Discovery of Rickettsia and Rickettsiella Intracellular Bacteria in Emerald Ash Borer Agrilus planipennis by Metagenomic Study of Larval Gut Microbiome in European Russia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Location and Sampling
2.2. DNA Extraction, Sequencing and Data Processing
3. Results and Discussion
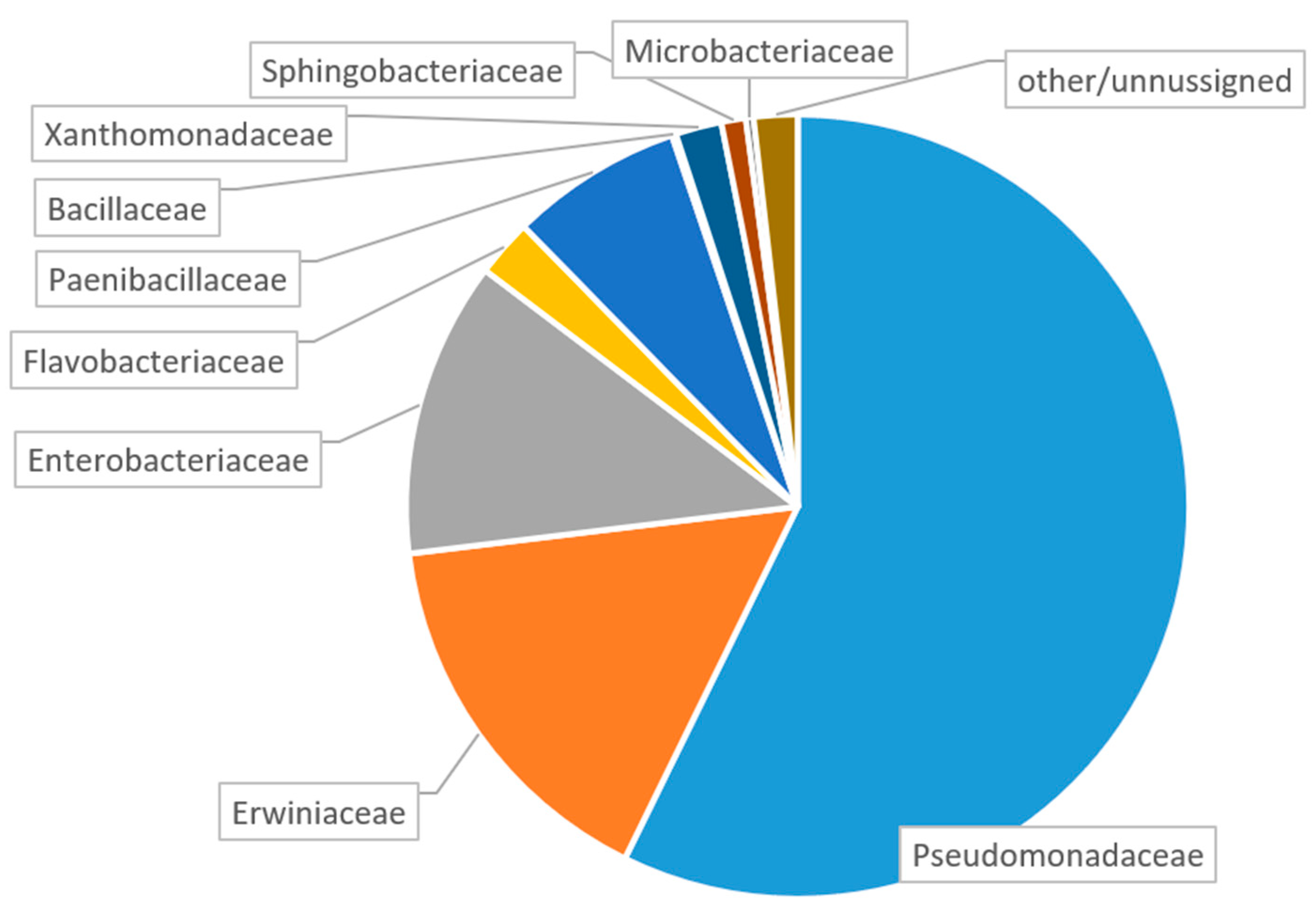
3.1. General Composition of the Larval Gut Microbiome
3.2. The First Record of Rickettsia
3.3. The First Record of Rickettsiella
4. Conclusions
- The dominant families in our samples from European Russia were Pseudomonadaceae, Erwinaceae and Enterobacteriacea. Since the same families are known to be dominant in the A. planipennis larval gut in the USA and China, these families probably belong to the core microbiome typical for A. planipennis;
- Obligate intracellular parasites were first recorded in A. planipennis, namely representatives of the genus Rickettsia, which are known to be in mutualistic symbiosis with some phytophagous insects, and Rickettsiella, which are known to be pathogens of a wide range of arthropods;
- The finding of Rickettsia and Rickettsiella opens perspectives for future research on the interactions between these bacteria and A. planipennis. There is hope that if “natural enemies” or “natural allies” of the pest can be found, it could be used for the development of biological control measures.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schans, J.; Schrader, G.; Delbianco, A.; Graziosi, I.; Vos, S. Pest survey card on Agrilus planipennis. EFSA Support. Publ. 2020, 17, 1945E. [Google Scholar] [CrossRef]
- Orlova–Bienkowskaja, M.J.; Volkovitsh, M.G. Are native ranges of the most destructive invasive pests well known? A case Study of the native range of the emerald ash borer, Agrilus planipennis (Coleoptera: Buprestidae). Biol. Invasions 2018, 20, 1275–1286. [Google Scholar] [CrossRef]
- Haack, R.A.; Jendek, E.; Liu, H.; Marchant, K.R.; Petrice, T.R.; Poland, T.M.; Ye, H. The Emerald Ash Borer: A new exotic pest in North America. Newsl. Mich. Entomol. Soc. 2002, 47, 1–5. [Google Scholar]
