Effective Methods for Adventitious Root Regeneration on Weeping Fig Stems
Abstract
:1. Introduction
2. Materials and Methods
2.1. 2019
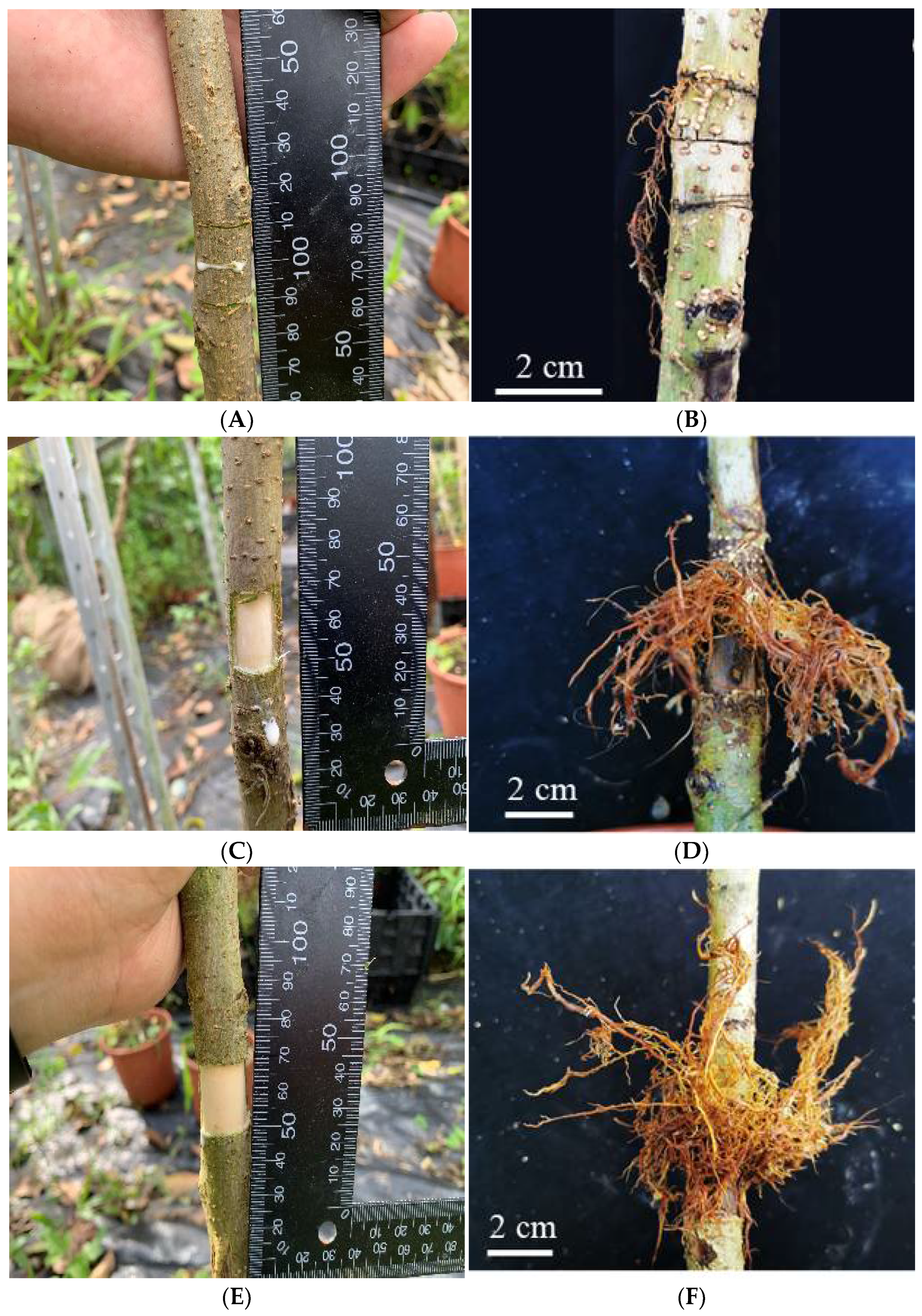
- (1)
- 3LC: three lines were cut on the tree stem deep into the xylem at 60 cm above the ground by a sharp knife. Lines were 1 cm apart, half the length of the stem perimeter, and parallel to each other. The wound width was the thickness of the blade.
