Abstract
The objective of this study was to evaluate the long-term effects of piling secondary forest residue (after 3 decades) on soil chemical properties, growth, and nutrition of Pinus taeda and weeds at three locations. After secondary forest removal and residue piling, areas were cultivated with P. taeda (22 years), followed by eucalyptus (7 years), and returned to P. taeda. At 2 years of age, tree height and needle-nutrient levels of ongoing P. taeda from areas influenced by residue piling and areas outside the piling zone were evaluated. Biomass and nutrient levels of herbaceous and shrub weeds, NDVI indices (via a drone), and soil chemistry were also evaluated. Residue-piled areas displayed a decrease in soil pH and an increase in available soil P and K. Although herbaceous and shrub-weed biomass increased 2.5 to 10 times in residue-piling areas, this did not compromise pine growth. While residue piling had little impact on the nutritional status of pine and weeds, NDVI values indicated greater plant growth in piling areas. In general, the long-term effect of residue piling was an important factor associated with the large variation in tree growth and weed incidence after 3 decades.
1. Introduction
The Pinus genus was introduced into Brazil in 1948 by the State of São Paulo Forest Service and has been widely planted with significant expansion in the 1960s [1]. Currently, Pinus plantations represent ~20% of total planted forest area in Brazil, primarily concentrated in the states of Paraná (721.6 th ha) and Santa Catarina (542.4 th ha) [2].
Over time, areas with primary and secondary native vegetation were converted to commercial-forest stands. During forest expansion, the concepts of no-tillage and minimum tillage were not practiced; thus, classic silviculture residue practices [3] used chains to removed native vegetation [4] and accumulated residue pilings were spaced ~40 to 60 m apart [5]. After accumulation, residue converts to necromass, which is composed of different portions of dead vegetation at various stages of decomposition [6]. In most cases, pilings were burned to facilitate seedling establishment [7]. However, burning was not always practiced due to environmental restrictions (Law 4.771/65, old Brazilian Forest Code).
In the absence of burning, decomposition of woody material is generally a slow process [8,9]. The sequence of decompositional processes varies over time due to changes in physical climate and the chemical and physical makeup of wood over the decay cycle [6]. In addition to a constant input of energy and nutrients, this slow decomposition process can impact organic matter addition, habitat for decomposing organisms, water retention during drought periods, and establishment of ecosystem nutrients pools [10]. Several studies have evaluated median- to long-term residual effects of nutrients added as industrial residue by-products or fertilizer to agroforestry systems, especially in regards to nutrient cycling [11,12,13,14].
Nutrients added to soil from the decay of residue pilings depend on nutrient concentrations and biomass amounts. Tropical and subtropical forests can have more than 200 t ha−1 of biomass consisting of timber, branches, bark, and foliage [15] and can accumulate large amounts of N, Ca, K, Mg, P, and other nutrients aboveground. Nutrients added by deforestation can be estimated from amounts found in standing biomass; this can be substantial for some nutrients. A subtropical forest in southern Brazil with 232 t ha−1 of biomass had 1626, 1620, 1364, 224, 115, and 103 kg ha−1 of K, N, Ca, Mg, S, and P, respectively [16]. Similar results were reported by others, where 210 t ha−1 of biomass contained 1929, 1515, 926, 386, 275, and 80 kg ha−1 for Ca, N, K, Mg, S, and P, respectively [17]. Clearly, quantities can vary across sites. When pilings are burned, nutrient amounts can differ due to losses via volatilization or fly ash powder [18,19]; residue type/quantity and environmental conditions can influence nutrient-loss variability. Loss of N and S can be high, with some authors reporting most N and S being lost [20,21], while others reported ~50% lost [22]. However, losses of P, Ca, and K are generally less than 40% [20,21,22].
Destruction of organic structures by burning can accelerate the release of nutrients remaining in ash [7], while unburned residue pilings can result in the very slow release of some nutrients, especially nutrients contained in timber. Although residue burning has been reported to decrease soil acidity [8,23] and increase the availability of some nutrients (Ca, K, Mg, sulfate, and nitrate) [8], soil organic matter levels have exhibited no change [23] or increases [8]. Effects of forest slash pilings on nutrient deposition and tree growth were reported by [24] for a 22-month-old Eucalyptus dunnii forest in southern Brazil. These authors reported 7.2 and 4.1 m tree heights inside and outside piling areas, respectively. In addition, they found higher foliar N and P and higher soil P and K inside piling areas.
While weeds can also benefit from addition of nutrients to forest systems [25,26], the response can differ among herbaceous and shrub weeds [26]. In one year of a three-year evaluation, [27] found an influence of fertilization on total vegetation soil coverage, but no differences among vegetation groups (i.e., graminoids, forbs, ferns, woody plants, and woody vines). There are reports that fertilization and weed control has an additive effect on Pinus growth in North America [28,29,30]. However, no additive effect was found in Argentina, where only weed control enhanced P. taeda yield [31]. Others found that weed control during initial growth stages of Pinus greatly enhanced seedling height (59%) and diameter (103%) [32,33]. Lack of weed control prior to planting resulted in a ~60% reduction in P. taeda biomass accumulation during the first year [32]. Another observation showed that weed control conducted over three periods resulted in significant differences in Pinus growth; the average height difference (relative to the control) was 16% or 0.88 m [34]. Others have reported that increased weed growth with fertilizer application resulted in increased mortality of pine seedlings [26].
Studies that have evaluated the long-term effects of necromass accumulation on the soil properties, growth, and nutrition of P. taeda and interaction with weeds are sparse. We hypothesized that the great variability in tree growth observed by satellite imagery was an artifact of piling residue without burning, which resulted in enhanced soil properties and tree growth. This information can be of great importance to foresters seeking to adopt management practices that increase soil nutrition, reduce negative environmental effects, and guarantee forest productivity and sustainability. Thus, the aim of this study was to survey the long-term effects (after 30 years) on soil chemical properties and growth and nutrition of Pinus taeda and weeds by piling secondary-forest residues.
2. Materials and Methods
2.1. General Caracterization
The study site was located in the Rio Negrinho municipality of Santa Catarina, Brazil; this was a commercial-forest area managed by the pulp industry (Companhia Volta Grande Papel). The region was Cfb (Köppen classification) and had a humid temperate climate with summer temperatures around 16.5 °C and uniformly distributed annual precipitation of 1600 mm [35]. This plateau region was located at geographic coordinates of 26.499984 S and 49.541919 W (area center) with altitudes of 950 to 980 m. Shale is the parent material for the study sites soil that displayed strong acidic reactions, low base sums and saturation, and high levels of organic carbon and Al, which are reflective of a humid, cold climate that favors intense leaching of soils [36].
