Abstract
Climate change increases global average temperatures worldwide. We tested whether temperature during seed maturation in a broadleaved woody perennial may influence the phenological performance of the seedlings. We performed two controlled crosses of Prunus padus L. in two subsequent years (2015 and 2016). Clonal mother shrubs were subjected to a cold and a warm condition during seed maturation. In the first year after germination, the seedlings from the warm seed maturation condition burst their buds earlier compared with the cold condition seedlings. In contrast, in the second and third years, these seedlings burst their buds later. A temporary maternal effect may have advanced bud burst for the warm condition seedlings in the first year, whereas a delay of bud burst in the following years may be caused by a transgenerational epigenetic memory, putatively expressing a stress reaction upon the suboptimal elevated temperature during seed maturation. A warm spring treatment in 2020 enlarged the difference in timing of bud burst between the cold and warm seed maturation conditions in offspring of both crosses, suggesting that the epigenetic memory is more strongly expressed in a warmer spring environment. The timing of the autumnal leaf senescence in the seedlings was not influenced by the temperature during seed maturation in all observation years, suggesting that autumnal senescence is less (epi)genetically determined compared with bud burst and more sensitive to ambient temperatures.
1. Introduction
Climate change becomes a challenge for forest ecosystems. To a certain degree, trees can adapt to a changing environment as they harbor high levels of genetic diversity, allowing natural selection processes, and because of their ability to alter their phenotype upon environmental change (phenotypic plasticity). Local adaptation in populations of trees is a trade-off between gene flow and selective pressure [1]. In addition, being sessile organisms, and because of their long life span and long generation times, local adaptation in trees may be a slower process compared with annuals. Therefore, phenotypic plasticity may be more important for woody species [2].
Bud burst and leaf senescence in woody plants, as naturally recurring events, are two phenophases marking the beginning and the end of the growing season in temperate and boreal zones. The phenophases are strongly influenced by temperature, proving the ability to adapt plastically to variable growth conditions. The phenotypic plasticity in these phenophases allows woody species to acclimate to inter-annual fluctuations in the local climate [3,4,5]. Bud burst and leaf senescence can also adapt to longer-term changes in local climate by natural selection. However, because of their long generation times, this type of adaptation in woody perennials is not expected to keep pace with the predicted speed of climate change [6,7]. Many plant species have already advanced their bud burst due to the current climate warming [8,9,10]. In contrast, the autumnal leaf senescence displays variable responses, both delaying, and advancing upon climate warming [11]. The order of magnitude of these responses is species dependent and also genotype-dependent [12,13].
The local environment and genetics of parental plants as driving factors for the phenological responses of plants have been thoroughly studied in herbaceous plants. It is well known that a mother plant can influence seed traits, including seed size, dormancy, and germination success [14]. Apart from genetics and developmental aspects of the mother plant, the environment during seed production can also play a role in seed germinability, with the temperature being a dominant signal across species [14,15]. Temperature experienced by a parental herbaceous plant, even while still in a vegetative phase, can be remembered and affect later seed properties. In Arabidopsis thaliana, seed dormancy and germination are influenced by a long-term temperature memory, established before fertilization and imprinted in the silique (fruit tissue) [16]. The combined action of temperature and light during seed maturation is well studied, which affects dormancy in Arabidopsis thaliana [17].
Variation in the maternal environment may be transferred by epigenetic mechanisms to the developing embryos, resulting in affected offspring phenotypes. In this way, phenotypic plasticity across generations is also called epigenetic memory [18]. Environmental stress can also induce epigenetic marks in plants that can be inherited by the offspring as a form of pre-adaption [19]. Epigenetic modification, a change in gene expression without any alteration in the DNA sequence, can allow woody species to adapt quickly to a varying environment [18,19]. Because of their ability to adapt quickly to a changing environment, ecologists and evolutionary biologists are mostly interested in transgenerational epigenetic changes [20].
In general, epigenetic modifications through variations in DNA methylation patterns have been difficult to link with phenotypic variation in trees [18]. A valuable example is the study of heritable epigenetic modification, also called adaptive epigenetic memory, in Norway spruce (Picea abies). Budset phenology in Norway spruce was found to be influenced by the temperature during embryogenesis and seed maturation, with a warmer maternal environment during embryogenesis delaying the formation of terminal buds of the next generation [21,22]. The effect is caused by epigenetic modification during embryogenesis [23]. The effect persisted up to 20 years after germination [24]. Temperature changes during somatic embryogenesis triggered alterations in the methylation of DNA and histones [23]. As conifers are characterized by deviating life history traits compared with broadleaves, it has been questioned whether maternal temperature during seed maturation could result in similar responses in deciduous species. In Populus nigra, seedlings resulting from a warmer maternal environment displayed later bud burst and earlier bud set in one of the three controlled crossings [25].
We performed controlled crosses in the common shrub species Prunus padus L. and studied the response of the full-sib seedlings to the maternal temperature during seed development. We hypothesized that the seedlings grown from the seeds that experienced a different maternal temperature during seed maturation (i) would display a different timing of bud burst and leaf senescence and (ii) would respond differently upon a spring temperature treatment.