- (2)
- RP13: a girdle knife was used to cut a rectangular window into the xylem layer on the stem (approximately 2 × 2 cm) at 60 cm above the ground with a length of one third of the perimeter. The bark on the windows was then peeled off.
2.2. 2020
3. Results
3.1. 2019
3.2. 2020
3.3. AR Distribution in Wounds
4. Discussion
4.1. Wounding Method
4.2. Preformed Root Initials
4.3. Auxin Solution
4.4. Interaction between Wounding and Auxin
4.5. Rooting Locations
4.6. Tree Age and Juvenility
4.7. Application on the Mature Heritage Trees
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Benson, A.; Koeser, A.; Morgenroth, J. A test of tree protection zones: Responses of Quercus virginiana Mill trees to root severance treatments. Urban For. Urban Green. 2019, 38, 54–63. [Google Scholar] [CrossRef]
- Day, S.; Wiseman, E.; Dickinson, S.; Harris, R. Tree root ecology in the urban environment and implications for a sustainable rhizosphere. J. Arboric. 2010, 36, 193–204. [Google Scholar] [CrossRef]
- Allen, K.; Harper, R.; Bayer, A.; Brazee, N. A review of nursery production systems and their influence on urban tree survival. Urban For. Urban Green. 2017, 21, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Mathers, H.M.; Lowe, S.B.; Scagel, C.; Struve, D.K.; Case, L.T. Abiotic factors influencing root growth of woody nursery plants in containers. HortTechnology 2007, 17, 151–162. [Google Scholar] [CrossRef] [Green Version]
- Watson, T. Influence of tree size on transplant establishment and growth. HortTechnology 2005, 15, 118–122. [Google Scholar] [CrossRef] [Green Version]
- ANSI A300 Part 6; Tree, Shrub, and Other Woody Plant Management—Standard Practices (Planting and Transplanting). Tree Care Industry Association, Inc. (TCIA): Manchester, NH, USA, 2012; p. 31.
- Lily, S.J. Arborists’ Certification Study Guide, 7th ed.; International Society of Arboriculture: Altanta, GA, USA, 2010. [Google Scholar]
- Geisler, D.; Ferree, D. Response of plants to root pruning. Hortic. Rev. 1984, 6, 155–188. [Google Scholar]
- Gilman, E.F.; Anderson, P.J. Root Pruning and transplant success for Cathedral Oak® Live Oaks. J. Environ. Hortic. 2006, 24, 13–17. [Google Scholar] [CrossRef]
- Japan Greening Center Foundation (JPGreen). The Newest Tree Doctor Instructions, 3rd ed.; Japan Greening Center Foundation (JPGreen): Tokyo, Japan, 2008; pp. 525–668. [Google Scholar]
- Japan Park and Green Space Association (POSA). Landscaping Construction Management Technology (Japanese) Book; POSA: Tokyo, Japan, 2005; p. 249. [Google Scholar]
- Taipei City Government. Ordinance of Tree Transplant; Taipei City Government: Taipei, Taiwan, 2016. [Google Scholar]
- Lins, L.C.R.; Salomao, L.C.C.; Cecon, P.R.; de Siqueira, D.L. The lychee tree propagation by layering. Rev. Bras. Frutic. 2015, 37, 480–487. [Google Scholar] [CrossRef]
- Gawankar, M.S.; Haldankar, P.M.; Salvi, B.R.; Parulekar, Y.R.; Dalvi, N.V.; Kulkarni, M.M.; Saitwal, Y.S.; Nalage, N.A. Effect of girdling on induction of flowering and quality of fruits in horticultural crops—A review. Adv. Agric. Res. Technol. J. 2019, 3, 201–215. [Google Scholar]
