Improvement of Surface Coating and Interfacial Properties of Hot-Waxed Wood Using Maleic Anhydride Grafted Polypropylene Wax
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Modified Polypropylene Wax by Grafting Maleic Anhydride
2.3. Preparation of Hot-Waxed Woods
2.4. Properties of Hot-Waxed Woods
2.4.1. Melting Behaviors of Waxes and Heat Resistances of Hot-Waxed Woods Tests
2.4.2. Adhesion Strength and Scratch Hardness Tests
2.4.3. Water Contact Angle Test
2.4.4. Surface Color and Gloss Tests
2.4.5. Characterization of Hot-Waxed Wood
3. Results and Discussion
3.1. Acid Value and Characterization of MAH-Modified PPW
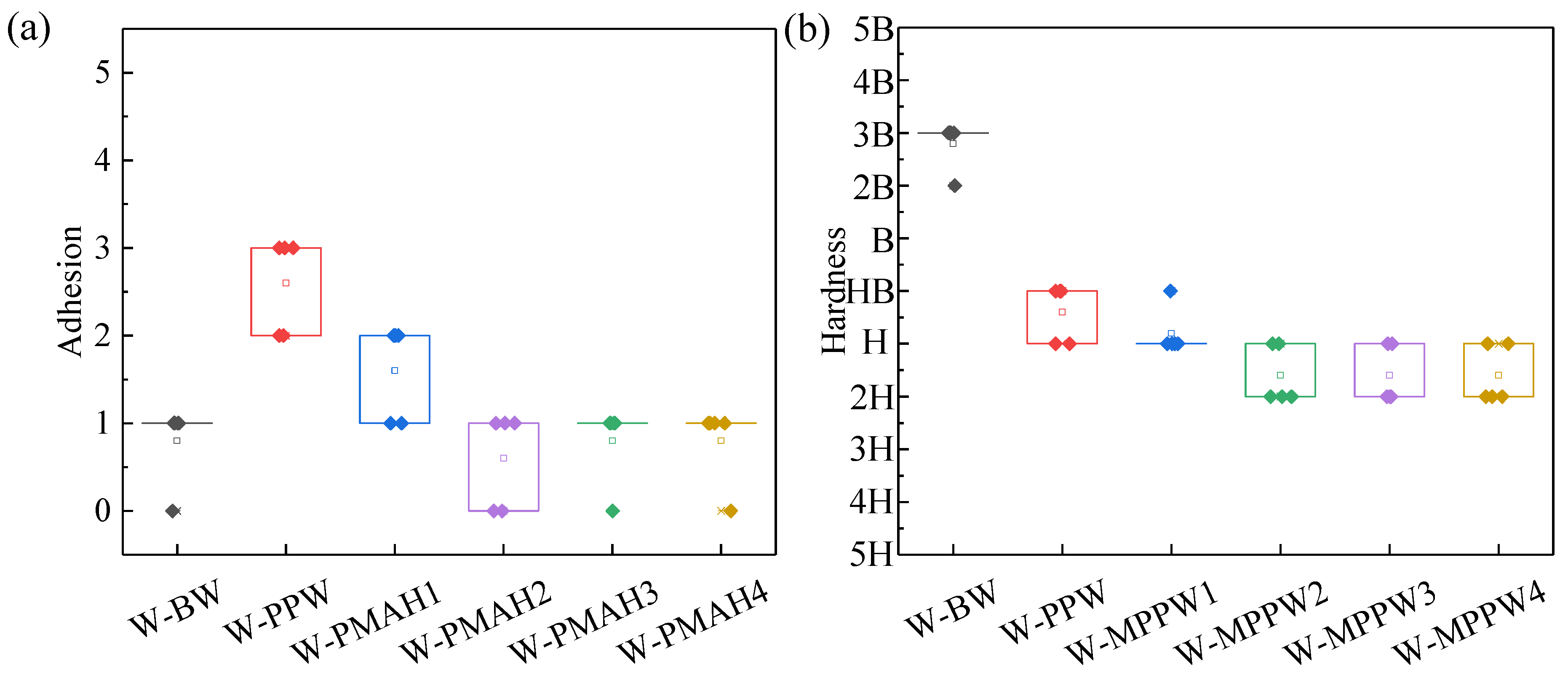
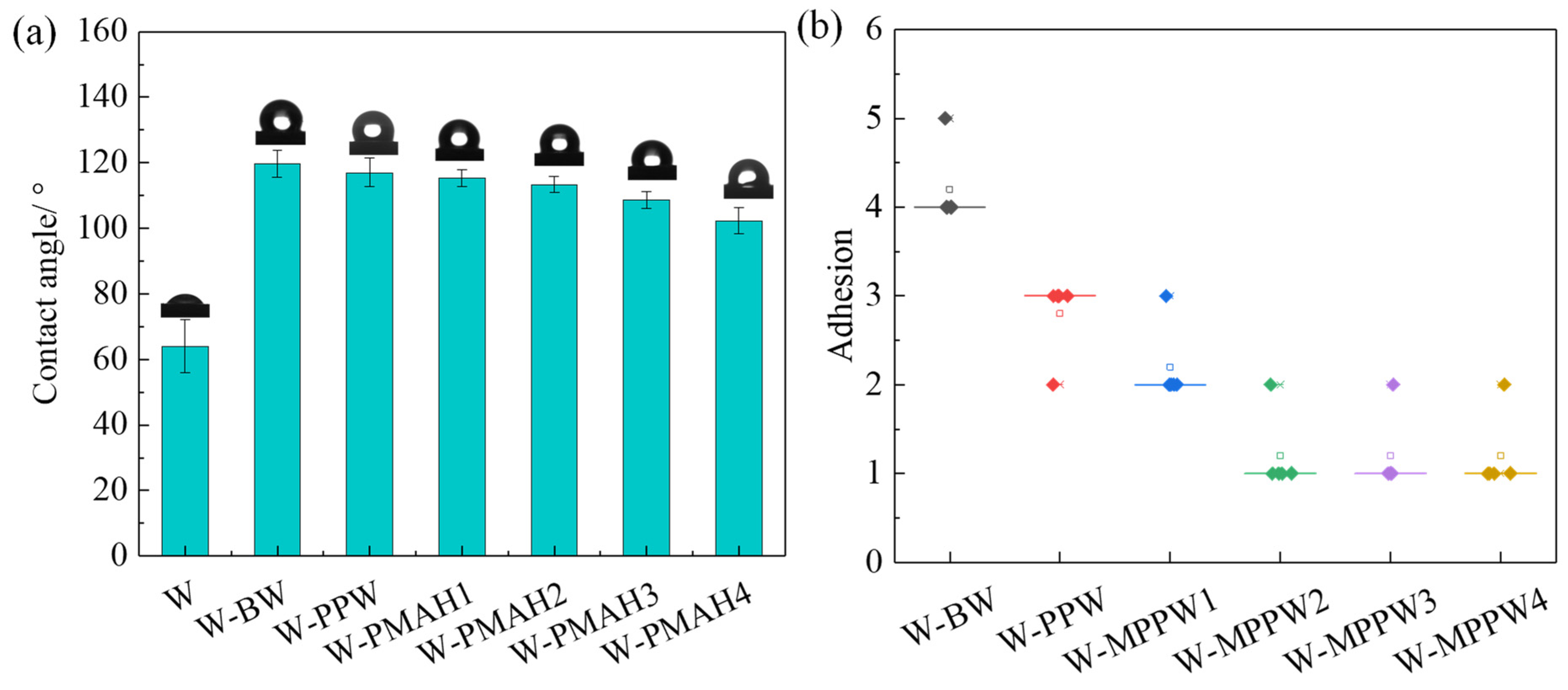
3.2. Adhesion and Hardness of Hot-Waxed Wood
3.3. Hydrophobicity of Hot-Waxed Wood
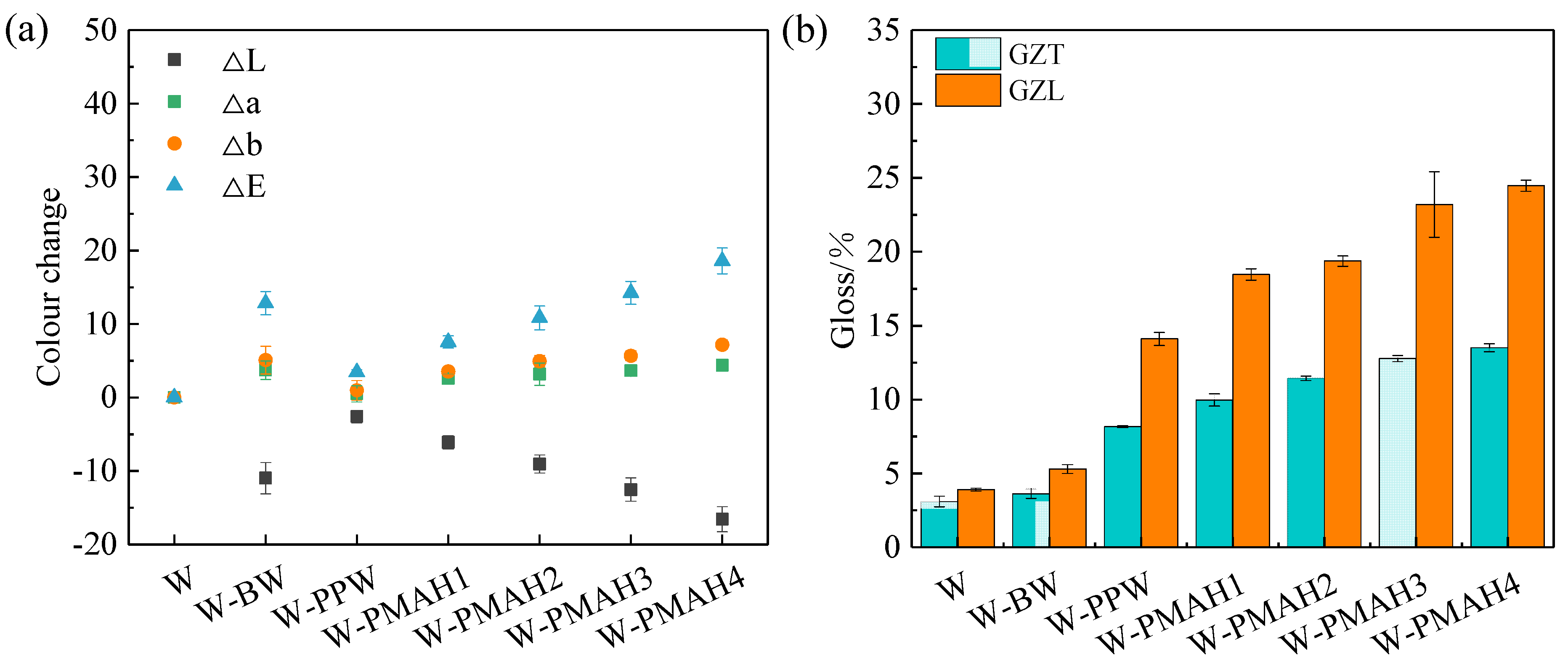
3.4. Color and Gloss Measurement of Hot-Waxed Wood
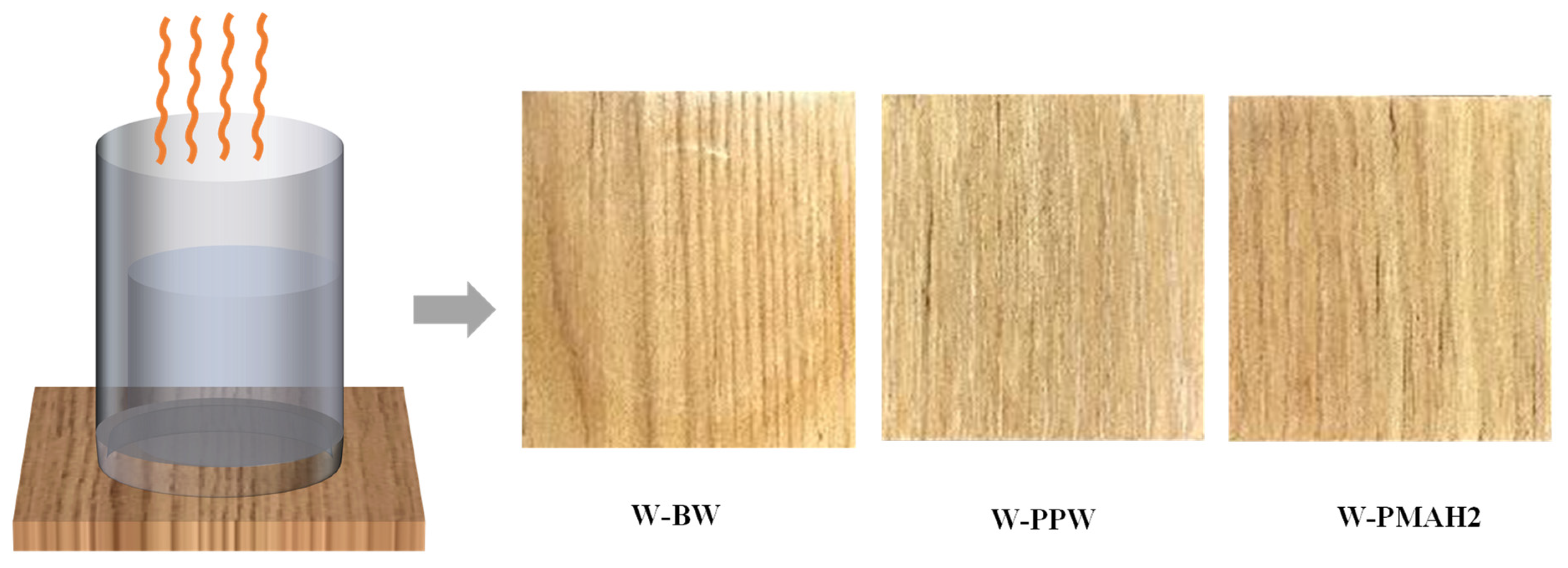
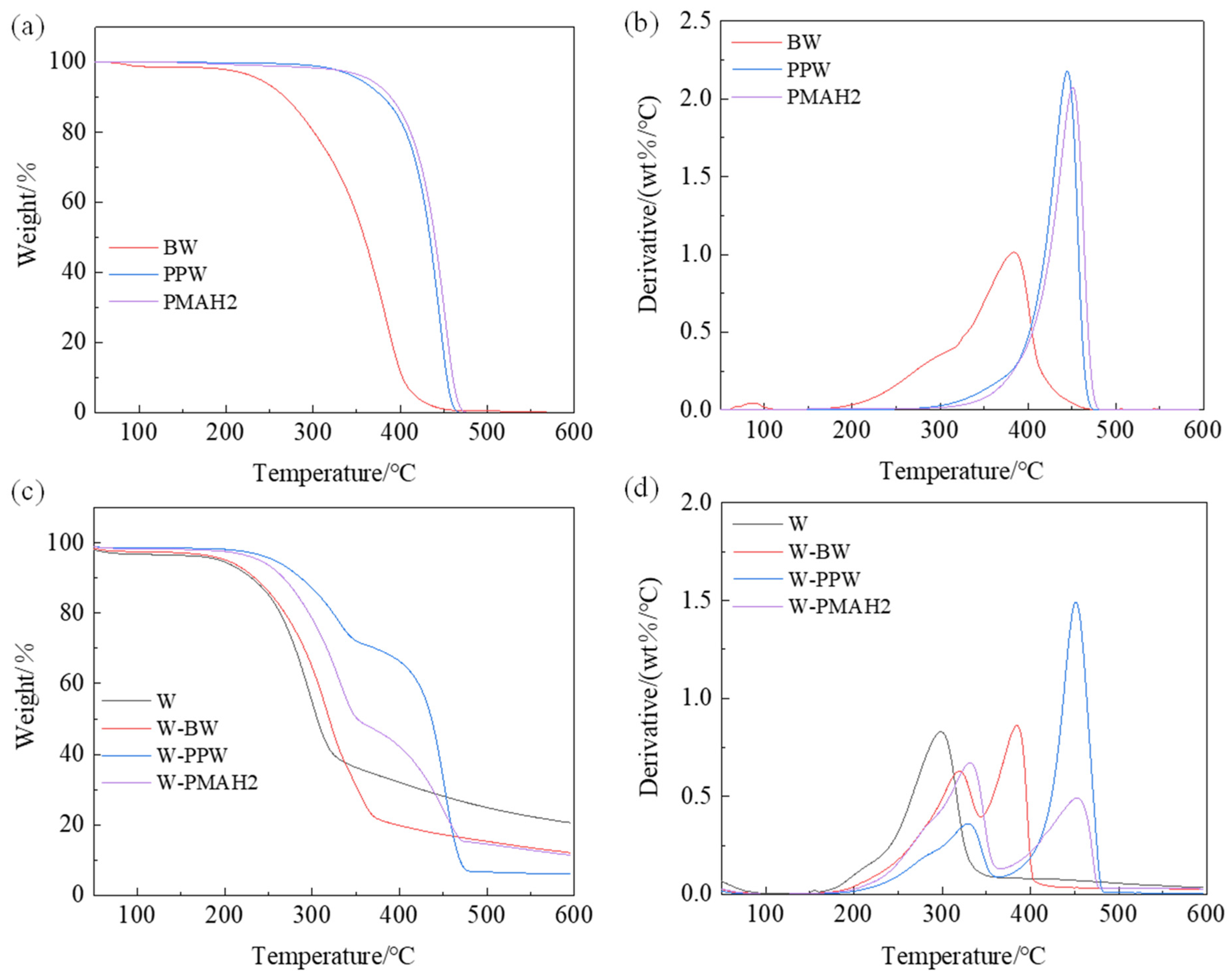
3.5. Thermal Stability of Hot-Waxed Wood
3.6. Characterization
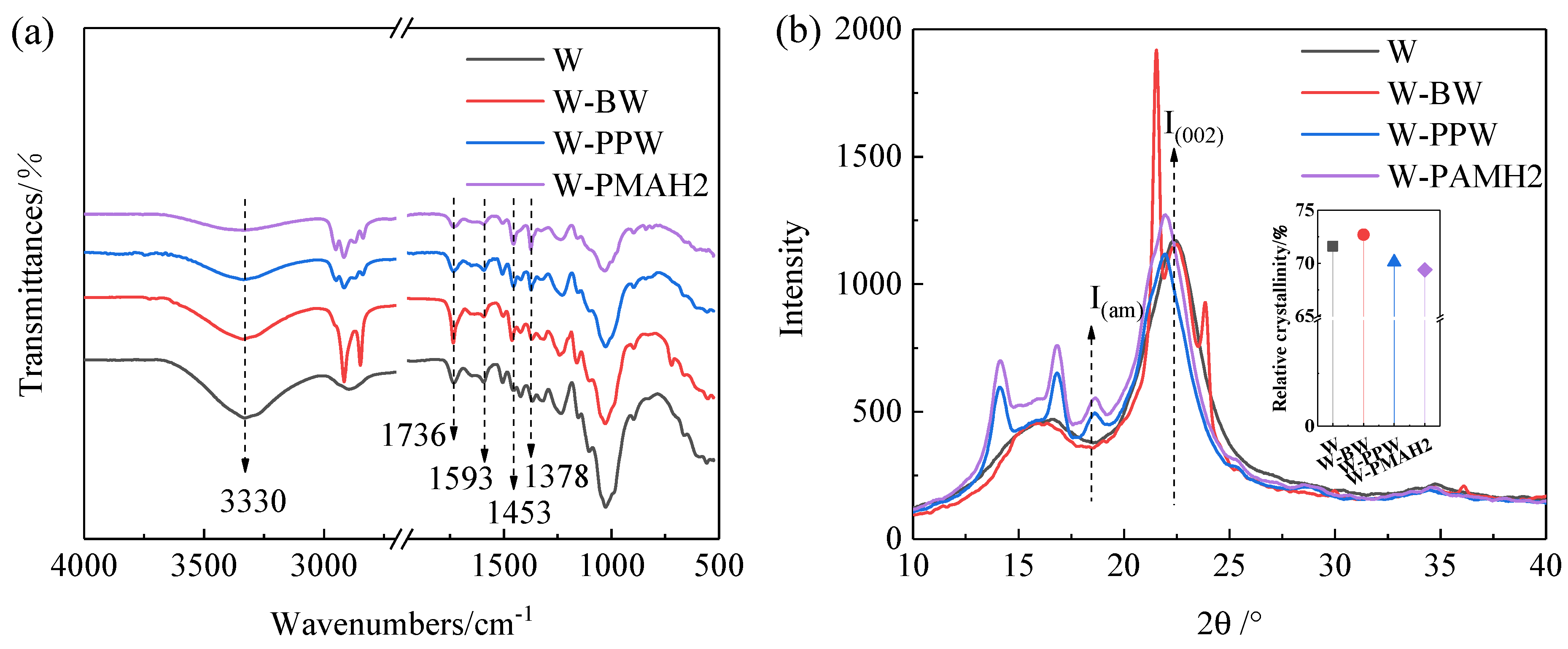
3.6.1. Fourier Transform Infrared Spectra (FTIR) Analysis
3.6.2. X-ray Diffraction (XRD) Analysis
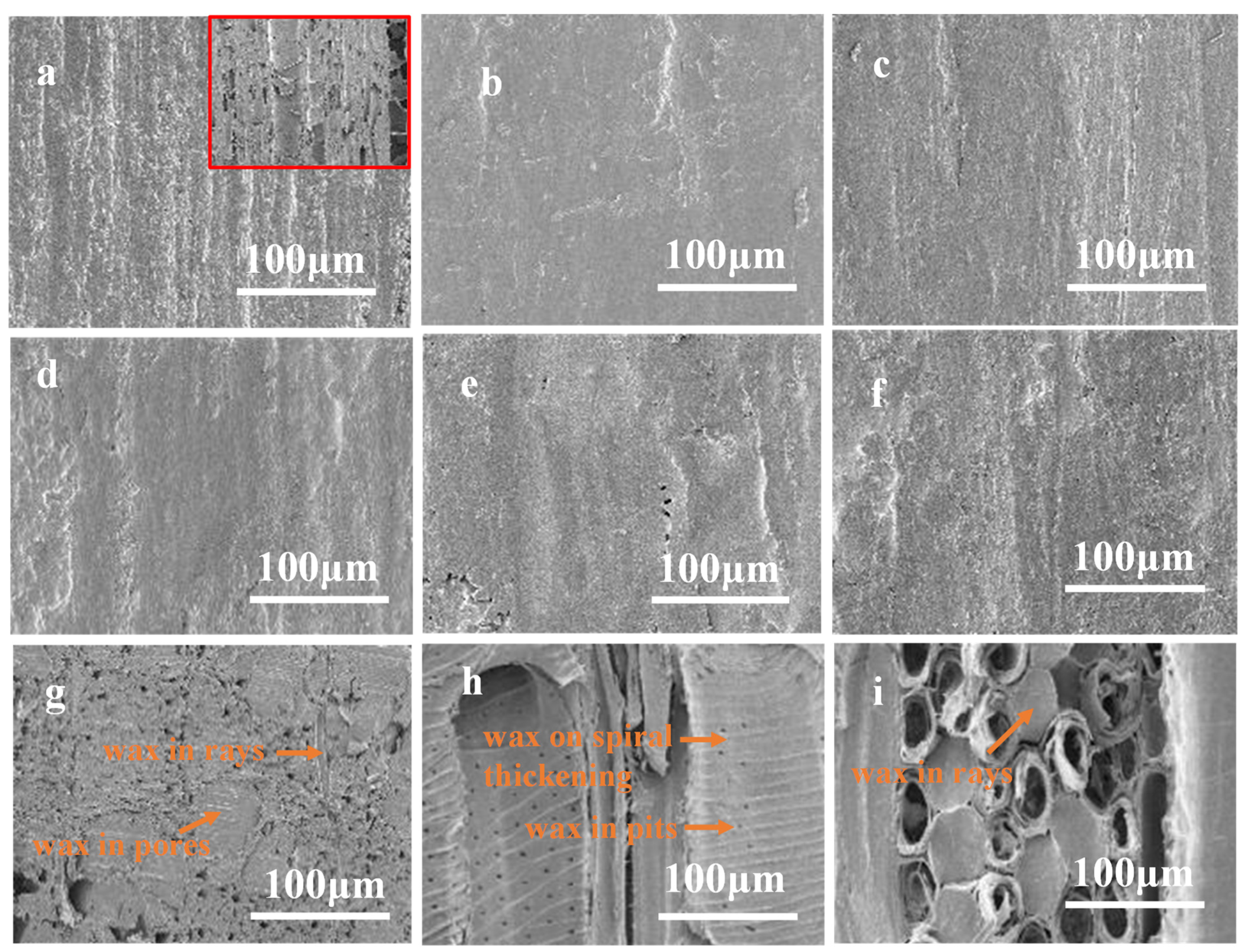
3.6.3. Scanning Electron Microscope (SEM) Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brischke, C.; Alfredsen, G. Wood-water relationships and their role for wood susceptibility to fungal decay. Appl. Microbiol. Biotechnol. 2020, 104, 3781–3795. [Google Scholar] [CrossRef]
- Nagarajappa, G.B.; Pandey, K.K. UV resistance and dimensional stability of wood modified with isopropenyl acetate. J. Photochem. Photobiol. B-Biol. 2016, 155, 20–27. [Google Scholar] [CrossRef]