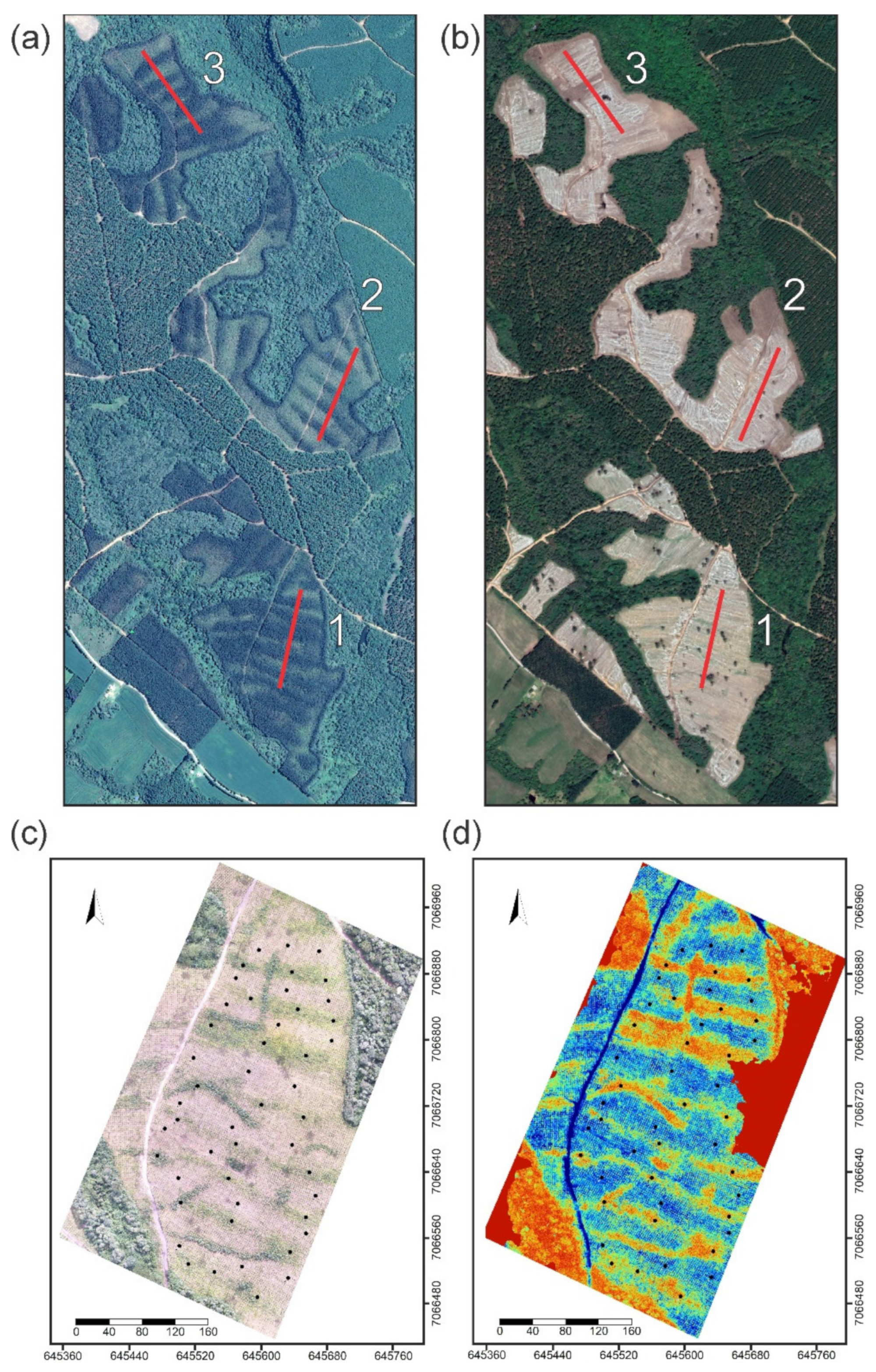
Three sites identified by the commercial-forest company were used for this study (Figure 1a). These sites historically displayed large spatial variability in tree growth as result of initial piling management. These sites were initially managed by piling secondary-forest residues (without burning) prior to commercial-forest planting. The first commercial planting used P. taeda (22-year cycle, with two thinnings at 7 and 14 years, without fertilization and liming; space 2.5 m × 2.5 m spaces). Then, Eucalyptus (7-year cycle) was planted (3.0 × 2.5 m) for biomass production and received only fertilizer, receiving 30, 120, and 45 kg/ha of N, P2O5, and K2O at planting and 16 and 48 kg/ha of N and K2O kg/ha of sidedressing, respectively. Due to very low eucalyptus yields, the areas were replanted with P. taeda on 2.5 by 2.5 m spacings. Study samplings were conducted when trees were 2 years old. The three study sites also received a broadcast rate of 20 Mg ha−1 of paper residue in 2016 (Figure 1b) and had the following chemical properties: pH—8.6; C—238 g/kg; N—0.3 g/kg; S—0.4 g/kg; Ca—150 g/kg; P—2.3 g/kg; Mg—1.7 g/kg; K—0.14 g/kg; Fe—2480 mg/kg; Zn—265 mg/kg; Mn—44.3 mg/kg; and Cu—49 mg/kg. Approximately 31 years had passed since initial forest clearing, before areas were assessed for long-term impacts of residue piling.
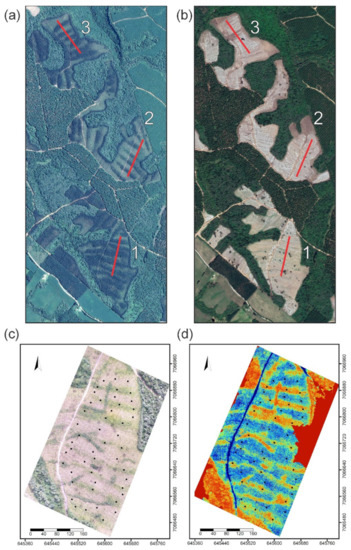
Figure 1.
Satellite image of three sites on 8 February 2014 before eucalyptus harvest (a); satellite image of Pinus taeda on 5 December 2016 after paper-residue application (b); DRONE (c) and NDVI (d) imagery of the third site at sampling on 1 April 2018. 1;2;3—Transect lines are shown in red (a,b).
