Abstract
The CBL-interacting protein kinases’ (CIPKs) gene family plays an important role in plants under salt stress. In this study, a total of 31 PtrCIPK genes were identified in poplar. CIPKs’ gene family was divided into two categories, few intron classes and multi-intron classes. They all have the core components of the kinase domain and regulatory domain unique to the CIPK gene family and share most of the same motifs. PtrCIPKs have 17 fragment repeat events and have high homology with Arabidopsis thaliana and Betula platyphylla, and partial homology with Zea mays. Prediction of cis-acting elements found that the PtrCIPK gene family has the most elements in terms of stress. Under NaCl stress, all members of poplar CIPKs’ gene family were significantly expressed. There were fourteen up-regulated genes and four down-regulated genes. Candidate gene expression was significantly higher in the phloem than in other tissues. In this study, characterization of CBL-interacting protein kinases’ gene family and expression pattern reveal their important roles in response to salt stress in poplar.
1. Introduction
CIPKs are plant-specific serine/threonine protein kinases and belong to the plant SnRK3 protein kinase subfamily. CIPKs proteins specifically bind to calcineurin B-like proteins (CBLs) and constitute a network of regulating plant growth and development and signal response to stress under the mediation of Ca2+ [1,2].
Salt stress can lead to ionic stress, osmotic stress and secondary stresses in plants. Therefore, to adapt to salt stress, plants rely on signals and pathways that re-establish cellular ionic, osmotic and reactive oxygen species (ROS) homeostasis [3]. CIPK proteins are key regulators of various ion channel proteins. Plants change ion content and distribution in response to salt stress [4]. In Arabidopsis the complex formed by CBL1/9-CIPK23 controls the K+ channel protein AKT1 [5] and the NO3− ion channel protein CHL [6]. CBL2/3−CIPK3/9/26 and CIPK5 complexes maintain manganese homeostasis in vivo by controlling the ion transporter MTP8 on the plasma membrane [7]. The CBL−CIPK complex also regulates transporters on the tonoplast. The CBL10−CIPK24 complex regulates a Na+ transporter that transfers excess intracellular Na+ into the vacuole [8].
It has been confirmed that CIPK genes have a certain response to NaCl treatment in some plants. SlCIPKs of tomatoes (Solanum lycopersicum) were up-regulated to varying degrees under 200 mM NaCl treatment compared with the control group [9]. Eighteen MtCIPKs in Medicago (Medicago truncatula) were significantly changed in gene expression levels under NaCl treatment [10]. In sugarcane (Saccharum spontaneum) the expression levels of SsCIPK1, SsCIPK2 and SsCIPK28 were significantly up-regulated under salt stress [11]. Therefore, study of the CIPKs’ gene family is essential to understanding ion content imbalance in plants under salt stress. However, since CIPKs’ gene family has many members with different gene functions, we need to identify salt tolerance genes from family members and explore the possibility of creating salt tolerant varieties using genetic engineering. Therefore, understanding the gene function of CIPK protein families has become a hot research topic.
Poplar is mostly used in wood processing, pulp production and environmental protection, and has important economic and ecological value. However, most varieties are sensitive to salt stress and unable to plant in large areas of saline−alkali land. Although CIPKs’ gene family has been studied on Arabidopsis and other annual crops, few studies have been done on woody plants under salt stress. Therefore, we studied CIPKs’ gene family in poplar and compared its gene structure, promoter elements and evolutionary relationship with other species. We examined the response of CIPK gene expression levels to salt stress. These significantly up-regulated genes could be used to create salt tolerant cultivars and provide insight into understanding the role of CIPKs’ family in response to salt stress.
2. Materials and Methods
2.1. Plant Materials
The differentiated tissue culture plants of Populus. alba × P. berolinensis were rooted in 1/2 MS medium with 0.5 mg/L IBA. After 21 days, rooted plants were transplanted and grown in a mixture substrate (V peat soil: V vermiculite: V perlite = 6:3:1) supplied with MS nutrient solution. After another 21 days, plants with the same growth vigor were selected for subsequent NaCl stress treatment. The untreated plants were transplanted and grown in 16 cm × 16 cm flowerpots with the same substrate. After 2 months, plants with a height of 50 cm were selected and the young leaves, mature leaves, petioles, xylem, phloem and roots (Figure S1) were stored at −80 °C. Each sample was replicated three times. The growing condition of light time was 16 h light/8 h dark with a light intensity of 1800 Lx and temperature of 25 °C. As for NaCl stress treatment, plants were treated with three replications of 30 mL of 200 mM NaCl solution at 0 h, 3 h, 6 h, 12 h and 24 h. The treated whole plant was used for qRT-PCR. Samples were collected in foil packs and snap-frozen in liquid nitrogen for subsequent RNA extraction and qRT-PCR analysis.
2.2. Identification of PtrCIPK Gene Family Members and Structural Analysis
The protein sequences of CIPKs’ gene family, obtained from Arabidopsis as queries, were used to search against the P. trichocarpa genome (https://www.ncbi.nlm.nih.gov/genome/98 (accessed on 20 January 2022)) by BLASTP. The genes with both kinase and regulatory domains were identified as members of the poplar CIPK family. The Arabidopsis genome was downloaded from TAIR (https://www.arabidopsis.org/ (accessed on 20 January 2022)).
A phylogenetic tree was constructed from the identified protein sequences of Arabidopsis and poplar CIPKs using MEGA7 software [12]. The protein sequences of PtrCIPKs were aligned using Clustal X [13]. The motif structure of PtrCIPKs was analyzed on the MEME website (https://meme-suite.org/meme/doc/meme.html (accessed on 24 January 2022)), and the number of motifs was set to 15. Visual analysis of gene structure was done by using TBtools [14].
Collinearity analysis was performed with McScanX software [15], and visualization was performed with TBtools. The maize genome used for the inter-species collinearity analysis was downloaded from NCBI (https://www.ncbi.nlm.nih.gov/genome/?term=Zea+mays (accessed on 25 January 2022)), and the birch genome was downloaded from Phytozome (https://phytozome-next.jgi.doe.gov/info/Bplatyphylla_v1_1 (accessed on 25 January 2022)).
The promoter selection range was the first 1 kb of the gene, and then the PlantCARE website (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/ (accessed on 26 January 2022)) was used to predict cis-acting elements; the predicted elements were classified and then TBtools were used to create the graph.
2.3. qRT-PCR Analysis
Total RNA was extracted using the extraction kit (Beijing Biotech Biotechnology Co., Ltd., Beijing, China). The quality of total RNA was examined by 1% agarose gel electrophoresis and NanoDrop 10,000 (Thermo Company, Waltham, MA, USA). RNA was reverse-transcribed into cDNA with the reverse transcription kit (Shanghai Toyobo Biotechnology Co., Ltd., Shanghai, China). Real-time quantitative PCR was used 2 × SYBR-Green (Toyobo, Osaka, Japan). UBQ was used as a reference gene. Significance analysis was performed using the one-way ANOVA and results were analyzed using Duncan. Experiments were performed in three biological replicates. The specific primer sequences are shown in the supplementary material (Table S1). The relative expression was calculated by 2−ΔΔCT. The expression histogram was made by Prism8.