- Fredriksson, M.; Wadso, L.; Johansson, P. Small resistive wood moisture sensors: A method for moisture content determination in wood structures. Eur. J. Wood Wood Prod. 2013, 71, 515–524. [Google Scholar] [CrossRef]
- Liu, X.Y.; Timar, M.C.; Varodi, A.M. A comparative study on the artificial UV and natural aging of beeswax and Chinese wax and influence of wax finishing on the ageing of Chinese Ash (Fraxinus mandshurica) wood surfaces. J. Photochem. Photobiol. B-Biol. 2019, 201, 111607. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F.; Sparacino, V. Thermal and dynamic mechanical properties of beeswax-halloysite nanocomposites for consolidating waterlogged archaeological woods. Polym. Degrad. Stab. 2015, 120, 220–225. [Google Scholar] [CrossRef]
- Janesch, J.; Arminger, B.; Gindl-Altmutter, W.; Hansmann, C. Superhydrophobic coatings on wood made of plant oil and natural wax. Prog. Org. Coat. 2020, 148, 105891. [Google Scholar] [CrossRef]
- Chen, J.Y.; Wang, Y.J.; Cao, J.Z.; Wang, W. Improved Water Repellency and Dimensional Stability of Wood via Impregnation with an Epoxidized Linseed Oil and Carnauba Wax Complex Emulsion. Forests 2020, 11, 271. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Chen, J.Y.; Zhang, S.D.; Cao, J.Z.; Wang, W. Forming textured hydrophobic surface coatings via mixed wax emulsion impregnation and drying of poplar wood. Wood Sci. Technol. 2020, 54, 421–439. [Google Scholar] [CrossRef]
- Wang, W.; Ran, Y.Y.; Wang, J.M. Improved performance of thermally modified wood via impregnation with carnauba wax/organoclay emulsion. Constr. Build. Mater. 2020, 247, 118586. [Google Scholar] [CrossRef]