Google Earth satellite images of the three sites were used to check for variation in tree growth. Historic images of the previous eucalyptus forest identified the occurrence pale green and dark green strips; residual effects of residue piling were associated with the dark green areas (Figure 1a).
2.2. Experimental Design
The experimental design was an adaptation of the Linear Intercept Sampling (LIS) method developed by Candfield in 1941 [37] that is based on measuring plants intercepting horizontal transects [38]. The length and width of transects are adjustable according to research interests [39]. This method is typically used in biodiversity and ecology work focused on studying plant communities and structures. This method is particularly useful for assessing transition gradients between communities [39]. Although our work does not focus on biodiversity or ecology, the existence of gradients (soil and plant cover) supports the use of this method as being appropriate to the research. Three transect lines measuring 300 m length intersected 5 residue piling lines (Figure 1a,b). Transects were initiated from the base of a line of trees; starting points were randomly selected and tree height measurements and soil samples (two depths) were systematically sampled every 7.5 m (Figure 1c). Samples were collected regardless of whether or not the collection point fell in the residue-piling area.
2.3. Plant Data Collection
Transect lines were established in order to collect soil and tree parameters (height and foliar tissue samples) outside (OP) or inside (IP) the influence of piling in each area of study. Transects were composed of three 300 m lines that transposed five piling areas and five outside areas. Lines crossing the transects were systematically established every 7.5 m along a 300 m transect; this resulted in 41 crossing lines with each having five associated trees (Figure 1a,b). At each of the 41 crossing lines, heights of individual pines were measured using a stretch ruler (total of 205 trees measurements). Mean height was calculated using heights of the five trees at each crossing line. At the time of tree height determination, 4 branches were collected from the upper third of each tree canopy to form one sample composited of 20 branches (5 trees × 4 branches per tree) per crossing line. Needles were sampled from branches based on methods of [40], where newly mature needles were collected (usually the penultimate needle flush of the last 12 months). After collection, needle samples were dried (60 °C) for ~7 days and 100 needles from each sample were weighed on a precision scale.
It is important to note that a satellite image was used as guide to establish transects and piling-area locations. Soil properties and the abundance of weeds and residue were used to indicate if the crossing line stayed in the piling area or outside the piling area. Each of the 41 crossing lines were classified as outside (OP) or inside (IP) the piling areas according to its position relative to piling areas. This sampling methodology resulted in unbalanced data for soil and tree parameters (OP n = 81; IP n = 42).
Using the 300 m transect from previously tree and soil sampling, one month after collecting pine height and branches, weeds samples were collected. Weeds were separated into two categories: herbaceous plants, considering all flexible stem species; and shrub weeds, represented by “wild tobacco” (Solanum mauritianum Scop.), since it was by far the most abundant shrub weed in study site. In meadow areas (OP and IP), 56 m2 sample areas were demarcated for weed collection; five OP and five IP samples areas were in each site.
To assess herbaceous weeds, a 0.5 × 0.5 m template was randomly placed inside the demarcated 56 m2 sample areas, and all herbaceous plants within the template were collected, by cutting ~5 cm above the soil surface, and placed into plastic bags. This procedure was repeated four times per sample area, for a total of 40 herbaceous weed samples (5 OP × 4 samples and 5 IP × 4 samples). After herbaceous sampling, the number of “wild tobacco” plants inside the 56 m2 area was counted, and 4 representative plants were cut 5 cm above the soil surface and placed in separated plastic bags for a total of 40 shrub weed samples (5 OP × 4 samples and 5 IP × 4 samples). Harvested plants were stored in plastic bags for transport back to the laboratory. Wild tobacco was separated into leaves and stems for green-weight measurements. All samples were transfer to paper bags and dried in a forced-air-circulation oven (65 °C) for one week. Dried materials were weighed to obtain total dry mass. The total number of samples for OP and IP were the same (balanced).
Dry needles, herbaceous weeds, and wild tobacco leaves were ground in a Wiley mill. Ground plant tissues were ashed (500 °C) and solubilized by HCl, prior to determination of P, K, Ca, Mg, Cu, Fe, Mn, and Zn by an Optical Emission Spectrophotometer equipped with Plasma Induction (ICP-OES) (Varian, 720-ES—Mulgrave, Victoria, Australia).
2.4. Soil Sample Collection
Along each transect (as describe above), five soil samples (0–20-cm and 20–40-cm depths) associated with the five trees measured for height were collected using a standard soil probe and mixed, totaling 82 soil samples for site (41 crossing line × 2 depths). After collection, soil samples were dried (40 °C) for ~4 days. Afterwards, soil was ground to pass a 2 mm sieve and analyzed for pH in 0.01 M CaCl2 (1:2.5 soil:solution), Al, Ca, Mg, K, P, Zn, Mn, Fe, Cu, and C; 1 M KCl was used for extraction of Al, Ca, and Mg; and Mehlich I (0.05 M HCl and 0.0125 M H2SO4) was used for K, P, and micronutrients. Total C and N were determined using a Perkin-Elmer CHNS/OPEZ 400 series Elemental Analyzer. Based on granulometry testing, soil from the sites was classified as clay loam with 385 and 400 g kg−1 of clay and 380 and 370 g kg−1 of silt for the 0–20- and 20–40-cm depths, respectively.
2.5. NDVI Collection
Drone images were obtained to evaluate the piling effect at the three selected sites. After image acquisition, the Normalized Difference Vegetation Index (NDVI) was determined for OP and IP areas (Figure 1). The drone was a Phantom 4 (DJI) quad-copter (four-propeller) equipped with a 4K-resolution camera that captured high spatial-resolution images. Flight settings (sensors, camera, GPS, etc.) were defined in the DJI app. For the present study, autonomous flight was performed (i.e., previously programmed flight plan) using the Drone Deploy application. The flight occurred on 1 April 2018 under favorable weather conditions (no rain, with wind speeds less than 20 km h−1). Flight preparation was previously performed using the Drone Deploy application and a delimited polygon around the study area. Within the application, flight height (120 m) and lateral (60%) and longitudinal (90%) overlap between images were selected to produce an image with a spatial resolution of ~4 cm.
The Mapir Survey 3 camera coupled to the drone captured infrared wavelengths. Field targets with known coordinates were used for image adjustment. After image acquisition and adjustment, NDVI was calculated from red (R) and infrared (IR) bands, according to the following equation:
2.6. Data Analysis
Soil and plant data associated with OP and IP areas were compared using the t-test. Each of the three evaluated sites was tested individually. Statistical analyses were performed using R software version 4.2.0 (Vienna, Austria).