3. Results
3.1. Identification of PtrCIPK Family Genes
To identify PtrCIPK family genes from poplar, using Arabidopsis CIPK gene families as queries and 31 protein sequences of CIPK candidate genes, PtrCIPKs were obtained after alignment and screening in the P. trichocarpa genome. These sequences contain both kinase and regulatory domains and the length is between 386 and 549 amino acids. The theoretical isoelectric point is between 6.228 and 9.429. The amino acid molecular mass is between 43.79 and 61.39 kDa (Table 1).

Table 1.
Features of PtrCIPK genes in poplar.
3.2. Analysis of PtrCIPKs’ Phylogenetic Tree, Gene Structure, Motif and Conserved Domain
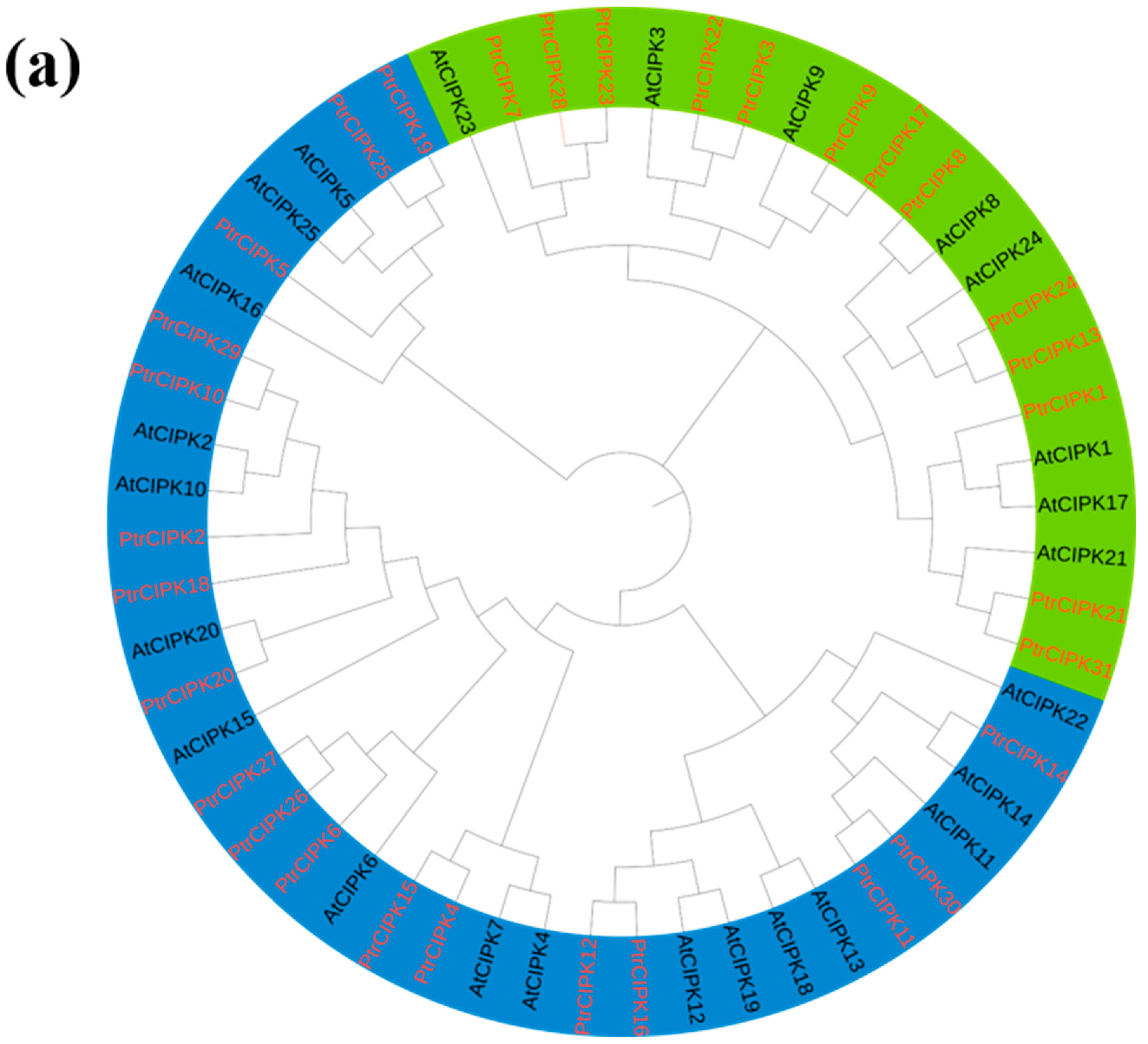
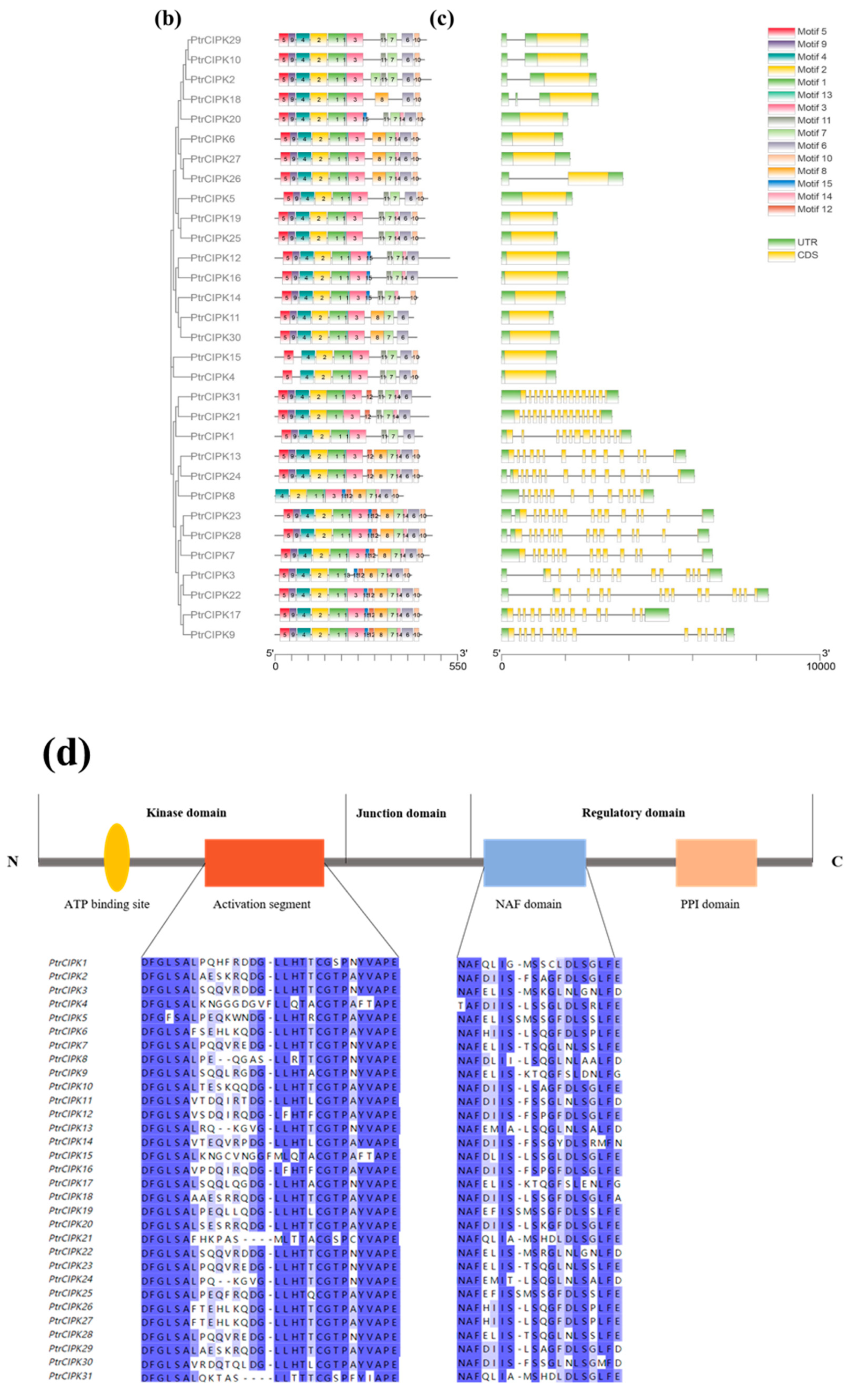
To further study the evolutionary relationship of the PtrCIPKs gene family, a phylogenetic tree was constructed using protein sequences of the Arabidopsis and poplar CIPK gene families (Figure 1a). PtrCIPKs were divided into two groups consistent with the gene structure grouping (Figure 1c). The first group of gene structures with multiple introns includes PtrCIPK1, 3, 7, 8, 9, 13, 17, 21, 22, 23, 24, 28, 31, and the second group of gene structures without introns includes PtrCIPK2, 4, 5, 6, 10, 11, 12, 14, 15, 16, 18, 19, 20, 25, 26, 27, 29, 30.
