Effects of Nutrient Elements on Growth and Expression of Insect-Defense Response Genes in Zanthoxylum bungeanum Maxim
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Plant Materials and Treatment
2.3. Measurement and Sampling
2.4. Total RNA Extraction and cDNA Library Construction for RNA Sequencing
2.5. Transcriptome and Functional Annotation
2.6. Differentially Expressed Gene (DEG) Analysis
2.7. Screening of Insect-Desense Response Genes
2.8. Statistical Analyses
3. Results
3.1. Effects of Fertilizer on Growth Indicators
3.2. Z. bungeanum Sequencing Analysis and Annotation of Transcriptomes
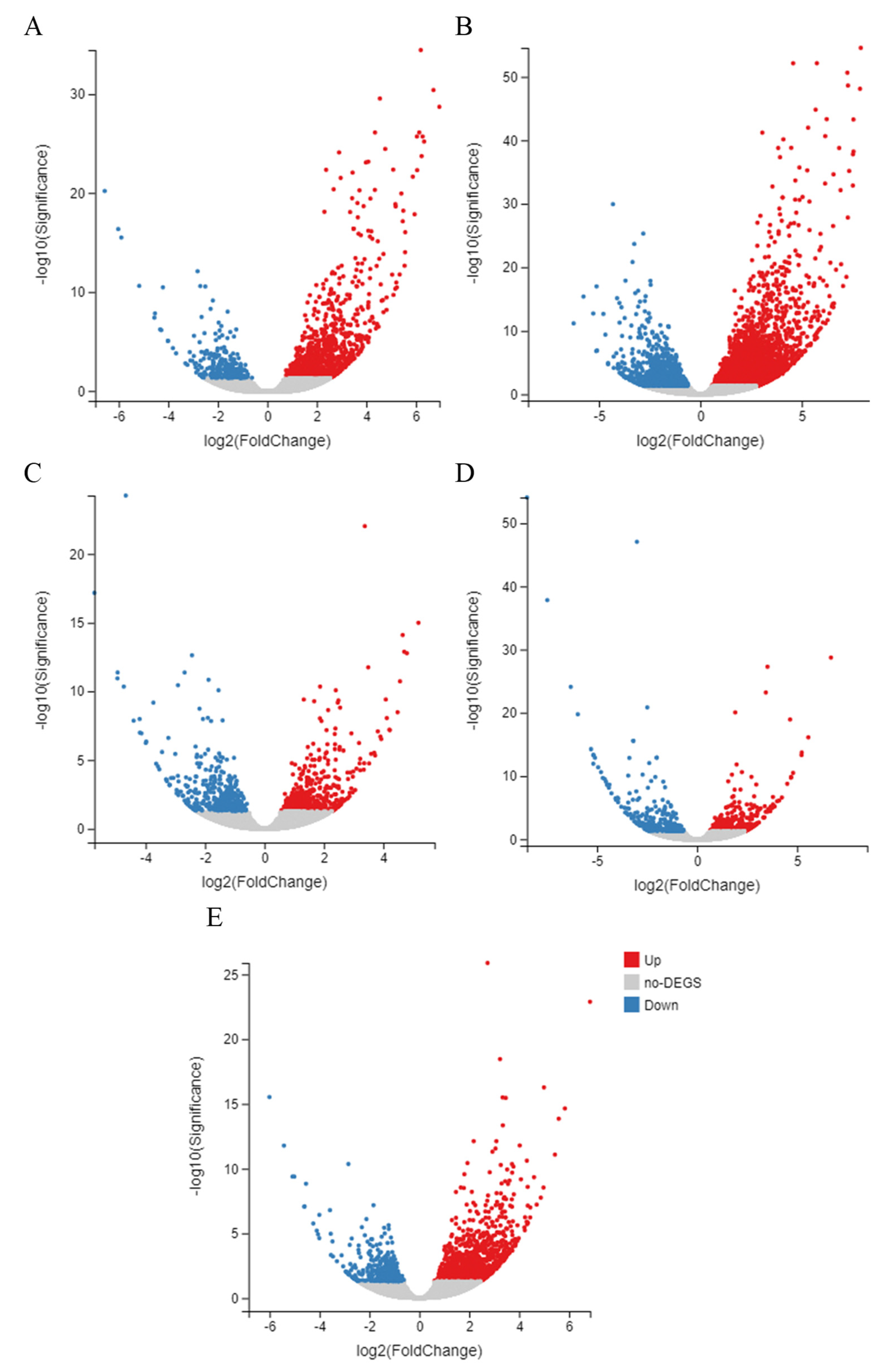
3.3. Clustering and Dynamics of DEGs between Treated and Control Groups
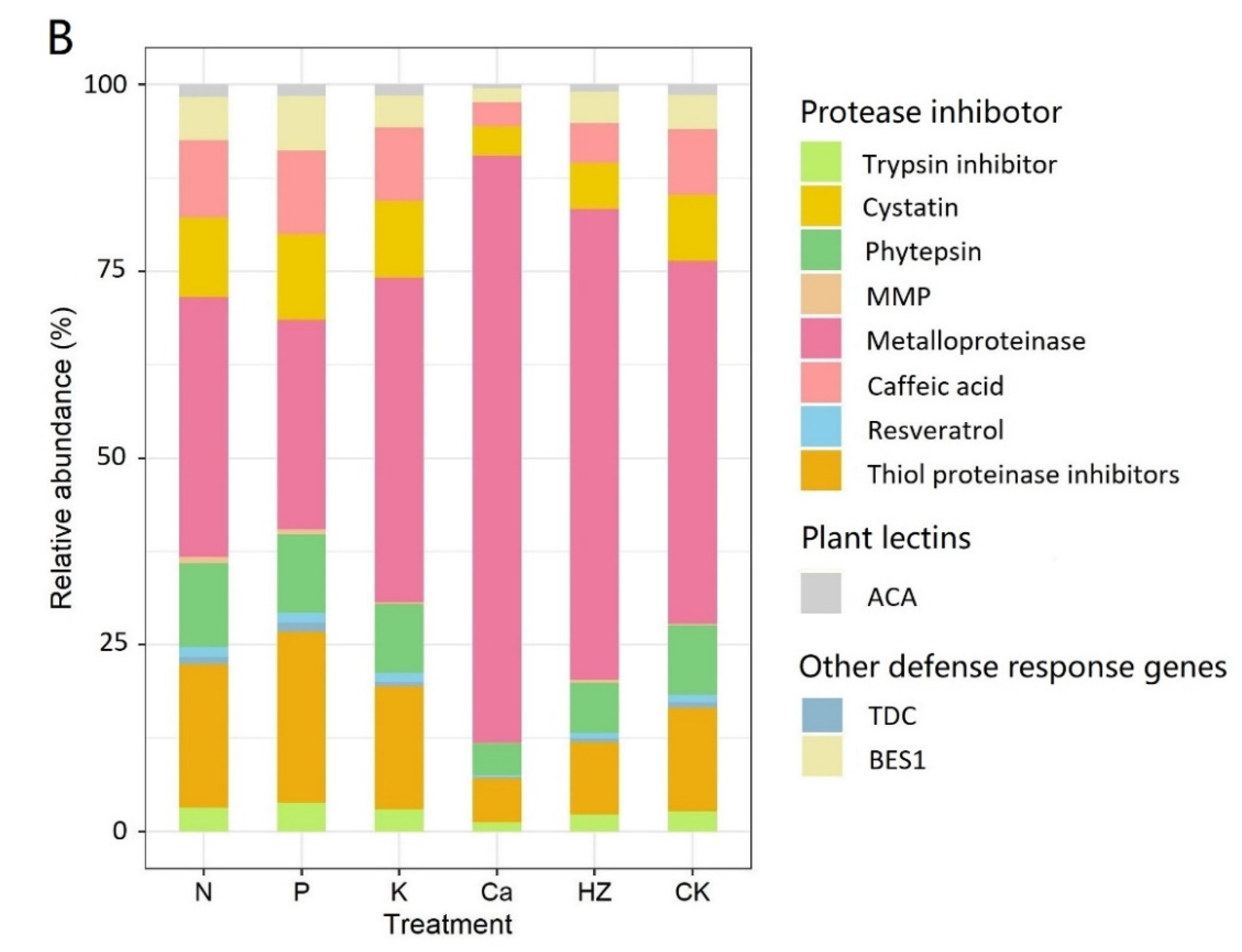
3.4. Annotation of Insect-Defense Response Genes under Different Fertilizer Treatments
3.5. Identification of DEGs
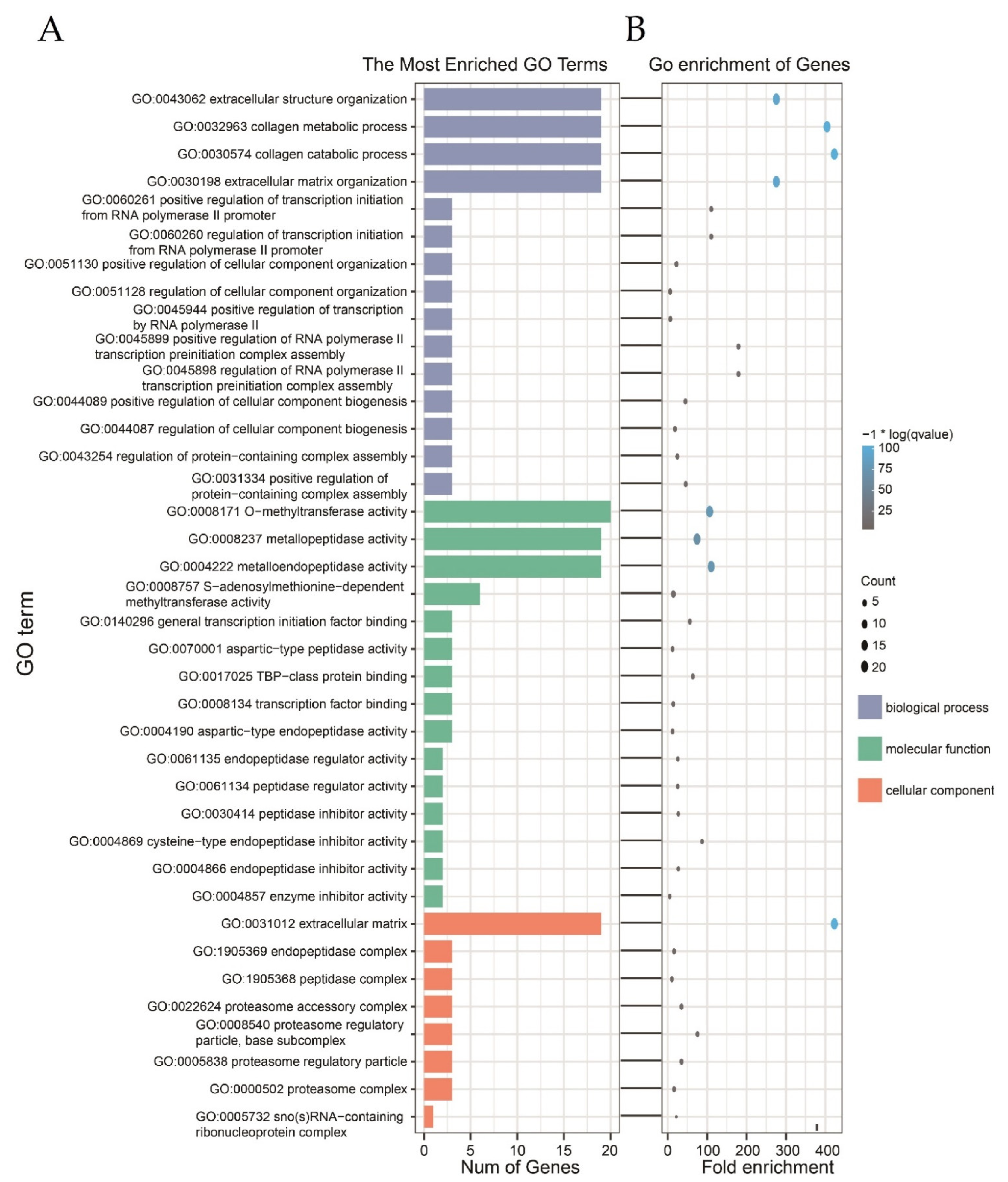
3.6. Functional Classification of Differentially Expressed Insect-Defense Response Genes by GO Analysis
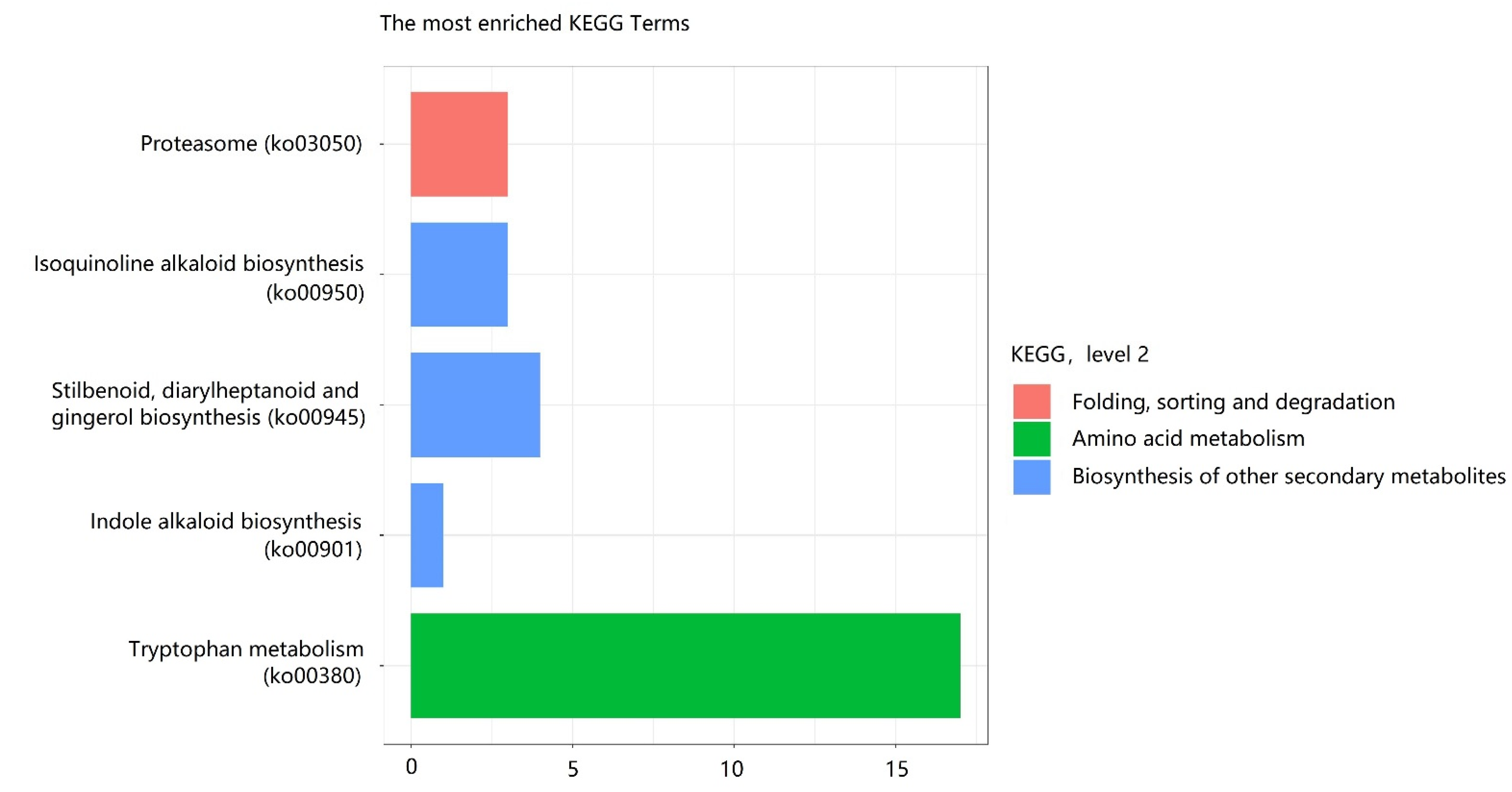
3.7. Functional Classification of Differential Expressed Insect-Defense Response Genes by KEGG Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Hu, Y.; Tian, L.; Shi, J.; Tian, J.; Zhao, L.; Feng, S.; Wei, A. Genetic structure of cultivated Zanthoxylum species investigated with SSR markers. Tree Genet. Genomes 2018, 14, 89. [Google Scholar] [CrossRef]
- Fei, X.; Shi, Q.; Yang, T.; Fei, Z.; Wei, A. Expression Stabilities of Ten Candidate Reference Genes for RT-qPCR in Zanthoxylum bungeanum Maxim. Molecules 2018, 23, 802. [Google Scholar] [CrossRef]
- Sun, J.; Sun, B.; Ren, F.; Chen, H.; Zhang, N.; Zhang, Y.; Zhang, H. Effects of Storage Conditions on the Flavor Stability of Fried Pepper (Zanthoxylum bungeanum) Oil. Foods 2021, 10, 1292. [Google Scholar] [CrossRef]
- Yu, X.; Zheng, X.; Kan, J.; Guo, J. Evaluation of specific quality of Zanthoxylum bungeanum Maxim and Zanthoxylum schinifolium Sieb. et Zucc. Food Sci. 2009, 30, 45–48. [Google Scholar]
- Yang, F.X.; Su, Y.Q.; Li, X.H.; Zhang, Q.; Sun, R.C. Studies on the preparation of biodiesel from Zanthoxylum bungeanum Maxim seed oil. J. Agric. Food Chem. 2008, 56, 7891–7896. [Google Scholar] [CrossRef]
