Abstract
Determining the mechanisms of pine wilt disease (PWD) resistance in Pinus is a popular research topic, but information on volatile organic substances (VOS) and their role in PWD is lacking. Whether the difference in VOS among Pinus thunbergii parl. that have different levels of resistance with pine wood nematodes (PWNs) is the reason for the differing resistance needs to be studied. In this study, resistant P. thunbergii introduced from Japan and susceptible P. thunbergii native to China were used to investigate the effects of different lines inoculated with PWN. We determined the expression levels of the terpene synthesis-related genes geranylgeranyl diphosphate synthase (GGPPS), 3-hydroxy-3-methylglutaryl-coenzyme A reductase 1 (HMDH1), two kinds of alpha-farnesene synthase (PT) genes. The types and the relative percentage content of terpenoids in the pine needles were also determined by gas chromatography coupled with mass spectrometry (GC-MS). Results show that the growth, population size and migration of PWNs were significantly inhibited. The expression of terpene synthesis genes in the resistant P. thunbergii was higher than that in the susceptible one. The analysis of terpenoids revealed a total of 41 terpenoids, of which resistant P. thunbergii contained 39 and susceptible P. thunbergii only 28; 14 terpenoids were specific to resistant P. thunbergii, in which 8 of the terpenoids were constitutive terpenes and 6 were inducible terpenes. There were 3 terpenes unique to the susceptible P. thunbergii, and only 1 inducible terpene. Our results showed that the reduction in the expression of disease symptom and suppression of PWNs in resistant P. thunbergii was likely related to differences in the types and content of resistance-related substances in the trees. This study does not specifically connect elevated compounds in resistant P. thunbergii to resistance to PWN and assays should be conducted to establish direct effects of terpenoids on pinewood nematode activity and reproduction.
1. Introduction
Pine wilt disease (PWD) is a devastating disease of pine trees caused by Bursaphelenchus xylophilus (pine wood nematode; PWN) Nickel (Aphelenchida: Parasitaphelenchidae). The PWN is native to North America and is now found in the USA, Canada, Mexico, China, Korea, Japan, Portugal and Spain [1,2,3,4]. Since the introduction of PWN into China in 1982, despite the adoption of various measures to prevent its spread, the occurrence of PWD has exceeded expectations. PWD has occurred in 666 county-level administrative regions of 19 provinces (autonomous regions and municipalities directly under the Central Government), with a total area of 1,114,600 hm2 and 19,467,400 dead pine trees in 2019, which has resulted in severe direct economic losses and losses of ecological service value equal to hundreds of billions of yuan [5].
The control of PWD has been studied for decades, including chemical, physical and biological, but prevention spread has not yet been achieved [6,7,8]. In field studies on the control of PWD where a large number of trees had died, a small number of trees survived [9]. It is apparent that trees have some resistance to PWNs, and the underlying mechanism of this resistance has attracted the attention. Studies suggest that pine trees resist infection through a combination of internal tissue structural changes, physiological and biochemical responses [10,11,12,13], and gene regulation.
After the PWN infects a pine, the tree produces a series of defense responses. In previous studies, it was found that the main signaling molecules for plant defense responses are reactive oxygen species (ROS), Ca2+, phytohormones and volatile organic substances (VOS) [14,15]. Previous studies on pine resistance have mainly focused on ROS metabolism related substances and the regulation of plant hormones. The role of VOS in pine resistance to PWN has yet to be elucidated. Keeling and Bohlmann show that conifers produce terpenoids that negatively affect the physiology of invading species or offspring [16]. Cheng et al. confirmed the inhibitory effects of trans-2-hexenal on the growth, reproduction and efficacy against of PWNs [17]. In addition, coniferous trees emit more monoterpenoids and sesquiterpenoids [18], and terpenoid concentrations may vary among different provenances of trees [19], therefore, it has been speculated this differentiation may partly explain resistance to PWD. Further research is needed to determine the differences in VOS among the families of the same pine tree species exhibiting varying resistance. It has also been found that Pinus. sylvestris (L.) synthesizes and releases a large number of secondary metabolites, of which VOS account for a large proportion [20]. These VOS play an important role as chemical signals in the resistance of the host plant to disease. Dong et al. [21] found that the main VOS of the P. massoniana (Lamb) with different levels of resistance were terpenes such as α-pinene, camphene, β-pinene and β-watercressene, but the pines species that are highly resistant to the PWN released more types of odor components than the susceptible P. massoniana, indicating that there may be correlation between the VOS of P. massoniana and the ability to resist the PWN [21]. By measuring the major terpenes in two P. thunbergii (Parlatore) stands infected with PWD, Takeuchi and Futai [22] found that the release of some terpenes, such as α-pinene, began to increase in summer, overlapping with the egg-laying season of the vector beetle, which may influence the development of symptoms in trees. It has also been suggested that large amounts of volatile terpenes are produced in pine trees inoculated with PWN, which enter the canal cell of xylem and lead to the formation of canal cell cavities that impede the water transport process, thus causing the plant to wilt and die [23]. However, VOS and their role in PWD is an understudied area; the differences between resistant and susceptible P. thunbergii and the relationship between VOS and resistance to the PWN is relatively scarce, and this relationship needs to be investigated.
With the rapid development of analytical detection technology and bioinformatics, genomics has become an important technology in systematic biology research. In a process of mutual selection and synergy with the PWN, pine trees employ a variety of resistance mechanisms to safeguard their physiological metabolism [13,24]. However, the mechanisms of resistance are extremely complex, i.e., diverse and idiosyncratic. Previous studies on PWN resistance mainly focused on P. massoniana, P. taeda and P. densiflora, thus, since few research studies focused on PWN resistance of P. thunbergii, we introduced resistant P. thunbergii germplasm from Japan for planting in 2004. Based on observation of PWD incidence under natural conditions in the field, we found that the resistant P. thunbergii #40 line [9] has resistance to the PWD, but with regard to its resistance mechanism and how it differs from that of the native P. thunbergii, it is not clear at present if this contributes to disease resistance.
In this paper, first, the growth and population size of PWNs and their ability to secrete cellulase in trees were measured after inoculation in resistant P. thunbergii from Japan and the local susceptible P. thunbergii, and the expression of terpenoid synthesis-related genes in trees was compared. In addition, the study of the differences in the variation of resistance-related VOS in P. thunbergii with different resistance levels can help to reveal the mechanisms of resistance to PWD.
2. Materials and Methods
2.1. Plant and PWN Sources
Plant source: Resistant P. thunbergii #40 [25] introduced from the Japanese Forest Tree Breeding Centre, and local susceptible P. thunbergii, were planted in February 2004 in the Jurong Forest Farm, Jiangsu, China.
PWN: AMA3, a highly virulent strain of PWNs, was isolated from naturally susceptible P. massoniana in Maanshan City, Anhui Province, China, and stored at the Forest Pathology Laboratory of Nanjing Forestry University. The PWNs obtained by isolation were purified and placed in pure culture on Botrytis cinerea plates in an incubator at 25 °C (5–7 d). The PWNs were collected by the Baermann funnel method after the mycelia had been consumed [26].
2.2. Resistant and Susceptible P. thunbergii Branches Inoculated with PWN
In greenhouse trials, three two-year-old branches of each of (#) resistant and susceptible P. thunbergii trees were inoculated with 3000 PWNs. Mock-inoculated plants were inoculated with sterile water. The time required for disease symptoms to appear was recorded and disease incidence was assessed (each experiment was replicated three times). The degree of susceptibility of pine trees was classified into the following three classes: greenish, reddish-brown, and discolored. The morbidity and disease severity index (DSI) were calculated according to the following formulae [27]:
Morbidity = number of susceptible plants/total number of plants × 100%
DSI = ∑(number of plants at each level × number of levels)/(total number of plants × highest level of disease) × 100
2.3. Migration, Population Size and Morphological Observations of the PWNs
Fourteen days after inoculation with PWNs, inoculated branches of different resistant pine trees (from Section 2.2) were cut and divided into 5 and 10 cm sections above the inoculation site and 5 and 10 cm sections below the inoculation site, and the PWNs were collected from each part using the Baermann funnel method. The differences in population size and sex ratio of PWNs in different P. thunbergii samples were also summarized and measured (summarized and measured in triplicate) [23]. Mock-inoculated P. thunbergii branches were used as the controls (CKs).