- Wang, W.; Chen, C.; Cao, J.Z.; Zhu, Y. Improved properties of thermally modified wood (TMW) by combined treatment with disodium octoborate tetrahydrate (DOT) and wax emulsion (WE). Holzforschung 2018, 72, 243–250. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, Y.P.; Cao, J.Z.; Mei, C.T. Improved Hydrophobicity and Dimensional Stability of Wood Treated with Paraffin/Acrylate Compound Emulsion through Response Surface Methodology Optimization. Polymers 2020, 12, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lisuzzo, L.; Hueckel, T.; Cavallaro, G.; Sacanna, S.; Lazzara, G. Pickering Emulsions Based on Wax and Halloysite Nanotubes: An Ecofriendly Protocol for the Treatment of Archeological Woods. ACS Appl. Mater. Interfaces 2021, 13, 1651–1661. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, H.H. Effect of a Combination of Moderate-Temperature Heat Treatment and Subsequent Wax Impregnation on Wood Hygroscopicity, Dimensional Stability, and Mechanical Properties. Forests 2020, 11, 920. [Google Scholar] [CrossRef]
- Wang, J.M.; Zhong, H.; Ma, E.N.; Cao, J.Z. Properties of wood treated with compound systems of paraffin wax emulsion and copper azole. Eur. J. Wood Wood Prod. 2018, 76, 315–323. [Google Scholar] [CrossRef]
- Liu, M.; Zhong, H.; Ma, E.N.; Liu, R. Resistance to fungal decay of paraffin wax emulsion/copper azole compound system treated wood. Int. Biodeterior. Biodegrad. 2018, 129, 61–66. [Google Scholar] [CrossRef]
- Lesar, B.; Straze, A.; Humar, M. Sorption Properties of Wood Impregnated with Aqueous Solution of Boric Acid and Montan Wax Emulsion. J. Appl. Polym. Sci. 2011, 120, 1337–1345. [Google Scholar] [CrossRef]