- Artaria, C.; Maramaldi, G.; Bonfigli, A.; Rigano, L.; Appendino, G. Lifting properties of the alkamide fraction from the fruit husks of Zanthoxylum bungeanum. Int. J. Cosmet. Sci. 2011, 33, 328–333. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, J.; Zhu, L.; Li, T.; Jiang, W.; Zhou, J.; Peng, W.; Wu, C. Zanthoxylum bungeanum Maxim. (Rutaceae): A Systematic Review of Its Traditional Uses, Botany, Phytochemistry, Pharmacology, Pharmacokinetics, and Toxicology. Int. J. Mol. Sci. 2017, 18, 2172. [Google Scholar] [CrossRef]
- He, W.; Puyvelde, L.V.; Kimpe, N.D.; Verbruggen, L.; Anthonissen, K.; Flaas, M.V.d.; Bosselaers, J.; Mathenge, S.G.; Mudida, F.P. Chemical constituents and biological activities of Zanthoxylum usambarense. Phytother. Res. 2002, 16, 66–70. [Google Scholar] [CrossRef]
- Chen, J.-J.; Lin, Y.-H.; Day, S.-H.; Hwang, T.-L.; Chen, I.-S. New benzenoids and anti-inflammatory constituents from Zanthoxylum nitidum. Food Chem. 2011, 125, 282–287. [Google Scholar] [CrossRef]
- Doh, E.J.; Yang, N.; Ji, M.-G.; Lee, G.-S.; Ju, Y.-S.; Lee, J.H.; Ko, B.S.; Oh, S.-E. Discrimination of Kalopanax pictus from its varieties and other ‘Kalopanacis Cortex’plants by multiplex polymerase chain reaction (PCR). Genes Genom. 2011, 33, 711–719. [Google Scholar] [CrossRef]
- Wei, S.; Zhang, H.; Wang, Y.; Wang, L.; Li, X.; Wang, Y.; Zhang, H.; Xu, X.; Shi, Y. Ultrasonic nebulization extraction-heating gas flow transfer-headspace single drop microextraction of essential oil from pericarp of Zanthoxylum bungeanum Maxim. J. Chromatogr. A 2011, 1218, 4599–4605. [Google Scholar] [CrossRef] [PubMed]
- Tantapakul, C.; Phakhodee, W.; Ritthiwigrom, T.; Yossathera, K.; Deachathai, S.; Laphookhieo, S. Antibacterial compounds from Zanthoxylum rhetsa. Arch. Pharmacal Res. 2012, 35, 1139–1142. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Yang, T.; Li, X.; Chen, L.; Liu, Z.; Wei, A. Genetic relationships of Chinese prickly ash as revealed by ISSR markers. Biologia 2015, 70, 45–51. [Google Scholar] [CrossRef]
