Environmental and Plant-Derived Controls on the Seasonality and Partitioning of Soil Respiration in an American Sycamore (Platanus occidentalis) Bioenergy Plantation Grown at Different Planting Densities
Abstract
:1. Introduction
2. Materials and Methods
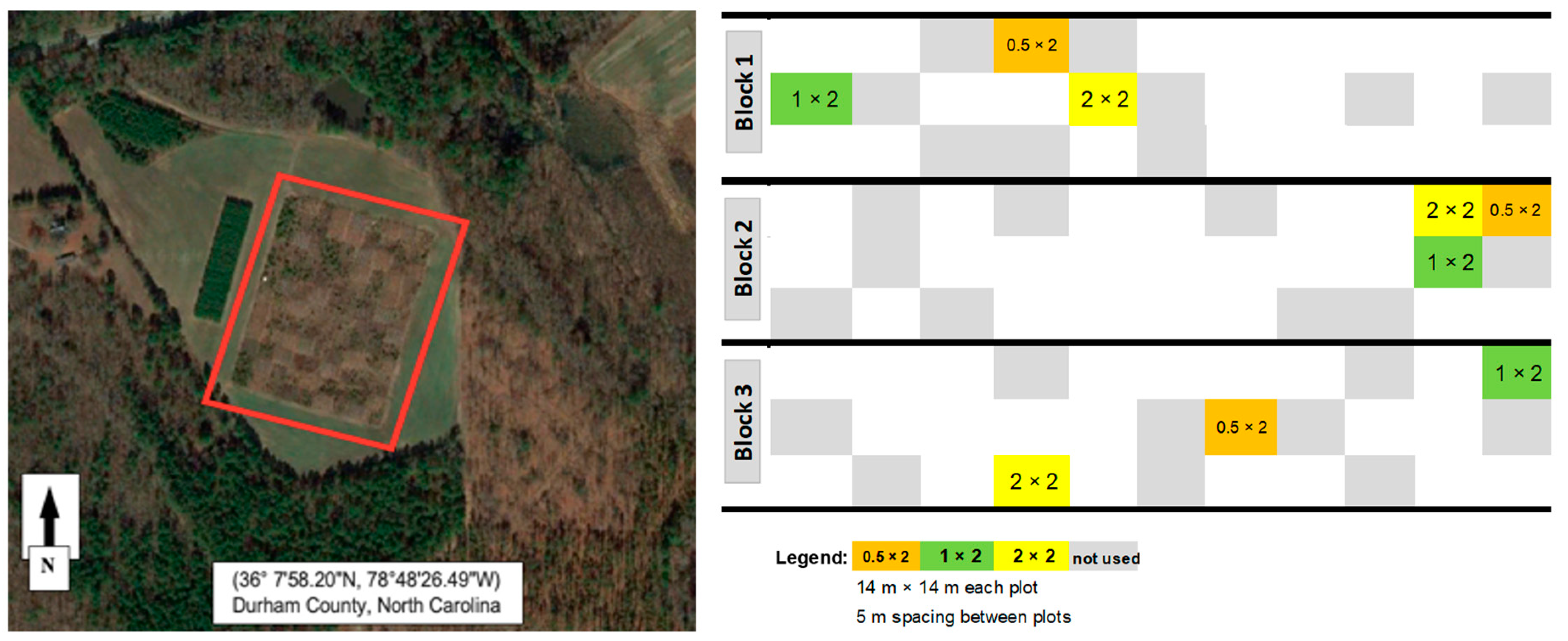
2.1. Site Description
2.2. Experimental Design
2.3. Soil Respiration Measurements
2.4. Meteorological Measurements
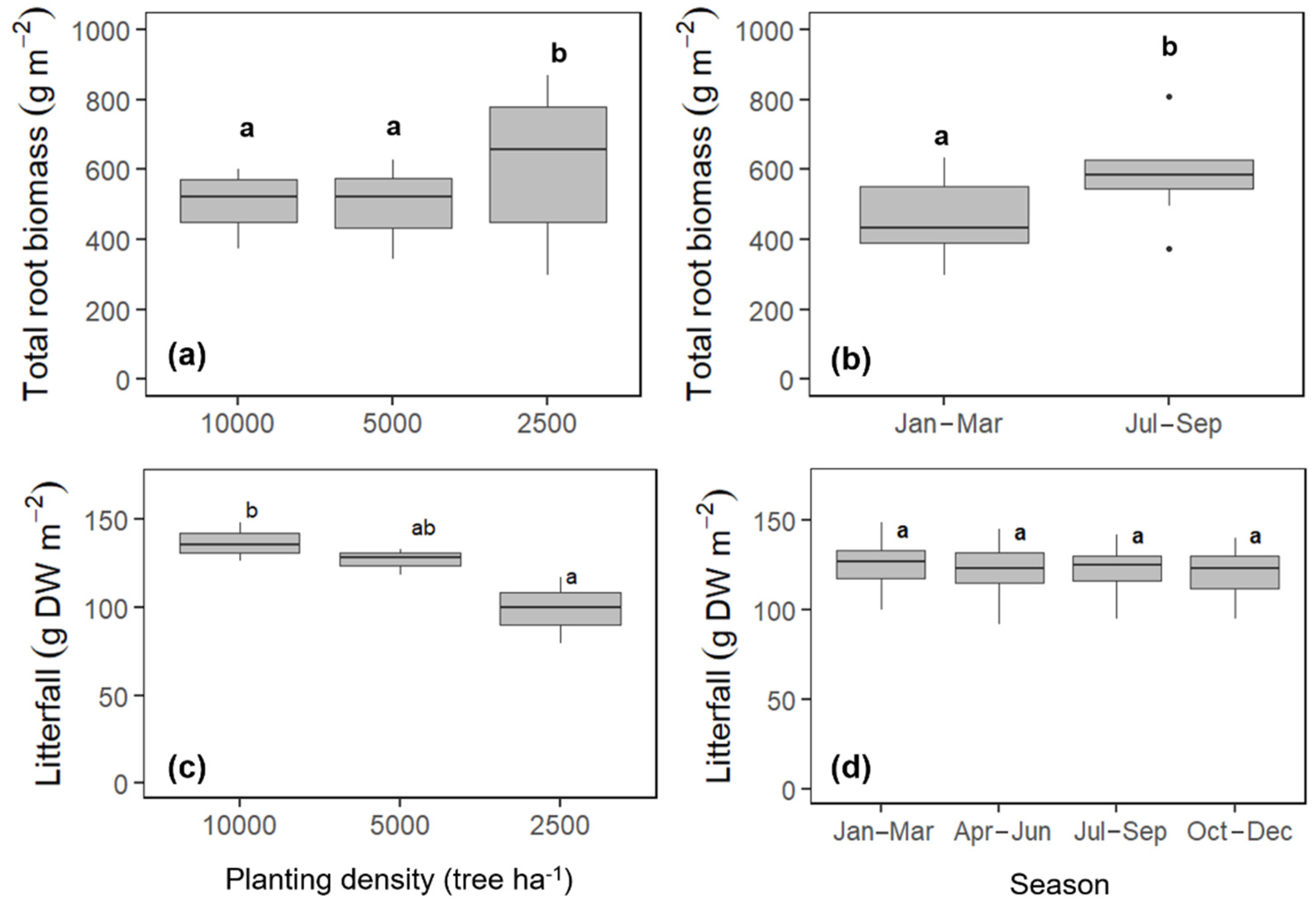
2.5. Root Biomass and Litterfall
2.6. Data Analysis
3. Results
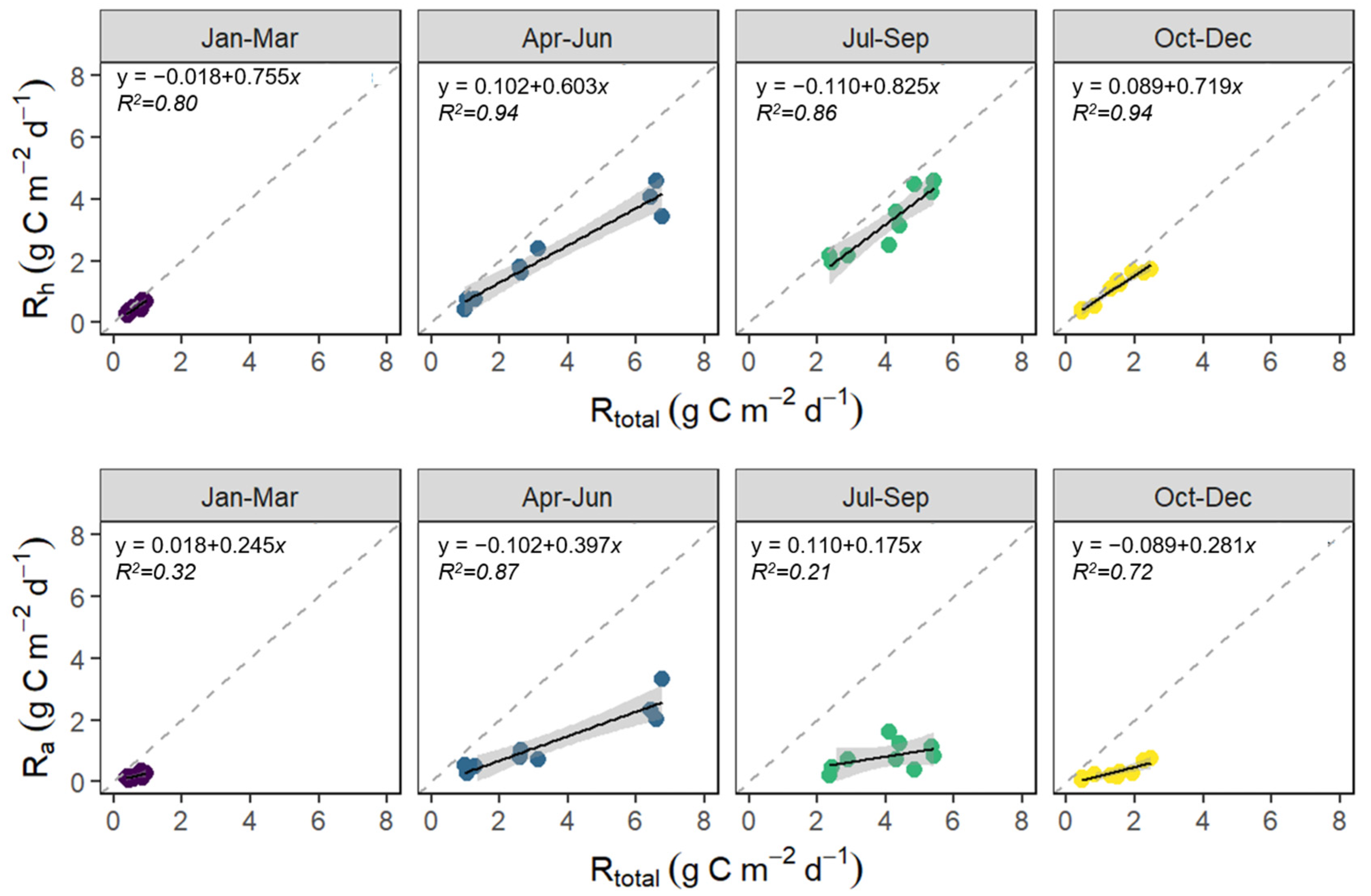
3.1. Seasonal Variation in Rtotal, Rh, and Ra
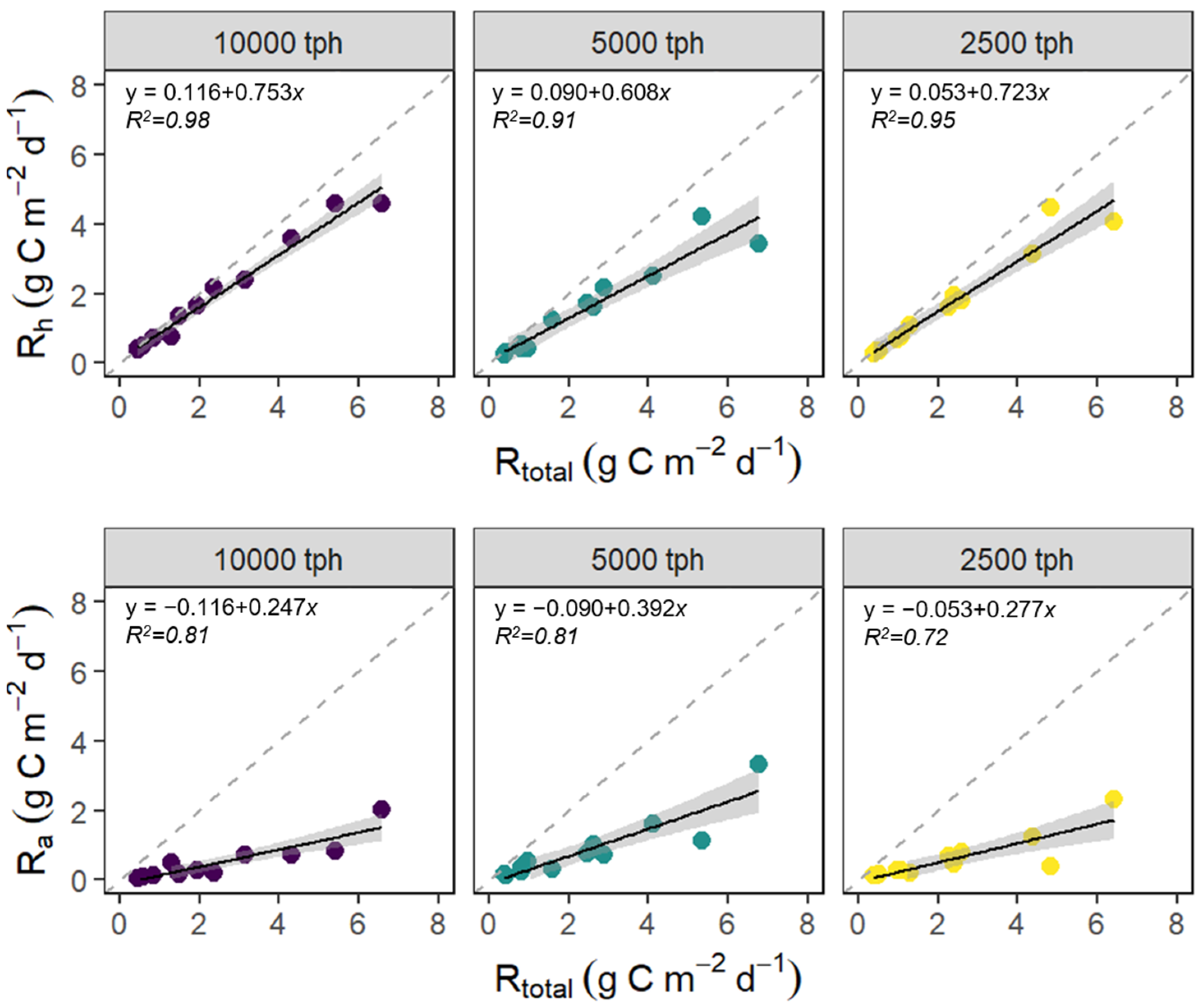
3.2. Variation in Rtotal, Rh, and Ra due to Planting Density
3.3. The Contribution of Rh and Ra to Rtotal
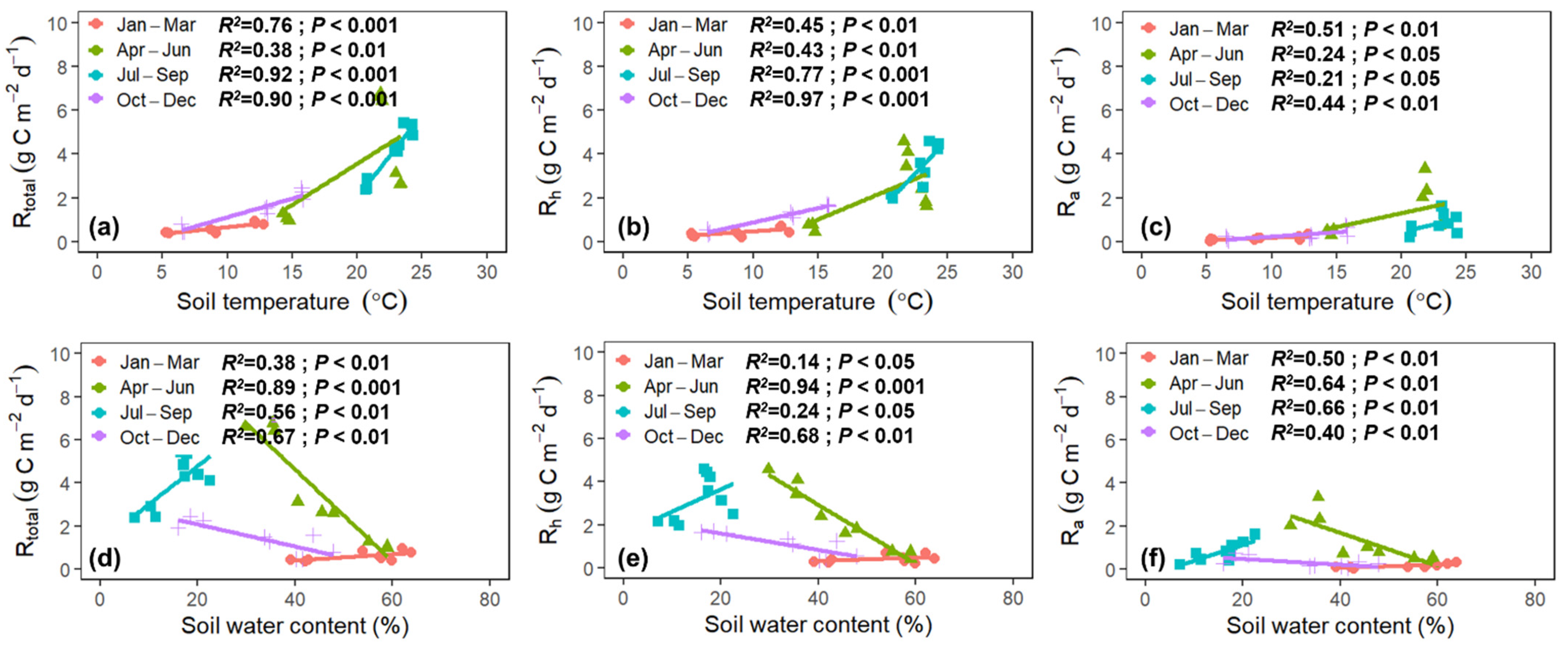
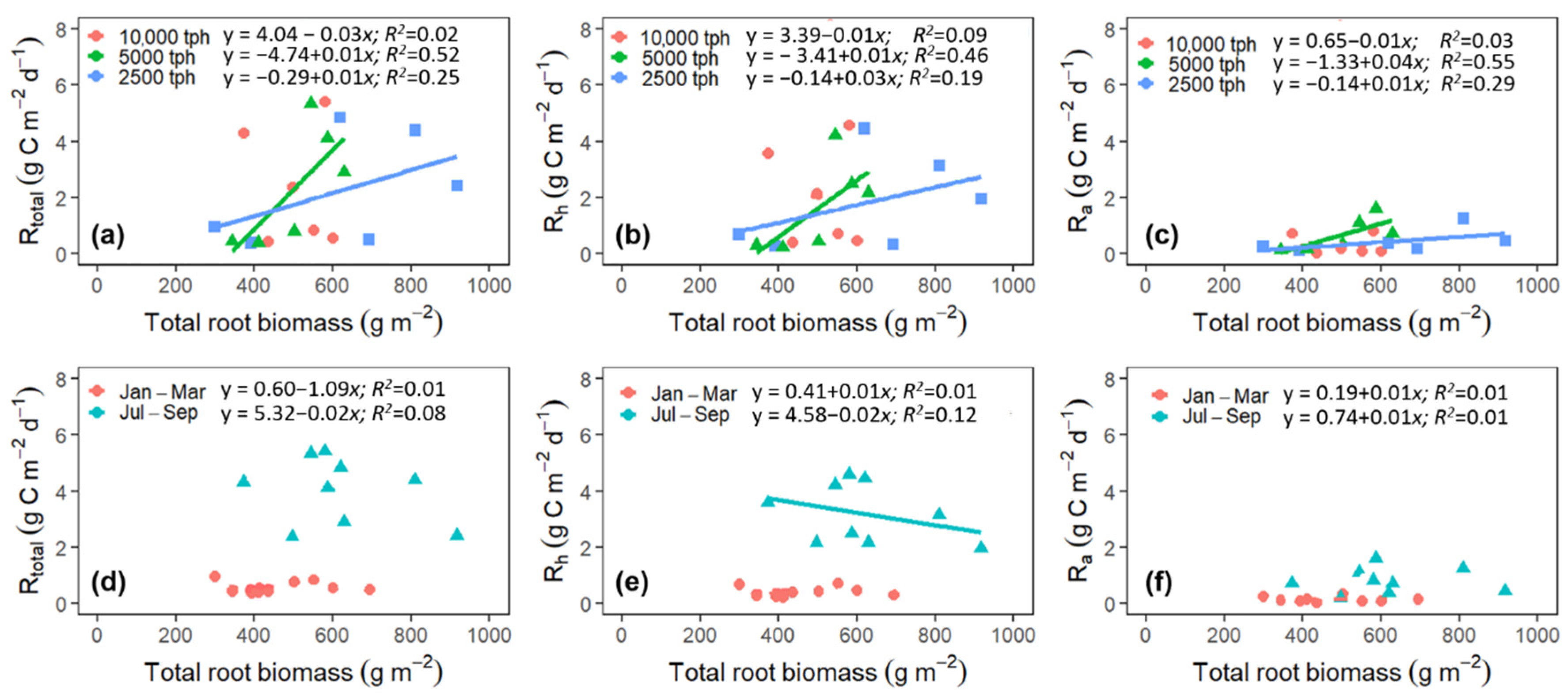
3.4. Environmental Drivers of Rtotal, Rh, and Ra
3.5. Plant-Mediated Drivers of Rtotal, Rh, and Ra
4. Discussion
4.1. Limitations and Uncertainties
4.2. Seasonal Variation in Rtotal, Rh, and Ra
4.3. Variation in Rtotal, Rh, and Ra as Affected by Planting Density
4.4. The Contribution of Rh and Ra to Rtotal
4.5. Responses of Rtotal, Rh, and Ra to Soil Temperature and Water Content
4.6. Responses of Rtotal, Rh, and Ra to Root Biomass and Litterfall
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manolis, E.N.; Zagas, T.D.; Poravou, C.A.; Zagas, D.T. Biomass Assessment for Sustainable Bioenergy Utilization in a Mediterranean Forest Ecosystem in Northwest Greece. Ecol. Eng. 2016, 91, 537–544. [Google Scholar] [CrossRef]
- Lee, R.A.; Lavoie, J.M. From First- to Third-Generation Biofuels: Challenges of Producing a Commodity from a Biomass of Increasing Complexity. Anim. Front. 2013, 3, 6–11. [Google Scholar] [CrossRef]
- Ross, D.J.; Tate, K.R.; Scott, N.A.; Wilde, R.H.; Rodda, N.J.; Townsend, J.A. Afforestation of Pastures with Pinus Radiata Influences Soil Carbon and Nitrogen. Aust. J. Soil Res. 2002, 40, 1303–1318. [Google Scholar] [CrossRef]
- McLaughlin, S.B.; Walsh, M.E. Evaluating Environmental Consequences of Producing Herbaceous Crops for Bioenergy. Biomass Bioenergy 1998, 14, 317–324. [Google Scholar] [CrossRef]
- Liptow, C.; Tillman, A.M.; Janssen, M.; Wallberg, O.; Taylor, G.A. Ethylene Based on Woody Biomass—What Are Environmental Key Issues of a Possible Future Swedish Production on Industrial Scale. Int. J. Life Cycle Assess. 2013, 18, 1071–1081. [Google Scholar] [CrossRef]
- Brinks, J.S.; Lhotka, J.M.; Barton, C.D.; Warner, R.C.; Agouridis, C.T. Effects of Fertilization and Irrigation on American Sycamore and Black Locust Planted on a Reclaimed Surface Mine in Appalachia. For. Ecol. Manag. 2011, 261, 640–648. [Google Scholar] [CrossRef]