- Izhevskii, S.S. Threatening Findings of the Emerald ash Borer Agrilus planipennis in the Moscow Region. Available online: http://www.zin.ru/Animalia/Coleoptera/rus/agrplaiz.htm (accessed on 4 May 2022).
- Emerald Ash Borer Informative Network. Available online: http://www.emeraldashborer.info/ (accessed on 4 May 2022).
- Orlova–Bienkowskaja, M.J.; Drogvalenko, A.N.; Zabaluev, I.A.; Sazhnev, A.S.; Peregudova, E.Y.; Mazurov, S.G.; Komarov, E.V.; Struchaev, V.V.; Martynov, V.V.; Nikulina, T.V. Current range of Agrilus planipennis Fairmaire, an alien pest of ash trees, in European Russia and Ukraine. Ann. For. Sci. 2020, 77, 29. [Google Scholar] [CrossRef]
- Volkovitsh, M.G.; Bieńkowski, A.O.; Orlova-Bienkowskaja, M.J. Emerald Ash Borer Approaches the Borders of the European Union and Kazakhstan and Is Confirmed to Infest European Ash. Forests 2021, 12, 691. [Google Scholar] [CrossRef]
- Davydenko, K.; Skrylnyk, Y.; Borysenko, O.; Menkis, A.; Vysotska, N.; Meshkova, V.; Olson, Å.; Elfstrand, M.; Vasaitis, R. Invasion of Emerald Ash Borer Agrilus planipennis and Ash Dieback Pathogen Hymenoscyphus fraxineus in Ukraine—A Concerted Action. Forests 2022, 13, 789. [Google Scholar] [CrossRef]
- Orlova-Bienkowskaja, M.J.; Bieńkowski, A.O. Southern Range Expansion of the Emerald Ash Borer, Agrilus planipennis, in Russia Threatens Ash and Olive Trees in the Middle East and Southern Europe. Forests 2022, 13, 541. [Google Scholar] [CrossRef]
- Selikhovkin, A.V.; Musolin, D.L.; Popovichev, B.G.; Merkuryev, S.A.; Volkovitsh, M.G.; Vasaitis, R. Invasive populations of the emerald ash borer Agrilus planipennis Fairmaire, 1888 (Coleoptera: Buprestidae) in Saint Petersburg, Russia: A Hitchhiker? Insects 2022, 13, 191. [Google Scholar] [CrossRef]
- Caudullo, G.; Welk, E.; San-Miguel-Ayanz, J. Chorological maps for the main European woody species. Data Brief 2017, 12, 662–666. [Google Scholar] [CrossRef]
- Orlova-Bienkowskaja, M.J.; Bieńkowski, A.O. Minimum winter temperature as a limiting factor of the potential spread of Agrilus planipennis, an alien pest of ash trees, in Europe. Insects 2020, 11, 258. [Google Scholar] [CrossRef]
- Orlova-Bienkowskaja, M.J.; Bieńkowski, A.O. Low heat availability could limit the potential spread of the emerald ash borer to Northern Europe (prognosis based on growing degree days per year). Insects 2022, 13, 52. [Google Scholar] [CrossRef] [PubMed]