2. Materials and Methods
2.1. Plant Material and Controlled Crosses
Two P. padus plants from a natural population in Lennik (lat: 50.806017, long: 4.153938), Belgium, were propagated vegetatively by cuttings in 2010. From one plant, 6 clonal replicates were propagated, whereas, from the other plant, only one plant was propagated. For the propagation, vigorous young shoots were cut from the mother plants at the beginning of July. In a standard greenhouse of the Research Institute for Nature and Forest (Geraardsbergen, Belgium), shoots were partitioned into cuttings of 10 cm in length, having at least one leaf with an axillary bud. The cuttings were put in plant propagation trays filled with standard propagation soil (20% organic matter, pH 5.0–6.5, EC 200 µS/cm, dry matter 25%, 1.5 kg/m3 powdered compound fertilizer NPK 12 + 14 + 24). The cuttings were given plentiful water and were covered with thin transparent plastic. To limit the potential growth of fungi, the cover was opened twice a week for two hours in the morning. Once rooted, the plastic cover was taken away. The cuttings stayed in the trays during the winter. In the spring of 2011, the rooted cuttings were transferred to 1 L pots using standard nursery potting soil (20% organic matter, pH 5.0–6.5, EC 450 µS/cm, dry matter 25%, 1.5 kg/m3 powdered compound fertilizer NPK 12 + 14 + 24). They were placed on a container field with automatic watering at the Research Institute for Nature and Forest (Geraardsbergen, Belgium). In the summer of 2011, the young plants were pruned at 30 cm height to stimulate a shrubby habitus. In the spring of 2012, plants were transferred to 4 L pots; in the spring of 2014, they were transferred to 6 L pots, all using standard nursery potting soil.
The six clonal plants were considered mother plants, whereas the single plant was considered the father plant. All plants were transferred to a standard greenhouse in the spring of 2015. Two of the mother plants and the father plant flowered. Before the flowers opened, perforated and transparent plastic bags were put over each inflorescence to keep any insect away from the flowers. Except for the handling of the controlled cross-pollination itself, these plastic bags remained over the inflorescences until all flowers finished blooming. From each individual flower on the mother plants, the petals were manually removed just before the flower opened, and all the stamen were manually removed using a pincet (emasculation). For the next four days, the stigma were gently rubbed with ripe anthers from flowers on the father plant that was flowering at the same time as the mother plants. As not all flowers ripened on the same day, this process was repeated on a daily basis. When all flowers finished blooming, and the controlled crossings were ended, one mother plant remained in the standard greenhouse, whereas the other plant was placed in an adjacent open greenhouse without walls. Grey shade nets operated in both greenhouses, in the same way to protect the plants from heavy insolation. Both conditions are referred to as “cold” and “warm” seed maturation. The plants stayed in these places until the berries ripened, which took place at the end of June and the beginning of July. Mature berries were collected, and the seeds were cleaned and stratified according to standard nursery practices. Seeds were put in plant propagation trays filled with an equal mixture of sand and standard propagation soil, one seed in each cell. This first cross is further called cross X1.
After berries were collected, the two plants were transferred to the container field, where they stayed until the spring of 2016, together with the father plant and the other mother plants. In the spring of 2016, all the plants developed inflorescences. The plants were again moved into the greenhouse, and the second round of crossings was performed in exactly the same way as described above. Six control inflorescences, one on each mother shrub, were included in the experiment. For these control inflorescences, the petals and stamen were removed from the flowers, but no pollination took place. None of the ovaries of these control inflorescences developed into berries. After the controlled crossings, three mother plants were placed in the “warm” standard greenhouse and the other three in the adjacent “cold” open greenhouse. During the seed development and maturation of the mother plants, the temperature difference between the two greenhouses was, on average, 2.74 °C (Figure S1). Because of a technical problem, greenhouse temperatures in 2015 during the cross X1 were not available. However, because it concerned exactly the same greenhouses, we assumed that the temperature difference between the two greenhouses in 2015 was from the same order of magnitude as in 2016. The second cross is referred to as cross X2.
2.2. Growth of Seedlings and Temperature Treatment in the Spring of 2020
In the springs of 2016 and 2017, 10 (6 “cold” and 4 “warm”) and 44 (28 “cold” and 16 ”warm”) seeds germinated from cross X1 and cross X2, respectively. Germinated seedlings stayed in the propagation trays during the growing season. The trays were placed in the open greenhouse. In the succeeding spring (2017 for cross X1 and 2018 for cross X2), the seedlings were transferred to 1 L pots using standard nursery potting soil and remained in the open greenhouse. Plants were randomly intermingled. In the springs of 2018 (cross X1) and 2019 (cross X2), plants were transferred to 4 L pots using standard nursery potting soil. Plants were again randomly intermingled. Because the plants from cross X1 were growing big, they were pruned in June 2018 at 20 cm height.