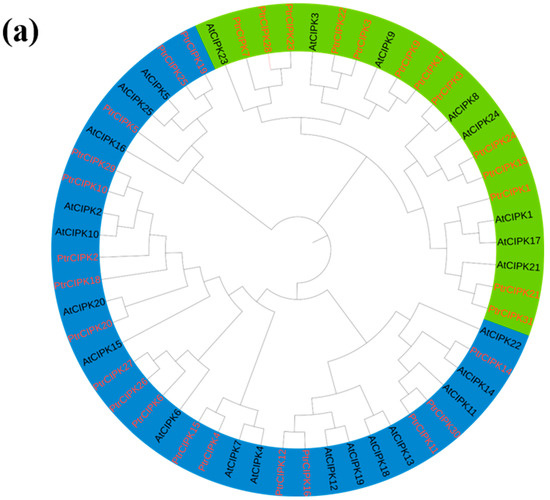
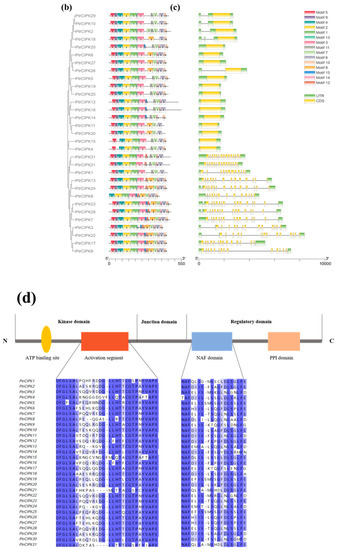
Figure 1.
PtrCIPKs’ phylogenetic tree and basic structure. (a) Poplar and Arabidopsis CIPKs’ phylogenetic tree. (b) PtrCIPKs’ motif structure analysis results. (c) PtrCIPKs’ gene structure. Gene lengths and motif distributions of PtrCIPKs are shown to scale. (d) Conserved domains of amino acid sequences of PtCIPKs.
To understand the structure of PtrCIPKs, we further studied their gene structure, motifs and conserved domains. The motif structures of PtrCIPKs were analyzed by the MEME online website, and each gene contained 10 to 15 motifs (Figure 1b). We found that 1, 2, 3 and 4 motifs were present in all PtrCIPKs, and 5, 6 and 7 motifs were present in 30 motifs. PtrCIPK8, PtrCIPK14 and PtrCIPK18 did not contain motif5, motif6 and motif7, respectively. Analysis of the conserved domains of PtrCIPKs revealed that PtrCIPKs contained the activation loop portion of the kinase domain of the CIPK gene family and the NAF structure of the regulatory domain (Figure 1d). It suggests that the identified 31 genes have similar structures and perform similar functions.
3.3. Chromosome Localization and Collinearity Analysis
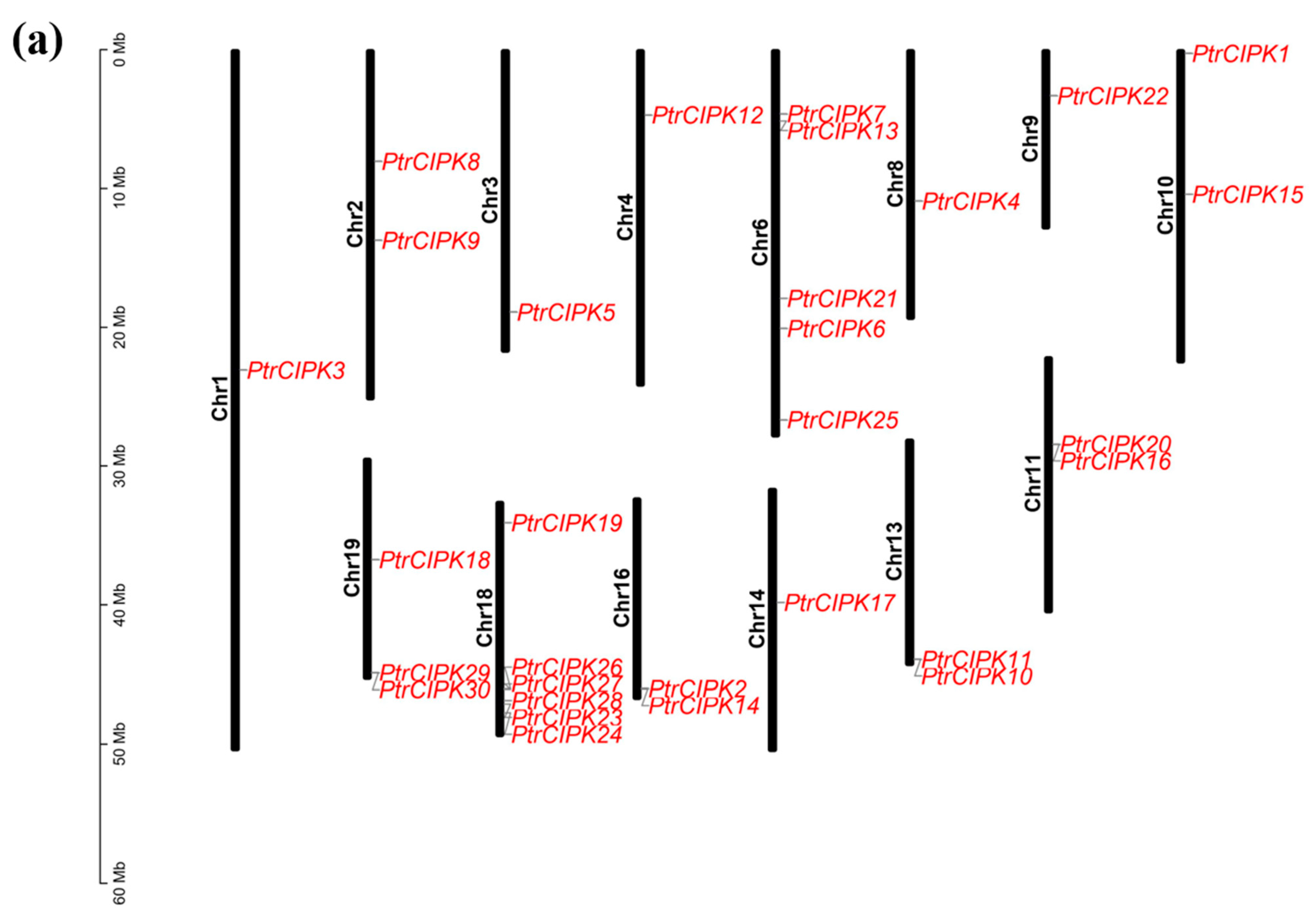
To study the positional relationship of PtrCIPK genes on chromosomes, identification of gene locations on chromosomes was carried out. PtrCIPKs are unevenly distributed on 14 chromosomes, of which Chr1, 3, 4, 8, 9 and 14 have one gene, Chr2, 10, 11, 13 and 16 have two genes, Chr19 has three genes, Chr6 has five genes and Chr18 contains six PtrCIPK genes (Figure 2a).