- Ghezehei, S.B.; Nichols, E.G.; Hazel, D.W. Early Clonal Survival and Growth of Poplars Grown on North Carolina Piedmont and Mountain Marginal Lands. Bioenergy Res. 2016, 9, 548–558. [Google Scholar] [CrossRef]
- Davis, A.A.; Trettin, C.C. Sycamore and Sweetgum Plantation Productivity on Former Agricultural Land in South Carolina. Biomass Bioenergy 2006, 30, 769–777. [Google Scholar] [CrossRef]
- Bouwman, A.F. Biology and Fertility of Soils: Editorial. Biol. Fertil. Soils 1998, 30, 269. [Google Scholar] [CrossRef]
- Veenendaal, E.M.; Kolle, O.; Lloyd, J. Seasonal Variation in Energy Fluxes and Carbon Dioxide Exchange for a Broad-Leaved Semi-Arid Savanna (Mopane Woodland) in Southern Africa. Glob. Change Biol. 2004, 10, 318–328. [Google Scholar] [CrossRef]
- Kane, E.S.; Valentine, D.W.; Schuur, E.A.G.; Dutta, K. Soil Carbon Stabilization along Climate and Stand Productivity Gradients in Black Spruce Forests of Interior Alaska. Can. J. For. Res. 2005, 35, 2118–2129. [Google Scholar] [CrossRef]
- Boone, R.D.; Nadelhoffert, K.J.; Canary, J.D.; Kaye, J.P. Sensitivity of Soil Respiration. Nature 1998, 396, 570–572. [Google Scholar] [CrossRef]
- Epron, D.; Le Dantec, V.; Dufrene, E.; Granier, A. Seasonal Dynamics of Soil Carbon Dioxide Efflux and Simulated Rhizosphere Respiration in a Beech Forest. Tree Physiol. 2001, 21, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Hanson, P.J.; Edwards, N.T.; Garten, C.T.; Andrews, J.A. Separating Root and Soil Microbial Contributions to Soil Respiration: A Review of Mehtods and Observations. Biogeochemistry 2000, 48, 115–146. [Google Scholar] [CrossRef]
- Raich, J.W.; Tufekcioglu, A. Vegetation and Soil Respiration: Correlations and Controls. Biogeochemistry 2000, 48, 71–90. [Google Scholar] [CrossRef]
- Li, X.; Fu, H.; Guo, D.; Li, X.; Wan, C. Partitioning Soil Respiration and Assessing the Carbon Balance in a Setaria Italica (L.) Beauv. Cropland on the Loess Plateau, Northern China. Soil Biol. Biochem. 2010, 42, 337–346. [Google Scholar] [CrossRef]
- Jia, B.; Zhou, G.; Wang, F.; Wang, Y.; Yuan, W.; Zhou, L. Partitioning Root and Microbial Contributions to Soil Respiration in Leymus Chinensis Populations. Soil Biol. Biochem. 2006, 38, 653–660. [Google Scholar] [CrossRef]
- Subke, J.A.; Inglima, I.; Cotrufo, M.F. Trends and Methodological Impacts in Soil CO2 Efflux Partitioning: A Metaanalytical Review. Glob. Change Biol. 2006, 12, 921–943. [Google Scholar] [CrossRef]
- Aguilos, M.; Takagi, K.; Liang, N.; Watanabe, Y.; Teramoto, M.; Goto, S.; Takahashi, Y.; Mukai, H.; Sasa, K. Sustained Large Stimulation of Soil Heterotrophic Respiration Rate and Its Temperature Sensitivity by Soil Warming in a Cool-Temperate Forested Peatland. Tellus Ser. B Chem. Phys. Meteorol. 2013, 65, 20792. [Google Scholar] [CrossRef]
- Lloyd, J.; Taylor, J.A. On the Temperature Dependence of Soil Respiration. 1994. Available online: http://www.Jstor.Org/Stable/2389824 (accessed on 15 December 2021).
