, 161, 187–198. [Google Scholar] [CrossRef]
- Linderholm, H.W. Growing season changes in the last century. Agric. For. Meteorol. 2006, 137, 1–14. [Google Scholar] [CrossRef]
- Zhu, W.; Tian, H.; Xu, X.; Pan, Y.; Chen, G.; Lin, W. Extension of the growing season due to delayed autumn over mid and high latitudes in North America during 1982–2006. Glob. Ecol. Biogeogr. 2012, 21, 260–271. [Google Scholar] [CrossRef]
- Kolářová, E.; Nekovář, J.; Adamík, P. Long-term temporal changes in central European tree phenology (1946− 2010) confirm the recent extension of growing seasons. Int. J. Biometeorol. 2014, 58, 1739–1748. [Google Scholar] [CrossRef] [PubMed]
- Estiarte, M.; Peñuelas, J. Alteration of the phenology of leaf senescence and fall in winter deciduous species by climate change: Effects on nutrient proficiency. Glob. Chang. Biol. 2015, 21, 1005–1017. [Google Scholar] [CrossRef] [Green Version]
- Jensen, J.S.; Hansen, J.K. Geographical variation in phenology of Quercus petraea (Matt.) Liebl and Quercus robur L. oak grown in a greenhouse. Scand. J. For. Res. 2008, 23, 179–188. [Google Scholar] [CrossRef]
- Gill, A.L.; Gallinat, A.S.; Sanders-DeMott, R.; Rigden, A.J.; Short Gianotti, D.J.; Mantooth, J.A.; Templer, P.H. Changes in autumn senescence in northern hemisphere deciduous trees: A meta-analysis of autumn phenology studies. Ann. Bot. 2015, 116, 875–888. [Google Scholar] [CrossRef] [Green Version]
- Vitasse, Y.; Delzon, S.; Bresson, C.C.; Michalet, R.; Kremer, A. Altitudinal differentiation in growth and phenology among populations of temperate-zone tree species growing in a common garden. Can. J. For. Res. 2009, 39, 1259–1269. [Google Scholar] [CrossRef] [Green Version]
- Wesołowski, T.; Rowiński, P. Timing of bud burst and tree-leaf development in a multispecies temperate forest. For. Ecol. Manag. 2006, 237, 387–393. [Google Scholar] [CrossRef]