- Commission Delegated Regulation (EU) 2019/1702 of 1 August 2019 Supplementing Regulation (EU) 2016/2031 of the European Parliament and of the Council by Establishing the List of Priority Pests. OJ L 260:8–10. Available online: http://data.europa.eu/eli/reg_del/2019/1702/oj (accessed on 21 June 2022).
- Cipollini, D.; Rigsby, C.M.; Peterson, D.L. Feeding and development of emerald ash borer (Coleoptera: Buprestidae) on cultivated olive, Olea europaea. J. Econ. Entomol. 2017, 110, 1935–1937. [Google Scholar] [CrossRef] [PubMed]
- EPPO Global Database. Available online: https://gd.eppo.int/taxon/AGRLPL/categorization (accessed on 4 May 2022).
- Popa, V.; Déziel, E.; Lavallée, R.; Bauce, E.; Guertin, C. The complex symbiotic relationships of bark beetles with microorganisms: A potential practical approach for biological control in forestry. Pest Manag. Sci. 2012, 68, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Gibson, C.M.; Hunter, M.S. Extraordinarily widespread and fantastically complex: Comparative biology of endosymbiotic bacterial and fungal mutualists of insects. Ecol. Lett. 2010, 13, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Feng, R.; Fu, X.; Zhao, J.; Zhang, S.; Wang, J.; Wang, X.; Wei, J. Lignans dramatically enhance the resistance of Fraxinus velutina Torr. by adjusting advantage bacterium group of Agrilus planipennis Fairmaire. Pest Manag. Sci. 2021, 78, 1386–1397. [Google Scholar] [CrossRef]
- Vasanthakumar, A.; Handelsman, J.O.; Schloss, P.D.; Bauer, L.S.; Raffa, K.F. Gut microbiota of an invasive subcortical beetle, Agrilus planipennis Fairmaire, across various life stages. Environ. Entomol. 2008, 37, 1344–1353. [Google Scholar] [CrossRef]
- Bergeron, A. Caractérisation de la communauté bactérienne associée à l’agrile du frêne, Agrilus planipennis Fairmaire (Coleoptera: Buprestidae). Doctoral Dissertation, Université du Québec, Institut National de la Recherche Scientifique, 2016. Available online: https://123dok.net/document/dzxx2o4z-caracterisation-communaute-bacterienne-associee-planipennis-fairmaire-coleoptera-buprestidae.html (accessed on 4 May 2022).