- Raich, J.W.; Schlesinger, W.H. The Global Carbon Dioxide Flux in Soil Respiration and Its Relationship to Vegetation and Climate. Tellus B 1992, 44, 81–99. [Google Scholar] [CrossRef]
- Aguilos, M.; Takagi, K.; Liang, N.; Watanabe, Y.; Goto, S.; Takahashi, Y.; Mukai, H.; Sasa, K. Soil Warming in a Cool-Temperate Mixed Forest with Peat Soil Enhanced Heterotrophic and Basal Respiration Rates but Q10 Remained Unchanged. Biogeosciences Discuss. 2011, 8, 6415–6445. [Google Scholar] [CrossRef]
- Davidson, E.A.; Verchot, L.V.; Cattanio, J.H.; Ackerman, I.L.; Carvalho, J.E.M. Effects of Soil Water Content on Soil Respiration in Forests and Cattle Pastures of Eastern Amazonia. Biogeochemistry 2000, 48, 53–69. [Google Scholar] [CrossRef]
- Curiel Yuste, J.; Ma, S.; Baldocchi, D.D. Plant-Soil Interactions and Acclimation to Temperature of Microbial-Mediated Soil Respiration May Affect Predictions of Soil CO2 Efflux. Biogeochemistry 2009, 98, 127–138. [Google Scholar] [CrossRef]
- Xu, M.; Qi, Y. Spatial and Seasonal Variations of Q10 Determined by Soil Respiration Measurements at a Sierra Nevadan Forest. Glob. Biogeochem. Cycles 2001, 15, 687–696. [Google Scholar] [CrossRef]
- Gaumont-Guay, D.; Black, T.A.; Griffis, T.J.; Barr, A.G.; Jassal, R.S.; Nesic, Z. Interpreting the Dependence of Soil Respiration on Soil Temperature and Water Content in a Boreal Aspen Stand. Agric. For. Meteorol. 2006, 140, 220–235. [Google Scholar] [CrossRef]
- Reichstein, M.; Beer, C. Soil Respiration across Scales: The Importance of a Model-Data Integration Framework for Data Interpretation. J. Plant Nutr. Soil Sci. 2008, 171, 344–354. [Google Scholar] [CrossRef]
- Sheng, H.; Yang, Y.; Yang, Z.; Chen, G.; Xie, J.; Guo, J.; Zou, S. The Dynamic Response of Soil Respiration to Land-Use Changes in Subtropical China. Glob. Change Biol. 2010, 16, 1107–1121. [Google Scholar] [CrossRef]
- Reichstein, M.; Rey, A.; Freibauer, A.; Tenhunen, J.; Valentini, R.; Banza, J.; Casals, P.; Cheng, Y.; Grünzweig, J.M.; Irvine, J.; et al. Modeling Temporal and Large-Scale Spatial Variability of Soil Respiration from Soil Water Availability, Temperature and Vegetation Productivity Indices. Glob. Biogeochem. Cycles 2003, 17, 1–15. [Google Scholar] [CrossRef]
- Raich, J.W.; Potter, C.S.; Bhagawati, D. Interannual Variability in Global Soil Respiration, 1980–1994. Glob. Change Biol. 2002, 8, 800–812. [Google Scholar] [CrossRef]
- Janssens, I.A.; Lankreijer, H.; Matteucci, G.; Kowalski, A.S.; Buchmann, N.; Epron, D.; Pilegaard, K.; Kutsch, W.; Longdoz, B.; Grünwald, T.; et al. Productivity Overshadows Temperature in Determining Soil and Ecosystem Respiration across European Forests. Glob. Change Biol. 2001, 7, 269–278. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Bronson, D.; Bladyka, E.; Gower, S.T. Corrigendum to “A Comparison of Trenched Plot Techniques for Partitioning Soil Respiration” [Soil Biol. Biochem. 43 (10) (2011) 2108-2114]. Soil Biol. Biochem. 2012, 47, 220. [Google Scholar] [CrossRef]