- Scholz, G.; Nothnick, E.; Avramidis, G.; Krause, A.; Militz, H.; Viol, W.; Wolkenhauer, A. Adhesion of wax impregnated solid beech wood with different glues and by plasma treatment. Eur. J. Wood Wood Prod. 2010, 68, 315–321. [Google Scholar] [CrossRef] [Green Version]
- Lesar, B.; Humar, M. Use of wax emulsions for improvement of wood durability and sorption properties. Eur. J. Wood Wood Prod. 2021, 69, 231–238. [Google Scholar] [CrossRef] [Green Version]
- Avramidis, G.; Scholz, G.; Nothnick, E.; Militz, H.; Viol, W.; Wolkenhauer, A. Improved bondability of wax-treated wood following plasma treatment. Wood Sci. Technol. 2011, 45, 359–368. [Google Scholar] [CrossRef]
- Mngomezulu, M.E.; Luyt, A.S.; Krupa, I. Structure and Properties of Phase-Change Materials Based on High-Density Polyethylene, Hard Fischer-Tropsch Paraffin Wax, and Wood Flour. Polym. Compos. 2011, 32, 1155–1163. [Google Scholar] [CrossRef]
- Mngomezulu, M.E.; Luyt, A.S.; Krupa, I. Structure and Properties of Phase Change Materials Based on HDPE, Soft Fischer-Tropsch Paraffin Wax, and Wood Flour. J. Appl. Polym. Sci. 2010, 118, 1541–1551. [Google Scholar] [CrossRef]
- Poletto, M. Effect of styrene maleic anhydride on physical and mechanical properties of recycled polystyrene wood flour composites. Maderas-Cienc. Tecnol. 2016, 18, 533–542. [Google Scholar] [CrossRef] [Green Version]
- Estan-Cerezo, G.; Martin-Martinez, J.M. Thermal, viscoelastic and adhesion properties of EVA (ethylene-co-vinyl acetate) hot melts containing polypropylene waxes of different nature. J. Adhes. Sci. Technol. 2015, 298, 75–88. [Google Scholar] [CrossRef]