3. Results and Discussion
3.1. Soil
The soil had very low (<4.0) to low (4.0–4.4) pH with high values of Al (>2.5 cmolc dm−3) common to weathered soils of southern Brazil cultivated with pine [41]. Acidity parameters indicated pH change between the OP and IP areas, with lower values for IP areas at 0–20 cm (site 3) and 20–40 cm (sites 1 and 2). The IP areas also displayed increased Al at sites 1 and 3 in the 20–40-cm layer (Table 1). High values of Al indicated increased buffering power likely associated with more total soil C.

Table 1.
Soil chemical analysis of two soil layers collected outside (OP) and inside (IP) piling areas in a two-year-old Pinus taeda forest at three sites in southern Brazil. Bolded text indicates overall site average or averages across depths within OP or IP areas.
Our results differed from those normally observed with pile burning, where pH generally increases (short- or long-term) due to the addition of ash-containing bases in the form of alkaline oxides, alkaline earth oxides, and carbonates [42,43]. The addition of residues can increase soil pH, when there are higher levels of bases in relation to anions [44]. In our study, the decrease in pH may be related to the maintenance of N and S anions from a lack of residue burning [45]. Furthermore, added residue was likely high in N, which is common in secondary forests in regions with a predominance of native Fabaceae (Leguminosae) species such as Bracatinga (Mimosa scabrella Benth.). Since our soil had high buffering power, large amounts of acidic components were necessary for pH change. In contrast to our study, [8,23] found an enhancement of pH. However, high correlations of increased soil pH with net N mineralization and net nitrification of forest residues indicate that this aspect requires further investigation [23].
The accumulation of residue in pilings led to a trend for increased C and N at sites 1 and 2 and an increase in the 20–40-cm layer at site 3 (Table 2), as seen for Al at this depth. In contrast, [23] found no organic matter change in the 5-cm layer. Others have reported that residue piling could increase decomposition in the organic horizon, while possibly enhancing organic-matter inputs via decomposition or from improved root and mycorrhizae colonization [46]. High values in lower soil layers may be indicative of high biological activity, which is well-known to increase beneath residue pilings [10]. In addition, significant bioturbation activity has been reported in pine plantations of southern Brazil [47].

Table 2.
Nitrogen and carbon in two soil layers collected outside (OP) and inside (IP) piling areas in a two-year-old Pinus taeda forest at three sites in southern Brazil.
According to state recommendations for fertilizer and lime additions, Ca levels were high [48]. Calcium is the first- or second-most-abundant element found in the native biomass of subtropical forests [17]. While some have indicated that Ca can be the element showing the greatest increase in piling areas (with or without burning) [8,42], others did not report an increase in Ca availability due to piling and burning [42]. In our case, availability of Ca was not influenced by the residual effects of piling (Table 1), since Ca-rich alkaline residue (a forest industry by-product) was applied before planting. In addition, previously planted eucalyptus may have exported significant amounts of Ca (i.e., 270 kg of Ca for 100 t of trunk [49]).
Unlike Ca, Mg values (<0.2 cmolc dm−3) were well below the ideal range of 0.2–0.4 cmolc dm−3, which confirms the low availability observed in soil [48] and the low concentration in the applied by-product residue (Table 1). Availability of Mg may have been affected by the accumulation of necromass in pilings, since levels were slightly higher in the 0–20-cm soil layer at site 1. However, this increase was not sufficient to attain a medium level (0.5–1.0 cmolc dm−3).
Differences were even greater for available K, given the high additions and efficient maintenance in the system. Under some conditions, K is the most abundant [16] or the third-most abundant [17] nutrient cation in native forest biomass. The greatest effects were observed in the upper soil layer, likely due to nutrient cycling in pilings. Differences were observed for the 0–20-cm soil layer at sites 2 and 3 and for the 20–40-cm soil layer at site 1 (Table 1). Effects on base maintenance, as a function of piling, have been reported with and without burning [8,42,43,50]. Decade-long maintenance of K has also been reported under forest conditions [11,43].
Piling increased availability of P in the 0–20-cm layer at all sites and in the 20–40-cm layer at site 3 (Table 1), which is very significant since P has a high capacity for adsorption with low pH and an abundance of exchangeable Al [51,52]. Phosphorus has also been shown to be the element most limiting pine growth in Brazil [14,53,54,55] and several other countries [56]. In addition, residue piling enhanced extractable Fe, Mn, and Cu, which could be related to nutrient addition from residues and/or pH decreases.
3.2. Plant Growth and NDVI
Increased P. taeda height at the second and third sites indicated that pilings induced better growth conditions and soil chemical properties after 30 years (Table 3). Residual effects of piling were clearly seen with the biomass of IP herbaceous weeds increasing by 3.2, 2.5, and 2.7 times at the first, second, and third sites, respectively (Table 4). Larger variations were observed for the shrub S. mauritianum, primarily at the first site. A statistical comparison was not possible for the third site, since S. mauritianum only occurred in pilings. Overall, weeds showed greater sensitivity to residual effect of pilings compared to pine.

Table 3.
Tree height (m) and NDVI obtained outside (OP) and inside (IP) piling areas in a two-year-old Pinus taeda forest at three sites in southern Brazil.

Table 4.
Biomass of herbaceous and shrub (wild tobacco, Solanum mauritianum) weeds growing outside (OP) and inside (IP) piling areas in a two-year-old Pinus taeda forest at three sites in southern Brazil.
Increased growth of pine and weeds may be due to increased availability of soil P and K (Table 1), since these nutrients have been reported to play an important role in weed growth [57,58]. In a longleaf pine (Pinus palustris P. Mill.) establishment study, [26] reported that fertilization increased herbaceous (1467 to 1958 kg ha−1) and shrub (85 to 156 kg ha−1) weed mass; however, after two years, they found no difference between fertilized and non-fertilized areas. Other studies have reported more than a 50% reduction in Pinus ssp. growth parameters with no weed control [32,33,59]. In our study, a large decrease in pine growth was expected at this early growth stage, but this did not occur. Pines appeared to overcome weed competition and exhibited satisfactory growth, probably due to improved soil chemical properties combined with favorable climatic conditions, abundance of well-distributed rain, and deep soil that can help eliminate competition for soil water.