- Aguilos, M.; Takagi, K.; Liang, N.; Ueyama, M.; Fukuzawa, K.; Nomura, M.; Kishida, O.; Fukazawa, T.; Takahashi, H.; Kotsuka, C.; et al. Dynamics of Ecosystem Carbon Balance Recovering from a Clear-Cutting in a Cool-Temperate Forest. Agric. For. Meteorol. 2014, 197, 26–39. [Google Scholar] [CrossRef]
- Li, X.; King, J.S. An Improved Method for Measuring the Production, Mortality and Decomposition of Extramatrical Mycelia of Ectomycorrhizal Fungi in Forests. Soil Biol. Biochem. 2018, 116, 360–368. [Google Scholar] [CrossRef]
- Remaury, A.; Guittonny, M.; Rickson, J. The Effect of Tree Planting Density on the Relative Development of Weeds and Hybrid Poplars on Revegetated Mine Slopes Vulnerable to Erosion. New For. 2019, 50, 555–572. [Google Scholar] [CrossRef]
- Mead, D.J. Opportunities for Improving Plantation Productivity. How Much? How Quickly? How Realistic? Biomass Bioenergy 2005, 28, 249–266. [Google Scholar] [CrossRef]
- Rabot, E.; Wiesmeier, M.; Schlüter, S.; Vogel, H.J. Soil Structure as an Indicator of Soil Functions: A Review. Geoderma 2018, 314, 122–137. [Google Scholar] [CrossRef]
- McIvor, I.R.; Douglas, G.B.; Benavides, R. Coarse Root Growth of Veronese Poplar Trees Varies with Position on an Erodible Slope in New Zealand. Agrofor. Syst. 2009, 76, 251–264. [Google Scholar] [CrossRef]
- Domec, J.C.; Ashley, E.; Fischer, M.; Noormets, A.; Boone, J.; Williamson, J.C.; King, J.S. Productivity, Biomass Partitioning, and Energy Yield of Low-Input Short-Rotation American Sycamore (Platanus Occidentalis, L.) Grown on Marginal Land: Effects of Planting Density and Simulated Drought. Bioenergy Res. 2017, 10, 903–914. [Google Scholar] [CrossRef]
- Berhongaray, G.; Janssens, I.A.; King, J.S.; Ceulemans, R. Fine Root Biomass and Turnover of Two Fast-Growing Poplar Genotypes in a Short-Rotation Coppice Culture. Plant Soil 2013, 373, 269–283. [Google Scholar] [CrossRef]
- McElligott, K.M.; Seiler, J.R.; Strahm, B.D. Partitioning Soil Respiration across Four Age Classes of Loblolly Pine (Pinus Taeda, L.) on the Virginia Piedmont. For. Ecol. Manag. 2016, 378, 173–180. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2021. [Google Scholar]
- Fang, C.; Moncrieff, J.B.; Gholz, H.L.; Clark, K.L. Soil CO2 Efflux and Its Spatial Variation in a Florida Slash Pine Plantation. Plant Soil 1998, 205, 135–146. [Google Scholar] [CrossRef]
- Xu, M.; Qi, Y. Soil-Surface CO2 Efflux and Its Spatial and Temporal Variations in a Young Ponderosa Pine Plantation in Northern California. Glob. Change Biol. 2001, 7, 667–677. [Google Scholar] [CrossRef]