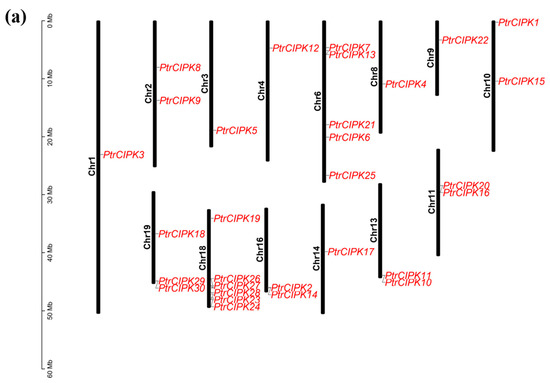
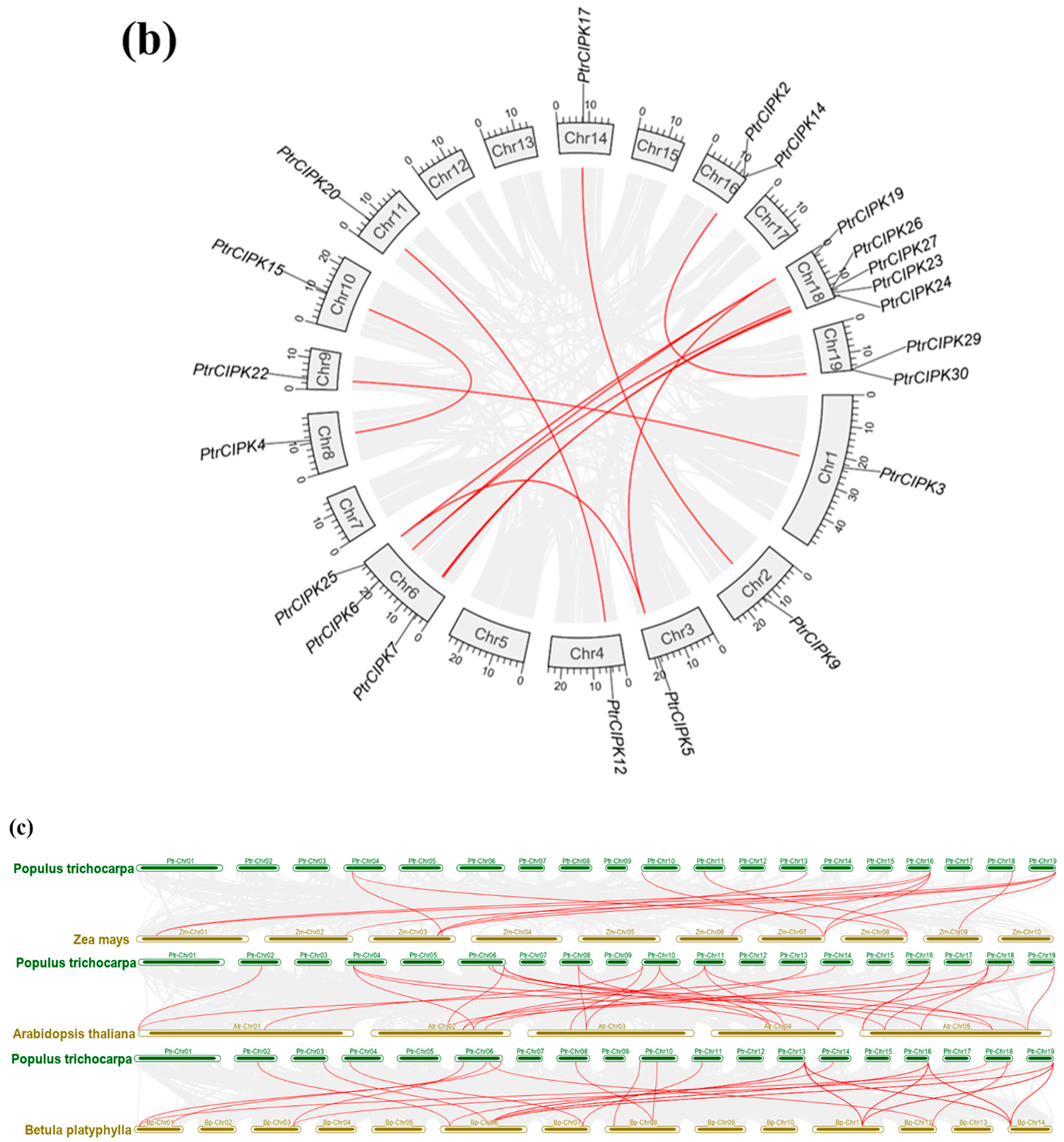
Figure 2.
Collinearity analysis results. (a) Chromosome location map. Gene names are in red font. Chromosome lengths are shown to scale. (b) Intra-species collinearity analysis. The gray lines represent segment duplication events across the genome, and the red lines represent segment duplication events in the CIPK gene family. (c). Inter-species collinearity analysis. The grey lines represent fragment duplication events between the two genomes, and the red lines represent fragment duplication events in the CIPK gene family.
To study the duplication of the PtrCIPK gene family, 31 PtrCIPKs were found to have 17 segment duplications (Figure 2b) and five segment duplications between Chr6 and Chr18 by intra-species collinearity analysis. PtrCIPKs have multiple segmental duplications in the whole genome, indicating that segmental duplication is one of the main expansion methods of the PtrCIPK gene family.
Colinear alignment was done between species using Arabidopsis, birch and maize together with poplar genomes. Results showed that the homology of CIPK genes in birch, Arabidopsis and poplar was high. There were 27 homologous genes between poplar and Arabidopsis, 24 homologous genes between poplar and birch and 12 homologous genes between maize and poplar (Figure 2c). It shows that the CIPK gene family is relatively conservative and formed before the differentiation of monocotyledonous plants, but some genes are also divergent between monocotyledonous plants and dicotyledonous plants.