On the 12th of February 2020, all saplings from the two controlled crosses were divided into two groups (Table 1). Half of the plants were put in a non-heated but frost-free greenhouse, whereas the other group was transferred to a warmed greenhouse at the Flanders Research Institute for Agriculture, Fisheries and Food in Melle, Belgium. The two greenhouses are further referred to as the “cold” and the “warm” condition. During the bud burst process, the temperature difference between the two greenhouses was, on average, 2.71 °C (Figure S2). Plants were watered to full capacity twice a week and remained in the two greenhouses until leaves were fully developed. Then, plants were transferred to a container field and randomly intermingled. Plants were watered with automatic rotating sprinklers.

Table 1.
Observation dates of the phenophases bud burst and leaf senescence.
Because the plants were getting large, they were pruned at 20 cm height at the beginning of June 2020. To bring all saplings to a similar habitus, all side branches were removed so that only a leafless stem remained. On the 28th of June, a single phenological observation of the stage of reflushing from dormant buds was performed (0: no reflushing yet, 1: buds swelling and starting to open, 2: leaves protruding from the buds, 3: leaves unfolding). In the spring of 2021, all plants were transferred to 6 L pots using standard nursery potting soil.
2.3. Phenological Observations
Phenological observations on the plants included both spring bud burst and autumnal leaf senescence. Different developmental stages of bud burst were scored with 1: buds in winter rest, 2: buds swelling, 3: leaves protruding the bud but not yet unfolding, 4: leaves unfolding, and 5: leaves fully unfolded. In addition, for leaf senescence, five stages were scored with 1: leaves green, 2: leaves light green with or without rose color, 3: leaves turning rose to red, 4: leaves turning dark red, and 5: leaves falling off. The phenological observations were performed each year starting from the year of germination (2016 for cross X1 and 2017 for cross X2) until 2021 (Figure 1). The actual dates of observation are indicated in Table 1.
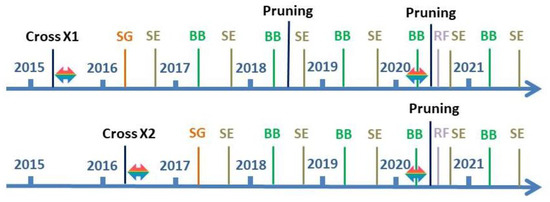
Figure 1.
Timelines indicating the controlled crossings X1 and X2, the temperature treatments during the seed maturation (horizontal two-pointed arrow), seed germination, the phenological observations on the plants, the pruning of the plants, and the temperature treatment in 2020 (horizontal two-pointed arrow). SG: seed germination (orange lines); SE: leaf senescence (brown-green lines); BB: bud burst (green lines); RF: reflushing of dormant buds (light purple lines).
2.4. Statistical Analysis
All statistical analyses were performed in the open-source software R (version 3.6.1, R Core Team, Vienna, Austria) [26]. The phenological scores belonged to an ordinal data type, having ordered levels. The data was modeled with cumulative logistic regression in the R package ordinal [27]. The cumulative probability (p) is the chance to have reached a given score of the phenological variable or a score below this score, so it is the chance to have maximally reached the given score. The bud burst scores were ordered in a reversed way, from the end of the developmental phase to the beginning, which was from fully unfolded leaves up to buds in winter rest (from 5 to 1). The leaf senescence scores were ordered in a normal chronological way, from green leaves to dark red leaves that were falling off (from 1 to 5). The reversed order of the bud burst scores allowed an easier understanding and interpretation of the modeled probabilities. A probability of having reached maximally a bud burst score of, e.g., 3 when the scores are ordered from 5 to 1 is the probability of reaching a score of 5, 4, or 3. Plants with an early bud burst timing have a higher score at a given time and therefore also have a higher modeled probability of having reached a score of 5, 4, or 3.
Mixed models were applied. In the fixed part of the models, the variable C indicated the cross (X1 or X2), the variable Y indicated the number of years after the year in which the seedlings germinated (0, 1, 2, and 3), Ts indicated the temperature during the seed maturation after the controlled crossing of the mother plants (“cold” and “warm”), T20 indicated the temperature treatment of the seedlings in the spring of 2020 (“cold” and “warm”) and D indicated the day of the phenological observations. A unique identity code was present in the random part of the models to account for the different observations on the same plants.
Autumnal leaf senescence from 2016 until 2019 and bud burst from 2017 until 2019 (2 models):
log(p1/(1 − p1)) = αTrp1 − βCp1C − βYp1Y − βCYp1CY − βTsp1Ts − βTsp1TsC − βYTsp1TsY − βDp1D
αTrp1 is an estimated threshold value for passing on from one score of the leaf senescence or bud burst variable to the next. A significant interaction term between the cross C and the number of years after germination Y indicated that the timing of the phenophase in the two crosses differed depending on the number of years after germination. A significant interaction term between the seed maturation temperature Ts and the cross C indicated that the timing of the phenophase for the two temperature regimes during seed maturation differed between the two crosses. A significant interaction term between the number of years after germination Y and the seed maturation temperature Ts indicated that the difference in timing of the phenophase for the two temperature regimes during seed maturation differed between the number of years after germination.