- Wang, C.; Yang, J.; Zhang, Q. Soil Respiration in Six Temperate Forests in China. Glob. Change Biol. 2006, 12, 2103–2114. [Google Scholar] [CrossRef]
- Chang, S.X.; Shi, Z.; Thomas, B.R. Soil Respiration and Its Temperature Sensitivity in Agricultural and Afforested Poplar Plantation Systems in Northern Alberta. Biol. Fertil. Soils 2016, 52, 629–641. [Google Scholar] [CrossRef]
- Gaumont-Guay, D.; Black, T.A.; Griffis, T.J.; Barr, A.G.; Morgenstern, K.; Jassal, R.S.; Nesic, Z. Influence of Temperature and Drought on Seasonal and Interannual Variations of Soil, Bole and Ecosystem Respiration in a Boreal Aspen Stand. Agric. For. Meteorol. 2006, 140, 203–219. [Google Scholar] [CrossRef]
- Chander, K.; Goyal, S.; Nandal, D.P.; Kapoor, K.K. Soil Organic Matter, Microbial Biomass and Enzyme Activities in a Tropical Agroforestry System; Springer-Verlag: Berlin/Heidelberg, Germany, 1998; Volume 27. [Google Scholar]
- Larionova, A.A.; Yevdokimov, I.V.; Kurganova, I.N.; Sapronov, D.V.; Kuznetsova, L.G.; de Lopes Gerenju, V.O. Root Respiration and Its Contribution to the CO2 Emission from Soil. Eurasian Soil Sci. 2003, 36, 173–184. [Google Scholar]
- Chen, C.R.; Condron, L.M.; Xu, Z.H.; Davis, M.R.; Sherlock, R.R. Root, Rhizosphere and Root-Free Respiration in Soils under Grassland and Forest Plants. Eur. J. Soil Sci. 2006, 57, 58–66. [Google Scholar] [CrossRef]
- King, J.S.; Albaugh, T.J.; Lee Allen, H.; Buford, M.; Strain, B.R.; Dougherty, P. Below-Ground Carbon Input to Soil Is Controlled by Nutrient Availability and Fine Root Dynamics in Loblolly Pine. New Phytol. 2002, 154, 389–398. [Google Scholar] [CrossRef]
- Aguilos, M.; Sun, G.; Noormets, A.; Domec, J.C.; McNulty, S.; Gavazzi, M.; Prajapati, P.; Minick, K.J.; Mitra, B.; King, J. Ecosystem Productivity and Evapotranspiration Are Tightly Coupled in Loblolly Pine (Pinus Taeda l.) Plantations along the Coastal Plain of the Southeastern, U.S. Forests 2021, 12, 1123. [Google Scholar] [CrossRef]
- Hutyra, L.R.; Munger, J.W.; Hammond-Pyle, E.; Saleska, S.R.; Restrepo-Coupe, N.; Daube, B.C.; de Camargo, P.B.; Wofsy, S.C. Resolving Systematic Errors in Estimates of Net Ecosystem Exchange of CO2 and Ecosystem Respiration in a Tropical Forest Biome. Agric. For. Meteorol. 2008, 148, 1266–1279. [Google Scholar] [CrossRef]
- Moyano, F.E.; Kutsch, W.L.; Rebmann, C. Soil Respiration Fluxes in Relation to Photosynthetic Activity in Broad-Leaf and Needle-Leaf Forest Stands. Agric. For. Meteorol. 2008, 148, 135–143. [Google Scholar] [CrossRef]
- Liguori, G.; Gugliuzza, G.; Inglese, P. Evaluating Carbon Fluxes in Orange Orchards in Relation to Planting Density. J. Agric. Sci. 2009, 147, 637–645. [Google Scholar] [CrossRef]
- Martin, D.; Beringer, J.; Hutley, L.B.; McHugh, I. Carbon Cycling in a Mountain Ash Forest: Analysis of below Ground Respiration. Agric. For. Meteorol. 2007, 147, 58–70. [Google Scholar] [CrossRef]