- Kishor, K.; Upreti, B.M.; Pangtey, Y.; Tewari, A.; Tewari, L.M. Propagation and conservation of Himalayan yew (Taxus baccata L.) through air layering: A simple method of clonal propagation. Ann. Plant Sci. 2015, 4, 1064–1067. [Google Scholar]
- Kozlowski, T.T. Carbohydrate sources and sinks in woody plants. Bot. Rev. 1992, 58, 107–222. [Google Scholar] [CrossRef]
- Li, N.; Tu, P.C.; Lo, K.C.; Chang, Y.S. The Induction of adventitious roots regeneration before transplanting rootless Ficus elastica heritage tree. Forests 2020, 11, 1057. [Google Scholar] [CrossRef]
- Matosevich, R.; Cohen, I.; Gil-Yarom, N.; Modrego, A.; Friedlander-Shani, L.; Verna, C.; Scarpella, E.; Efron, I. Local auxin biosynthesis is required for root regeneration after wounding. Nat. Plants 2020, 6, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Overvoorde, P.; Fukaki, H.; Beeckman, T. Auxin control of root development. Cold Spring Harb. Perspect. Biol. 2010, 2, a001537. [Google Scholar] [CrossRef] [Green Version]
- Lucas, M.; Guedon, Y.; Jay-Allemand, C.; Godin, C.; Laplaze, L. An auxin transport-based model of root branching in Arabidopsis thaliana. PLoS ONE 2008, 3, e3673. [Google Scholar] [CrossRef] [Green Version]
- Benková, B.; Michniewicz, E.M.; Sauer, M.; Seifertova, D.; Jurgens, D.; Friml, J. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 2003, 115, 591–602. [Google Scholar] [CrossRef] [Green Version]
- Dubrovsky, J.; Sauer, M.; Napsucialy-Mendivil, S.; Ivanchenko, M.; Frimi, J.; Shishkova, S.; Celenza, J.; Benkova, I. Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proc. Natl. Acad. Sci. USA 2008, 105, 8790–8794. [Google Scholar] [CrossRef] [Green Version]
- Blythe, E.K.; Sibley, J.L.; Ruter, J.M.; Tilt, K.M. Cutting propagation of foliage crops using a foliar application of auxin. Sci. Hortic. 2004, 103, 31–37. [Google Scholar] [CrossRef]
- Gilani, S.; Shah, K.; Ahmed, I.; Basit, A.; Sajid, M.; Bano, A.; Ara, G.; Shahid, U. Influence of indole butyric acid (IBA) concentrations on air layerage in guava (Psidium guajava L.) cv. Sufeda. Pure Appl. Biol. 2019, 8, 355–362. [Google Scholar] [CrossRef]
- Moles, A.; Iagdish, A.; Wu, Y.; Gooley, S.; Dalrymple, D.; Feng, P.; Auld, J. From dangerous branches to urban banyan: Facilitating aerial root growth of Ficus rubiginosa. PLoS ONE 2019, 14, e0226845. [Google Scholar] [CrossRef] [Green Version]
- Alegre, J.; Toledo, J.L.; Martínez, A.; Mora, O.; De Andres, E.F. Rooting ability of Dorycnium spp. under different conditions. Sci. Hortic. 1998, 76, 123–129. [Google Scholar] [CrossRef]
- Al-Saqri, F.; Alderson, P.G. Effects of IBA, cutting type and rooting media on rooting of Rosa centifolia. J. Hortic. Sci. 1996, 71, 729–737. [Google Scholar] [CrossRef]
- Abdulqader, S.; Abdulrhman, A.; Ibrahim, Z. Effect of wounding and different concentration of IBA on the rooting and vegetative growth of stem cutting of three olive cultivars (Olea europaea L.). Kufa J. Agric. Sci. 2017, 9, 203–225. [Google Scholar]