Bud burst and autumnal leaf senescence in 2020 and 2021 (4 models):
log(p2/(2 − p2)) = αTrp2 − βCp2C − βTsp2Ts − βTsp2CTs − βT20p2T20 − βCT20p2CT20 − βTsT20p2TsT20 − βDp2D
αTrp2 is an estimated threshold value for passing on from one score of the bud burst or leaf senescence variable to the next. A significant interaction term between the seed maturation temperature Ts and the spring temperature in 2020 T20 indicated that the difference in timing of the phenophase for the two seed maturation temperature regimes (“cold” and “warm”) differed depending on the spring temperature condition in 2020 (also “cold” and “warm”). A significant interaction term between the seed maturation temperature Ts and the cross C indicated that the difference in timing of the phenophase for the two temperature regimes during seed maturation differed between the two crosses. A significant interaction term between the cross C and the spring temperature in 2020 T20 indicated that the difference in timing of the phenophase for the two crosses differed depending on the spring temperature in 2020.
3. Results
3.1. Influence of Maternal Seed Maturation Temperature in the First Years of the Seedlings
The timing of leaf senescence in the year of germination (2016 for cross X1 and 2017 for cross X2) and in the following years, until 2019, was influenced by the number of years after germination (p-value = 0.025 for Y_1 and p-value = 0.002 for Y_3, Table 2) and by the cross in a year-dependent way (p-value < 0.001 for C_X2:Y_2, Table 2, Figure S3a,b). The timing of leaf senescence did not depend on the seed maturation temperature (no significant p-values for Ts_warm, C_X2:Ts_warm, Y_1:Ts_warm, Y_2:Ts_warm, nor Y_3:Ts_warm, Table 2).

Table 2.
Estimates and p-values for the modeled leaf senescence (2016–2019) and bud burst (2017–2019). The first controlled cross, X1 (C_X1), is the standard to which the second cross is compared (C_X2). The “cold” condition of the seed maturation temperature is the standard condition to which the “warm” (Ts_warm) are compared. For leaf senescence, the year of germination of the seedlings Y_0 (2016 for cross X1 and 2017 for cross X2) is the standard to which the years after germination are compared (Y_1, Y_2, and Y_3). For bud burst, the first year after germination of the seedlings Y_1 (2017 for cross X1 and 2018 for cross X2) is the standard to which the years after this year are compared (Y_2 and Y_3). D is the day of observation.
The timing of bud burst in the second and third year after germination was significantly different from the first year after germination (p-value = 0.003 for second year Y_2 and p-value < 0.001 for third year Y_3, Table 2). The timing of bud burst for the cross X2 differs significantly from X1 (p-value < 0.001 for C_X2, Table 2). More importantly and remarkably, the interaction term between the year after germination and temperature during seed maturation was significant for the second year and the third year after germination (p-value < 0.001 for Y_2:Ts_warm and p-value = 0.027 for Y_3:Ts_warm, Table 2). This indicates that the difference in timing of the bud burst between the cold and the warm seed maturation conditions in the first year after germination was significantly different from this difference in the second and the third year after germination. Figure 2 shows that the timing of bud burst for the warm seed maturation condition is advanced compared with the cold condition in the first year after germination (2017 for cross X1 and 2018 for cross X2). In contrast, in the second and the third year after germination, this is reversed (2018 and 2019 for cross X1 and 2019 for cross X2), with the warm condition displaying a delayed bud burst. Independent of the seed maturation temperature condition, the difference in timing of bud burst in cross X1 and cross X2 is also different between the first and the second year after germination (p-value < 0.001 for C_X2:Y_2, Table 2). This is visible in Figure S4 with, e.g., the blue line (cold seed maturation condition) in the first year after germination for cross X1 being advanced in comparison to the second year after germination, whereas the blue line for cross X2 in the first year after germination is delayed in comparison to the second year after germination.
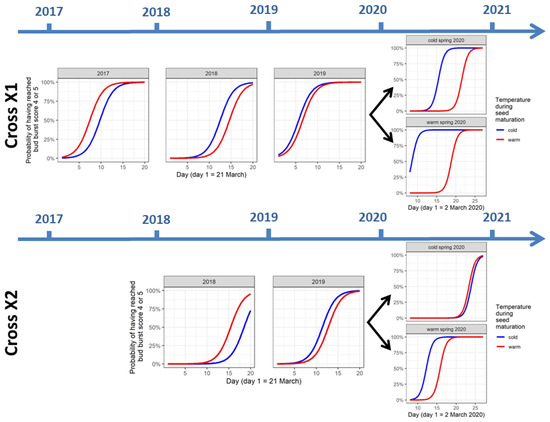
Figure 2.
Modeled timing of bud burst of the seedlings from the crosses X1 and X2 for the first three (X1: 2017, 2018, and 2019) or first two (X2: 2018 and 2019) years after germination and during the spring temperature treatment in 2020, depending on the temperature during seed maturation.