The PWNs were obtained in the above experiments, placed in formalin, and their morphology was observed using an Environmental Scanning Electron Microscope (FEI Quanta 200, Waltham, MA, USA) [28].
2.4. Determination of Cellulase Activity Secreted by PWNs
PWN whole-protein extracts: First, 1 μL each of phosphatase inhibitor, protease inhibitor and phenylmethylsulfonyl fluoride was added to 100 μL of lysate and set aside on ice; then, 1000 nematodes from each plant were added to a centrifuge tube, which was previously frozen in liquid nitrogen, and then the nematode-containing liquid was ground into powder using a high-speed tissue grinder. The supernatant of the tubes was taken as the nematode whole protein extract, and the protein concentration was determined by bicinchoninic acid (BCA) [29] and adjusted to the same concentration for each sample. Each extraction procedure was replicated 3 times.
According to the method of Hu and Jiang [30,31], we used the above protein extract to determine the cellulase activity secreted by PWNs. The cellulose plate assay results were measured by a ruler.
2.5. Real-Time qRT-PCR of Terpene Synthesis-Related Genes
Pine needles of P. thunbergii with different levels of resistance after inoculation with PWNs for 0 and 24 h were selected as experimental materials (P. thunbergii uninoculated with PWNs was the CK. According to the manufacturer’s instructions, total RNA was extracted from P. thunbergii using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA). The RNA concentration and purity were analyzed by a Nano Drop ND-2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). cDNA was synthesized using 1 μg of total RNA by HiScript II Q RT SuperMix for qPCR according to the manufacturer’s instructions (Vazyme, Nanjing, China).
RT–qPCR specific primers were designed according to the coding sequence of pine trees. By the use the EF1a-F gene of P. thunbergii as an internal reference, RT–qPCR of geranylgeranyl diphosphate synthase (GGPPS) and 3-hydroxy-3-methylglutaryl-coenzyme A reductase 1 (HMDH1), two kinds of Alpha-farnesene synthase (PT), were performed by real-time fluorescence quantitative PCR (RT-qPCR) (American Applied Biosystems Company, Foster City, CA, USA). The primers for qPCR were designed on the NCBI website. Data were calculated and analyzed via 7500 Software V2.3 (American Applied Biosystems Company, Foster City, CA, USA). The sequences of the primers used are shown in Table S1. Each RT–qPCR procedure was replicated 3 times.
2.6. Terpene Species and Content Determination
Needles were collected from resistant and susceptible P. thunbergii groups 0, 24, 72 and 168 h after inoculation with PWNs, and terpenes were determined by gas chromatography–mass spectrometry (GC–MS) (using C30 as a standard) as follows: 0.5 g of the needles to be tested was weighed and cut into 1 cm sections in a headspace vial, the vial was placed in a water bath at 40 °C, the detachable solid-phase microextraction probe (SPME) (65 um PDMS/DVB) was inserted for 30 min, and then the extraction needle was transferred to a gas chromatograph-mass spectrometer (Tracel 300 ISO-LT GC 1300, Waltham, MA, USA; helium was used as a carrier gas) for 3 min and monitored with an electron bombardment source. The temperature program for terpenes was from 50 °C to 250 °C. The heating rate was 5 °C/min [32], and the chromatographic column that was used was a DB-5MS (30 × 0.25 × 0.25).
The resulting data from each of the three replicate experiments were calculated and analyzed against the information within NIST database to determine the substances represented by each peak in the mass spectra.
The formula is: RI = 100n + 100(tx − tn)/tn+1 − tn (where tx is the retention time of the compound of the compound to be tested; tn is the retention time of N-alkanes with n carbon atoms; tn+1 is the retention time of N-alkanes with n + 1 carbon atoms; and n is the number of carbon atoms). The change in VOS could reflect the different resistance responses of P. thunbergii with the extension of invasion time.
2.7. Analysis of Data
The data were analyzed using analysis of variance (ANOVA), in Excel and SPSS 23 software (SPSS Inc., Chicago, IL, USA). The results are expressed as percentages using nonparametric methods, and plots were constructed via Prism (Patterns & Practices., Redmond, WA, USA) and Adobe Photoshop CS6 (64 bit) software (Adobe, San Jose, CA, USA).
3. Results
3.1. Phenotypes of Resistant and Susceptible P. thunbergii after Inoculation with PWN
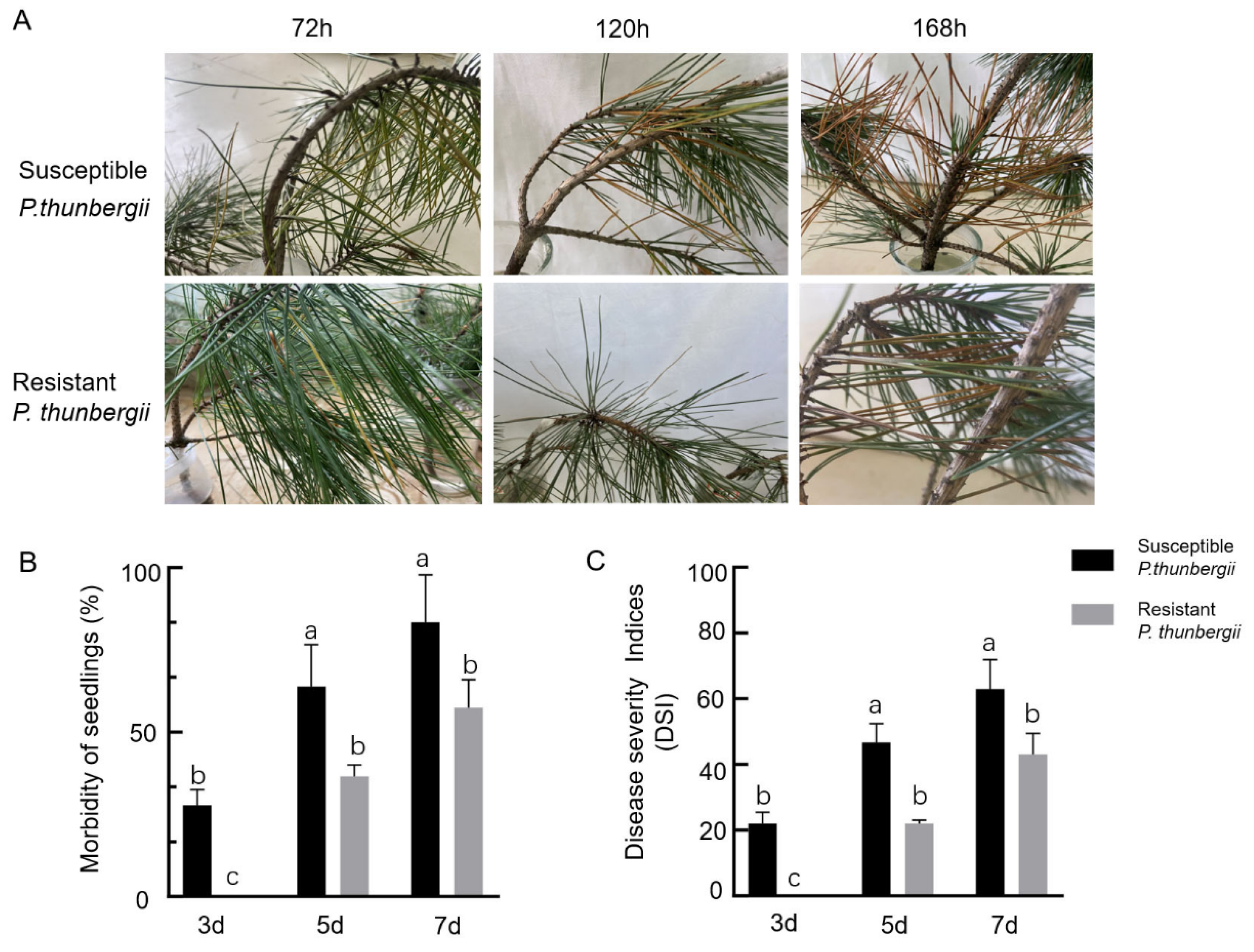
After the inoculation of P. thunbergii with PWNs, the needles of the susceptible P. thunbergii first appeared greenish, with an incident rate of 27.7% 72 h after inoculation, and there were no obvious external symptoms in the resistant P. thunbergii. At 120 h after inoculation, the number of greenish needles in the susceptible P. thunbergii increased, with a 63.5% incidence rate, while the needles of the resistant P. thunbergii appeared greenish, with a 36.5% incidence rate. Then 168 h after inoculation, the needles of the susceptible P. thunbergii were already pendulous and all of needles within the upper 5 cm of the inoculation site were reddish-brown, with an incidence rate of 83.3% and a disease index of 62.9. In contrast, the resistant P. thunbergii had faded green needles that began to appear reddish-brown, with fewer discolored needles than the susceptible P. thunbergii, as well as an incidence rate of 55.6% and a disease index of 41.7, indicating that the resistant P. thunbergii developed symptoms later than the susceptible P. thunbergii did (Figure 1B,C).