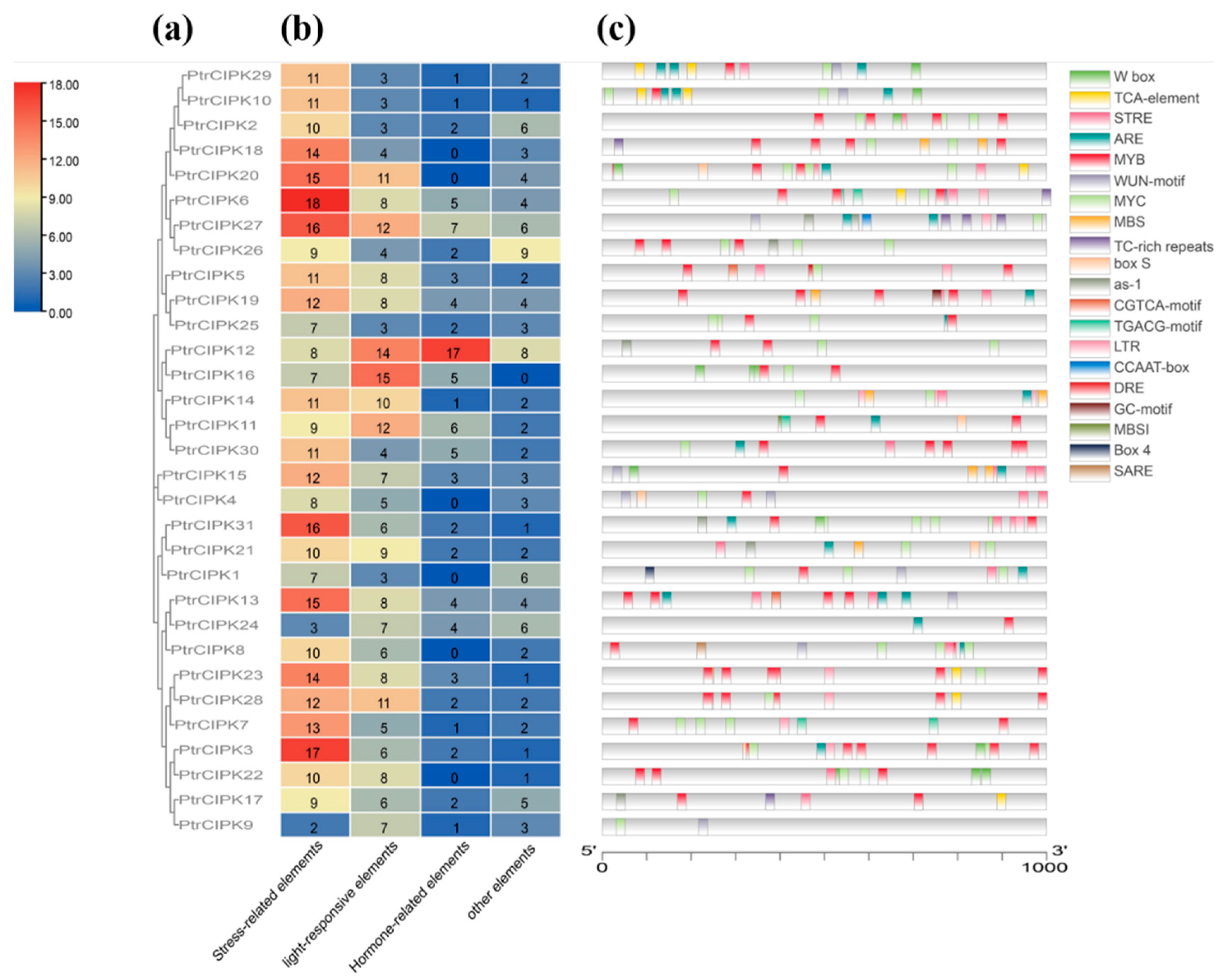
3.4. Prediction of Cis-Acting Elements
Prediction of cis-acting elements on the PlantCARE site using the first 1 kb of the promoter region. The obtained elements were divided into four parts: stress-related elements, light-responsive elements, hormone-responsive elements and other elements. According to the classification results, PtrCIPKs contained the majority of stress-related elements (Figure 3b), mainly MYB, MYC, TGACG-motif, TC-rich repeats and LTR elements (Figure 3c). A total of 749 effective elements were obtained, of which 338 were stress-related elements, accounting for 45%. The result indicated that PtrCIPKs responded to the stress environment.
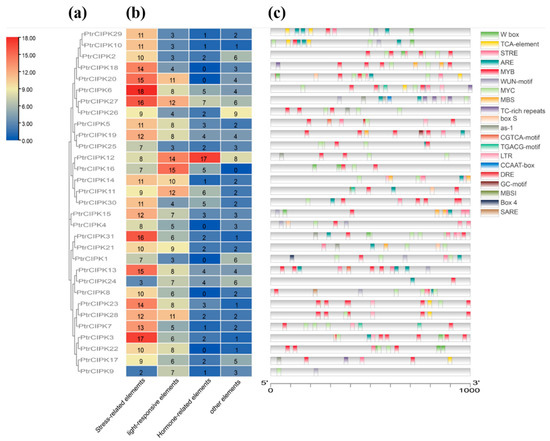
Figure 3.
PtrCIPKs’ promoter prediction analysis. (a) PtrCIPKs’ phylogenetic tree. (b) Heat map of promoter numbers. (c) Distribution of environmental stress-related components.
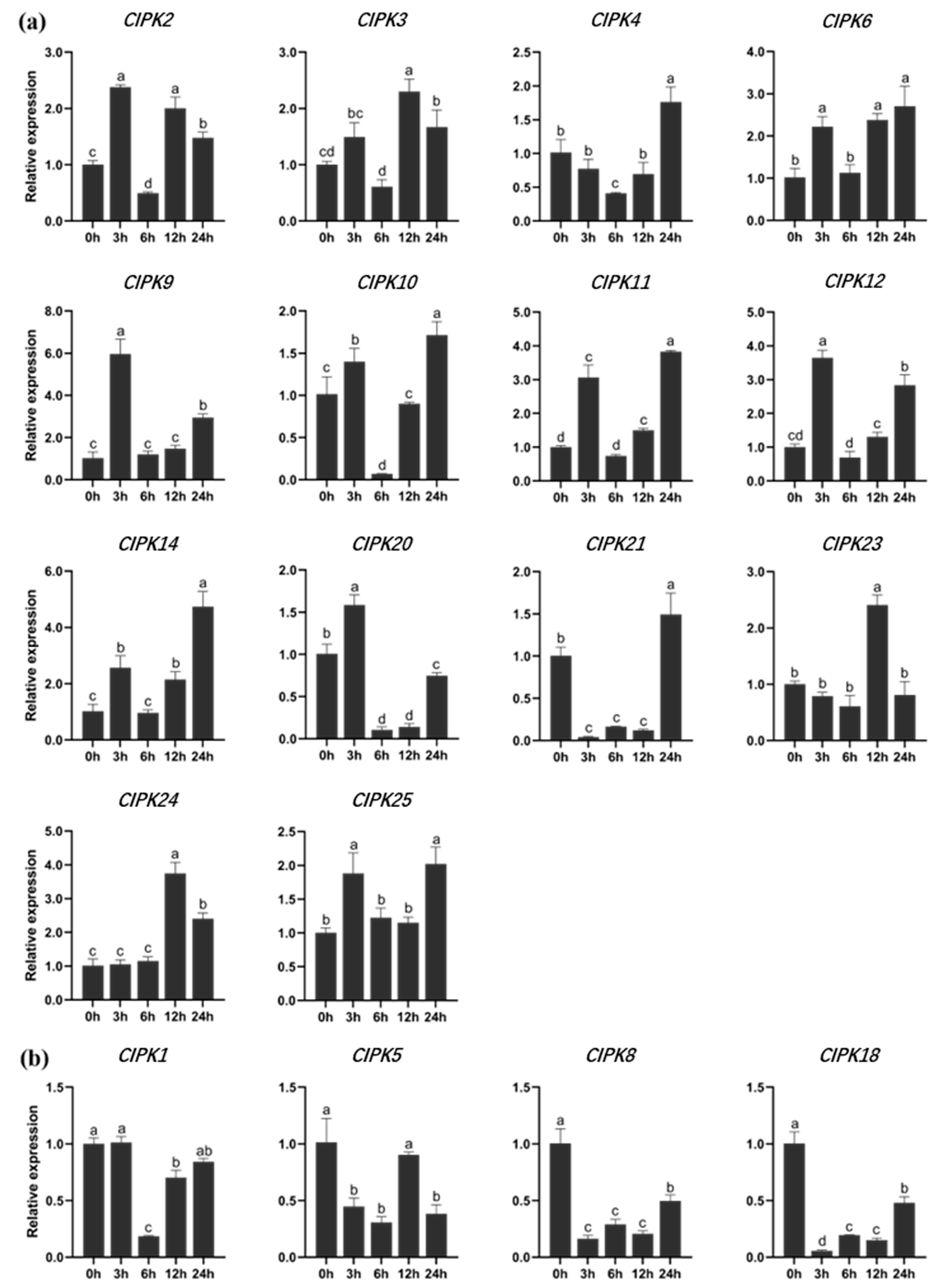
3.5. Response of PtrCIPKs under NaCl Stress
Eighteen genes were selected for gene expression analysis. Plants (P. alba × P. berolinensis) treated with NaCl solution showed that the gene expression of PtrCIPKs changed significantly. Among them, 14 genes were up-regulated (Figure 4a). The gene expression of CIPK9, CIPK11, CIPK12, CIPK14 and CIPK24 were increased more than 3.5 times. CIPK1, CIPK5, CIPK8 and CIPK18 showed an overall downward trend (Figure 4b). It indicated that PtrCIPKs had different degrees of response to salt stress.