- Cole, E.F.; Sheldon, B.C. The shifting phenological landscape: Within-and between-species variation in leaf emergence in a mixed-deciduous woodland. Ecol. Evol. 2017, 7, 1135–1147. [Google Scholar] [CrossRef]
- Franjic, J.; Sever, K.; Bogdan, S.; Skvorc, Z.; Krstonosic, D.; Aleskovic, I. Phenological asynchronization as a restrictive factor of efficient pollination in clonal seed orchads of Pedunculate Oak (Quercus robur L.)/Fenoloska neujednacenost kao ogranicavajuci cimbenik uspjesnoga oprasivanja u klonskim sjemenskim plantazama hrasta luznjaka (Quercus robur L.). Croat. J. For. Eng. 2011, 32, 141–157. [Google Scholar]
- Diggle, P.K.; Mulder, C.P. Diverse developmental responses to warming temperatures underlie changes in flowering phenologies. Integr. Comp. Biol. 2019, 59, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Marchand, L.J.; Dox, I.; Gričar, J.; Prislan, P.; Leys, S.; Van den Bulcke, J.; Fronti, P.; Lange, H.; Matthysen, E.; Peñuelas, J.; et al. Inter-individual variability in spring phenology of temperate deciduous trees depends on species, tree size and previous year autumn phenology. Agric. For. Meteorol. 2020, 290, 108031. [Google Scholar] [CrossRef] [PubMed]
- Keenan, T.F.; Richardson, A.D. The timing of autumn senescence is affected by the timing of spring phenology: Implications for predictive models. Glob. Chang. Biol. 2015, 21, 2634–2641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
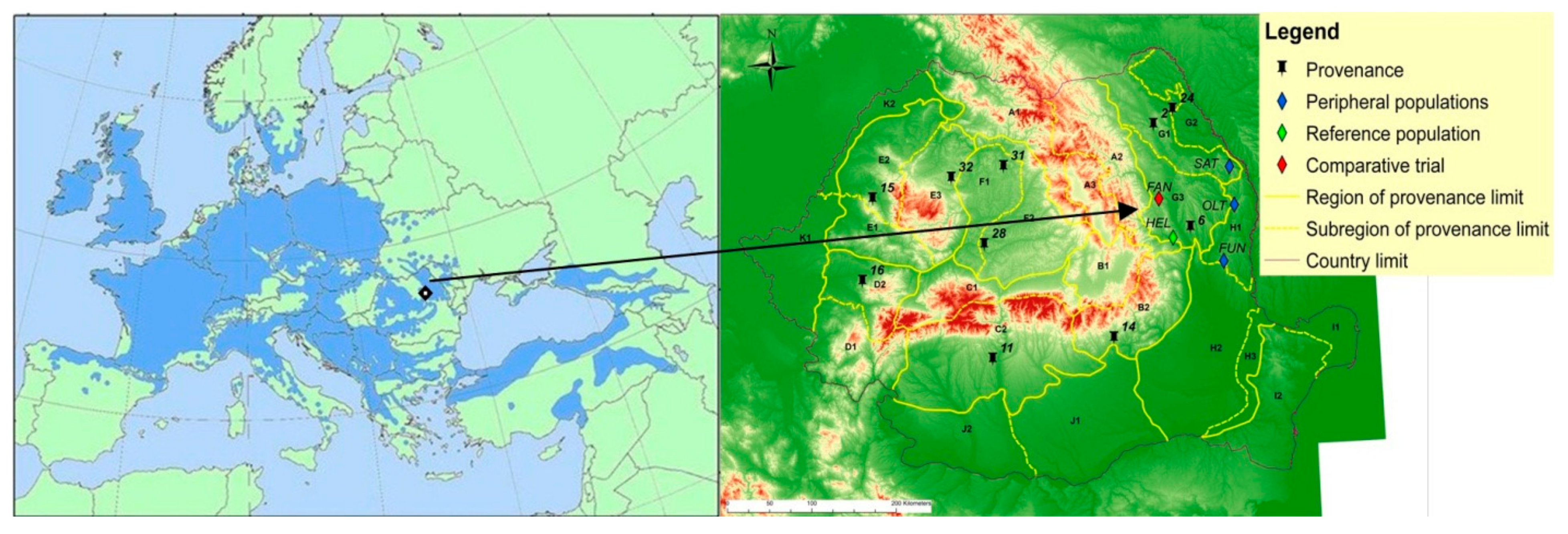
| Label | Population | No. of Trees | Trees Characteristics | Geographical Position | Climatic Conditions | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| DBH (cm) | Height (m) | Age | Latitude (N) | Longitude (E) | Altitude (m) | Annual Average Temperature (°C) | Annual Rainfall (mm) | |||