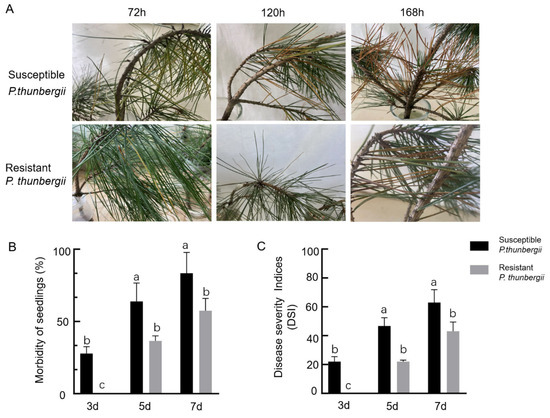
Figure 1.
Morbidity and disease severity index (DSI) of P. thunbergii with different levels of resistance after inoculation with PWNs. (A) Disease course of P. thunbergii with different levels of resistance after inoculation with PWN (B) Morbidity of P. thunbergii with different levels of resistance (C) DSI of P. thunbergii with different levels of resistance. The values represent the mean ± SD of three independent biological samples. The different letters above the error bars indicate statistically significant differences according to Duncan’s multiple range test (p < 0.05).
3.2. Differences in Morphology, Migration and Population Size of PWNs in P. thunbergii with Different Levels of Resistance
The PWN counts at 15 days after inoculation showed that the PWNs migrated mainly to the upper part of the inoculation site. There were differences in the numbers of PWNs in susceptible and resistant P. thunbergii at 5 and 10 cm below the inoculation point, with more PWNs in susceptible P. thunbergii and fewer PWNs in resistant P. thunbergii, but the differences were not significant; the differences in the numbers of PWNs in P. thunbergii were mainly above the inoculation point, with 160 PWNs in resistant P. thunbergii and 670 PWNs in susceptible P. thunbergii at 10 cm above the inoculation point (Table 1), indicating that the migration of PWNs was hindered in the resistant P. thunbergii.

Table 1.
Migration status of PWNs within P. thunbergii with different levels of resistance. The different letters above the error bars indicate statistically significant differences (p < 0.05) as measured by Duncan’s multiple range test.
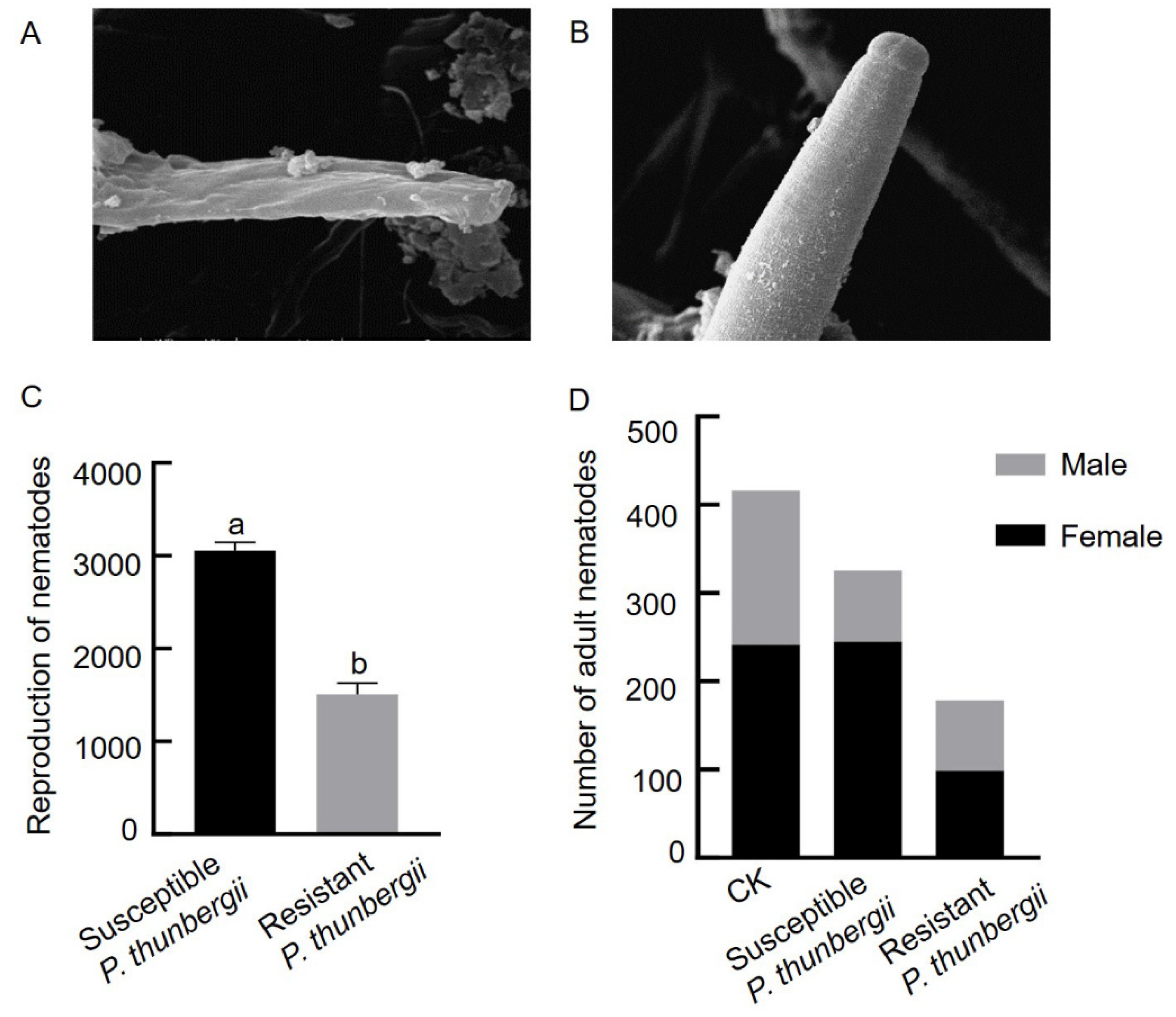
To explore whether pine trees with different resistance levels exert stress on the PWNs that invade the trees, we observed the morphological differences in PWNs from P. thunbergii with different levels of resistance after 15 days of inoculation by scanning electron microscopy. The morphological integrity was altered that the body surface of PWNs from resistant P. thunbergii was highly wrinkled, whereas that of PWNs from susceptible P. thunbergii was relatively intact (Figure 2A,B). The total number of PWNs within isolated branches 15 days after inoculation was counted to determine the PWN population multiplication or reduction within the tree, and the results showed that the number of PWNs in resistant and susceptible P. thunbergii differed significantly, with approximately 3053 PWNs in susceptible P. thunbergii and only approximately 1500 PWNs in resistant P. thunbergii, which was 1/2 the number of PWNs in susceptible P. thunbergii (Figure 2C).