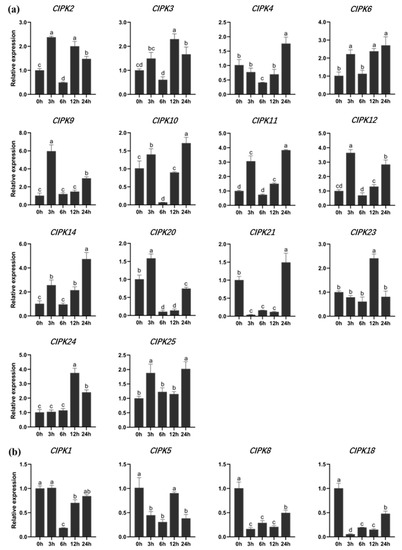
Figure 4.
Gene expression of CIPKs in response to salt stress in P. alba × P. berolinensis. (a) PtrCIPKs gene with high expression under salt stress. (b) PtrCIPKs gene with reduced expression under salt stress. Different lowercase letters indicate significant differences (p < 0.05).
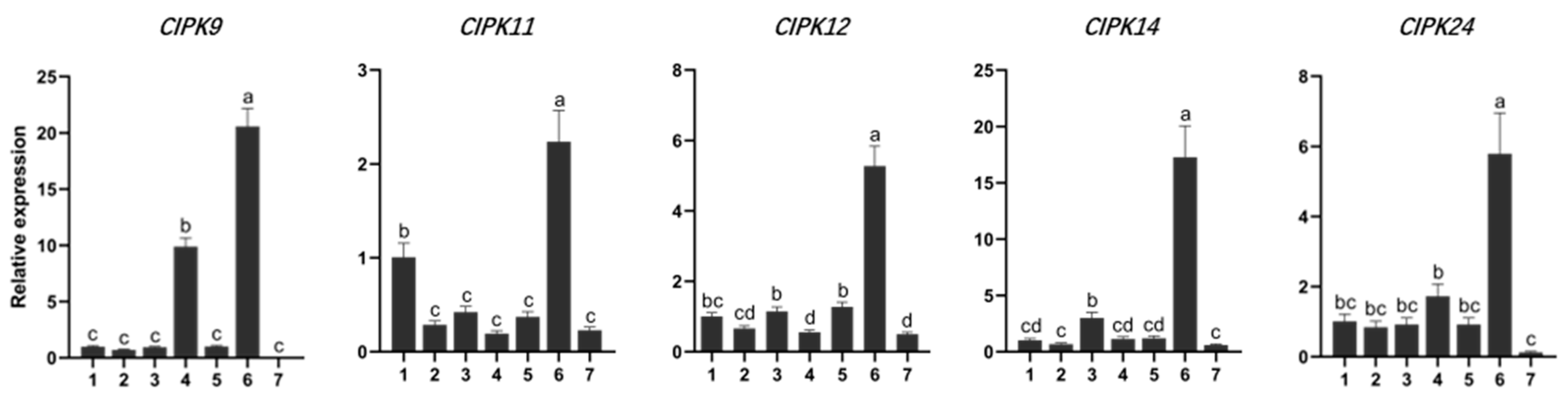
3.6. PtrCIPK Genes Were Expressed Differently in Different Types of Tissues
Tissue expression-specific analysis was performed on genes whose expression changed more than 3.5 times under salt stress. Five genes (CIPK9, 11, 12, 14, 24) were significantly changed in different tissues in response to salt stress. The expression level of CIPK9 in mature leaves was significantly higher than that in other tissues except for phloem (Figure 5). The expression level of CIPK11 in roots was also significantly higher than that in other tissues except phloem (Figure 5). This demonstrates that the CIPK gene family is involved in ion exchange transport in poplar phloem. Moreover, the expression level of CIPKs in young stems is not prominent, which may be due to more active CIPK genes in mature phloem tissue.
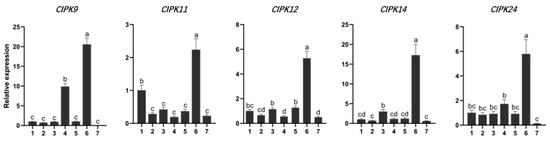
Figure 5.
Gene expression of CIPKs in different tissues. Root is the reference sample for the tissue-based qRT-PCR. One root, two stems, three young leaves, four mature leaves, five petioles, six phloem, seven xylem. Different lowercase letters indicate significant differences (p < 0.05).
4. Discussion
In this study, we found that poplar with the genome size of 380 Mb has 31 CIPK genes. Based on gene structure, the CIPK family was divided into the multi-intron class and intron-free class. Compared with few intron genes, multi-intron genes may have more potential in evolution and function, but the reasons for the differentiation of genes within the CIPK gene family into two categories need to be further explored. The numbers of genes in the CIPK family are 27, 31 and 34 for Arabidopsis, wheat and apple, respectively [16,17], and their genome sizes are 125 Mb, 14.5 Gb and 742 Mb [18,19,20], respectively. However, the CIPK family classification results of the above plants were consistent with that of poplar. Our results show that the number of CIPK genes is independent of genome size in different species.