- Garcia, R.A.; Cloutier, A.; Riedl, B. Dimensional stability of MDF panels produced from fibres treated with maleated polypropylene wax. Wood Sci. Technol. 2005, 39, 630–650. [Google Scholar] [CrossRef]
- Ameri, M.; Afshin, A.; Shiraz, M.E.; Yazdipanah, F. Effect of wax-based warm mix additives on fatigue and rutting performance of crumb rubber modified asphalt. Constr. Build. Mater. 2020, 262, 120882. [Google Scholar] [CrossRef]
- Wang, Z.H.; Zou, W.H.; Sun, D.L.; Ji, X.Q.; Yu, M.G. Fabrication and performance of in-depth hydrophobic wood modified by a silica/wax complex emulsion combined with thermal treatment. Wood Sci. Technol. 2020, 54, 1223–1239. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Wu, D.; Huang, C.X.; Zhang, M.; Umemura, K.; Yong, Q. Utilization of enzymatic hydrolysate from corn stover as a precursor to synthesize an eco-friendly adhesive for plywood II: Investigation of appropriate manufacturing conditions, curing behavior, and adhesion mechanism. J. Wood Sci. 2020, 66, 85. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Huang, C.X.; Wu, D.; Chen, Z.; Zhu, N.; Gui, C.S.; Zhang, M.; Umemura, K.; Yong, Q. Utilization of enzymatic hydrolysate from corn stover as a precursor to synthesize an eco-friendly plywood adhesive. Ind. Crop. Prod. 2020, 152, 112501. [Google Scholar] [CrossRef]
- Nagel, J.; Kroschwald, F.; Bellmann, C.; Schwarz, S.; Janke, A.; Heinrich, G. Immobilisation of different surface-modified silica nanoparticles on polymer surfaces via melt processing, Colloid Surf. A-Physicochem. Eng. Asp. 2017, 532, 208–212. [Google Scholar] [CrossRef]