Increased plant growth due to residue piling has also been reported for eucalyptus in Brazil [24] and Douglas fir [60]. The last authors reported greater tree growth and better competition with weeds close to pilings. Furthermore, they indicated that residue management (piling and dispersion) could reduce the impact of invasive plants on cultivated forests. In regards to Pinus, weed control for three periods resulted in significant differences in growth in relation to areas with no control [34]; the average difference was 16.4% (corresponding to 0.88 m).
The NDVI is considered an important tool for evaluating plant physiological status, where values close to 1 suggest good nutritional state and values between 0 and 0.33 suggest plant nutritional issues [61]. The higher NDVI values obtained in pilings indicate that this was a good tool for assessing the growth of pine/weeds. High NDVI was also observed at site 1, where no effect of piling on pine growth was found, suggesting that weeds contributed to higher NDVI. This confirms previous observations regarding the NDVI’s sensitivity in verifying a greater effect of weed growth over the main target plant [62,63].
Increases in total C in soil and weed biomass, without compromising tree growth, can be considered in relation to C sinks. This accumulation can be a direct effect of residue accumulation or an indirect effect by input additions from enhanced tree and weed growth. Establishment of forests is a major way to mitigate climate change, since these systems have been indicated to absorb 45% of gases released by human activity and constitute 85% to 90% of terrestrial biomass [64].
3.3. Plant Nutrition
All nutrient concentrations in pine needles were within ranges considered adequate (Table 5), except for Mg (third site), which agrees with low available values observed in soil. This may also be related to high available Ca, which can result in antagonistic interactions [55]. Low Mg levels have been reported in soil and plants in different Pinus ssp. regions [41,55,65,66,67]. On a clayey soil in the same region as our study, [68] found needle Mg concentrations below the critical level for trees that had received an application of an alkaline residue.

Table 5.
Foliar nutrient concentrations assessed outside (OP) and inside (IP) piling areas in a two-year-old Pinus taeda forest at three sites in southern Brazil.
Results indicated differences in Ca and Fe leaf concentrations, with lower values found in pilings at the second and third sites, respectively. This was unexpected, since no increase in Ca availability was found due to accumulation of necromass in pilings. Decreases in concentration in these two areas were associated with increased tree height in the pilings, suggesting a possible dilution effect. Furthermore, a trend towards higher K values at these sites may have also contributed to this effect. Increases in K were expected as a function of the increases in seen soil.
Assessment of herbaceous plants indicated much higher K values in piling areas (Table 6), which followed observed increases in soil availability and a trend in needle tissue. In addition, there were trends for increased P, Mn, and Zn in pilings. Greater availability of soil P only led to the trend for P increase in tissue. In contrast, lower values of Mg, Fe, Ni, and Al were found in at least one of the three sites evaluated. Lower Mg values may be related to increases in K. On the other hand, the lower values of toxic Al may be indicative of greater organic matter influence in piling areas.

Table 6.
Elemental concentration from total biomass of herbaceous weeds collected outside (OP) and inside (IP) piling areas in a two-year-old Pinus taeda forest at three sites in southern Brazil.
For wild tobacco, differences were only observed for Ca, with much lower values found in pilings (Table 7). This was probably associated with a dilution effect resulting from increased growth. In contrast, a trend for increased Zn, Cu, and Ba was observed.

Table 7.
Foliar elemental concentrations of wild tobacco (Solanum mauritianum) collected outside (OP) and inside (IP) piling areas in a two-year-old Pinus taeda forest at three sites in southern Brazil.
4. Conclusions
Large variations in tree growth can be traced to pilings from management practices that occurred more than 3 decades previously. A large residual effect of piling was observed on weed growth (herbaceous and shrub weeds) after 30 years, which did appear to limit growth of 2-year-old pine seedlings. Findings suggest that soil improvements outweighed the deleterious effects of weed competition on pine seedlings. Improvements in the P and K availability were observed even after 30 years. Total soil carbon was higher in piling areas due to direct or indirect factors. The large heterogeneity in tree growth in this area could be traced to the long-term effects of residue-piling management used in the establishment of this forest system.
Author Contributions
A.R.S.Z.—conceptualization, methodology, software, validation, formal analysis, investigation, data curation, writing—original draft preparation; G.Q.P.—formal analysis, investigation, writing—original draft preparation; L.F.G.—writing—original draft preparation, writing—review and editing; S.M.—conceptualization, investigation, resources, writing—review and editing, visualization, funding acquisition; M.V.M.B.—writing—original draft preparation, writing—review and editing, visualization; E.M.—conceptualization, methodology, software, validation, formal analysis, data curation, writing—review and editing, visualization; S.A.P.—data curation, methodology, writing—review and editing, visualization; L.C.d.P.S.—conceptualization, methodology, software, validation, formal analysis, data curation; J.C.d.O.J.—conceptualization, methodology, software, validation, formal analysis, writing—review and editing; A.C.V.M.—conceptualization, methodology, validation, investigation, data curation, writing—review and editing, supervision, project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Council for Scientific and Technological Development (CNPq) for financial support and by the Coordination for the Improvement of Higher Education Personnel (CAPES) for scholarship financial support.
Acknowledgments
The authors thank the Cahdan Volta Grande Brazilian paper companies and staff (Forest Eng. Daniel Maros) for field-work support. The authors thank Carla G. Albuquerque, Fabiana Gavelaki, and Henrique A. S. Ducheiko of the UFPR for laboratory analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sampaio, K.R.M. Análise do Desenvolvimento de Pinus taeda L. Através de Diferentes Técnicas de Plantio na Região de Piraí do Sul-PRI; Completion of Course Work; Universidade Federal do Paraná: Curitiba, Brazil, 2014. [Google Scholar]
- Indústria Brasileira de Árvores–IBÁ. Relatório Anual: Ano Base 2020; IBÁ: Brasília, Brazil, 2021. [Google Scholar]
- Gonçalves, J.L.M. Principais solos usados para plantações florestais. In Conservação e Cultivo de Solos Para Plantações Florestais; Gonçalves, J.L.M., Stape, J.L., Eds.; IPEF: Piracicaba, Brazil, 2002; Volume 1, pp. 1–46. [Google Scholar]
- Fonseca, S.M. Preparo de Solo Para Implantação de Florestas; ESALQ: Piracicaba, Brazil, 1978; pp. 1–30. [Google Scholar]
- Balloni, E.A.; Simões, J.W. Implantação de Povoamentos Florestais com Espécies do Gênero Eucalyptus; Circular técnica, n. 60; IPEF: Piracicaba, Brazil, 1979; pp. 1–14. [Google Scholar]
- Palace, M.; Keller, M.; Hurtt, G.; Frolking, S. A review of above ground necromass in tropical forests. In Tropical Forests; Sudarshana, P., Nageswara-Rao, M., Soneji, J.R., Eds.; Intech: London, UK, 2012; pp. 215–252. Available online: http://www.intechopen.com/books/tropical-forests (accessed on 3 June 2022).