3.2. Influence of a Variable Spring Temperature in 2020 on the Saplings
The temperature treatment in the spring of 2020 clearly influenced the timing of the bud burst of the saplings. As expected, the warm condition of 2020 advanced the bud burst (p-value < 0.001 for T20_warm, Table 3, Figure 2). The temperature during seed maturation still influenced the timing of the bud burst of the saplings in this year, with the warm seed maturation condition displaying a delayed bud burst compared with the cold condition (p-value = 0.003 for Ts_warm, Table 3, Figure 2). Strikingly, the difference in timing of bud burst between the saplings from the cold and warm seed maturation condition was larger in the warm spring temperature condition of 2020 (p-value = 0.013 for Ts_warm: T20_warm, Table 3, Figure 2) and was also larger for cross X1 than for cross X2 (p-value = 0.001 for Ts_warm: C_X2, Table 3, Figure 2). Finally, the difference in timing of bud burst between the two spring temperature conditions in 2020 was different between the saplings from cross X1 and the saplings from cross X2 (p-value < 0.001 for T20_warm: C_X2, Table 3).

Table 3.
Estimates and p-values for the modeled bud burst and leaf senescence in 2020 and 2021. The “cold” condition of the seed maturation temperature is the standard condition to which the “warm” (Ts_warm) conditions are compared. The “cold” temperature treatment of the saplings in 2020 is the standard condition to which the “warm” treatment (T20_warm) is compared. The first cross X1 is the standard to which the second cross (C_X2) is compared. D is the day of observation.
The timing of leaf senescence in 2020 was not influenced anymore by the temperature treatment in the spring of this year (p-value = 0.239 for T20_warm in Table 3). Only a small significant effect was present for the interaction between the maternal seed maturation temperature and the cross, indicating that depending on the cross, the seed maturation temperature still influenced the timing of the leaf senescence (p-value = 0.028 for Ts_warm: C_X2 in Table 3). As the plants from the warm seed maturation condition of cross X1 were reflushing more quickly after the pruning in the spring of 2020 than the plants from the cold seed maturation condition, and as this phenomenon was not observed among the plants from cross X2 (Table S1), this time lap in reflushing may have influenced the timing of leaf senescence. Therefore the minor effect on the timing of leaf senescence was considered to be caused by chance. In the timing of bud burst in 2021, there was only a small carry-over effect from the spring temperature treatment in the year before (p-value = 0.010 for T20_warm, Table 3), with the warm temperature plants still displaying an earlier bud burst. In the next years, timing of the autumnal leaf senescence, no significant effect of any treatment was apparent (Table 3).
4. Discussion
The variability in the occurrence of the bud burst process among the different observation years and between the two crosses is likely attributable to inter-annual variability in spring conditions. Remarkably, in the first year after germination (2017 for cross X1 and 2018 for cross X2), the seedlings from the warm seed maturation condition burst their buds earlier compared with the cold seed maturation seedlings. In contrast, in the second (2018 for cross X1 and 2019 for cross X2) and third year (2019 for cross X1) after germination, these warm seed maturation seedlings burst their buds later than the cold seed maturation seedlings. Accordingly, in the year of the spring temperature treatment (2020), both controlled crosses display a delayed bud burst for the warm seed maturation condition. A reversed order of timing of bud burst for the cold and warm seed maturation condition between the first year after germination and the following years may suggest that bud burst in the first year followed a temporary maternal effect, overruling in this year another mechanism which was expressed in its turn in the succeeding years. A possible explanation for the temporary maternal effect is that the warmer temperature during seed maturation may have influenced seed weight and/or timing of emergence in comparison with the cold temperature condition, hence influencing the phenophase bud burst one year after germination. In general, plant maternal tissues regulate photoassimilate import and sink strength of seeds [28]. Carbohydrate metabolism in maturing seeds may differ in varying maternal temperature conditions as plant carbohydrates are direct products of photosynthetic CO2 fixation, and a tight linkage between temperature sensing and adjustment of carbohydrate metabolism has been suggested [29]. Seed size and/or seed weight may influence the first year’s growth and phenology of the germinated seedlings in a species dependent way. In Juglans ailanthifolia, which is characterized by an indeterminate shoot development (similar to P. padus in our experiment), the timing of seedling emergence influenced seedling performance, whereas seed size displayed a negligible effect [30]. On the contrary, in Quercus mongolica, a species with determinate shoot development (delimited leaf flush), seed size influenced seedling height growth and survival, but seedling emergence time was negligibly affecting these traits [30].