- Novak, I.; Krupa, I.; Luyt, A.S. Modification of the polarity of isotactic polypropylene through blending with oxidized paraffin wax. J. Appl. Polym. Sci. 2004, 94, 529–533. [Google Scholar] [CrossRef]
- Novak, I.; Krupa, I.; Luyt, A.S. Modification of the polarity and adhesive properties of polyolefins through blending with maleic anhydride grafted Fischer-Tropsch paraffin wax. J. Appl. Polym. Sci. 2006, 100, 3069–3074. [Google Scholar] [CrossRef]
- Marek, A.A.; Zawadiak, J.; Piotrowski, T.; Hefczyc, B. A new efficient method for the processing of post-consumer polypropylene and other polyolefin wastes into polar waxes. Waste Manag. 2015, 46, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.F.; Ding, Y.F.; Wang, T.W.; Yang, J.; Guan, Y.; Zheng, A.N. Zheng, Preparation of nonleaching antimicrobial polypropylene wax and its application in polypropylene. J. Appl. Polym. Sci. 2017, 134, 44190. [Google Scholar] [CrossRef]
- Niu, K.R.; Song, K.Y. Surface coating and interfacial properties of hot-waxed wood using modified polyethylene wax. Prog. Org. Coat. 2021, 150, 105947. [Google Scholar] [CrossRef]
- Zhang, Y.; Simpson, B.K.; Dumont, M.J. Dumont, Effect of beeswax and carnauba wax addition on properties of gelatin films: A comparative study. Food Biosci. 2018, 26, 88–95. [Google Scholar] [CrossRef]
- Kucera, F.; Petrus, J.; Balkova, R.; Jancar, J. Solid-state grafting of maleic anhydride onto polypropylene: The influence of morphology of polypropylene on heterogeneous reaction. Polym. Eng. Sci. 2020, 60, 1076–1082. [Google Scholar] [CrossRef]