- López-Arredondo, D.L.; Sánchez-Calderón, L.; Yong-Villalobos, L. Molecular and genetic basis of plant macronutrient use efficiency: Concepts, opportunities, and challenges. In Plant Macronutrient Use Efficiency; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–29. [Google Scholar]
- Hillel, D.; Hatfield, J.L. Encyclopedia of Soils in the Environment; Elsevier: Amsterdam, The Netherlands, 2005; Volume 3. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). FAOSTAT Statistics Database; FAO: Rome, Italy, 2017. [Google Scholar]
- López-Arredondo, D.L.; Leyva-González, M.A.; González-Morales, S.I.; López-Bucio, J.; Herrera-Estrella, L. Phosphate nutrition: Improving low-phosphate tolerance in crops. Annu. Rev. Plant Biol. 2014, 65, 95–123. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, W.-H. Potassium transport and signaling in higher plants. Annu. Rev. Plant Biol. 2013, 64, 451–476. [Google Scholar] [CrossRef] [PubMed]
- Bass, J.; Lazar, M.A. Circadian time signatures of fitness and disease. Science 2016, 354, 994–999. [Google Scholar] [CrossRef]
- Zhu, J.-K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Pandey, P.; Irulappan, V.; Bagavathiannan, M.V.; Senthil-Kumar, M. Impact of combined abiotic and biotic stresses on plant growth and avenues for crop improvement by exploiting physio-morphological traits. Front. Plant Sci. 2017, 8, 537. [Google Scholar] [CrossRef]
- Liu, Y.; He, C. A review of redox signaling and the control of MAP kinase pathway in plants. Redox Biol. 2017, 11, 192–204. [Google Scholar] [CrossRef]
- Qi, J.; Wang, J.; Gong, Z.; Zhou, J.-M. Apoplastic ROS signaling in plant immunity. Curr. Opin. Plant Biol. 2017, 38, 92–100. [Google Scholar] [CrossRef]
- Chen, L.-L.; Wang, H.-Y.; Gong, X.-C.; Zeng, Z.-H.; Xue, X.-Z.; Hu, Y.-G. Transcriptome analysis reveals effects of red and blue light-emitting diodes (LEDs) on the growth, chlorophyll fluorescence and endogenous plant hormones of potato (Solanum tuberosum L.) plantlets cultured in vitro. J. Integr. Agric. 2021, 20, 2914–2931. [Google Scholar] [CrossRef]