| FUN | Fundeanu | 34 | 30.3 | 16.6 | 88 | 45°58′ | 27° 41’ | 210 | 9.6 | 470 |
| OLT | Oltenesti | 50 | 27.6 | 19.9 | 73 | 46°34′ | 27° 53’ | 305 | 9.2 | 530 |
| SAT | Satu Nou | 50 | 59.6 | 25.3 | 107 | 46°58′ | 27° 49’ | 300 | 9.2 | 535 |
| HEL | Heltiu | 43 | 34.5 | 25.0 | 90 | 46°14′ | 26° 55’ | 490 | 8.9 | 544 |
| FAN * | Fantanele | 50 | 18.2 | 16.1 | 34 | 46°39′ | 26° 43’ | 260 | 9.6 | 590 |
| Provenance | Ecological Subregion of Provenance | Provenance Location | ||||
|---|---|---|---|---|---|---|
| Code * | Name | Forest District Production Unit Amenajistic Unit | Latitude (N) | Longitude (E) | Altitude (m) | |
| 2 | G150 | Suceava Tableland and Siret and Iasi hills | Dolhasca V Dolhasca 20 A | 47°07′ | 26°37′ | 325 |
| 6 | G350 | Barlad Tableland and Bacau hills | Sascut IV Gaiceana 52A | 46°10′ | 27°05′ | 300 |
| 11 | C250 | Outer Southern Carpathians | Babeni III Sirineasa 10 A, 26 B | 44°55′ | 24°14′ | 290 |
| 14 | B250 | Outer Curvature Carpathians | Valeni III Slanic 45 A | 45°23′ | 23°03′ | 490 |
| 15 | E250 | Western Apuseni Mountains | Beius VII Cerbu 201 C,202 B | 46°40′ | 22°22′ | 300 |
| 16 | D250 | Banat Mountains: Tarcu Poiana Ruscai | Faget V Vladeana 90 B, 91 B | 45°45′ | 22°05′ | 410 |
| 24 | G26C | Jijia Plain | Botosani IV Poienita-Turbuleni 46 B | 47°45′ | 26°40′ | 130 |
| 28 | F250 | Transylvania Tableland | Blaj II Valea Lunga 19 G | 46°12′ | 23°54′ | 420 |
| 31 | F150 | Transylvania Plain | Lechinta V Matei 16 | 47°02′ | 24°20′ | 425 |
| 32 | E350 | Eastern Apuseni Mountains | Cluj IX Mara 38 | 46°45′ | 23°34′ | 600 |
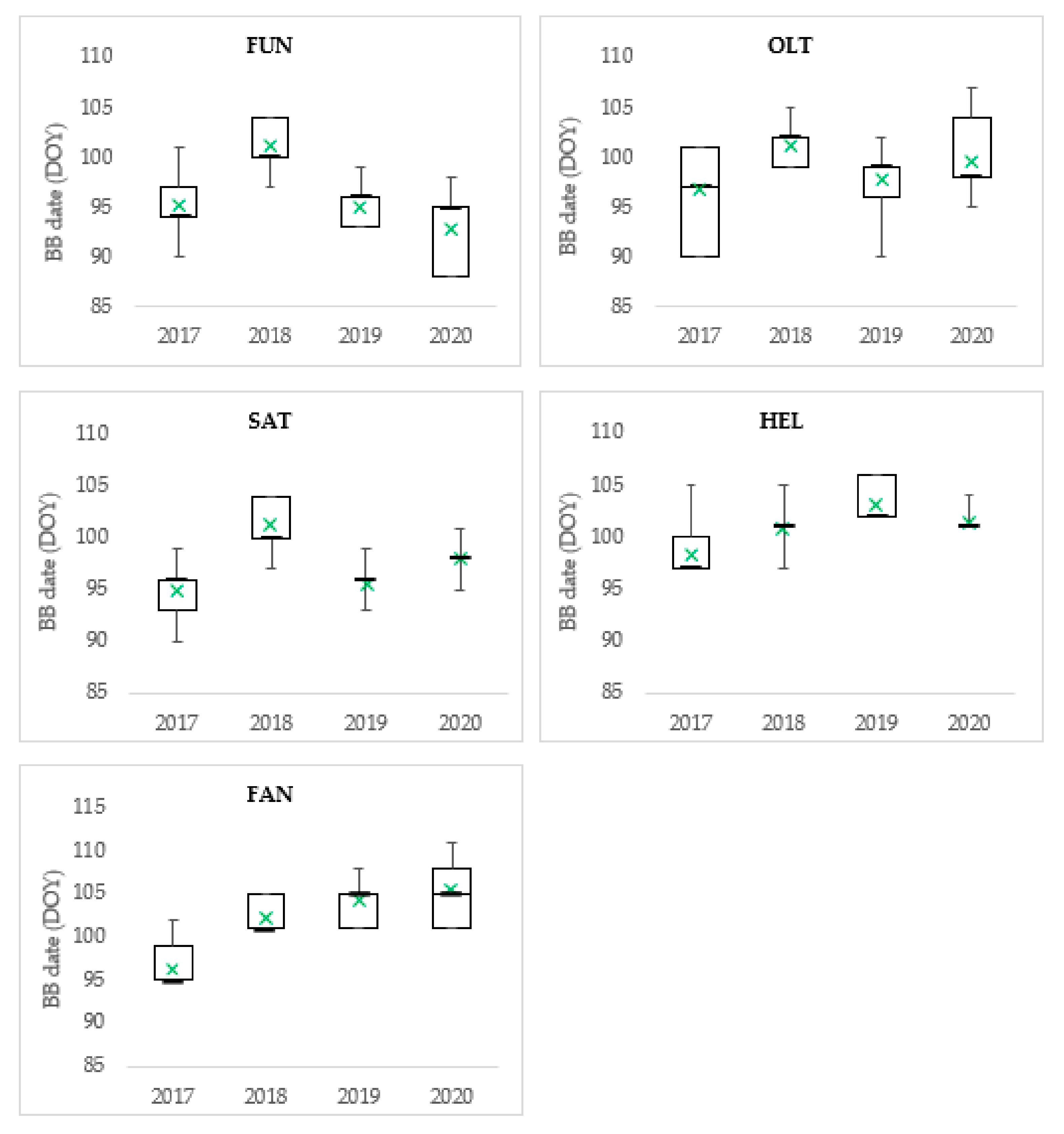
| Population | Budburst (DOY)—Stage I | Leaf Development (DOY)—Stage III | BB-LD Dynamic | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean ± SE | Range | SD | Min | Max | Mean ± SE | Range | SD | No. of Days | Average T (°C) | ∑ T (°C) | |