- Mayer, N.A.; Pereira, F.M. Effect of wounds applied to the bases of herbaceous cuttings on the rooting of four Japanese apricot clones (Prunus mume Sieb. et Zucc.) in an intermittent mist system. Acta Hortic. 2004, 658, 655–659. [Google Scholar] [CrossRef]
- Robbins, J.; Kays, S.; Dirr, M. Enhanced rooting of wounded mung bean cuttings by wounding and ethephon. J. Am. Soc. Hortic. Sci. 1983, 108, 325–329. [Google Scholar]
- Geiss, G.; Gutierrez, L.; Bellini, C. Adventitious root formation: New insights and perspectives. Root Dev. 2009, 37, 127–156. [Google Scholar]
- Koyuncu, F.; Balta, F. Adventitious root formation in leaf-bud cuttings of tea (Camellia sinensis L.). Pak. J. Bot. 2004, 36, 763–768. [Google Scholar]
- Igić, D.; Borišev, M.; Vilotić, D.; Šijačić-Nikolić, M.; Ćuk, M.; Ilić, M.; Kovačević, B. Variability and relationships among rooting characteristics for white poplar hardwood cuttings. Arch. Biol. Sci. 2020, 72, 153–163. [Google Scholar] [CrossRef]
- Kovacevic, B.; Igic, D.; Novcic, Z.; Orlovic, S. Survival and growth of white poplar rooted cuttings regarding term of planting. Topola/Poplar 2020, 205, 33–46. [Google Scholar]
- Ahkami, A.H.; Melzer, M.; Ghaffari, M.R.; Pollmann, S.; Javid, M.G.; Shahinnia, F.; Hajirezaei, M.R.; Druege, U. Distribution of indole-3-acetic acid in Petunia hybrida shoot tip cuttings and relationship between auxin transport, carbohydrate metabolism and adventitious root formation. Planta 2013, 238, 499–517. [Google Scholar] [CrossRef] [Green Version]
- Steffens, B.; Rasmussen, A. The physiology of adventitious roots. Plant Physiol. 2016, 170, 603–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, V.R.; Mudry, C.D.; Bettoni, M.M.; Zuffellato-Ribas, K.C. Rooting of stem cuttings of Ficus benjamina L. on different concentrations of indole butyric acid. Sci. Agrar. 2011, 12, 179–183. [Google Scholar]
- Babaie, H.; Zarei, H.; Hemmati, K. Propagation of Ficus benjamina var. Starlight by stenting technique under different concentration of IBA in various time of taking cutting. J. Ornam. Plants 2014, 4, 75–79. [Google Scholar]
- Ghehsareh, M.G.; Khosh-Khui, M. Effect of indole-3-butyric acid, putrescine and benzyl adenine on rooting and lateral bud growth of Ficus elastica Roxb. ex Hornem leaf-bud cuttings. Indian J. Hortic. 2016, 73, 25–29. [Google Scholar] [CrossRef]
- Mewar, D.; Naithani, D.C. Effect of different IBA concentrations and planting time on stem cuttings of wild fig (Ficus pamata Forsk.). Plant Arch. 2016, 15, 959–962. [Google Scholar]
- Shirzad, M.; Sedahathoor, S.; Hashemabadi, D. Effect of media and different concentrations of IBA on rooting of Ficus benjamina L. cutting. J. Ornam. Plants 2015, 2, 61–64. [Google Scholar]
- Siddiqui, M.I.; Hussain, S.A.; Bhande, M.H. Effect of indole butyric acid and types of cuttings on root initiation of Ficus hawaii. Sarhad J. Agric. 2007, 23, 919–926. [Google Scholar]
- Mansour, H.; Khalil, N. Effect of wounding and IBA on rooting of aerial and ground offshoots of date palm (Phoenix dactylifera L. Medjool cultivar). Plant Arch. 2019, 19, 685–689. [Google Scholar]