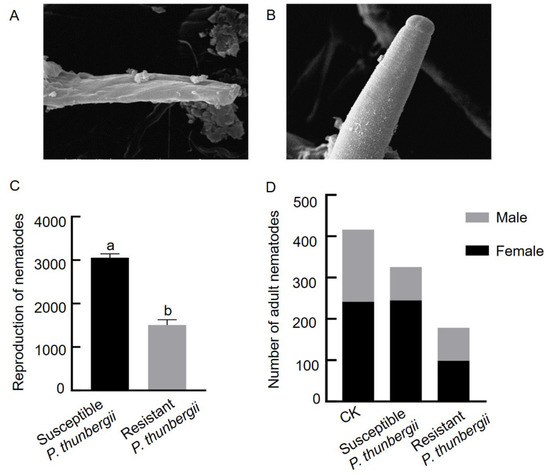
Figure 2.
Differences in morphology, population size and sex ratio of PWNs in P. thunbergii with different levels of resistance. (A) Head of PWNs in resistant P. thunbergii. (B) Head of PWNs in susceptible P. thunbergii. (C) Population size of PWNs in P. thunbergii with different levels of resistance. (D) Number of female and male PWNs in P. thunbergii with different levels of resistance. The different letters above the error bars indicate statistically significant differences (p < 0.05) as measured by Duncan’s multiple range test. CK: PWN before inoculation.
A comparative analysis of the number of female and male PWNs in branches of resistant and susceptible P. thunbergii showed no significant difference in the number of females but a significant in the number of males in the susceptible P. thunbergii at 15 days after inoculation compared to the before inoculation. The number of both females and males in resistant P. thunbergii was reduced and differed significantly from the preinoculation numbers. The female to male sex ratio after inoculation increased in susceptible P. thunbergii (3.1:1) compared to the CK (1.38:1), while the female to male sex ratio decreased in resistant P. thunbergii (1.2:1) (Figure 2D).
3.3. Differences in Cellulase Secretion by PWNs in P. thunbergii with Different Levels of Resistance
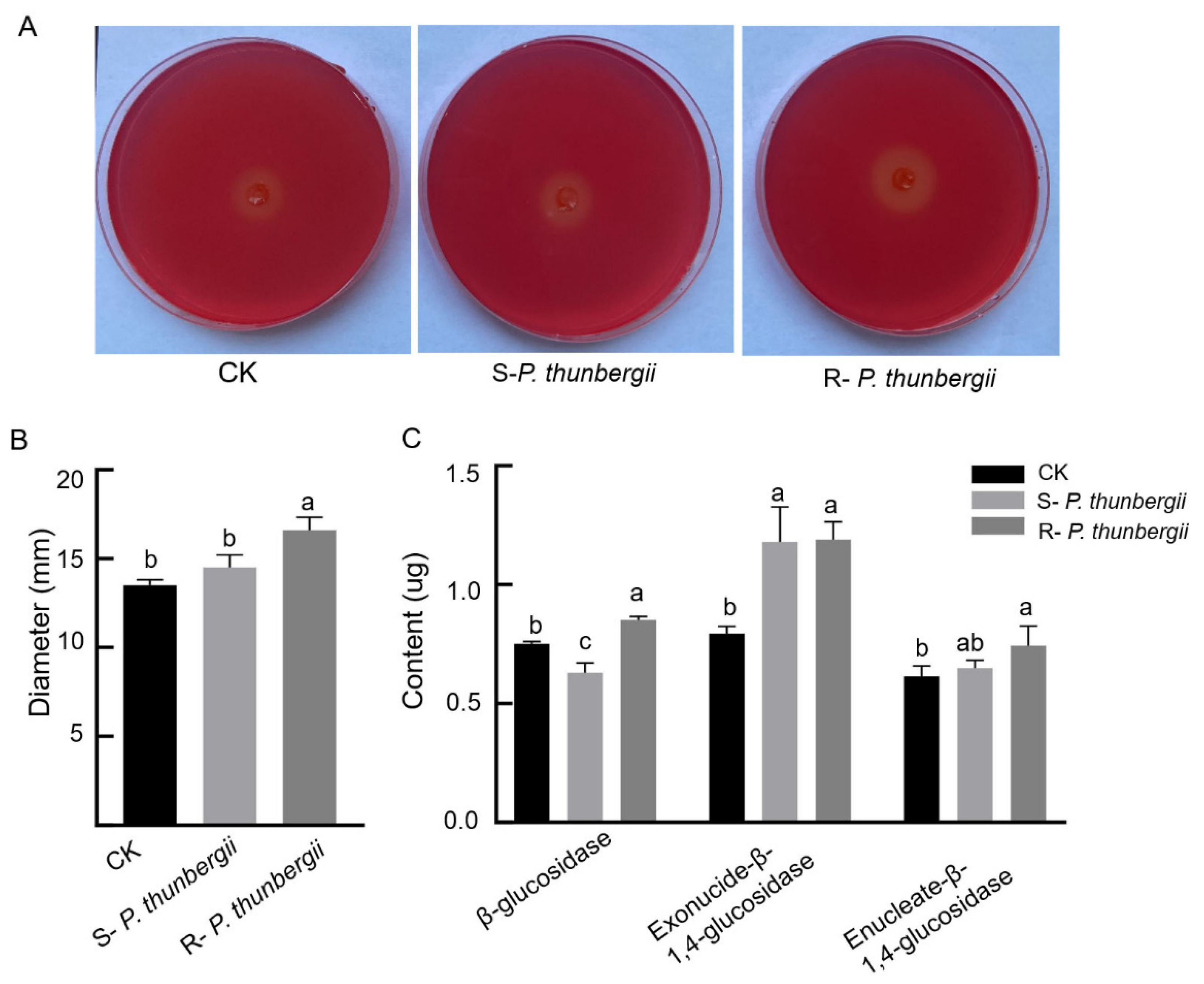
The cellulase contents secreted by PWNs from resistant and susceptible P. thunbergii were determined. Fifteen days after inoculation, the ability of PWNs from inoculated pine to secrete cellulase was found to be stronger than that of uninoculated PWNs, and PWNs from resistant P. thunbergii had stronger cellulase activity than those from susceptible P. thunbergii, with significant differences, as determined by the cellulase plate assay (Figure 3).
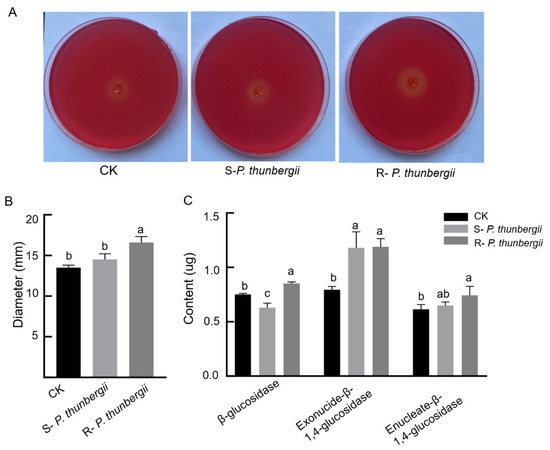
Figure 3.