| Peripheral populations | |||||||||||||
| FUN | 88 | 104 | 96 ± 0.38 | 16 | 4.4 | 101 | 114 | 109 ± 0.27 | 13 | 3.2 | 13.2 | 11.1 | 230.4 |
| OLT | 90 | 107 | 98 ± 0.27 | 17 | 3.7 | 104 | 120 | 111 ± 0.26 | 16 | 3.8 | 13.2 | 10.9 | 217.8 |
| SAT | 90 | 104 | 98 ± 0.23 | 14 | 3.1 | 106 | 120 | 112 ± 0.26 | 14 | 3.5 | 14.0 | 10.5 | 217.1 |
| Mean of peripheral populations | 89 | 105 | 98 ± 0.16 | 16 | 3.7 | 104 | 118 | 111 ± 0.16 | 14 | 3.5 | 13.5 | 10.8 | 221.8 |
| Reference population | |||||||||||||
| HEL | 97 | 106 | 101 ± 0.20 | 9 | 2.4 | 106 | 120 | 116 ± 0.25 | 14 | 3.3 | 15.4 | 10.4 | 214.5 |
| Comparative trial | |||||||||||||
| FAN | 95 | 112 | 103 ± 0.32 | 17 | 4.6 | 109 | 124 | 117 ± 0.29 | 15 | 3.9 | 15.4 | 10.4 | 236.9 |
| Population | FUN | OLT | SAT | HEL | FAN | |
|---|---|---|---|---|---|---|
| ∑ T (°C) ± SD | ||||||
| (A) 1 January-BB DOY | 191.5 ± 5.5 | 188.0 ± 5.6 | 173.0 ± 5.6 | 195.1 ± 5.5 | 203.8 ± 5.6 | |
| (B) LS DOY-BB DOY | 438.4 ± 5.0 | 445.3 ± 5.3 | 447.0 ± 5.1 | 500.2 ± 4.7 | 500.8 ± 5.0 | |
| Phenophase | Year of Observation | Altitude (m) | Latitude (N) | Ecophysiological Latitude | Longitude (E) |
|---|---|---|---|---|---|
| Budburst | 2017 | 0.310 * | −0.104 | 0.251 | −0.379 * |
| 0.012 | 0.481 | 0.079 | 0.022 | ||
| 2018 | 0.322 * | −0.254 | 0.179 | −0.322 * | |
| 0.022 | 0.075 | 0.213 | 0.020 | ||
| 2019 | 0.300 * | −0.049 | 0.210 | −0.493 *** | |
| 0.034 | 0.736 | 0.142 | <0.001 | ||
| 2020 | 0.386 ** | −0.239 | 0.253 | −0.581 *** | |
| 0.006 | 0.095 | 0.077 | <0.001 | ||
| Flowering | 2017 | 0.276 | 0.027 | 0.223 | −0.334 * |
| 0.067 | 0.860 | 0.141 | 0.025 | ||
| 2018 | 0.204 | −0.463 *** | 0.046 | −0.419 ** | |
| 0.155 | <0.001 | 0.751 | 0.002 | ||
| 2019 | 0.283 * | −0.304 * | 0.183 | −0.574 *** | |