- Baginsky, S.; Hennig, L.; Zimmermann, P.; Gruissem, W. Gene Expression Analysis, Proteomics, and Network Discovery. Plant Physiol. 2010, 152, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Huang, J.; Huang, X.; Chen, S.; Zou, P.; Li, W.; Yu, K.; Liu, Y. Generation of expressed sequence tags from a cDNA library of Coleus forskohlii for identification of genes involved in terpene biosynthesis. Biol. Plant. 2015, 59, 463–468. [Google Scholar] [CrossRef]
- Liao, J.-L.; Zhou, H.-W.; Peng, Q.; Zhong, P.-A.; Zhang, H.-Y.; He, C.; Huang, Y.-J. Transcriptome changes in rice (Oryza sativa L.) in response to high night temperature stress at the early milky stage. BMC Genom. 2015, 16, 18. [Google Scholar] [CrossRef]
- Hou, D.-Y.; Shi, L.-C.; Yang, M.-M.; Li, J.; Zhou, S.; Zhang, H.-X.; Xu, H.-W. De novo transcriptomic analysis of leaf and fruit tissue of Cornus officinalis using Illumina platform. PLoS ONE 2018, 13, e0192610. [Google Scholar] [CrossRef]
- Yu, X.; Wang, T.; Zhu, M.; Zhang, L.; Zhang, F.; Jing, E.; Ren, Y.; Wang, Z.; Xin, Z.; Lin, T. Transcriptome and physiological analyses for revealing genes involved in wheat response to endoplasmic reticulum stress. BMC Plant Biol. 2019, 19, 193. [Google Scholar] [CrossRef]
- Mu, W.; Wei, J.; Yang, T.; Fan, Y.; Cheng, L.; Yang, J.; Mu, R.; Liu, J.; Zhao, J.; Sun, W.; et al. RNA Extraction for Plant Samples Using CTAB-pBIOZOL; Protocols.io: Berkeley, CA, USA, 2017. [Google Scholar] [CrossRef]
- Niu, J.-Z.; Dou, W.; Ding, T.-B.; Shen, G.-M.; Zhang, K.; Smagghe, G.; Wang, J.-J. Transcriptome analysis of the citrus red mite, Panonychus citri, and its gene expression by exposure to insecticide/acaricide. Insect Mol. Biol. 2012, 21, 422–436. [Google Scholar] [CrossRef]
- Fang, C.; Zhong, H.; Lin, Y.; Chen, B.; Han, M.; Ren, H.; Lu, H.; Luber, J.M.; Xia, M.; Li, W.; et al. Assessment of the cPAS-based BGISEQ-500 platform for metagenomic sequencing. Gigascience 2018, 7, gix133. [Google Scholar] [CrossRef]
- Zhang, G.-H.; Ma, C.-H.; Zhang, J.-J.; Chen, J.-W.; Tang, Q.-Y.; He, M.-H.; Xu, X.-Z.; Jiang, N.-H.; Yang, S.-C. Transcriptome analysis of Panax vietnamensis var. fuscidicus discovers putative ocotillol-type ginsenosides biosynthesis genes and genetic markers. BMC Genom. 2015, 16, 159. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Zhang, Y.; Cui, H.; Liu, J.; Wu, Y.; Cheng, Y.; Xu, H.; Huang, X.; Li, S.; Zhou, A.; et al. WEGO 2.0: A web tool for analyzing and plotting GO annotations, 2018 update. Nucleic Acids Res. 2018, 46, W71–W75. [Google Scholar] [CrossRef]