Contents of cellulase secretions by PWNs within P. thunbergii with different levels of resistance. (A) Cellulose plate assay (measured by a ruler). (B) Cellulase plate assay clear circle diameter (different letters above the error bars indicate statistically significant differences using Duncan’s multiple range test (p < 0.05)). (C) Differences in cellulase content (different letters above the error bars indicate statistically significant differences according to Duncan’s multiple range test (p < 0.05)). Mock-inoculated P. thunbergii was used as a CK.
The main components of cellulases secreted by PWN were β-glucosidase, exonuclease-β-1,4-glucosidase and enucleate-β-1,4-glucosidase. The content of these three cellulases showed that the levels of secreted β-glucosidase in PWNs from P. thunbergii with different levels of resistance differed significantly. The exonuclease-β-1,4-glucosidase and enucleate-β-1,4-glucosidase secreted by PWNs from P. thunbergii with different levels of resistance increased obviously. The exonuclease-β-1,4-glucosidase had no obvious difference, and the enucleate -β-1,4-glucosidase had a difference but was not significant (Figure 3C).
3.4. Expression of Terpene Synthesis-Related Genes in P. thunbergii Inoculated with PWNs
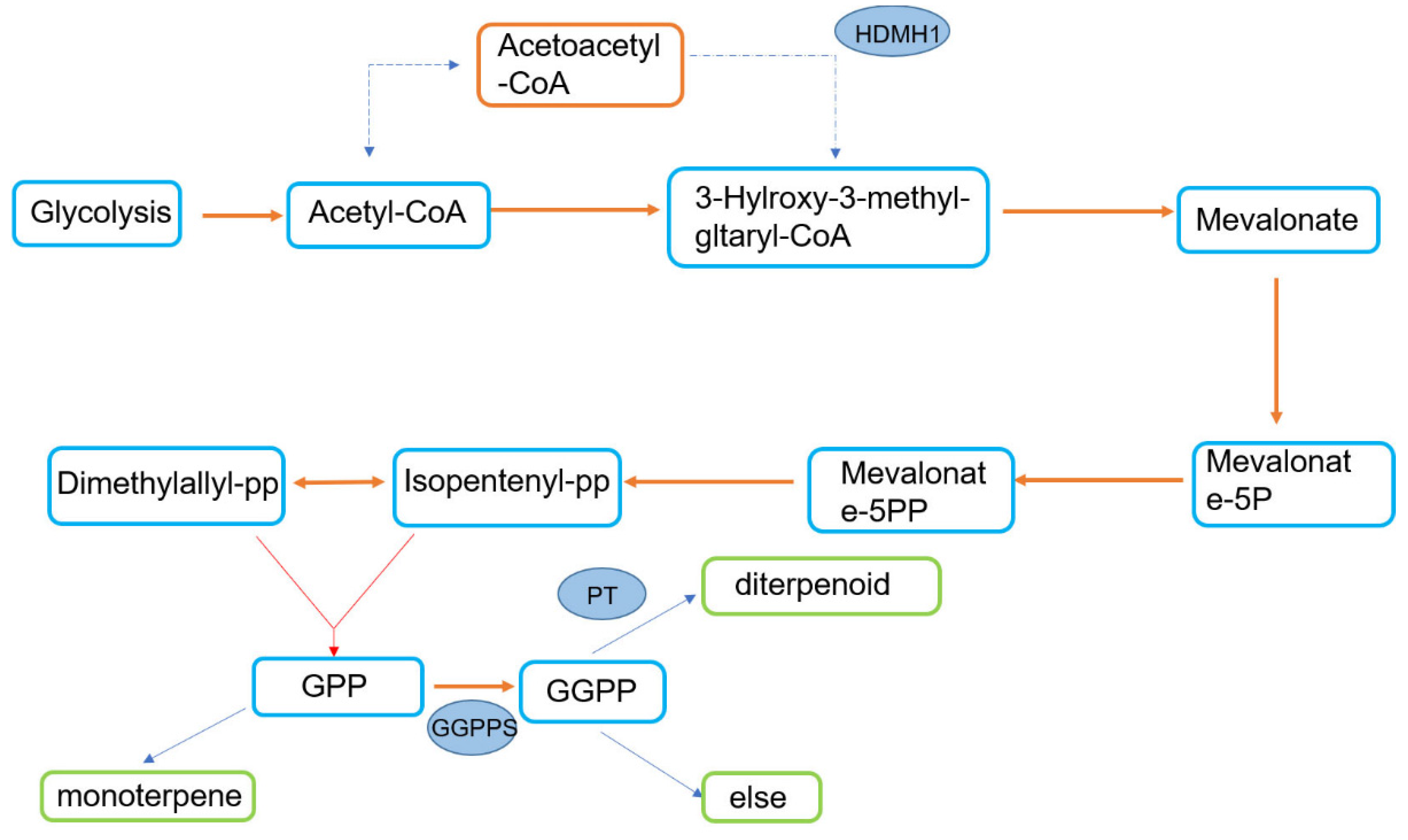
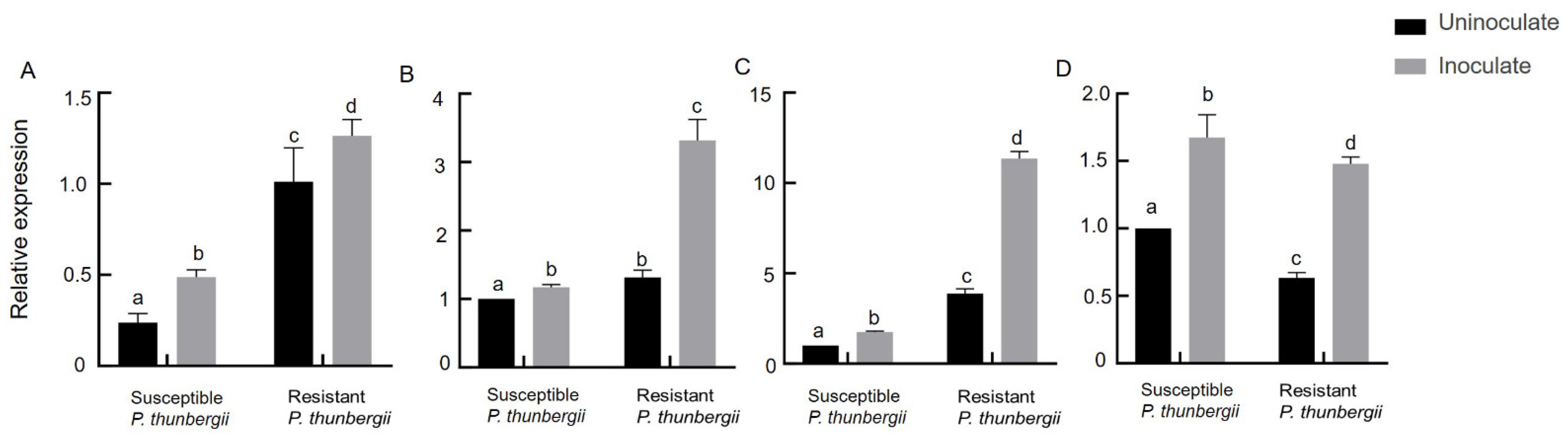
According to previous studies, the mevalonate pathway is an important way for terpene synthesis (Figure 4). To further reveal the resistance mechanism of P. thunbergii and verify the role of terpenoids in the resistance of trees to PWNs, we measured the relative expression of the GGPPS, HMDH1, PT1 and PT2 genes of P. thunbergii with different levels of resistance after inoculation with PWNs. It was found that after inoculation with PWNs, that the expression multiples of the GGPPS, HDMH 1 and PT 1 genes in resistant P. thunbergii were 1.3, 3, 3 and 3, while those in susceptible P. thunbergii were 2, 1.1, 1.5 and 1.6, respectively (Figure 5). The expression levels of HMDH1 were significantly upregulated in resistant P. thunbergii, but GGPPS was not significantly different in either type of P. thunbergii.