| 0.046 | 0.032 | 0.234 | <0.001 | ||
| 2020 | 0.132 | −0.349 * | −0.038 | −0.322 * | |
| 0.363 | 0.013 | 0.795 | 0.022 | ||
| Senescence | 2017 | −0.391 ** | −0.256 | −0.457 ** | 0.331 * |
| 0.005 | 0.073 | 0.001 | 0.019 | ||
| 2018 | −0.034 | −0.260 | −0.262 | 0.262 | |
| 0.814 | 0.068 | 0.054 | 0.066 | ||
| 2019 | −0.184 | −0.277 | −0.243 | 0.089 | |
| 0.202 | 0.051 | 0.090 | 0.538 |
| Population | Flowering (DOY) | Temperature °C | ||||||
|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean ± SE | Range | SD | Average | ∑T > 5 °C | No. of Days a | |
| Peripheral populations | ||||||||
| FUN | 101 | 114 | 109 ± 0.31 | 13 | 3.7 | 12.4 | 80.0 | 7.0 |
| OLT | 104 | 117 | 110 ± 0.22 | 13 | 3.1 | 11.8 | 96.6 | 8.3 |
| SAT | 106 | 120 | 111 ± 0.23 | 14 | 3.4 | 11.4 | 94.3 | 8,5 |
| Mean of peripheral populations | 104 | 117 | 110 ± 0.15 | 13 | 3.4 | 11.9 | 90.3 | 7.9 |
| Reference population | ||||||||
| HEL | 106 | 120 | 113 ± 0.28 | 14 | 3.1 | 11.1 | 90.5 | 8.0 |
| Comparative trial | ||||||||
| FAN | 109 | 124 | 117 ± 0.24 | 15 | 3.4 | 10.1 | 107.9 | 9.5 |
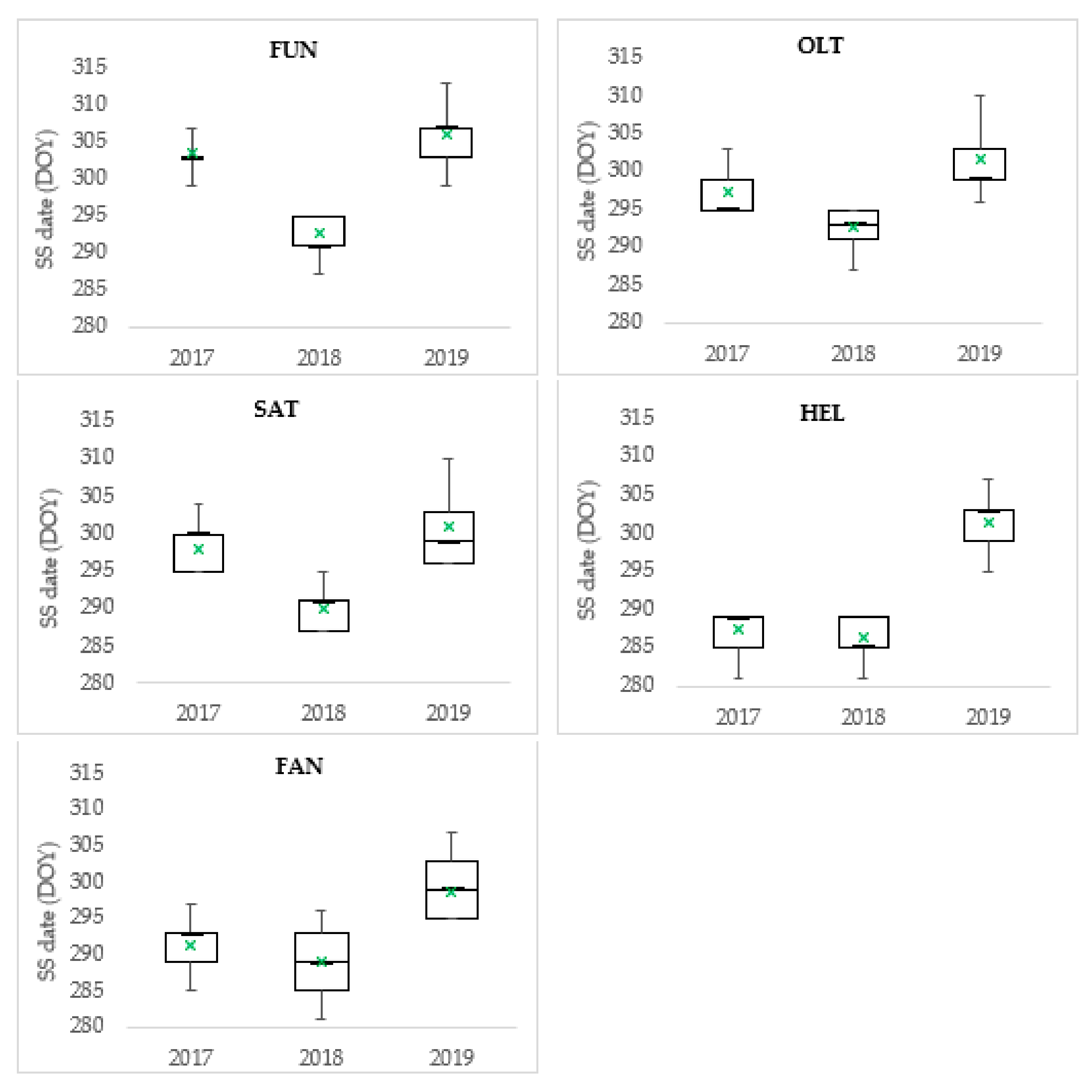
| Population | SS (DOY) | Bioactive Season | SD | |||||
|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean ± SE | Range | No. of Days a | Climate Conditions | |||
| Average T (°C) | ∑ T (°C) | |||||||
| Peripheral populations | ||||||||
| FUN | 287 | 313 | 301 ± 0.65 | 26 | 225 | 17.8 | 3806.7 | 6.5 |
| OLT | 287 | 310 | 297 ± 0.40 | 23 | 220 | 17.5 | 3576.3 | 4.9 |
| SAT | 287 | 310 | 296 ± 0.48 | 23 | 220 | 17.4 | 3500.4 | 5.8 |
| Mean of peripheral populations | 287 | 311 | 298 ± 0.29 | 24 | 222 | 17.6 | 3627.8 | 5.7 |
| Reference population | ||||||||
| HEL | 281 | 307 | 282 ± 0.66 | 26 | 210 | 17.1 | 3271.2 | 7.5 |
| Comparative trial | ||||||||