- Conesa, A.; Götz, S.; García-Gómez, J.M.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef]
- Haynes, W. Benjamini–Hochberg Method. In Encyclopedia of Systems Biology; Dubitzky, W., Wolkenhauer, O., Cho, K.-H., Yokota, H., Eds.; Springer: New York, NY, USA, 2013; p. 78. [Google Scholar]
- Murtagh, F.; Legendre, P. Ward’s Hierarchical Agglomerative Clustering Method: Which Algorithms Implement Ward’s Criterion? J. Classif. 2014, 31, 274–295. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Hou, R.; Bao, Z.; Wang, S.; Su, H.; Li, Y.; Du, H.; Hu, J.; Wang, S.; Hu, X. Transcriptome sequencing and de novo analysis for Yesso scallop (Patinopecten yessoensis) using 454 GS FLX. PLoS ONE 2011, 6, e21560. [Google Scholar] [CrossRef]
- Moilanen, M.; Hökkä, H. The growth response of Scots pine to PK fertilization depends on the nutrient status of the stand on drained peatlands. Suo 2009, 60, 111–119. [Google Scholar]
- Guo, J.; Wu, Y.; Wang, B.; Lu, Y.; Cao, F.; Wang, G. The effects of fertilization on the growth and physiological characteristics of Ginkgo biloba L. Forests 2016, 7, 293. [Google Scholar]
- Herms, D.A. Effects of Fertilization on Insect Resistance of Woody Ornamental Plants: Reassessing an Entrenched Paradigm. Environ. Entomol. 2002, 31, 923–933. [Google Scholar] [CrossRef]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Elekwachi, C.O.; Wang, Z.; Wu, X.; Rabee, A.; Forster, R.J. Total rRNA-Seq analysis gives insight into bacterial, fungal, protozoal and archaeal communities in the rumen using an optimized RNA isolation method. Front. Microbiol. 2017, 8, 1814. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, R.; Wang, R.; Tian, Y.; Shao, C.; Jia, X.; Chen, S. The integrated analysis of RNA-seq and microRNA-seq depicts miRNA-mRNA networks involved in Japanese flounder (Paralichthys olivaceus) albinism. PLoS ONE 2017, 12, e0181761. [Google Scholar]
- Chung, M.; Bruno, V.M.; Rasko, D.A.; Cuomo, C.A.; Muñoz, J.F.; Livny, J.; Shetty, A.C.; Mahurkar, A.; Dunning Hotopp, J.C. Best practices on the differential expression analysis of multi-species RNA-seq. Genome Biol. 2021, 22, 121. [Google Scholar] [CrossRef]