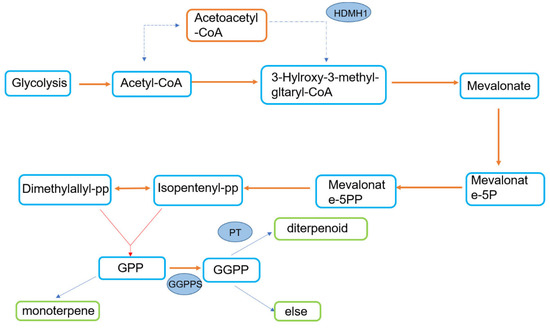
Figure 4.
Terpene synthesis pathway.
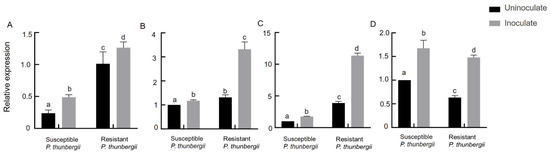
Figure 5.
Expression levels of GGPPS, HDMH1 and PT genes of P. thunbergii with different levels of resistance after inoculation with PWNs (A) GGPPS (B) HDMH1 (C) PT1, and (D) PT2. The different letters above the error bars indicate statistically significant differences (p < 0.05) as measured by Duncan’s multiple range test.
3.5. Changes in Terpenoids in P. thunbergii after Inoculation with PWNs
To further study the influence of the terpenoid synthesis pathway on the resistance of Pinus thunbergii, we chose 0, 24, 72 and 168 h after inoculation with PWNs in P. thunbergii with different levels of resistance, to determine the types and contents of terpenoids. A total of 41 volatiles were detected in resistant and susceptible P. thunbergii. The pine needles over all time periods were measured (Supplementary Table S2, Figure 1), 39 terpenoids were detected in resistant P. thunbergii, 28 were detected in susceptible P. thunbergii, and 25 were detected in both.
A comparison of the main terpenoids detected in P. thunbergii with different levels of resistance at various time periods after inoculation by the PWN showed that, among the terpenoids common to P. thunbergii with different levels of resistance, the relative percentage of caryophyllene was higher in susceptible P. thunbergii than in the resistant one at all inoculation stages. Additionally, the relative percentage of α-pinene, β-pinene and germacrene Dwas higher, which are the key VOS of resistance in resistant and susceptible P. thunbergii. However, the relative percentage of β-pinene and germacrene D were higher in susceptible P. thunbergii than in the resistant one. The relative percentage of γ-cadinene was consistently higher in resistant P. thunbergii than in the susceptible one, but it showed a decreasing trend in resistant P. thunbergii and the susceptible one. Seventy-two hours after inoculation, β-cis-ocimene appeared simultaneously in P. thunbergii with different levels of resistance and occupied a higher relative percentage (17.28% in susceptible and 11.07% in resistant P. thunbergii), and 168 h of inoculation the relative percentage of β-cis-ocimene increased in resistant P. thunbergii but decreased in susceptible P. thunbergii, its relative content was consistently higher than that of resistant P. thunbergii at all times (Table 2).

Table 2.
Changes in the content of major terpenoids in P. thunbergii with different levels of resistance following inoculation with PWNs.
The analysis revealed 14 terpenes specific to resistant P. thunbergii, of which eight terpenes before and after inoculation with PWNs were cis-γ-bisabolene, linalool, alloaromadendrene, germacrene B, bicylogermacrene, dehydrosabinene, pseudolimonen, and α-thujene. Furthermore, six of the 14 specific terpenes appeared only after inoculation (i.e., inducible), including α-terpineol, epi-β-caryophyllen, myrtenal, benzene, n-butyl, caryophyllene oxide and sabinene. In contrast, only three terpenes were unique to susceptible P. thunbergii, including β-cadinene and α-muurolene, which were present both before and after inoculation with PWNs, and borneol was detected after inoculation with PWNs (Table 3). Analysis of the content of the main specific terpenoids in resistant P. thunbergii revealed that the relative percentage of sabinene (after inoculation with PWNs in resistant P. thunbergia) was higher, with values of 15.97%, 72 h after inoculation with PWNs and 18.89% 168 h after inoculation; the relative percentage of pseudolimonen (in resistant P. thunbergia) was 10.37% before inoculation, decreased gradually after inoculation to 7.63% at 72 h, and was no longer detected 168 h after inoculation (Table 3). The relative percentage of α-thujene (common to P. thunbergii with different levels of resistance) was also large, 10.82% before inoculation, 14.14% after 72 h of inoculation and 9.1% after 168 h inoculation in resistant P. thunbergii, but only 0.22% before inoculation and 5.52% 24 h after inoculation in the susceptible one (Supplementary Table S2).

Table 3.
Specific and induced terpenoids and their contents (%) in P. thunbergii with different levels of resistance in the early stages of PWN infestation.
4. Discussion
The tree produces a series of defensive responses to infection, such as resistance-related enzymes, hormones, and ROS, and synthesizes and releases a large number of VOS, which play an important role as a chemical signal in the host plant’s resistance to the disease. This may be one of the reasons for the differences in the variation in resistance substances in pine tree with different resistance levels [33,34]. In this study, we found that resistant P. thunbergii showed substantially fewer PWD-related symptoms and that the migration rate of PWNs in resistant P. thunbergii was lower than that in susceptible P. thunbergii, indicating that resistant P. thunbergii had a stronger barrier to PWN migration than the susceptible one. PWN populations were faster in susceptible P. thunbergii than in resistant P. thunbergii. Studies by Keiko and Dai showed that PWN-resistant P. thunbergii and P. densiflora inhibited the population size and migration of PWNs, revealing advantages over susceptible pine trees [35,36]. Further results showed that cellulase activity in PWNs from resistant P. thunbergii was higher than that from susceptible P. thunbergii. According to Xue et al. [37], the pathogenicity of PWNs is positively correlated with their cellulase activity, while resistant pine trees develop resistance to PWNs; therefore, more cellulase is secreted by PWNs in response. It is important to examine the changes in the resistant material in differentially resistant P. thunbergii inoculated with PWNs to help the underlying resistance mechanisms.
Previous studies have shown that terpenoids are closely related to resistance in pine. Earlier studies on pine resistance to PWNs mainly focused on ROS-related metabolites in the tree, but terpenes play an important role in plant chemosensory effects, signaling, the onset of defense mechanisms, and the inhibition of microbial growth [33]. Terpenoid concentrations may vary among different tissues of trees [38], and strong positive correlation was found between proportional quantities of several terpenes of the needles and wood, with the highest total monoterpene concentrations in the needles and the wood.
Liu et al. [39] found that there are many differentially expressed genes (DEGs) in the terpenoid biosynthesis pathway in the transcriptome analysis of P. massoniana with different levels of resistance. The regulation of this pathway contributes to PWN resistance. However, there is little research on how terpenoids affect P. thunbergii with different levels of resistance. According to Liu et al., GGPPS is an important target for terpenoid synthesis [40,41], and the PT gene is involved in diterpenoid production. Therefore, we related the genes GGPPS, HMDH1, and PT to the types and contents of terpene VOS in P. thunbergii with different levels of resistance after inoculation and compared their differences. Our results show that the genes related to terpene synthesis of resistant P. thunbergii and susceptible P. thunbergii inoculated with PWNs were significantly upregulated (except GGPPS in susceptible P. thunbergii), but the gene expression levels of resistant P. thunbergii were higher. Monoterpenes, sesquiterpenes and diterpenes are formed by the condensation of dimethylallyl diphosphate (DMAPP) with isopentenyl diphosphates (IPP) catalyzed by specific prenyltransferases [40]. HMDH1 is an upstream gene regulating DMAPP and IPP, while GGPPS is an upstream gene regulating diterpenoid synthesis (Figure 4). The results indicated that the differences in terpene synthesis pathways between P. thunbergii with different levels of resistance may be concentrated in monoterpene and sesquiterpene pathways. This may be a potential explanation for the variations in terpenoid synthesis-related gene expression in P. thunbergii of different resistances. It was found that the terpenoids in P. thunbergii with different levels of resistance were mostly monoterpenoids and sesquiterpenoids, which was consistent with our prediction of terpenoid gene expression.