- Dietrich, S.T.; Mackenzie, M.D. Comparing spatial heterogeneity of bioavailable nutrients and soil respiration in boreal sites recovering from natural and anthropogenic disturbance. Front. Environ. Sci. 2018, 6, 126. [Google Scholar] [CrossRef]
- Gotou, J.I.; Nishimura, T. Pile movement and nutrient distribution in the soil around slash piles produced during whole-tree logging by a yarder and a processor. J. For. Res. 2002, 7, 179–184. [Google Scholar] [CrossRef]
- Gama-Rodrigues, A.C.; Barros, N.F. Ciclagem de nutrientes em floresta natural e em plantios de eucalipto e de dandá no sudoeste da Bahia, Brasil. Rev. Árvore 2002, 26, 193–207. [Google Scholar]
- Stevenson, R.J. Scale-dependent determinants and consequences of benthic algal heterogeneity. J. N. Am. Bethological Soc. 1997, 16, 248–262. [Google Scholar] [CrossRef]
- Shepard, J.P.; Mitchell, M.J. Nutrient cycling in a red pine plantation thirty-nine years after potassium fertilization. Soil Sci. Soc. Am. J. 1990, 54, 1433–1440. [Google Scholar] [CrossRef]
- Bakker, M.R.; Jolicoeur, E.; Trichet, P.; Augusto, L.; Plassard, C.; Guinberteau, J.; Loustau, D. Adaptation of fine roots to annual fertilization and irrigation in a 13-year-old Pinus pinaster stand. Tree Physiol. 2009, 29, 229–238. [Google Scholar] [CrossRef]
- Sass, A.L.; Bassaco, M.V.M.; Motta, A.C.V.; Maeda, S.; Barbosa, J.Z.; Bognola, I.A.; Gomes, J.B.V.; Goularte, G.D.; Prior, S.A. Cellulosic industrial waste to enhance Pinus taeda nutrition and growth: A study in subtropical Brazil. Sci. For. 2020, 48, e3165. [Google Scholar] [CrossRef]
- Consalter, R.; Barbosa, J.Z.; Prior, S.A.; Vezzani, F.M.; Bassaco, M.V.M.; Pedreira, G.Q.; Motta, A.C.V. Mid-rotation fertilization and liming efects on nutrient dynamics of Pinus taeda L. in subtropical Brazil. Eur. J. For. Res. 2009, 140, 19–35. [Google Scholar] [CrossRef]
- Alves, L.F.; Vieira, S.A.; Scaranello, M.A.; Camargo, P.B.; Santos, F.A.; Joly, C.A.; Martinelli, L.A. Forest structure and live aboveground biomass variation along an elevational gradient of tropical Atlantic moist forest (Brazil). For. Ecol. Manag. 2010, 260, 679–691. [Google Scholar] [CrossRef]
- Caldeira, M.V.W. Determinação de Biomassa e Nutrientes em uma Floresta Ombrófila Mista Montana em General Carneiro, Paraná. Ph.D. Thesis, Agricultural Sciences Sector, Federal University of Paraná, Curitiba, Brazil, 2003. [Google Scholar]
- Vogel, H.L.M.; Spathelf, P.; Schumacher, M.V.; Trüby, P. Biomass, Litterfall and nutrients of tree species in a native forest in South Brazil. Austrian J. For. Sci. 2008, 125, 157–182. [Google Scholar]
- Knoepp, J.D.; Debano, L.F.; Neary, D.G. Soil chemistry. In Wildland Free in Ecosystems: Effects of Fire on Soil and Water; Neary, D.G., Ryan, K.C., Debano, L.F., Eds.; Forest Service, Rocky Mountain Research Station, US Department of Agriculture: Fort Collins, CO, USA, 2005; Volume 4, pp. 53–71. [Google Scholar]
- Massman, W.J.; Frank, J.M.; Mooney, S.J. Advancing investigation and physical modeling of first-order fire effects on soils. Fire Ecol. 2010, 6, 36–54. [Google Scholar] [CrossRef]
- Heard, J.; Cavers, C.; Adrian, G. Up in smoke-nutrient loss with straw burning. Better Crops 2006, 90, 10–11. [Google Scholar]
- Kauffman, J.B.; Sanford Junior, R.L.; Cummings, D.L.; Salcedo, I.H.; Sampaio, E.V.S.B. Biomass and nutrient dynamics associated with slash fires in neotropical dry forests. Ecology 1993, 74, 140–151. [Google Scholar] [CrossRef]
- Kauffman, J.B.; Cummings, D.L.; Ward, D.E.; Babbitt, R. Fire in the Brazilian Amazon: Biomass, nutrient pools, and losses in slashed primary forests. Oecologia 1995, 104, 397–408. [Google Scholar] [CrossRef]
- Törmänen, T.; Kitunen, V.; Lindroos, A.J.; Heikkinen, J.; Smolander, A. How do logging residues of different tree species affect soil N cycling after final felling? For. Ecol. Manag. 2018, 427, 182–189. [Google Scholar] [CrossRef]
- Reissmann, C.B.; Abrao, H.R.S.; Brito, M.A.R. Efeito da Leira no crescimento, resistência a geada e níveis de N, P e K em Eucalyptus Dunnii, Maiden. Rev. Set. Ciências Agrárias 1991, 11, 121–125. [Google Scholar]
- Woods, P.V.; Nambiar, E.K.S.; Smethurst, P.J. Effect of annual weeds on water and nitrogen availability to Pinus radiata trees in a young plantation. For. Ecol. Manag. 1992, 48, 145–163. [Google Scholar] [CrossRef]
- Haywood, J.D. Influence of herbicides and felling, fertilization, and prescribed fire on longleaf pine establishment and growth through six growing seasons. New For. 2007, 33, 257–279. [Google Scholar] [CrossRef]
- Knapp, B.O.; Walker, J.L.; Wang, G.G.; Hu, H.; Addington, R.N. Effects of overstory retention, herbicides, and fertilization on sub-canopy vegetation structure and functional group composition in loblolly pine forests restored to longleaf pine. For. Ecol. Manag. 2014, 320, 149–160. [Google Scholar] [CrossRef]
- Neary, D.G.; Klopatek, C.C.; DeBano, L.F.; Ffolliott, P.F. Fire effects on belowground sustainability: A review and synthesis. For. Ecol. Manag. 1999, 122, 51–71. [Google Scholar] [CrossRef]