- Michaud, D. Recombinant Protease Inhibitors in Plants; CRC Press: Sainte-Foy, QC, Canada, 2001. [Google Scholar]
- Haq, S.K.; Atif, S.M.; Khan, R.H. Protein proteinase inhibitor genes in combat against insects, pests, and pathogens: Natural and engineered phytoprotection. Arch. Biochem. Biophys. 2004, 431, 145–159. [Google Scholar] [CrossRef]
- Stevens, J.A.; Dunse, K.M.; Guarino, R.F.; Barbeta, B.L.; Evans, S.C.; West, J.A.; Anderson, M.A. The impact of ingested potato type II inhibitors on the production of the major serine proteases in the gut of Helicoverpa armigera. Insect Biochem. Mol. Biol. 2013, 43, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Cai, Y.M.; Bonneau, L.; Rotari, V.; Danon, A.; McKenzie, E.A.; McLellan, H.; Mach, L.; Gallois, P. Inhibition of cathepsin B by caspase-3 inhibitors blocks programmed cell death in Arabidopsis. Cell Death Differ. 2016, 23, 1493–1501. [Google Scholar] [CrossRef]
- Swathi, M.; Mishra, P.K.; Lokya, V.; Swaroop, V.; Mallikarjuna, N.; Dutta-Gupta, A.; Padmasree, K. Purification and Partial Characterization of Trypsin-Specific Proteinase Inhibitors from Pigeonpea Wild Relative Cajanus platycarpus L. (Fabaceae) Active against Gut Proteases of Lepidopteran Pest Helicoverpa armigera. Front. Physiol. 2016, 7, 388. [Google Scholar] [CrossRef]
- Hilder, V.A.; Gatehouse, A.M.; Sheerman, S.E.; Barker, R.F.; Boulter, D. A novel mechanism of insect resistance engineered into tobacco. Nature 1987, 330, 160–163. [Google Scholar] [CrossRef]
- Duan, X.; Li, X.; Xue, Q.; Abo-EI-Saad, M.; Xu, D.; Wu, R. Transgenic rice plants harboring an introduced potato proteinase inhibitor II gene are insect resistant. Nat. Biotechnol. 1996, 14, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.; Narvaez, J.; An, G.; Ryan, C. Expression of proteinase inhibitors I and II in transgenic tobacco plants: Effects on natural defense against Manduca sexta larvae. Proc. Natl. Acad. Sci. USA 1989, 86, 9871–9875. [Google Scholar] [CrossRef]