- Mogouong, J.; Constant, P.; Lavallée, R.; Guertin, C. Gut microbiome of the emerald ash borer, Agrilus planipennis Fairmaire, and its relationship with insect population density. FEMS Microbiol. Ecol. 2020, 96, fiaa141. [Google Scholar] [CrossRef]
- Mogouong, J.; Constant, P.; Legendre, P.; Guertin, C. The phyllosphere microbiome of host trees contributes more than leaf phytochemicals to variation in the Agrilus planipennis Fairmaire gut microbiome structure. Sci. Rep. 2021, 11, 15911. [Google Scholar] [CrossRef]
- Musolin, D.L.; Selikhovkin, A.V.; Peregudova, E.Y.; Popovichev, B.G.; Mandelshtam, M.Y.; Baranchikov, Y.N.; Vasaitis, R. North-Westward expansion of the invasive range of emerald ash borer, Agrilus planipennis Fairmaire (Coleoptera: Buprestidae) towards the EU: From Moscow to Saint Petersburg. Forests 2021, 12, 502. [Google Scholar] [CrossRef]
- Majorov, S.R.; Bochkin, V.D.; Nasimovich, Y.A.; Shcherbakov, A.V. Alien Flora of Moscow and Moscow Region; KMK Publishing house: Moscow, Russia, 2012. [Google Scholar]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Russell, J.A.; Funaro, C.F.; Giraldo, Y.M.; Goldman-Huertas, B.; Suh, D.; Kronauer, D.J.C.; Moreau, C.S.; Pierce, N.E. A veritable menagerie of heritable bacteria from ants, butterflies, and beyond: Broad molecular surveys and a systematic review. PLoS ONE 2012, 7, e51027. [Google Scholar] [CrossRef] [PubMed]
- Mittapalli, O.; Bai, X.; Mamidala, P.; Rajarapu, S.P.; Bonello, P.; Herms, D.A. Tissue-specific transcriptomics of the exotic invasive insect pest emerald ash borer (Agrilus planipennis). PLoS ONE 2010, 5, e13708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolasa, M.; Kubisz, D.; Gutowski, J.M.; Oecibor, R.; Mazur, M.A.; Holekova, M.; Kajtoch, L. Infection by endosymbiotic “male-killing” bacteria in Coleoptera. Folia Biologica (Kraków) Folia Biologica (Kraków). Folia Biol. 2018, 66, 165–177. [Google Scholar] [CrossRef]
- Lawson, E.T.; Mousseau, T.A.; Klaper, R.; Hunter, M.D.; Werren, J.H. Rickettsia associated with male-killing in a buprestid beetle. Heredity 2001, 86, 497–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, P.Q.; Chen, X.Y.; Chen, X.S.; Lv, N.; Liu, Y.; Qiu, B.L. Rickettsia increases its infection and spread in whitefly populations by manipulating the defense patterns of the host plant. FEMS Microbiol. Ecol. 2021, 97, fiab032. [Google Scholar] [CrossRef]
- Mahadav, A.; Gerling, D.; Gottlieb, Y.; Czosnek, H.; Ghanim, M. Parasitization by the wasp Eretmocerus mundus induces transcription of genes related to immune re-sponse and symbiotic bacteria proliferation in the whitefly Bemisia tabaci. BMC Genom. 2008, 9, 342. [Google Scholar] [CrossRef] [Green Version]
- Brumin, M.; Ghanim, M.; Kontsedalov, S. Rickettsia influences thermotolerance in the whitefly Bemisia tabaci B biotype. Insect Sci. 2011, 18, 57–66. [Google Scholar] [CrossRef]
- Himler, A.G.; Adachi-Hagimori, T.; Bergen, J.E.; Kozuch, A.; Kelly, S.E.; Tabashnik, B.E.; Chiel, E.; Duckworth, V.E.; Dennehy, T.J.; Zchori-Feinand, E.; et al. Rapid spread of a bacterial symbiont in an invasive whitefly is driven by fitness benefits and femalebias. Science 2011, 332, 254–256. [Google Scholar] [CrossRef] [Green Version]