- Jokela, E.J.; Martin, T.A. Effects of ontogeny and soil nutrient supply on production, allocation, and leaf area efficiency in loblolly and slash pine stands. Can. J. For. Res. 2000, 30, 1511–1524. [Google Scholar] [CrossRef]
- Jokela, E.J.; Martin, T.A.; Vogel, J.G. Twenty-five years of intensive forest management with southern pines: Important lessons learned. J. For. 2010, 108, 338–347. [Google Scholar] [CrossRef]
- Schulte, M.L.; Cook, R.L.; Albaugh, T.J.; Allen, H.L.; Rubilar, R.A.; Pezzutti, R.; Caldato, S.L.; Campoe, O.; Carter, D.R. Mid-rotation response of Pinus taeda to early silvicultural treatments in subtropical Argentina. For. Ecol. Manag. 2020, 473, 118317. [Google Scholar] [CrossRef]
- Kogan, M.; Figueroa, R. Control de malezas herbáceas en primer y segundo año de desarrollo del Pinus radiata D. In Don. Agroanálisis “Edición Forestal”; Santiago, Chile, 1997; pp. 14–18. [Google Scholar]
- Cantarelli, E.B.C.; Machado, S.L.O.; Costa, E.C.; Pezzutti, R. Efeito do manejo de plantas daninhas no desenvolvimento inicial de Pinus taeda em várzeas na Argentina. Rev. Árvore 2006, 30, 711–718. [Google Scholar] [CrossRef]
- Pezzutti, R.V.; Caldato, S.L. Efecto del control de malezas em el crecimiento de plantaciones de Pinus taeda, Pinus elliottii var. elliottii y Pinus elliottii var. elliottii x Pinus caribaea var. Hondurensis. Bosque 2004, 25, 77–87. [Google Scholar]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Moraes Gonçalves, J.L.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Almeida, J.A.; Freitas Ribeiro, C.; Ribeiro de Oliveira, M.V.; Sequinatto, L. Mineralogia da argila e propriedades químicas de solos do Planalto Norte Catarinense. Rev. Ciências Agroveterinárias 2018, 17, 267–277. Available online: https://revistas.udesc.br/index.php/agroveterinaria/article/view/8815 (accessed on 27 January 2022). [CrossRef]
- Corrow, A.L. Double Sampling for Coarse Woody Debris Estimations following Line Intersect Sampling. Master’s Thesis, Western Washington University, Bellingham, WA, USA, 2007. [Google Scholar]
- Coulloudon, B.; Eshelman, K.; Gianola, J.; Habich, N.; Hughes, L.; Johnson, C.; Pellant, M.; Podborny, P.; Rasmussen, A.; Robles, B. Sampling Vegetation Attributes: Interagency Technical Reference, 2nd ed.; USDI Bureau of Land Management National Applied Resource Sciences Center: Denver, CO, USA, 1999; pp. 1–163. [Google Scholar]
- Garcia, P.O.; Lobo-Faria, P.C. Metodologias para Levantamentos da Biodiversidade Brasileira. Available online: http://www.acszanzini.net/DISCIPLINAS_2012/ARDB%202012%20-1%20TXT/METODOLOGIA%20PARA%20LEVANTAMENTOS%20DA%20BIODIVERSIDADE%20BRASILEIRA.pdf (accessed on 9 January 2022).
- Gonçalves, J.L.M. Recomendações de adubação para Eucalyptus, Pinus e espécies típicas da mata atlântica. Doc. Florestais ESALQ 2005, 15, 1–23. [Google Scholar]
- Rodrigues, A.N.A.; Motta, A.C.V.; Melo, V.F.; Goularte, G.D.; Prior, S.A. Forms and buffering potential of aluminum in tropical and subtropical acid soils cultivated with Pinus taeda L. J. Soils Sediments 2019, 19, 1355–1366. [Google Scholar] [CrossRef]
- Seymour, G.; Tecle, A. Impact of slash pile size and burning on soil chemical characteristics in ponderosa pine forests. J. Ariz. -Nev. Acad. Sci. 2005, 38, 6–20. [Google Scholar] [CrossRef]
- Rhoades, C.C.; Fegel, T.S.; Zaman, T.; Fornwalt, P.J.; Miller, S.P. Are soil changes responsible for persistent slash pile burn scars in lodgepole pine forests? For. Ecol. Manag. 2021, 490, 119090. [Google Scholar] [CrossRef]
- Noble, A.D.; Zennec, I.; Randall, P.J. Leaf litter ash alkalinity and neutralisation of soil acidity. Plant Soil 1996, 179, 293–302. [Google Scholar] [CrossRef]
- Sakala, G.M.; Rowell, D.L.; Pilbeam, C.J. Acid–base reactions between an acidic soil and plant residues. Geoderma 2004, 123, 219–232. [Google Scholar] [CrossRef]
- Ojanen, P.; Mäkiranta, P.; Penttilä, T.; Minkkinen, K. Do logging residue piles trigger extra decomposition of soil organic matter? For. Ecol. Manag. 2017, 405, 367–380. [Google Scholar] [CrossRef]
- de Quadros, L.P.; Ducheiko, H.A.S.; Maeda, S.; Prior, S.A.; Araújo, E.M.; Gomes, J.B.V.; Motta, A.C.V. Effects of wood ash application on tree nutrition and soil dynamics in a Pinus taeda system. For. Sci. 2021, 67, 618–628. [Google Scholar] [CrossRef]
- Pauletti, V.; Motta, A.C.V. Manual de Adubação e Calagem para o Estado do Paraná, 2nd ed.; SBCS, Núcleo Estadual Paraná: Curitiba, Brazil, 2019; 482p. [Google Scholar]
- Santana, R.C. Predição de Biomassa e Alocação de Nutrientes em Povoamentos de Eucalipto no Brasil. Ph.D. Thesis, Universidade Federal de Viçosa, Viçosa, Brazil, 2000. [Google Scholar]
- Reissmann, C.B.; Radomski, M.I.; Quadros, R.M.B.D. Chemical composition of Ilex paraguariensis St. Hil. under different management conditions in seven localities of Paraná State. Braz. Arch. Biol. Technol. 1999, 42. [Google Scholar] [CrossRef]
- Sousa, A.F.; Braga, T.P.; Gomes, E.C.C.; Valentini, A.; Longhinotti, E. Adsorption of phosphate using mesoporous spheres containing iron and aluminum oxide. Chem. Eng. J. 2012, 210, 143–149. [Google Scholar] [CrossRef]