- Hartl, M.; Giri, A.P.; Kaur, H.; Baldwin, I.T. Serine protease inhibitors specifically defend Solanum nigrum against generalist herbivores but do not influence plant growth and development. Plant Cell 2010, 22, 4158–4175. [Google Scholar] [CrossRef] [Green Version]
- Fürstenberg-Hägg, J.; Zagrobelny, M.; Bak, S. Plant Defense against Insect Herbivores. Int. J. Mol. Sci. 2013, 14, 10242–10297. [Google Scholar] [CrossRef]
- da Silva, D.S.; de Oliveira, C.F.; Parra, J.R.; Marangoni, S.; Macedo, M.L. Short and long-term antinutritional effect of the trypsin inhibitor ApTI for biological control of sugarcane borer. J. Insect Physiol. 2014, 61, 1–7. [Google Scholar] [CrossRef]
- Joshi, R.S.; Wagh, T.P.; Sharma, N.; Mulani, F.A.; Sonavane, U.; Thulasiram, H.V.; Joshi, R.; Gupta, V.S.; Giri, A.P. Way toward “dietary pesticides”: Molecular investigation of insecticidal action of caffeic acid against Helicoverpa armigera. J. Agric. Food Chem. 2014, 62, 10847–10854. [Google Scholar] [CrossRef]
- Peres, L.L.S.; Sobreiro, A.I.; Couto, I.F.S.; Silva, R.M.; Pereira, F.F.; Heredia-Vieira, S.C.; Cardoso, C.A.L.; Mauad, M.; Scalon, S.P.Q.; Verza, S.S.; et al. Chemical Compounds and Bioactivity of Aqueous Extracts of Alibertia spp. in the Control of Plutella xylostella L. (Lepidoptera: Plutellidae). Insects 2017, 8, 125. [Google Scholar] [CrossRef]
- Murdock, L.L.; Shade, R.E. Lectins and protease inhibitors as plant defenses against insects. J. Agric. Food Chem. 2002, 50, 6605–6611. [Google Scholar] [CrossRef]
- Cotabarren, J.; Tellechea, M.E.; Tanco, S.M.; Lorenzo, J.; Garcia-Pardo, J.; Avilés, F.X.; Obregón, W.D. Biochemical and MALDI-TOF Mass Spectrometric Characterization of a Novel Native and Recombinant Cystine Knot Miniprotein from Solanum tuberosum subsp. andigenum cv. Churqueña. Int. J. Mol. Sci. 2018, 19, 678. [Google Scholar] [CrossRef]
- Macedo, M.L.R.; Oliveira, C.F.; Oliveira, C.T. Insecticidal activity of plant lectins and potential application in crop protection. Molecules 2015, 20, 2014–2033. [Google Scholar] [CrossRef]
- Vandenborre, G.; Smagghe, G.; Van Damme, E.J. Plant lectins as defense proteins against phytophagous insects. Phytochemistry 2011, 72, 1538–1550. [Google Scholar] [CrossRef]