| FAN | 281 | 307 | 293 ± 0.47 | 26 | 212 | 17.2 | 3347.1 | 5.7 |
| Phenophase | Population | Years of Observation | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2017–2018 | 2018–2019 | 2019–2020 | 2017–2019 | 2017–2020 | 2018–2020 | ||||||||
| rs | p | rs | p | rs | p | rs | p | rs | p | rs | p | ||
| BB | FUN | 0.347 * | 0.044 | 0.343 * | 0.047 | 0.440 ** | 0.009 | 0.433 * | 0.011 | 0.656 *** | <0.001 | 0.402 * | 0.018 |
| OLT | 0.585 *** | <0.001 | 0.304 * | 0.043 | 0.471 *** | <0.001 | 0.487 * | 0.014 | 0.487 *** | <0.001 | 0.416 ** | 0.002 | |
| SAT | 0.417 ** | 0.003 | 0.397 ** | 0.005 | 0.340 * | 0.018 | 0.586 *** | <0.001 | 0.348 * | 0.015 | 0.596 *** | <0.001 | |
| HEL | 0.474 ** | 0.002 | 0.321 * | 0.038 | 0.375 * | 0.015 | 0.341 * | 0.027 | 0.495 *** | <0.001 | 0.310 * | 0.045 | |
| FAN | 0.533 *** | <0.001 | 0.370 ** | 0.008 | 0.438 ** | 0.001 | 0.301 * | 0.033 | 0.479 *** | <0.001 | 0.448 ** | 0.001 | |
| LD | FUN | 0.063 | >0.05 | 0.165 | >0.05 | 0.403 * | 0.018 | 0.252 | 0.150 | 0.450 ** | 0.008 | 0.165 | >0.05 |
| OLT | 0.175 | >0.05 | 0.149 | >0.05 | 0.237 | >0.05 | 0.214 | >0.05 | 0.197 | >0.05 | 0.063 | >0.05 | |
| SAT | 0.355 * | 0.013 | 0.580 *** | <0.001 | 0.394 ** | 0.006 | 0.329 * | 0.022 | 0.173 | >0.05 | 0.361 * | 0.012 | |
| HEL | 0.235 | >0.05 | 0.174 | >0.05 | 0.305 * | 0.049 | 0.400 ** | 0.009 | 0.375 * | 0.014 | 0.225 | >0.05 | |
| FAN | 0.442 ** | 0.001 | 0.347 * | 0.013 | 0.308 * | 0.030 | 0.354 * | 0.012 | 0.273 | >0.05 | 0.399 ** | 0.004 | |
| FL | FUN | 0.358 * | 0.037 | 0.340 * | 0.049 | 0.539 ** | 0.001 | 0.523 ** | 0.001 | 0.527 ** | 0.001 | 0.361 * | 0.036 |
| OLT | 0.372 * | 0.012 | 0.428 ** | 0.002 | 0.395 ** | 0.005 | 0.320 * | 0.034 | 0.375 * | 0.012 | 0.417 ** | 0.003 | |
| SAT | 0.353 * | 0.035 | 0.331 * | 0.022 | 0.389 ** | 0.006 | 0.347 * | 0.038 | 0.372 * | 0.026 | 0.421 ** | 0.003 | |