Bud burst in woody species can occur in spring when the plants have received sufficient chilling during the winter and when ambient temperatures in spring have become favorable for growth [31]. The later bud burst in seedlings from the warm seed maturation condition, observed from the second year after germination onward, is counter-intuitive as it suggests an adaptation to a later start of the growing season, which can be expected in a colder climate rather than a warmer climate. Earlier bud burst in provenances originating from a warmer climate is often observed in common gardens of woody species where provenances from colder and warmer climate origins are planted together (e.g., for the shrub species Crataegus monogyna [32]). As the experiment was performed in a greenhouse environment, the cold maternal temperature condition (non-heated greenhouse) was closer to the natural conditions to which plants are adapted compared with the warm condition (heated greenhouse). The delayed bud burst in the seedlings from the warm seed maturation condition from the second year after germination onward could be an expression of the exposure of the mother plants to environmental temperatures above the optimal temperature to which these mother plants were adapted, causing a stress reaction in the mother plants. It can be hypothesized that this stress response is transferred in an epigenetic way to the developing seeds, rendering an adjustment, such as DNA methylation, that may have affected circadian clock genes. The circadian clock regulates plant metabolism, which is related to normal plant development and also in response to abiotic stress [33]. Specific components of the circadian clock in plants have been shown to function as thermosensors in plants [29], and genes involved in the circadian clock of Populus tremula × Populus tremuloides were shown to play a role in the temperature-dependent processes of dormancy, including the timing of bud burst [34]. Similar to our results, seedlings from controlled crosses in Populus nigra, resulting from a warmer maternal environment, displayed later bud burst in one out of three controlled crossings [27].
In addition, the warm spring temperature treatment in 2020, when compared with the cold spring temperature, enlarged the difference in timing of bud burst between the cold and warm seed maturation conditions in both crosses, suggesting that the putative epigenetic memory is triggered by a warmer spring environment. The putative epigenetic memory effect was not detectable anymore in the year after the spring temperature treatment.
The timing of the autumnal leaf senescence in the seedlings was not influenced by the temperature during seed maturation. In addition, the timing of leaf senescence for the two crosses concurred well when compared in the actual year of observation. A comparison of the number of years after germination rendered a lesser concurrence of the two crosses. Together, these results suggest that autumnal senescence is less (epi)genetically determined in comparison to spring bud burst and more sensitive to ambient temperatures. Several results demonstrated the sensitivity of autumnal senescence in woody species to temperature variation [35,36]. In addition, results of variance analysis of seasonal phenophases in two plantations of P. padus in Belgium consisting of clonal replicates of different genotypes demonstrated that bud burst is more genetically controlled than leaf senescence in this species [37].
5. Conclusions
We demonstrate a putative epigenetic memory of the temperature during seed maturation in seedlings and saplings of the shrub P. padus. Moreover, we demonstrate that the observed effect on the phenophase bud burst is more strongly expressed when the offspring is confronted with an elevated spring temperature. Further research is needed to confirm the suggested epigenetic mechanism and to characterize the order of magnitude of these putative transgenerational epigenetic effects and the way in which they may help woody species cope with climate warming.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f13091362/s1, Figure S1: Mean daily temperatures after the controlled cross and during the seed development in the “cold” (open greenhouse) and “warm” (standard greenhouse) conditions in 2016; Figure S2: Mean daily temperatures in the spring of 2020 for the “cold” and the “warm” greenhouse conditions; Figure S3: Modeled autumnal leaf senescence for the seedlings resulting from the crosses X1 and X2, according to the actual year of observation (a) and according to the number of years after germination (b); Figure S4: Modeled timing of bud burst of the seedlings from the crosses X1 and X2 for the first three (X1) or first two (X2) years after germination, depending on the temperature during seed maturation; Table S1: Number of plants in the different reflushing scores on the 28 June 2020, for the crosses X1 and X2 and according to the maternal seed maturation temperature (Ts).
Author Contributions
Conceptualization of experiment: K.V.M. and S.M.; methodology: K.V.M., S.M. and Y.A.G.; measurements and observations: K.V.M., S.M. and Y.A.G.; validation of data: K.V.M. and S.B.; statistical analysis: K.V.M., S.B. and Y.A.G.; preparation of draft manuscript: K.V.M.; editing and reviewing of the manuscript: K.V.M., S.B. and Y.A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are available at https://zenodo.org/record/6727646#.YwmUQxxByUk, accessed on 28 June 2022.