- Fernández-Pazos, M.T.; Garrido-Rodriguez, B.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Fernández-Sanjurjo, M.J.; Núñez-Delgado, A.; Álvarez, E. Cr(VI) adsorption and desorption on soils and biosorbents. Water Air Soil Pollut. 2012, 224, 1366. [Google Scholar] [CrossRef]
- Stahl, J.; Ernani, P.R.; Gatiboni, L.C.; Chaves, D.M.; Neves, C.U. Produção de massa seca e eficiência nutricional de clones de Eucalyptus dunnii e Eucalyptus benthamii em função da adição de doses de fósforo ao solo. Ciência Florest. 2013, 23, 287–295. [Google Scholar] [CrossRef]
- Moro, L.; Gatiboni, L.C.; Simonete, M.A.; Cassol, P.C.; Chaves, D.M. Response of one-, five-, and nine-year-old Pinus taeda to NPK fertilization in southern Brazil. Braz. J. Soil Sci. 2014, 38, 1181–1189. [Google Scholar]
- Motta, A.C.V.; Barbosa, J.Z.; Consalter, R.; Reissmann, C.B. Nutrição e Adubação da Cultura de Pínus. In Nutrição e Adubação de Espécies Florestais e Palmeiras, 1st ed.; Prado, R.d.M., Wadt, P.G.S., Eds.; FUNEP: Jaboticabal, Brazil, 2014; pp. 383–426. [Google Scholar]
- Fox, T.R.; Lee Allen, H.; Albaugh, T.J.; Rubilar, R.; Carlson, C.A. Tree nutrition and forest fertilization of pine plantations in the southern United States. South. J. Appl. For. 2007, 31, 5–11. [Google Scholar] [CrossRef]
- da Silva, W.; da Silva, A.A.; Sediyama, T.; de Freitas, R.S. Absorção de nutrientes por mudas de duas espécies de eucalipto em resposta a diferentes teores de água no solo e competição com plantas de Brachiaria brizantha. Ciência Agrotecnol. 2000, 24, 147–159. [Google Scholar]
- Medeiro, W.N.; Melo, C.A.D.; Tiburcio, R.A.S.; da Silva, G.S.; Machado, A.F.L.; Santos, L.D.T.; Ferreira, F.A. Crescimento inicial e concentração de nutrientes em clones de Eucalyptus urophylla x Eucalyptus grandis sob interferência de plantas daninhas. Ciência Florest. 2016, 26, 147–157. [Google Scholar] [CrossRef]
- Maclaren, J.P. Environmental effects of planted forests in New Zealand: The implications of continued afforestation of pasture. For. Res. Inst. Bull. For. Res. Inst. Rotorua 1996, 198, 180. [Google Scholar]
- Harrington, T.B.; Slesak, R.A.; Dollins, J.P.; Schoenholtz, S.H.; Peter, D.H. Logging-debris and vegetation-control treatments influence competitive relationships to limit 15-year productivity of coast Douglas-fir in western Washington and Oregon. For. Ecol. Manag. 2020, 473, 118288. [Google Scholar] [CrossRef]
- Pompa-García, M.; Camarero, J.J.; Colangelo, M.; González-Cásares, M. Inter and intra-annual links between climate, tree growth and NDVI: Improving the resolution of drought proxies in conifer forests. Int. J. Biometeorol. 2021, 65, 2111–2121. [Google Scholar] [CrossRef]
- Xing, F.; An, R.; Wang, B.; Miao, J.; Jiang, T.; Huang, X.; Hu, Y. Mapping the occurrence and spatial distribution of noxious weed species with multisource data in degraded grasslands in the Three-River Headwaters Region, China. Sci. Total Environ. 2021, 801, 149714. [Google Scholar] [CrossRef]
- Rasmussen, J.; Azim, S.; Nielsen, J. Pre-harvest weed mapping of Cirsium arvense L. based on free satellite imagery—The importance of weed aggregation and image resolution. Eur. J. Agron. 2021, 130, 126373. [Google Scholar] [CrossRef]
- Wang, F.; Harindintwali, J.D.; Yuan, Z.; Wang, M.; Wang, F.; Li, S.; Yin, Z.; Huang, L.; Fu, Y.; Li, L.; et al. Technologies and perspectives for achieving carbon neutrality. Innovation 2021, 2, 100180. [Google Scholar] [CrossRef]
- Chaves, R.D.Q.; Corrêa, G.F. Micronutrientes no sistema solo-Pinus caribaea Morelet em plantios apresentando amarelecimento das acículas e morte de plantas. Rev. Árvore 2003, 27, 769–778. [Google Scholar] [CrossRef]
- Batista, A.H.; Motta, A.C.V.; Reissmann, C.B.; Schneider, T.; Martins, I.L.; Hashimoto, M. Liming and fertilisation in Pinus taeda plantations with severe nutrient deficiency in Savanna soils. Acta Sci. Agron. 2015, 37, 117–125. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Langer, S.; Carrasquinho, I.; Bergström, E.; Larson, T.; Thomas-Oates, J.; António, C. Pinus pinaster early hormonal defence responses to pinewood nematode (Bursaphelenchus typophiles) infection. Metabolites 2021, 11, 227. [Google Scholar] [CrossRef]
- Rabel, D.O.; Maeda, S.; Araujo, E.M.; Gomes, J.B.; Bognolla, I.A.; Prior, S.A.; Magri, E.; Frigo, C.; Brasileiro, B.P.; Santos, M.C.; et al. Recycled alkaline paper waste influenced growth and structure of Pinus taeda L. forest. New For. 2020, 52, 249–270. [Google Scholar] [CrossRef]
- Albaugh, J.M.; Blevins, L.; Allen, H.L.; Albaugh, T.J.; Fox, T.R.; Stape, J.L.; Rubilar, R.A. Characterization of foliar macro- and micronutrient concentrations and ratios in loblolly pine plantations in the southeastern United States. South. J. Appl. For. 2010, 34, 53–64. [Google Scholar] [CrossRef]
- Sypert, R.H. Diagnosis of Loblolly Pine (Pinus taeda L.) Nutrient Deficiencies by Foliar Methods; Polytechnic Institute and State University: Blacksburg, VA, USA, 2006; 115p. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).