| HEL | 0.417 * | 0.048 | 0.488 ** | 0.001 | 0.405 ** | 0.008 | 0.425 * | 0.043 | 0.401 | >0.05 | 0.479 ** | 0.001 | |
| FAN | 0.317 * | 0.034 | 0.560 *** | <0.001 | 0.388 ** | 0.005 | 0.314 * | 0.036 | 0.300 * | 0.045 | 0.327 * | 0.020 | |
| SS | FUN | 0.110 | >0.05 | 0.171 | >0.05 | - | - | 0.266 | >0.05 | - | - | - | - |
| OLT | 0.282 * | 0.047 | 0.170 | >0.05 | - | - | 0.180 | >0.05 | - | - | - | - | |
| SAT | 0.396 ** | 0.005 | 0.208 | >0.05 | - | - | 0.172 | >0.05 | - | - | - | - | |
| HEL | 0.448 ** | 0.003 | 0.015 | >0.05 | - | - | 0.091 | >0.05 | - | - | - | - | |
| FAN | 0.632 *** | <0.001 | 0.412 ** | 0.003 | - | - | 0.562 *** | <0.001 | - | - | - | - | |
| Phenophase | Population | Years of Observation | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2017 | 2018 | 2019 | 2020 | ||||||
| rs | p | rs | p | rs | p | rs | p | ||
| BB-LD | FUN | 0.605 *** | <0.001 | 0.551 ** | <0.001 | 0.348 * | 0.044 | 0.740 *** | <0.001 |
| OLT | 0.363 ** | 0.009 | 0.293 * | 0.039 | 0.327 * | 0.020 | 0.488 *** | <0.001 | |
| SAT | 0.292 * | 0.044 | 0.371 ** | 0.009 | 0.333 * | 0.021 | 0.427 ** | 0.002 | |
| HEL | 0.319 * | 0.039 | 0.361 * | 0.019 | 0.376 * | 0.014 | 0.332 * | 0.031 | |
| FAN | 0.066 ** | 0.008 | 0.557 *** | <0.001 | 0.463 *** | <0.001 | 0.567 *** | <0.001 | |
| BB-FL | FUN | 0.562 ** | 0.001 | 0.348 * | 0.044 | 0.618 *** | <0.001 | 0.648 *** | <0.001 |
| OLT | 0.344 * | 0.022 | 0.403 ** | 0.004 | 0.723 *** | <0.001 | 0.702 *** | <0.001 | |
| SAT | 0.328 * | 0.044 | 0.291 * | 0.045 | 0.399 ** | 0.005 | 0.336 * | 0.020 | |
| HEL | 0.423 * | 0.045 | 0.329 * | 0.033 | 0.327 * | 0.034 | 0.485 ** | 0.001 | |
| FAN | 0.441 ** | 0.002 | 0.503 *** | <0.001 | 0.446 ** | 0.001 | 0.743 *** | <0.001 | |
| BB-SS | FUN | 0.528 ** | 0.001 | 0.188 | >0.05 | 0.231 | >0.05 | - | - |