Acknowledgments
We thank Lisa Carnal, Amy Lauwers, Cédric Van Dun, Segolene Bauduin, Stijn De Leenheer, Denis Cattoir, and Matthieu Gallin for their contribution to the data acquisition. Marc Schouppe and Nico De Regge helped in taking care of the plants.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Savolainen, O.; Pyhäjärvi, T.; Knürr, T. Gene Flow and Local Adaptation in Trees. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 595–619. [Google Scholar] [CrossRef]
- Bräutigam, K.; Vining, K.J.; Lafon-Placette, C.; Fossdal, C.G.; Mirouze, M.; Marcos, J.G.; Fluch, S.; Fraga, M.F.; Guevara, M.Á.; Abarca, D.; et al. Epigenetic regulation of adaptive responses of forest tree species to the environment. Ecol. Evol. 2013, 3, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Nicotra, A.B.; Atkin, O.K.; Bonser, S.P.; Davidson, A.M.; Finnegan, E.J.; Mathesius, U.; Poot, P.; Purugganan, M.D.; Richards, C.L.; Valladares, F.; et al. Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 2010, 15, 684–692. [Google Scholar] [CrossRef]
- Jump, A.S.; Peñuelas, J. Running to stand still: Adaptation and the response of plants to rapid climate change. Ecol. Lett. 2005, 8, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Valladares, F.; Matesanz, S.; Guilhaumon, F.; Araújo, M.B.; Balaguer, L.; Benito-Garzón, M.; Cornwell, W.; Gianoli, E.; van Kleunen, M.; Naya, D.E.; et al. The effects of phenotypic plasticity and local adaptation on forecasts of species range shifts under climate change. Ecol. Lett. 2014, 17, 1351–1364. [Google Scholar] [CrossRef] [PubMed]
- Piao, S.; Liu, Q.; Chen, A.; Janssens, I.A.; Fu, Y.; Dai, J.; Liu, L.; Lian, X.; Shen, M.; Zhu, X. Plant phenology and global climate change: Current progresses and challenges. Glob. Change Biol. 2019, 25, 1922–1940. [Google Scholar] [CrossRef]
- Franks, S.J.; Weber, J.J.; Aitken, S.N. Evolutionary and plastic responses to climate change in terrestrial plant populations. Evol. Appl. 2014, 7, 123–139. [Google Scholar] [CrossRef]
- Zhang, X.; Tarpley, D.; Sullivan, J.T. Diverse responses of vegetation phenology to a warming climate. Geophys. Res. Lett. 2007, 34. [Google Scholar] [CrossRef]
- Prieto, P.; Peñuelas, J.; Niinemets, Ü.; Ogaya, R.; Schmidt, I.K.; Beier, C.; Tietema, A.; Sowerby, A.; Emmett, B.A.; Láng, E.K.; et al. Changes in the onset of spring growth in shrubland species in response to experimental warming along a north–south gradient in Europe. Glob. Ecol. Biogeogr. 2009, 18, 473–484. [Google Scholar] [CrossRef]
- Menzel, A.; Sparks, T.H.; Estrella, N.; Koch, E.; Aasa, A.; Ahas, R.; Alm-Kubler, K.; Bissolli, P.; Braslavska, O.; Briede, A.; et al. European phenological response to climate change matches the warming pattern. Glob. Change Biol. 2006, 12, 1969–1976. [Google Scholar] [CrossRef]
- Chen, L.; Huang, J.G.; Ma, Q.Q.; Hanninen, H.; Tremblay, F.; Bergeron, Y. Long-term changes in the impacts of global warming on leaf phenology of four temperate tree species. Glob. Change Biol. 2019, 25, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Vitasse, Y.; Delzon, S.; Dufrêne, E.; Pontailler, J.-Y.; Louvet, J.-M.; Kremer, A.; Michalet, R. Leaf phenology sensitivity to temperature in European trees: Do within-species populations exhibit similar responses? Agric. For. Meteorol. 2009, 149, 735–744. [Google Scholar] [CrossRef]
- Fu, Y.H.; Campioli, M.; Deckmyn, G.; Janssens, I.A. Sensitivity of leaf unfolding to experimental warming in three temperate tree species. Agric. For. Meteorol. 2013, 181, 125–132. [Google Scholar] [CrossRef]
- Penfield, S.; MacGregor, D.R. Effects of environmental variation during seed production on seed dormancy and germination. J. Exp. Bot. 2016, 68, 819–825. [Google Scholar] [CrossRef]
- Springthorpe, V.; Penfield, S. Flowering time and seed dormancy control use external coincidence to generate life history strategy. Elife 2015, 4, e05557. [Google Scholar] [CrossRef]
- Chen, M.; MacGregor, D.R.; Dave, A.; Florance, H.; Moore, K.; Paszkiewicz, K.; Smirnoff, N.; Graham, I.A.; Penfield, S. Maternal temperature history activates Flowering Locus T in fruits to control progeny dormancy according to time of year. Proc. Natl. Acad. Sci. USA 2014, 111, 18787–18792. [Google Scholar] [CrossRef]
- He, H.; Willems, L.A.J.; Batushansky, A.; Fait, A.; Hanson, J.; Nijveen, H.; Hilhorst, H.W.M.; Bentsink, L. Effects of Parental Temperature and Nitrate on Seed Performance are Reflected by Partly Overlapping Genetic and Metabolic Pathways. Plant Cell Physiol. 2016, 57, 473–487. [Google Scholar] [CrossRef]