- Lukasik, P.; van Asch, M.; Guo, H.; Ferrari, J.; Charles, J.; Godfray, H. Unrelated facultative endosymbionts protect aphids against a fungal pathogen. Ecol. Lett. 2013, 16, 214–218. [Google Scholar] [CrossRef]
- Li, Y.H.; Ahmed, M.Z.; Li, S.J.; Lv, N.; Shi, P.Q.; Chen, X.S.; Qiu, B.L. Plant-mediated horizontal transmission of Rickettsia endosymbiont between different whitefly species. FEMS Microbiol. Ecol. 2017, 93, fix138. [Google Scholar] [CrossRef] [Green Version]
- Duron, O.; Cremaschi, J.; McCoy, K.D. The high diversity and global distribution of the intracellular bacterium Rickettsiella in the polar seabird tick Ixodes uriae. Microb. Ecol. 2016, 71, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Thu, M.J.; Qiu, Y.; Kataoka-Nakamura, C.; Sugimoto, C.; Katakura, K.; Isoda, N.; Nakao, R. Isolation of Rickettsia, Rickettsiella, and Spiroplasma from questing ticks in Japan using arthropod cells. Vector Borne Zoonotic Dis. 2019, 19, 474–485. [Google Scholar] [CrossRef]
- Bouchon, D.; Cordaux, R.; Grève, P. Rickettsiella, intracellular pathogens of arthropods. In Manipulative tenants: Bacteria Associated with Arthropods; Zchori-Fein, E., Bourtzis, K., Eds.; CRC Press: London, UK; New York, NY, USA, 2011; pp. 127–148. [Google Scholar]
- Delmas, F.; Timon-David, P. Effect of invertebrate rickettsiae on vertebrates: Experimental infection of mice by Rickettsiella grylli. Comptes Rendus De L’academie Des Sciences. Ser. III Sci. De La Vie 1985, 300, 115–117. [Google Scholar]
- Zhang, X.C.; Zhang, F. Chapter Four—The potential control strategies based on the interaction between indoor cockroaches and their symbionts in China. Adv. Insect Physiol. 2018, 55, 55–122. [Google Scholar]
| Phylum | Family | Genus |
|---|---|---|
| Acidobacteria | Acidobacteriaceae | Terriglobus |
| Actinobacteria | Actinomycetaceae | Actinomyces |
| Actinobacteria | Brevibacteriaceae | Brevibacterium |
| Actinobacteria | Corynebacteriaceae | Corynebacterium 1 |
| Actinobacteria | Lawsonellaceae | Lawsonella |
| Actinobacteria | Microbacteriaceae | Curtobacterium |
| Actinobacteria | Microbacteriaceae | Microbacterium |
| Actinobacteria | Micrococcaceae | Kocuria |
| Actinobacteria | Micrococcaceae | Micrococcus |
| Actinobacteria | Micrococcaceae | Rothia |
| Actinobacteria | Micromonosporaceae | Micromonospora |
| Actinobacteria | Nocardioidaceae | Nocardioides |
| Actinobacteria | Propionibacteriaceae | Cutibacterium |
| Actinobacteria | Pseudonocardiaceae | Actinomycetospora |
| Bacteroidetes | Chitinophagaceae | Segetibacter |
| Bacteroidetes | Dysgonomonadaceae | Dysgonomonas |
| Bacteroidetes | Flavobacteriaceae | Myroides |
| Bacteroidetes | Hymenobacteraceae | Hymenobacter |
| Bacteroidetes | Sphingobacteriaceae | Sphingobacterium |
| Firmicutes | Aerococcaceae | Globicatella |
| Firmicutes | Bacillaceae | Bacillus |
| Firmicutes | Bacillaceae | Virgibacillus |
| Firmicutes | Lactobacillaceae | Lactobacillus |