- Lakehal, A.; Bellini, C. Control of adventitious root formation: Insights into synergistic and antagonistic hormonal interactions. Physiol. Plant. 2019, 165, 90–100. [Google Scholar] [CrossRef] [Green Version]
- Gonin, M.; Bergougnoux, V.; Nguyen, T.D.; Gantet, P.; Champion, A. What makes adventitious roots? Plants 2019, 8, 240. [Google Scholar] [CrossRef] [Green Version]
- Olatunji, D.; Geelen, D.; Verstraeten, I. Control of endogenous auxin levels in plant root development. Int. J. Mol. Sci. 2017, 18, 2587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Klerk, G.J.; van der Krieken, W.; de Jong, J.C. Review the formation of adventitious roots: New concepts, new possibilities. Vitro Cell. Dev. Biol. Plant 1999, 35, 189–199. [Google Scholar] [CrossRef]
- Geiss, G.; Gutierrez, L.; Bellini, C. Adventitious root formation: New insights and perspectives. In Annual Plant Reviews Online; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 127–156. [Google Scholar]
- Heide, O.M. Juvenility, maturation and rejuvenation in plants: Adventitious bud formation as a novel rejuvenation process. J. Hortic. Sci. Biotechnol. 2019, 94, 2–11. [Google Scholar] [CrossRef]
- Bonga, J.M. Vegetative Propagation in Relation to Juvenility, Maturity and Rejuvenation. In Tissue Culture in Forestry; Bonga, J.M., Durzan, D.J., Eds.; Martinus Nijhofl: Boston, MA, USA, 1982; pp. 78–90. [Google Scholar]
- Hackett, W.P. Juvenility, maturation and rejuvenation in woody plants. Hortic. Rev. 1985, 7, 109–155. [Google Scholar]
- Hartmann, H.; Kester, D.; Davies, F., Jr.; Geneve, R. Principles of Propagation by Cuttings. In Hartmann & Kester’s Plant Propagation Principles and Practices, 8th ed.; Pearson Education Limited: Harlow, UK, 2014; pp. 293–359. [Google Scholar]
- Vielba, J.M.; Vidal, N.; Jose, M.C.S.; Rico, S.; Sanchez, C. Recent Advances in Adventitious Root Formation in Chestnut. Plants 2020, 9, 1543. [Google Scholar] [CrossRef]
- Diaz-Sala, C.; Hutchison, K.W.; Goldfarb, B.; Greenwood, M.S. Maturation-related loss in rooting competence by loblolly pine stem cuttings: The role of auxin transport, metabolism and tissue sensitivity. Physiol. Plant. 1996, 97, 481–490. [Google Scholar] [CrossRef]
- Leakey, R.R.B. Physiology of vegetative reproduction. In Encyclopedia of Forest Sciences; Burley, J., Evans, J., Youngquist, J.A., Eds.; Academic Press: London, UK, 2004; pp. 1655–1668. [Google Scholar]
- Dick, J.M.; Leakey, R. Differentiation of the dynamic variables affecting rooting ability in juvenile and mature cuttings of cherry (Prunus avium). J. Hortic. Sci. Biotechnol. 2006, 81, 296–302. [Google Scholar] [CrossRef]
- Treegarden. The Story of #1900 Ficus microcarpa Heritage Tree Transplant. YouTube. 2021. Available online: https://www.youtube.com/watch?v=wKQS9TJwV28&t=106s (accessed on 18 July 2022).
- Treegarden. The Transplant of 4 Heritage Trees at Site of Old Taipei City Council. YouTube. 2021. Available online: https://www.youtube.com/watch?v=kN0zLuhSCvA (accessed on 18 July 2022).