Among the specific terpenoids of resistant P. thunbergii, although myrtenal, linalool, α-thujene, caryophyllene oxide and sabinene have bactericidal, insecticidal and anti-inflammatory effects, there is no report regarding their lethality against PWNs [40,41,42]. In addition, the analysis of common and specific terpenoids indicates that resistant P. thunbergii can contain more terpenoids (resistant P. thunbergii: 39; susceptible P. thunbergii: 28). According to our analysis of the differences in the contents of terpenes shared by the differentially resistant P. thunbergii, we found that α-pinene, β-pinene and caryophyllene were all present at high relative percentages in P. thunbergii with different levels of resistance, with the contents in resistant P. thunbergii always higher than those in susceptible P. thunbergii, suggesting that shared terpenes may have an inhibitory effect on PWNs and that differences in their contents may be one of the reasons for the resistance in pine trees. There may be localized increases in terpene concentrations around the infection site which may explain PWN activity and reproduction that is not captured in needle proportions. The contents of α-pinene and β-pinene in highly resistant P. massoniana were higher than those in the low-resistance P. massoniana [21], but Zhao et al. demonstrated that the α- and β-pinene contents for highly resistant P. massoniana were lower [41]. In the present study, the differences in the α-pinene content were consistent with the former, while the differences in the β-pinene content were consistent with the latter. There were significantly more induced terpenoid in resistant P. thunbergii (6) than susceptible P. thunbergii (1). Among the terpenes specific to resistant P. thunbergii, pseudolimonene, α-thujene and sabinene were present in large percentages, with sabinene being an induced terpene. It is speculated that terpenoids uniquely to resistant P. thunbergii may inhibit the invasion, growth and population size of PWNs. Liu found that the major enzymatic products of α-pinene synthase (PmTPS4, primarily producing α-pinene) and longifolene synthase (PmTPS2, produced α-pinene) had inhibitory effects on PWNs in vitro [42], and α-pinene may have the ability to kill PWNs. Dong et al. [21] detected α-pinene only in moderately and highly resistant P. massoniana. Wu et al. [42] found that α-pinene has antibacterial and anti-inflammatory effects. Finally, Li found that B. xylophilus genes involved in transport, detoxification, and receptor activities were differentially expressed by α-pinene [39]. Further research is necessary to determine the role of terpene content on the differential levels of resistance in pines. This study does not specifically connect elevated compounds in esistant P. thunbergii to resistance to PWN and assays should be conducted to establish direct effects of terpenoids on pinewood nematode activity and reproduction. In the future, the odor composition of the host plant could be used as an indicator to assess resistance levels when selecting resistant P. thungergii strains [43,44], as well as to provide candidate criteria and plant materials for the breeding of resistant pine trees.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f13071140/s1, Figure S1: GC-MC chromatogram of P. thunbergii with different levels of resistance at 24 h after inoculation with PWN (A) resistant P. thunbergii (B) susceptible P. thunbergii.; Table S1: Sepcific primers used in this study; Table S2: Differences in volatile matter content of differentially resistant P. thunbergii after inoculation with PWNs.
Author Contributions
X.-Y.W. and X.-Q.W. designed the study; X.-Y.W., Y.-Q.F. and Y.Z. performed the experimental implementation and data analysis; X.-Y.W., X.-Q.W. and T.-Y.W. drafted the manuscript, and all authors contributed to revisions. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key R & D Program of China (2021YFD1400903), the Major Emergency Project in Science and Technology of the National Forestry and Grassland Administration (ZD202001), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
This work was supported by the National Key R&D Program of China (2021YFD1400903), the Major Emergency Project in Science and Technology of the National Forestry and Grassland Administration (ZD202001), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Branco, M.; Bragança, H.; Sousa, E.; Phillips, A.J. Pests and Diseases in Portuguese Forestry: Current and New Threats. In Forest Context and Policies in Portugal; Reboredo, F., Ed.; Springer: Lisbon, Portugal, 2014; Volume 19. [Google Scholar]
- Abelleira, A.; Picoaga, A.; Mansilla, J.P.; Aguin, O. Detection of Bursaphelenchus Xylophilus, Causal Agent of Pine Wilt Disease on Pinus pinaster in Northwestern Spain. Plant Dis. 2011, 96, 770–780. [Google Scholar] [CrossRef]
- Mamiya, Y. History of pine wilt disease in Japan. J. Nematol. 1988, 20, 219–226. [Google Scholar]
- Togashi, K.; Shigesada, N. Spread of the pinewood nematode vectored by the Japanese pine sawyer: Modeling and analytical approaches. Popul. Ecol. 2006, 48, 271–283. [Google Scholar] [CrossRef]
- National Forestry and Grassland Administration. Announcement of the National Forestry and Grassland Administration (No.6, 2022) (Pine Wood Nematode Disease Epidemic Area in 2022) [N]; Announcement of the National Forestry and Grassland Administration: Beijing, China, 2022. [Google Scholar]
- Choi, W.I.; Nam, Y.; Lee, C.Y.; Choi, B.K.; Shin, Y.J.; Lim, J.-H.; Koh, S.-H.; Park, Y.-S. Changes in Major Insect Pests of Pine Forests in Korea Over the Last 50 Years. Forests 2019, 10, 692. [Google Scholar] [CrossRef]
- De la Fuente, B.; Beck, P.S.A. Management measures to control pine wood nematode spreadin Europe. J. Appl. Ecol. 2019, 56, 2577–2580. [Google Scholar] [CrossRef]
- Liu, Y.; Ponpandian, L.N.; Kim, H.; Jeon, J.; Hwang, B.S.; Lee, S.K.; Park, S.-C.; Bae, H. Distribution and diversity of bacterial endophytes from four Pinus species and their efficacy as biocontrol agents for devastating pine wood nematodes. Sci. Rep. 2019, 9, 12461. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, X.-Q.; Wang, J. Determination and histopathological observation of resistance to PWD in Pinus densiflora. Natural Sciences Edition. J. Nanjing For. Univ. 2007, 31, 110–114. [Google Scholar]
- Lorio, P.L.; Stephen, F.M.; Paine, T.D. Environment and ontogeny modify loblolly pine response to induced acute water deficits and bark beetle attack. For. Ecol. Manag. 1995, 73, 97–110. [Google Scholar] [CrossRef]
- Lu, F.; Guo, K.; Chen, A.; Chen, S.; Lin, H.; Zhou, X. Transcriptomic profiling of effects of emamectin benzoate on the pine wood nematode Bursaphelenchus xylophilus. Pest Manag. Sci. 2020, 76, 747–757. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Q.; Zhou, Z.; Yin, H.; Wei, Y. Two terpene synthases in resistant Pinus massoniana contribute to defense against Bursaphelenchus xylophilus. Plant Cell Environ. 2020, 44, 257–274. [Google Scholar] [CrossRef]