| Firmicutes | Paenibacillaceae | Paenibacillus |
| Firmicutes | Peptoniphilaceae | Peptoniphilus |
| Firmicutes | Planococcaceae | Rummeliibacillus |
| Firmicutes | Staphylococcaceae | Macrococcus |
| Firmicutes | Staphylococcaceae | Staphylococcus |
| Firmicutes | Streptococcaceae | Streptococcus |
| Proteobacteria | Acetobacteraceae | Craurococcus |
| Proteobacteria | Alcaligenaceae | Paenalcaligenes |
| Proteobacteria | Aurantimonadaceae | Aureimonas |
| Proteobacteria | Brucellaceae | Paenochrobactrum |
| Proteobacteria | Comamonadaceae | Acidovorax |
| Proteobacteria | Diplorickettsiaceae | Rickettsiella |
| Proteobacteria | Enterobacteriaceae | Citrobacter |
| Proteobacteria | Enterobacteriaceae | Enterobacter |
| Proteobacteria | Enterobacteriaceae | Kluyvera |
| Proteobacteria | Enterobacteriaceae | Lelliottia |
| Proteobacteria | Erwiniaceae | Erwinia |
| Proteobacteria | Erwiniaceae | Pantoea |
| Proteobacteria | Hyphomicrobiaceae | Pedomicrobium |
| Proteobacteria | Methylobacteriaceae | Methylobacterium |
| Proteobacteria | Moraxellaceae | Acinetobacter |
| Proteobacteria | Morganellaceae | Providencia |
| Proteobacteria | Neisseriaceae | Roseomonas |
| Proteobacteria | Oxalobacteraceae | Massilia |
| Proteobacteria | Pseudomonadaceae | Pseudomonas |
| Proteobacteria | Rickettsiaceae | Candidatus Megaira |
| Proteobacteria | Rickettsiaceae | Rickettsia |
| Proteobacteria | Sphingomonadaceae | Sphingomonas |
| Proteobacteria | Weeksellaceae | Chryseobacterium |
| Proteobacteria | Xanthomonadaceae | Stenotrophomonas |
| Proteobacteria | Yersiniaceae | Serratia |
| Proteobacteria | Neisseriaceae | Neisseria |
| Verrucomicrobia | Chthoniobacteraceae | Candidatus Udaeobacter |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vecherskii, M.V.; Orlova-Bienkowskaja, M.J.; Kuznetsova, T.A.; Bieńkowski, A.O. Discovery of Rickettsia and Rickettsiella Intracellular Bacteria in Emerald Ash Borer Agrilus planipennis by Metagenomic Study of Larval Gut Microbiome in European Russia. Forests 2022, 13, 974. https://doi.org/10.3390/f13070974
Vecherskii MV, Orlova-Bienkowskaja MJ, Kuznetsova TA, Bieńkowski AO. Discovery of Rickettsia and Rickettsiella Intracellular Bacteria in Emerald Ash Borer Agrilus planipennis by Metagenomic Study of Larval Gut Microbiome in European Russia. Forests. 2022; 13(7):974. https://doi.org/10.3390/f13070974
Chicago/Turabian StyleVecherskii, Maxim V., Marina J. Orlova-Bienkowskaja, Tatyana A. Kuznetsova, and Andrzej O. Bieńkowski. 2022. "Discovery of Rickettsia and Rickettsiella Intracellular Bacteria in Emerald Ash Borer Agrilus planipennis by Metagenomic Study of Larval Gut Microbiome in European Russia" Forests 13, no. 7: 974. https://doi.org/10.3390/f13070974
APA StyleVecherskii, M. V., Orlova-Bienkowskaja, M. J., Kuznetsova, T. A., & Bieńkowski, A. O. (2022). Discovery of Rickettsia and Rickettsiella Intracellular Bacteria in Emerald Ash Borer Agrilus planipennis by Metagenomic Study of Larval Gut Microbiome in European Russia. Forests, 13(7), 974. https://doi.org/10.3390/f13070974