- Shi, Y.Q.; Liu, C.; Liu, L.; Fu, L.B.; Yu, B.; Lv, Y.C.; Yang, F.Q.; Song, P.G. Strengthening, toughing and thermally stable ultra-thin MXene nanosheets/polypropylene nanocomposites via nanoconfinement. J. Chem. Eng. J. 2019, 378, 122267. [Google Scholar] [CrossRef]
- Tan, X.M.; Xu, Y.S.; Jia, G.W.; Oian, J. Ultrasonically initiated grafting of maleic anhydride onto poly(propylene). Macromol. React. Eng. 2007, 1, 185–190. [Google Scholar] [CrossRef]
- Jana, S.; Martini, S. Phase behavior of binary blends of four different waxes. J. Am. Oil Chem. Soc. 2016, 93, 543–554. [Google Scholar] [CrossRef]
- Duce, C.; Orsini, S.; Spepi, A.; Colombini, M.P.; Tine, M.R.; Ribechini, E. Thermal degradation chemistry of archaeological pine pitch containing beeswax as an additive. J. Anal. Appl. Pyrolysis. 2015, 111, 254–264. [Google Scholar] [CrossRef]
- Sun, Z.M.; Zhang, Y.Z.; Zheng, S.L.; Park, Y.; Frost, R.L. Preparation and thermal energy storage properties of paraffin/calcined diatomite composites as form-stable phase change materials. Thermochim. Acta. 2013, 558, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Scholz, G.; Van den Bulcke, J.; Boone, M.; Zauer, M.; Baucker, E.; Van Acker, J.; Militz, H. Investigation on wax-impregnated wood. Part 1: Microscopic observations and 2D X-ray imaging of distinct wax types. Holzforschung 2010, 64, 581–585. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.J.; Yao, W.Y.; Chen, H.; Guo, W.H. Influence of Eva-g-mah Compatibilizer on Adhesion Strength, Mechanical Properties and Thermal Behavior of Eva/sbs Hot Melt Adhesive Blends. Mater. Res. Express. 2019, 6, 055319. [Google Scholar] [CrossRef]