- Zeng, X.; Song, Y.; Zeng, C.; Zhang, W.; He, S. Partitioning Soil Respiration in Two Typical Forests in Semi-Arid Regions, North China. Catena 2016, 147, 536–544. [Google Scholar] [CrossRef]
- Raich, J.W.; Tufekcioglu, A. Vegetation and Soil Respiration: Correlations and Controls; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; Volume 48. [Google Scholar]
- Gupta, S.R.; Singh, J.S. Soil Respiration in a Tropical Grassland Soil Biology and Biochemistry; Elsevier: Amsterdam, The Netherlands, 1981; Volume 13, 4, pp. 261–268. [Google Scholar] [CrossRef]
- Hashimoto, S.; Tanaka, N.; Suzuki, M.; Inoue, A.; Takizawa, H.; Kosaka, I.; Tanaka, K.; Tantasirin, C.; Tangtham, N. Soil Respiration and Soil CO2 Concentration in a Tropical Forest, Thailand. J. For. Res. 2004, 9, 75–79. [Google Scholar] [CrossRef]
- Rustad, L.E.; Fernandez, I.J. Experimental Soil Warming Effects on CO2 and CH4 Flux from a Low Elevation Spruce-Fir Forest Soil in Maine, USA. Glob. Change Biol. 1998, 4, 597–605. [Google Scholar] [CrossRef]
- Menichetti, L.; Reyes Ortigoza, A.L.; García, N.; Giagnoni, L.; Nannipieri, P.; Renella, G. Thermal Sensitivity of Enzyme Activity in Tropical Soils Assessed by the Q10 and Equilibrium Model. Biol. Fertil. Soils 2015, 51, 299–310. [Google Scholar] [CrossRef]
- Suseela, V.; Conant, R.T.; Wallenstein, M.D.; Dukes, J.S. Effects of Soil Moisture on the Temperature Sensitivity of Heterotrophic Respiration Vary Seasonally in an Old-Field Climate Change Experiment. Glob. Change Biol. 2012, 18, 336–348. [Google Scholar] [CrossRef]
- Yang, J.; Tian, H.; Pan, S.; Chen, G.; Zhang, B.; Dangal, S. Amazon Drought and Forest Response: Largely Reduced Forest Photosynthesis but Slightly Increased Canopy Greenness during the Extreme Drought of 2015/2016. Glob. Change Biol. 2018, 24, 1919–1934. [Google Scholar] [CrossRef]
- Aguilos, M.; Mitra, B.; Noormets, A.; Minick, K.; Prajapati, P.; Gavazzi, M.; Sun, G.; McNulty, S.; Li, X.; Domec, J.C.; et al. Long-Term Carbon Flux and Balance in Managed and Natural Coastal Forested Wetlands of the Southeastern USA. Agric. For. Meteorol. 2020, 288, 108022. [Google Scholar] [CrossRef]
- Raich, J.W.; Potter, S. Global Patterns of Carbon Dioxide Emissions from Soils. Glob. Biogeochem. Cycles 1995, 9, 23–36. [Google Scholar] [CrossRef]
- Aguilos, M.; Sun, G.; Noormets, A.; Domec, J.C.; McNulty, S.; Gavazzi, M.; Minick, K.; Mitra, B.; Prajapati, P.; Yang, Y.; et al. Effects of Land-Use Change and Drought on Decadal Evapotranspiration and Water Balance of Natural and Managed Forested Wetlands along the Southeastern US Lower Coastal Plain. Agric. For. Meteorol. 2021, 303, 108381. [Google Scholar] [CrossRef]