- Verhoeven, K.J.; vonHoldt, B.M.; Sork, V.L. Epigenetics in ecology and evolution: What we know and what we need to know. Mol. Ecol. 2016, 25, 1631–1638. [Google Scholar] [CrossRef]
- Pascual, J.; Cañal, M.J.; Correia, B.; Escandon, M.; Hasbún, R.; Meijón, M.; Pinto, G.; Valledor, L. Can Epigenetics Help Forest Plants to Adapt to Climate Change. In Epigenetics in Plants of Agronomic Importance: Fundamentals and Applications: Transcriptional Regulation and Chromatin Remodelling in Plants; Springer International Publishing: Cham, Switzerland, 2014; pp. 125–146. [Google Scholar]
- Richards, C.L.; Alonso, C.; Becker, C.; Bossdorf, O.; Bucher, E.; Colomé-Tatché, M.; Durka, W.; Engelhardt, J.; Gaspar, B.; Gogol-Döring, A.; et al. Ecological plant epigenetics: Evidence from model and non-model species, and the way forward. Ecol. Lett. 2017, 20, 1576–1590. [Google Scholar] [CrossRef]
- Kvaalen, H.; Johnsen, Ø. Timing of bud set in Picea abies is regulated by a memory of temperature during zygotic and somatic embryogenesis. New Phytol. 2008, 177, 49–59. [Google Scholar] [CrossRef]
- Johnsen, Ø.; Fossdal, C.G.; Nagy, N.; MØLmann, J.; DæHlen, O.G.; SkrØPpa, T. Climatic adaptation in Picea abies progenies is affected by the temperature during zygotic embryogenesis and seed maturation. Plant Cell Environ. 2005, 28, 1090–1102. [Google Scholar] [CrossRef]
- Yakovlev, I.A.; Carneros, E.; Lee, Y.; Olsen, J.E.; Fossdal, C.G. Transcriptional profiling of epigenetic regulators in somatic embryos during temperature induced formation of an epigenetic memory in Norway spruce. Planta 2016, 243, 1237–1249. [Google Scholar] [CrossRef] [PubMed]
- Yakovlev, I.; Fossdal, C.G.; Skrøppa, T.; Olsen, J.E.; Jahren, A.H.; Johnsen, Ø. An adaptive epigenetic memory in conifers with important implications for seed production. Seed Sci. Res. 2012, 22, 63–76. [Google Scholar] [CrossRef]
- Dewan, S.; Vander Mijnsbrugge, K.; De Frenne, P.; Steenackers, M.; Michiels, B.; Verheyen, K. Maternal temperature during seed maturation affects seed germination and timing of bud set in seedlings of European black poplar. For. Ecol. Manag. 2018, 410, 126–135. [Google Scholar] [CrossRef]
- R_Core_Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Christensen, R.H.B. Ordinal: Regression Models for Ordinal Data. R Package Version 2015.6-28. Available online: http://www.cran.r-project.org/package=ordinal/ (accessed on 20 June 2016).
- Aguirre, M.; Kiegle, E.; Leo, G.; Ezquer, I. Carbohydrate reserves and seed development: An overview. Plant Reprod. 2018, 31, 263–290. [Google Scholar] [CrossRef]
- Seydel, C.; Kitashova, A.; Fürtauer, L.; Nägele, T. Temperature-induced dynamics of plant carbohydrate metabolism. Physiol. Plant. 2022, 174, e13602. [Google Scholar] [CrossRef]
- Seiwa, K. Effects of seed size and emergence time on tree seedling establishment: Importance of developmental constraints. Oecologia 2000, 123, 208–215. [Google Scholar] [CrossRef]
- Rohde, A.; Bhalerao, R. Plant dormancy in the perennial context. Trends Plant Sci. 2007, 12, 217–223. [Google Scholar] [CrossRef]
- Vander Mijnsbrugge, K.; Onkelinx, T.; De Cuyper, B. Variation in bud burst and flower opening responses of local versus non-local provenances of hawthorn (Crataegus monogyna Jacq.) in Belgium. Plant Syst. Evol. 2014, 301, 1171–1179. [Google Scholar] [CrossRef]
- Kim, J.A.; Kim, H.-S.; Choi, S.-H.; Jang, J.-Y.; Jeong, M.-J.; Lee, S.I. The Importance of the Circadian Clock in Regulating Plant Metabolism. Int. J. Mol. Sci. 2017, 18, 2680. [Google Scholar] [CrossRef] [Green Version]
- IbÁñez, C.; Kozarewa, I.; Johansson, M.; Ögren, E.; Rohde, A.; Eriksson, M.E. Circadian Clock Components Regulate Entry and Affect Exit of Seasonal Dormancy as Well as Winter Hardiness in Populus Trees. Plant Physiol. 2010, 153, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, X.; Wang, H.; Ciais, P.; Peñuelas, J.; Myneni, R.B.; Desai, A.R.; Gough, C.M.; Gonsamo, A.; Black, A.T.; et al. Contrasting responses of autumn-leaf senescence to daytime and night-time warming. Nat. Clim. Change 2018, 8, 1092–1096. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.J.; Ho, C.H.; Gim, H.J.; Brown, M.E. Phenology shifts at start vs. end of growing season in temperate vegetation over the Northern Hemisphere for the period 1982–2008. Glob. Change Biol. 2011, 17, 2385–2399. [Google Scholar]
- Vander Mijnsbrugge, K.; Moreels, S. Varying Levels of Genetic Control and Phenotypic Plasticity in Timing of Bud Burst, Flower Opening, Leaf Senescence and Leaf Fall in Two Common Gardens of Prunus padus L. Forests 2020, 11, 1070. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).