- Kommling, A.; Jaunich, M.; Wolff, D. Revealing effects of chain scission during ageing of EPDM rubber using relaxation and recovery experiment. Polym. Test. 2016, 56, 261–268. [Google Scholar] [CrossRef]
- Scholz, G.; Krause, A.; Militz, H. Exploratory study on the impregnation of Scots pine sapwood (Pinus sylvestris L.) and European beech (Fagus sylvatica L.) with different hot melting waxes. Wood Sci. Technol. 2010, 44, 379–388. [Google Scholar] [CrossRef] [Green Version]
- Scholz, G.; Zauer, M.; Van den Bulcke, J.; Van Loo, D.; Pfriem, A.; Van Acker, J.; Militz, H. Investigation on wax-impregnated wood. Part 2: Study of void spaces filled with air by He pycnometry, Hg intrusion porosimetry, and 3D X-ray imaging. Holzforschung 2010, 64, 587–593. [Google Scholar] [CrossRef]
- Menyhard, A.; Faludi, G.; Varga, J. beta-crystallisation tendency and structure of polypropylene grafted by maleic anhydride and its blends with isotactic polypropylene. J. Therm. Anal. Calorim. 2008, 93, 937–945. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Song, K. Improvement of Surface Coating and Interfacial Properties of Hot-Waxed Wood Using Maleic Anhydride Grafted Polypropylene Wax. Forests 2022, 13, 1205. https://doi.org/10.3390/f13081205
Wang X, Song K. Improvement of Surface Coating and Interfacial Properties of Hot-Waxed Wood Using Maleic Anhydride Grafted Polypropylene Wax. Forests. 2022; 13(8):1205. https://doi.org/10.3390/f13081205
Chicago/Turabian StyleWang, Xuting, and Kuiyan Song. 2022. "Improvement of Surface Coating and Interfacial Properties of Hot-Waxed Wood Using Maleic Anhydride Grafted Polypropylene Wax" Forests 13, no. 8: 1205. https://doi.org/10.3390/f13081205