There are multiple fragment duplication events in PtrCIPKs, and the number of fragment duplications of CIPK gene families is different in plant species. In our study using intra-species collinear analysis, it was found that PtrCIPKs were unevenly distributed on 19 chromosomes of poplar and had 17 segmental duplications. This result was consistent with the chromosome segmental duplications in the whole genome [21]. In two cultivated allopolyploid cotton varieties, the CIPK gene family has a large number of segmental repeats that are basically present in all family genes [22]; the same is true in sugar cane and soybeans [11,23]. However, the number of repeats of CIPKs in grapevine (Vitis vinifera) and medicago is very small, only three to four [10,24]. This may be due to the rate of gene loss and fragment retention in whole genome replication as a result of environmental selection in different species.
Poplar CIPK gene families are highly homologous to Arabidopsis and birch and partially homologous to corn. Compared with the genomes of Arabidopsis thaliana, corn and birch, it was found that there were 27 pairs of homologous genes between poplar CIPKs and the Arabidopsis genome, and 24 pairs of homologous genes between the birch genome. This proves that CIPKs of poplar have high gene homology with Arabidopsis and birch. This may be because poplar, Arabidopsis and birch belong to the rose branch [25]. The previous results showed that overexpression of the PtrCIPK24 gene in Arabidopsis sos2 mutants can restore it to normal [26]. There are 43 CIPK genes in the corn genome, 12 CIPK genes are homologous to poplar and these 12 genes also have homologous genes in Arabidopsis and birch. However, whether these genes function similarly requires further study.
In this study, the gene expression levels of all poplar CIPKs were significantly changed under salt stress. There are many Wbox and MYB binding regions in the promoter region of most PtrCIPKs. Therefore, PtrCIPKs may be regulated by upstream WRKY and MYB genes under salt stress. There were two gene expression patterns: up-regulated and down-regulated expression. Among them, fourteen genes were up-regulated and four genes were down-regulated. This indicates that poplar CIPK genes are involved in the salt stress response. In addition, when CIPK functions as a protein kinase, it usually needs to be phosphorylated by the upstream CBL protein to function. The effect of phosphorylation on the function of CIPK proteins will be investigated in future experiments. In our tissue expression-specific study, five genes (CIPK9, 11, 12, 14, 24) were expressed more than 3.5 times. The gene expressions in phloem were higher than that in other tissues under salt stress. It may be due to the transport of concentrated ions and water in the phloem. Previous studies also found that the five CIPK genes directly or indirectly improve plant salt tolerance. For example, AtCIPK9 targets VDAC3 and regulates oxidative stress in Arabidopsis [27], and also regulates the high-affinity K+ transporter HAK5 [28]. In addition, high expression of NtCIPK9 in the halophyte white thorn increases the salt tolerance of Arabidopsis [29]. AtCIPK11 can enhance drought and salt tolerance in Arabidopsis [30,31]. AtCIPK14 is a key regulator of C/N nutrient responses in Arabidopsis [32]. CIPK24(SOS2) has been extensively studied in salt tolerance [33,34,35]. In apple, medicago, corn and other plants, CIPKs have a large number of gene expressions up-regulated under salt stress. Our results are consistent with these previous studies.
5. Conclusions
In conclusion, we characterized the CBL-interacting protein kinases’ gene family and expression pattern. The results reveal the expression pattern of CIPKs in response to salt stress in poplar. Some CIPKs genes could become excellent candidates for the creation of salt tolerant trees by using biotechnology tools.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f13091353/s1, Table S1: PtrCIPKs gene primers; Figure S1: Poplar tissue for qPCR.
Author Contributions
G.L. and X.B. conceived and designed the research. X.B. and J.J. (Jiaobao Ji) conducted the experiments. C.G. and W.W. analyzed the data. X.B. wrote the manuscript. J.J. (Jing Jiang) and Q.Y. checked the article and provided advice. C.Y. guided the research method. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Safety Monitoring of Pilotscale Experiment of Transgenic Populus simonii × P. nigra (KJZXSA202002) and Heilongjiang Touyan Innovation Team Program (Tree Genetics and Breeding Innovation Team).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Verma, P.; Sanyal, S.K.; Pandey, G.K. Ca2+-CBL-CIPK: A Modulator System for Efficient Nutrient Acquisition. Plant Cell Rep. 2021, 40, 2111–2122. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, Q.-H.; Yu, Y.-N.; Qiao, Y.-M.; Haq, S.U.; Gong, Z.-H. The CBL-CIPK Pathway in Plant Response to Stress Signals. Int. J. Mol. Sci. 2020, 21, 5668. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-F.; Han, G.-L.; Yang, Z.-R.; Li, Y.-X.; Wang, B.-S. Plant Salinity Sensors: Current Understanding and Future Directions. Front. Plant Sci. 2022, 13, 859224. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.-J.; Wang, C.; Li, K.; Luan, S. The CBL-CIPK Calcium Signaling Network: Unified Paradigm from 20 Years of Discoveries. Trends Plant Sci. 2020, 25, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; He, M.; Guo, J.; Zeng, H.; Wei, Y.; Liu, G.; Hu, W.; Shi, H. The CBL1/9-CIPK23-AKT1 Complex Is Essential for Low Potassium Response in Cassava. Plant Physiol. Biochem. PPB 2021, 167, 430–437. [Google Scholar] [CrossRef]
- Ho, C.H.; Frommer, W.B. Fluorescent sensors for activity and regulation of the nitrate transceptor CHL1/NRT1.1 and oligopeptide transporters. Elife 2014, 3, e01917. [Google Scholar] [CrossRef]
- Ju, C.; Zhang, Z.; Deng, J.; Miao, C.; Wang, Z.; Wallrad, L.; Javed, L.; Fu, D.; Zhang, T.; Kudla, J.; et al. Ca2+-Dependent Successive Phosphorylation of Vacuolar Transporter MTP8 by CBL2/3-CIPK3/9/26 and CPK5 Is Critical for Manganese Homeostasis in Arabidopsis. Mol. Plant 2022, 15, 419–437. [Google Scholar] [CrossRef]
- Kim, B.-G.; Waadt, R.; Cheong, Y.H.; Pandey, G.K.; Dominguez-Solis, J.R.; Schültke, S.; Lee, S.C.; Kudla, J.; Luan, S. The Calcium Sensor CBL10 Mediates Salt Tolerance by Regulating Ion Homeostasis in Arabidopsis. Plant J. Cell Mol. Biol. 2007, 52, 473–484. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, X.; Liu, S.; Yu, A.; Yang, C.; Chen, X.; Liu, J.; Wang, A. Identification and Functional Analysis of Tomato CIPK Gene Family. Int. J. Mol. Sci. 2019, 21, 110. [Google Scholar] [CrossRef]