- Linn, D.M.; Doran, J.W. Effect of Water-Filled Pore Space on Carbon Dioxide and Nitrous Oxide Production in Tilled and Nontilled Soils. Soil Sci. Soc. Am. J. 1984, 48, 1267–1272. [Google Scholar] [CrossRef]
- Bronson, D.R.; Gower, S.T.; Tanner, M.; Linder, S.; van Herk, I. Response of Soil Surface CO2 Flux in a Boreal Forest to Ecosystem Warming. Glob. Change Biol. 2008, 14, 856–867. [Google Scholar] [CrossRef]
- Ryan, M.G.; Law, B.E. Interpreting, Measuring, and Modeling Soil Respiration. Biogeochemistry 2005, 73, 3–27. [Google Scholar] [CrossRef]
- Tang, J.; Baldocchi, D.D.; Xu, L. Tree Photosynthesis Modulates Soil Respiration on a Diurnal Time Scale. Glob. Change Biol. 2005, 11, 1298–1304. [Google Scholar] [CrossRef]
- Aguilos, M.; Hérault, B.; Burban, B.; Wagner, F.; Bonal, D. What Drives Long-Term Variations in Carbon Flux and Balance in a Tropical Rainforest in French Guiana? Agric. For. Meteorol. 2018, 253, 114–123. [Google Scholar] [CrossRef]
- Brando, P.M.; Goetz, S.J.; Baccini, A.; Nepstad, D.C.; Beck, P.S.A.; Christman, M.C. Seasonal and Interannual Variability of Climate and Vegetation Indices across the Amazon. Proc. Natl. Acad. Sci. USA 2010, 107, 14685–14690. [Google Scholar] [CrossRef]
- Hutyra, L.R.; Munger, J.W.; Saleska, S.R.; Gottlieb, E.; Daube, B.C.; Dunn, A.L.; Amaral, D.F.; de Camargo, P.B.; Wofsy, S.C. Seasonal Controls on the Exchange of Carbon and Water in an Amazonian Rain Forest. J. Geophys. Res. Biogeosciences 2007, 112, 1–16. [Google Scholar] [CrossRef]
- Aguilos, M.; Stahl, C.; Burban, B.; Hérault, B.; Courtois, E.; Coste, S.; Wagner, F.; Ziegler, C.; Takagi, K.; Bonal, D. Interannual and Seasonal Variations in Ecosystem Transpiration and Water Use Efficiency in a Tropical Rainforest. Forests 2019, 10, 14. [Google Scholar] [CrossRef]
- Ile, O.J.; Aguilos, M.; Morkoc, S.; Minick, K.; Domec, J.C.; King, J.S. Productivity of Low-Input Short-Rotation Coppice American Sycamore (Platanus Occidentalis, L.) Grown at Different Planting Densities as a Bioenergy Feedstock over Two Rotation Cycles. Biomass Bioenergy 2021, 146, 105983. [Google Scholar] [CrossRef]
- Shi, W.Y.; Du, S.; Morina, J.C.; Guan, J.H.; Wang, K.B.; Ma, M.G.; Yamanaka, N.; Tateno, R. Physical and Biogeochemical Controls on Soil Respiration along a Topographical Gradient in a Semiarid Forest. Agric. For. Meteorol. 2017, 247, 1–11. [Google Scholar] [CrossRef]
- Davidson, E.A.; Savage, K.; Bolstad, P.; Clark, D.A.; Curtis, P.S.; Ellsworth, D.S.; Hanson, P.J.; Law, B.E.; Luo, Y.; Pregitzer, K.S.; et al. Belowground Carbon Allocation in Forests Estimated from Litterfall and IRGA-Based Soil Respiration Measurements. Agric. For. Meteorol. 2002, 113, 39–51. [Google Scholar] [CrossRef]
| Soil Respiration (g C m−2 d−1) | Climate variables | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rtotal | Soil temp. (°C) | SWC (%) | ||||||||||||||||||
| Season | ||||||||||||||||||||