- Lee, J.-P.; Sekhon, S.S.; Kim, J.H.; Kim, S.C.; Cho, B.-K.; Ahn, J.-Y.; Kim, Y.-H. The Pine Wood Nematode Bursaphelenchus xylophilus and Molecular Diagnostic Methods. Mol. Cell. Toxicol. 2020, 17, 1–13. [Google Scholar] [CrossRef]
- John, T.H.; Radhika, d.; Andrew, C.; Roger, D.H.; Steven, J.N. Cell signalling following plant/pathogen interactions involves the generation of reactive oxygen and reactive nitrogen species. Plant Physiol. Biochem. 2002, 40, 611–617. [Google Scholar]
- Wang, J.; Higgins, V.J. Nitric oxide modulates H2O2-mediated defenses in the Colletotrichum coccodes–tomato interaction. Physiol. Mol. Plant Pathol. 2005, 67, 131–137. [Google Scholar] [CrossRef]
- Keeling, C.I.; Bohlmann, J. Genes, enzymes and chemicals of terpenoid diversity in the constitutive and induced defence of conifers against insects and pathogens. New Phytol. 2006, 170, 657–675. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Xu, S.; Xu, C.; Lu, H.; Zhang, Z.; Zhang, D.; Mu, W.; Liu, F. Effects of trans-2-hexenal on reproduction, growth and behaviour and efficacy against the pinewood nematode, Bursaphelenchus xylophilus. Pest Manag. Sci. 2016, 73, 888–895. [Google Scholar] [CrossRef]
- Harman-Ware, A.E.; Davis, M.F.; Peter, G.F.; Wang, Y.; Sykes, R.W. Estimation of terpene content in loblolly pine biomass using a hybrid fast-GC and pyrolysis-molecular beam mass spectrometry method. J. Anal. Appl. Pyrolysis 2017, 124, 343–348. [Google Scholar] [CrossRef]
- Manninen, A.M.; Holopainen, M. Variation in Growth, Chemical Defense, and Herbivore Resistance in Scots Pine Provenances. J. Chem. Ecol. 1998, 24, 1315–1331. [Google Scholar] [CrossRef]
- Li, X.-F.; Jiao, H.; Yuan, Y.; Tian, S.-N. Allelopathy and chemical composition analysis of volatile compounds from branches and leaves of Cedrus. Ecol. Environ. Sci. 2015, 24, 263–269. [Google Scholar]
- Dong, G.-P.; Yang, L. Preliminary report on the correlation between volatile odor components of Pinus massoniana and its resistance to PWD. Anhui For. Sci. Technol. 2015, 41, 12–14. [Google Scholar]
- Takeuchi, Y.; Futai, K. Volatile compounds in pine stands suffering from pine wilt disease: Qualitative and quantitative evaluation. Nematology 2006, 8, 869–879. [Google Scholar]
- Ren, Q.; Hu, Y.-J.; Li, Z.-Y.; Jin, Y.-J. Lignin content and peroxidase activity in victimized Pinus massoniana. Acta Ecol. Sin. 2007, 27, 4895–4899. [Google Scholar]
- Sasaki, S.; Odani, K.; Nishiyama, Y. Development and Recovery of Pine Wilt Disease Studied by Tracing Ascending Sap Flow Marked with Water Soluble Stains. J. Jpn. For. Soc. 1987, 66, 141–148. [Google Scholar]
- Wu, X.-Q.; Zhang, Y.; Chen, W.-S. Resistance and histopathological observation of 13 resistant families of Pinus thunbergii to Bursaphelenchus xylophilus. Acta Phytopathol. Sin. 2008, 38, 44–50. [Google Scholar]
- Viglierchio, D.R.; Schmitt, R.V. On the methodology of nematode extraction from field samples: Comparison of methods for soil extraction. J. Nematol. 1983, 15, 450–454. [Google Scholar]
- Yang, X.; Wu, X.Q.; Zhou, A.D.; John, J. Bacterial Diversity and Community Structure in the Pine Wood Nematode Bursaphelenchus xylophilus and B. mucronatus with Different Virulence by High-Throughput Sequencing of the 16S rDNA. PLoS ONE 2015, 10, e0137386. [Google Scholar]
- Kusunoki, M. Symptom Development of Pine Wilt Disease-Histopathological Observations with Electron Microscopes. Jpn. J. Phytopathol. 1987, 53, 622–629. [Google Scholar] [CrossRef][Green Version]
- Walker, J.M. The bicinchoninic acid (BCA) assay for protein quantitation. In Basic Protein and Peptide Protocols, 1st ed.; Humana Press: Totowa, NJ, USA, 1994; pp. 11–15. [Google Scholar]
- Hu, K.-J.; Yang, B.-J. Studies on enzyme electrophoresis of different strains of Bursaphelenchus xylophilus and Bmucronatus. For. Res. 1995, 1, 73–77. [Google Scholar]
- Jiang, L.-Y.; Wang, X.-Y. Qualitative determination of cellulase in extracts and secretions of Bursaphelenchus xylophilus. For. Pest Dis. 1995, 3, 9–11. [Google Scholar]
- Manninen, A.M.; Tarhanen, S.; Vuorinen, M.; Kainulainen, P. Comparing the Variation of Needle and Wood Terpenoids in Scots Pine Provenances. J. Chem. Ecol. 2002, 28, 211–228. [Google Scholar] [CrossRef]
- Wu, Y.; Cheng, L. Advances in the pathogenesis of PWD. South China Agric. 2018, 6, 73–75. [Google Scholar]
- Li, Y.-X.; Zhang, X.-Y. Progress in pathogenesis of PWD. J. Environ. Entomol. 2018, 40, 231–241. [Google Scholar]
- Kusumoto, D.; Yonemichi, T.; Inoue, H.; Hirao, T.; Watanabe, A.; Yamada, T. Comparison of histological responses and tissue damage expansion between resistant and susceptible Pinus thunbergii infected with pine wood nematode Bursaphelenchus xylophilus. J. For. Res. 2017, 19, 285–294. [Google Scholar] [CrossRef]
- Kuroda, K. Inhibiting factors of symptom development in several Japanese red pine (Pinus densiflora) families selected as resistant to pine wilt. J. For. Res. 2004, 9, 217–224. [Google Scholar] [CrossRef]
- Xue, M.-J.; Li, Y.; Zhang, J. Relationship between cellulase activity and pathogenicity of Bursaphelenchus mucronatus. J. Biosaf. 2019, 28, 181–188. [Google Scholar]
- Futuyma, D.J.; Rosenthal, G.A.; Janzen, D.H. Herbivores: Their Interactions with Secondary Plant Metabolites. BioScience 1991, 30, 165–219. [Google Scholar]
- Liu, Q.; Wei, Y.; Xu, L.; Hao, Y.; Chen, X.; Zhou, Z. Transcriptomic Profiling Reveals Differentially Expressed Genes Associated with Pine Wood Nematode Resistance in Masson Pine (Pinus massoniana Lamb). Sci. Rep. 2017, 7, 4693. [Google Scholar] [CrossRef]
- Koyama, T.; Ogura, K. Isopentenyl diphosphate isomerase and prenyltransferases. In Isoprenoids, Including Carotenoids and Steroids; Comprehensive natural products chemistry, Elsevier: London, UK, 1999; Volume 2, pp. 69–96. [Google Scholar]
- Zhao, Z.-D.; Li, D.-M.; Hu, X.; Xu, F.-Y. Studies on the Chemical Constituents and resistance Mechanism of Pinus massoniana provenances to pine wood nematode Disease (Report iii). In Studies on the Relationship between the Content of Neutral Terpenes in Pinus massoniana Provenances Resistant to Pine Wood Nematode Inoculation; Chemistry and Industry of Forest Products: Nanjing, China, 2001. [Google Scholar]
- Wu, T.; Liu, J.; Li, M.; Zhang, G.; Liu, L.; Li, X.; Men, X.; Xian, M.; Zhang, H. Improvement of sabinene tolerance of Escherichia coli using adaptive laboratory evolution and omics technologies. Biotechnol. Biofuels 2020, 13, 79–93. [Google Scholar] [CrossRef]
- Cao, B.; Xu, W.-R.; Lu, W. Chemical composition analysis of several insect resistant tree species and study on insect resistance mechanism. For. Sci. Technol. 1998, 6, 27–29. [Google Scholar]
- Bing, C.; Zhong, L.Z.; Long, J.X.; Zeng, X.X. A Study on Scattering Effects of the Extracts from Ailanthus altissima on Anoplophora glabripennis. J. Nanjing For. Univ. 2004, 28, 47–49. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).