- Du, W.; Yang, J.; Ma, L.; Su, Q.; Pang, Y. Identification and Characterization of Abiotic Stress Responsive CBL-CIPK Family Genes in Medicago. Int. J. Mol. Sci. 2021, 22, 4634. [Google Scholar] [CrossRef]
- Su, W.; Ren, Y.; Wang, D.; Huang, L.; Fu, X.; Ling, H.; Su, Y.; Huang, N.; Tang, H.; Xu, L.; et al. New Insights into the Evolution and Functional Divergence of the CIPK Gene Family in Saccharum. BMC Genom. 2020, 21, 868. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X Version 2.0. Bioinform. Oxf. Engl. 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A Toolkit for Detection and Evolutionary Analysis of Gene Synteny and Collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Sun, T.; Wang, Y.; Wang, M.; Li, T.; Zhou, Y.; Wang, X.; Wei, S.; He, G.; Yang, G. Identification and Comprehensive Analyses of the CBL and CIPK Gene Families in Wheat (Triticum Aestivum L.). BMC Plant Biol. 2015, 15, 269. [Google Scholar] [CrossRef]
- Niu, L.; Dong, B.; Song, Z.; Meng, D.; Fu, Y. Genome-Wide Identification and Characterization of CIPK Family and Analysis Responses to Various Stresses in Apple (Malus domestica). Int. J. Mol. Sci. 2018, 19, 2131. [Google Scholar] [CrossRef]
- Zapata, L.; Ding, J.; Willing, E.-M.; Hartwig, B.; Bezdan, D.; Jiao, W.-B.; Patel, V.; James, G.V.; Koornneef, M.; Ossowski, S.; et al. Chromosome-Level Assembly of Arabidopsis Thaliana Ler Reveals the Extent of Translocation and Inversion Polymorphisms. Proc. Natl. Acad. Sci. USA 2016, 113, E4052–E4060. [Google Scholar] [CrossRef]
- Zhu, T.; Wang, L.; Rimbert, H.; Rodriguez, J.C.; Deal, K.R.; De Oliveira, R.; Choulet, F.; Keeble-Gagnère, G.; Tibbits, J.; Rogers, J.; et al. Optical Maps Refine the Bread Wheat Triticum Aestivum Cv. Chinese Spring Genome Assembly. Plant J. Cell Mol. Biol. 2021, 107, 303–314. [Google Scholar] [CrossRef]
- Velasco, R.; Zharkikh, A.; Affourtit, J.; Dhingra, A.; Cestaro, A.; Kalyanaraman, A.; Fontana, P.; Bhatnagar, S.K.; Troggio, M.; Pruss, D.; et al. The Genome of the Domesticated Apple (Malus × domestica Borkh.). Nat. Genet. 2010, 42, 833–839. [Google Scholar] [CrossRef]
- Tuskan, G.A.; Difazio, S.; Jansson, S.; Bohlmann, J.; Grigoriev, I.; Hellsten, U.; Putnam, N.; Ralph, S.; Rombauts, S.; Salamov, A.; et al. The Genome of Black Cottonwood, Populus Trichocarpa (Torr. & Gray). Science 2006, 313, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Su, Y.; Wang, J.; Jia, B.; Wu, M.; Pei, W.; Zhang, J.; Yu, J. Genome-Wide Characterization and Analysis of CIPK Gene Family in Two Cultivated Allopolyploid Cotton Species: Sequence Variation, Association with Seed Oil Content, and the Role of GhCIPK6. Int. J. Mol. Sci. 2020, 21, 863. [Google Scholar] [CrossRef]
- Zhu, K.; Chen, F.; Liu, J.; Chen, X.; Hewezi, T.; Cheng, Z.-M.M. Evolution of an Intron-Poor Cluster of the CIPK Gene Family and Expression in Response to Drought Stress in Soybean. Sci. Rep. 2016, 6, 28225. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Liu, J.; Dong, C.; Cheng, Z.M. The CBL and CIPK Gene Family in Grapevine (Vitis vinifera): Genome-Wide Analysis and Expression Profiles in Response to Various Abiotic Stresses. Front. Plant Sci. 2017, 9, 978. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, X.; Zhang, N.; Zhang, S.-D.; Yi, T.-S.; Ma, H.; Guo, Z.-H.; Li, D.-Z. Phylogenomic Analyses of Large-Scale Nuclear Genes Provide New Insights into the Evolutionary Relationships within the Rosids. Mol. Phylogenet. Evol. 2016, 105, 166–176. [Google Scholar] [CrossRef]
- Tang, R.-J.; Liu, H.; Bao, Y.; Lv, Q.-D.; Yang, L.; Zhang, H.-X. The Woody Plant Poplar Has a Functionally Conserved Salt Overly Sensitive Pathway in Response to Salinity Stress. Plant Mol. Biol. 2010, 74, 367–380. [Google Scholar] [CrossRef]
- Kanwar, P.; Sanyal, S.K.; Mahiwal, S.; Ravi, B.; Kaur, K.; Fernandes, J.L.; Yadav, A.K.; Tokas, I.; Srivastava, A.K.; Suprasanna, P.; et al. CIPK9 Targets VDAC3 and Modulates Oxidative Stress Responses in Arabidopsis. Plant J. Cell Mol. Biol. 2022, 109, 241–260. [Google Scholar] [CrossRef]
- Lara, A.; Ródenas, R.; Andrés, Z.; Martínez, V.; Quintero, F.J.; Nieves-Cordones, M.; Botella, M.A.; Rubio, F. Arabidopsis K+ Transporter HAK5-Mediated High-Affinity Root K+ Uptake Is Regulated by Protein Kinases CIPK1 and CIPK9. J. Exp. Bot. 2020, 71, 5053–5060. [Google Scholar] [CrossRef]
- Lu, L.; Chen, X.; Zhu, L.; Li, M.; Zhang, J.; Yang, X.; Wang, P.; Lu, Y.; Cheng, T.; Shi, J.; et al. NtCIPK9: A Calcineurin B-Like Protein-Interacting Protein Kinase From the Halophyte Nitraria tangutorum, Enhances Arabidopsis Salt Tolerance. Front. Plant Sci. 2020, 11, 1112. [Google Scholar] [CrossRef]
- Ma, Y.; Cao, J.; Chen, Q.; He, J.; Liu, Z.; Wang, J.; Li, X.; Yang, Y. The Kinase CIPK11 Functions as a Negative Regulator in Drought Stress Response in Arabidopsis. Int. J. Mol. Sci. 2019, 20, 2422. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Chen, X.; Wang, P.; Lu, Y.; Zhang, J.; Yang, X.; Cheng, T.; Shi, J.; Chen, J. CIPK11: A Calcineurin B-like Protein-Interacting Protein Kinase from Nitraria Tangutorum, Confers Tolerance to Salt and Drought in Arabidopsis. BMC Plant Biol. 2021, 21, 123. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, S.; Aoyama, S.; Hasegawa, Y.; Sato, T.; Yamaguchi, J. Arabidopsis CBL-Interacting Protein Kinases Regulate Carbon/Nitrogen-Nutrient Response by Phosphorylating Ubiquitin Ligase ATL31. Mol. Plant 2017, 10, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, C.; Tang, R.-J.; Xu, H.-X.; Lan, W.-Z.; Zhao, F.; Luan, S. Calcineurin B-Like Proteins CBL4 and CBL10 Mediate Two Independent Salt Tolerance Pathways in Arabidopsis. Int. J. Mol. Sci. 2019, 20, 2421. [Google Scholar] [CrossRef] [PubMed]
- Chaves-Sanjuan, A.; Sanchez-Barrena, M.J.; Gonzalez-Rubio, J.M.; Moreno, M.; Ragel, P.; Jimenez, M.; Pardo, J.M.; Martinez-Ripoll, M.; Quintero, F.J.; Albert, A. Structural Basis of the Regulatory Mechanism of the Plant CIPK Family of Protein Kinases Controlling Ion Homeostasis and Abiotic Stress. Proc. Natl. Acad. Sci. USA 2014, 111, E4532–E4541. [Google Scholar] [CrossRef]
- Kumar, G.; Basu, S.; Singla-Pareek, S.L.; Pareek, A. Unraveling the Contribution of OsSOS2 in Conferring Salinity and Drought Tolerance in a High-Yielding Rice. Physiol. Plant. 2022, 174, e13638. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).