| Treatments z | Wounding Methods y | Auxin Solution | Year | |
|---|---|---|---|---|
| 2019 | 2020 | |||
| Control | None | Water | V | V |
| C03 | 3LC | Water | V | V |
| C13 | RP13 | Water | V | V |
| C23 | RP23 | Water | V | |
| 2B03 | 3LC | 2000 mg·L−1 IBA | V | |
| 2B13 | RP13 | 2000 mg·L−1 IBA | V | V |
| 2B23 | RP23 | 2000 mg·L−1 IBA | V | |
| 2NB03 | 3LC | 2000 mg·L−1 IBA + 2000 mg·L−1 NAA | V | |
| 2NB13 | RP13 | 2000 mg·L−1 IBA + 2000 mg·L−1 NAA | V | V |
| 2NB23 | RP23 | 2000 mg·L−1 IBA + 2000 mg·L−1 NAA | V | |
| 4B03 | 3LC | 4000 mg·L−1 IBA | V | |
| 4B13 | RP13 | 4000 mg·L−1 IBA | V | V |
| 4B23 | RP23 | 4000 mg·L−1 IBA | V | |
| LSD Test | Treatment z | Rooting % | Root Number | Root Length | Dry Weight |
|---|---|---|---|---|---|
| in cm | in mg | ||||
| Significance | p = 0.03 * | p = 0.000 * | p = 0.008 ** | p = 0.001 ** | |
| Control | 0 | 0 | 0 | 0 | |
| C03 | 60 b | 1 b | 5.2 b | 17 c | |
| C13 | 67 ab | 2 b | 10.2 ab | 297 bc | |
| 2B13 | 100 a | 24.8 a | 11.7 a | 284 bc | |
| 2NB13 | 100 a | 23.5 a | 12.9 a | 656 a | |
| 4B13 | 100 a | 21 a | 13.3a | 498 ab | |
| Wounding methods | p = 0.06 ns | p = 0.005 *** | p = 0.000 *** | p = 0.0027 ** | |
| RP13 | 93 a | 30 a | 12.4 a | 457 a | |
| 3LC | 67 a | 6 b | 5.2 b | 17 b | |
| Auxin effect | p = 0.02 ** | p = 0.000 *** | p = 0.02 ** | p = 0.0036 ** | |
| Control | 67 b | 12 b | 7.7 b | 157 b | |
| Auxin | 100 a | 24 a | 12.8 a | 484 a |
| LSD Test | Treatment z | Rooting % | Root Number | Root Length | Dry Weight |
|---|---|---|---|---|---|
| Significance | |||||
| Wounding method | p = 0.58 ns | p = 0.024 * | p = 0.604 ns | p = 0.000 *** | |
| Auxin solution | p = 0.04 * | p = 0.000 *** | p = 0.000 *** | p = 0.86 ns | |
| Interaction | p = 0.27 ns | p = 0.022 * | p = 0.008 ** | p = 0.006 ** |
| LSD Test | Treatment z | Rooting % | Root Number | Root Lengthin cm | Dry Weightin mg |
|---|---|---|---|---|---|
| Significance | p = 0.11 ns | p = 0.000 *** | p = 0.000 *** | p = 0.001 *** | |
| C03 | 83 a | 6 gh | 15 bcde | 1209 bcd | |
| C13 | 83 a | 3 h | 10 g | 761 ef | |
| C23 | 100 a | 8 fg | 13 defg | 813 def | |
| 2B03 | 83 a | 12 def | 17 ab | 1392 ab | |
| 2B13 | 100 a | 19 ab | 17 abc | 1245 abc | |
| 2B23 | 83 a | 21 a | 16 abcd | 1292 ab | |
| 2NB03 | 100 a | 12 def | 11 fg | 846 cdef | |
| 2NB13 | 100 a | 11 ef | 14 cdef | 655 f | |
| 2NB23 | 100 a | 16 abcd | 12 efg | 1210 bcd | |
| 4B03 | 100 a | 16 bcd | 15 bcde | 1285 ab | |
| 4B13 | 100 a | 19 abc | 17 abc | 1642 a | |
| 4B23 | 100 a | 15 cde | 19 a | 1085 bcde |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Chang, Y. Effective Methods for Adventitious Root Regeneration on Weeping Fig Stems. Forests 2022, 13, 1165. https://doi.org/10.3390/f13081165
Li N, Chang Y. Effective Methods for Adventitious Root Regeneration on Weeping Fig Stems. Forests. 2022; 13(8):1165. https://doi.org/10.3390/f13081165
Chicago/Turabian StyleLi, Nelson, and Yusen Chang. 2022. "Effective Methods for Adventitious Root Regeneration on Weeping Fig Stems" Forests 13, no. 8: 1165. https://doi.org/10.3390/f13081165