| Jan–Mar | 0.60 | ± | 0.21 | b | 0.43 | ± | 0.17 | c | 0.17 | ± | 0.18 | b | 8.86 | ± | 0.85 | b | 53.11 | ± | 4.52 | a |
| Apr–Jun | 3.50 | ± | 1.09 | a | 2.21 | ± | 1.28 | ab | 1.28 | ± | 0.85 | a | 19.84 | ± | 0.99 | a | 45.30 | ± | 6.41 | a |
| Jul–Sep | 4.01 | ± | 1.11 | a | 3.20 | ± | 0.81 | ab | 0.18 | ± | 0.60 | ab | 22.62 | ± | 0.76 | a | 15.59 | ± | 7.07 | b |
| Oct–Dec | 1.42 | ± | 0.29 | b | 1.11 | ± | 0.31 | bc | 0.21 | ± | 0.20 | b | 11.77 | ± | 0.36 | b | 33.03 | ± | 3.88 | c |
| Mean | 2.38 | ± | 0.67 | 1.74 | ± | 0.64 | 0.47 | ± | 0.46 | 15.77 | ± | 0.74 | 36.76 | ± | 5.47 | |||||
| Planting Density | ||||||||||||||||||||
| 10,000 tph | 2.41 | ± | 0.75 | a | 1.93 | ± | 0.64 | a | 0.48 | ± | 0.34 | a | 15.61 | ± | 0.70 | a | 34.27 | ± | 4.11 | a |
| 5000 tph | 2.43 | ± | 0.64 | a | 1.57 | ± | 0.39 | b | 0.86 | ± | 0.51 | b | 15.87 | ± | 0.71 | a | 38.61 | ± | 5.98 | a |
| 2500 tph | 2.30 | ± | 0.63 | a | 1.72 | ± | 0.40 | ab | 0.59 | ± | 0.53 | ab | 15.83 | ± | 0.80 | a | 37.41 | ± | 6.33 | a |
| Mean | 2.38 | ± | 0.67 | 1.74 | ± | 0.47 | 0.64 | ± | 0.46 | 15.77 | ± | 0.74 | 36.76 | ± | 5.47 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morkoc, S.; Aguilos, M.; Noormets, A.; Minick, K.J.; Ile, O.; Dickey, D.A.; Hardesty, D.; Kerrigan, M.; Heitman, J.; King, J. Environmental and Plant-Derived Controls on the Seasonality and Partitioning of Soil Respiration in an American Sycamore (Platanus occidentalis) Bioenergy Plantation Grown at Different Planting Densities. Forests 2022, 13, 1286. https://doi.org/10.3390/f13081286
Morkoc S, Aguilos M, Noormets A, Minick KJ, Ile O, Dickey DA, Hardesty D, Kerrigan M, Heitman J, King J. Environmental and Plant-Derived Controls on the Seasonality and Partitioning of Soil Respiration in an American Sycamore (Platanus occidentalis) Bioenergy Plantation Grown at Different Planting Densities. Forests. 2022; 13(8):1286. https://doi.org/10.3390/f13081286
Chicago/Turabian StyleMorkoc, Suna, Maricar Aguilos, Asko Noormets, Kevan J. Minick, Omoyemeh Ile, David A. Dickey, Deanna Hardesty, Maccoy Kerrigan, Joshua Heitman, and John King. 2022. "Environmental and Plant-Derived Controls on the Seasonality and Partitioning of Soil Respiration in an American Sycamore (Platanus occidentalis) Bioenergy Plantation Grown at Different Planting Densities" Forests 13, no. 8: 1286. https://doi.org/10.3390/f13081286
APA StyleMorkoc, S., Aguilos, M., Noormets, A., Minick, K. J., Ile, O., Dickey, D. A., Hardesty, D., Kerrigan, M., Heitman, J., & King, J. (2022). Environmental and Plant-Derived Controls on the Seasonality and Partitioning of Soil Respiration in an American Sycamore (Platanus occidentalis) Bioenergy Plantation Grown at Different Planting Densities. Forests, 13(8), 1286. https://doi.org/10.3390/f13081286