| OLT | 0.282 * | 0.047 | 0.180 | >0.05 | 0.170 | 0.238 | - | - | |
| SAT | 0.130 | >0.05 | 0.224 | >0.05 | 0.319 * | 0.027 | - | - | |
| HEL | 0.001 | >0.05 | 0.380 * | 0.013 | 0.243 | >0.05 | - | - | |
| FAN | −0.220 | >0.05 | −0.078 | >0.05 | −0.187 | >0.05 | - | - | |
| Phenophase | Population | Years of Observation | |||||
|---|---|---|---|---|---|---|---|
| 2017–2018 | 2018–2019 | 2019–2020 | |||||
| rs | p | rs | p | rs | p | ||
| Previous year SS-BB | FUN | 0.256 | >0.05 | 0.202 | >0.05 | 0.32 | >0.05 |
| OLT | 0.045 | >0.05 | 0.079 | >0.05 | 0.114 | >0.05 | |
| SAT | 0.238 | >0.05 | 0.319 * | 0.027 | 0.017 | >0.05 | |
| HEL | 0.009 | >0.05 | 0.414 ** | 0.006 | 0.069 | >0.05 | |
| FAN | −0.085 | >0.05 | −0.229 | >0.05 | −0.239 | >0.05 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gafenco, I.M.; Pleșca, B.I.; Apostol, E.N.; Șofletea, N. Spring and Autumn Phenology in Sessile Oak (Quercus petraea) Near the Eastern Limit of Its Distribution Range. Forests 2022, 13, 1125. https://doi.org/10.3390/f13071125
Gafenco IM, Pleșca BI, Apostol EN, Șofletea N. Spring and Autumn Phenology in Sessile Oak (Quercus petraea) Near the Eastern Limit of Its Distribution Range. Forests. 2022; 13(7):1125. https://doi.org/10.3390/f13071125
Chicago/Turabian StyleGafenco (Pleșca), Ioana Maria, Bogdan Ionuț Pleșca, Ecaterina Nicoleta Apostol, and Neculae Șofletea. 2022. "Spring and Autumn Phenology in Sessile Oak (Quercus petraea) Near the Eastern Limit of Its Distribution Range" Forests 13, no. 7: 1125. https://doi.org/10.3390/f13071125
APA StyleGafenco, I. M., Pleșca, B. I., Apostol, E. N., & Șofletea, N. (2022). Spring and Autumn Phenology in Sessile Oak (Quercus petraea) Near the Eastern Limit of Its Distribution Range. Forests, 13(7), 1125. https://doi.org/10.3390/f13071125