- Chung, S.H.; Bigham, M.; Lappe, R.R.; Chan, B.; Nagalakshmi, U.; Whitham, S.A.; Dinesh-Kumar, S.P.; Jander, G. A sugarcane mosaic virus vector for rapid in planta screening of proteins that inhibit the growth of insect herbivores. Plant Biotechnol. J. 2021, 19, 1713–1724. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Zhi, J.-R.; Ye, M.; Xie, W.; Zhang, T.; Li, D.-Y.; Liu, L.; Wu, X.-B.; Cao, Y. Life Table and Preference Choice of Frankliniella occidentalis (Thysanoptera: Thripidae) for Kidney Bean Plants Treated by Exogenous Calcium. Insects 2021, 12, 838. [Google Scholar] [CrossRef]
- Ehret, D.; Utkhede, R.; Frey, B.; Menzies, J.; Bogdanoff, C. Foliar applications of fertilizer salts inhibit powdery mildew on tomato. Can. J. Plant Pathol. 2002, 24, 437–444. [Google Scholar] [CrossRef]
- Engelberth, J. Secondary metabolites and plant defense. New Phytol. 1994, 127, 617–633. [Google Scholar]
- Rosenthal, G.A.; Janzen, D.H. Herbivores: Their Interaction with Secondary Plant Metabolites; Academic Press: New York, NY, USA, 1979. [Google Scholar]
- Singh, R.; Verma, S.; Mohanpuria, P.; Yadav, I.S.; Sarao, N.K. Comprehensive characterization of Allium cepa leaf lectin (ACAL1) for its potential use against sap-sucking plant pests: In silico study. J. Proteins Proteom. 2021, 12, 185–199. [Google Scholar] [CrossRef]
- Houston, K.; Tucker, M.R.; Chowdhury, J.; Shirley, N.; Little, A. The Plant Cell Wall: A Complex and Dynamic Structure As Revealed by the Responses of Genes under Stress Conditions. Front. Plant Sci. 2016, 7, 984. [Google Scholar] [CrossRef]
- Stavolone, L.; Lionetti, V. Extracellular matrix in plants and animals: Hooks and locks for viruses. Front. Microbiol. 2017, 8, 1760. [Google Scholar] [CrossRef]
- Kular, J.K.; Basu, S.; Sharma, R.I. The extracellular matrix: Structure, composition, age-related differences, tools for analysis and applications for tissue engineering. J. Tissue Eng. 2014, 5, 2041731414557112. [Google Scholar] [CrossRef]
- Rojas, C.; Senthil-Kumar, M.; Tzin, V.; Mysore, K. Regulation of primary plant metabolism during plant-pathogen interactions and its contribution to plant defense. Front Plant Sci 2014, 5, 17. [Google Scholar] [CrossRef]
- Zeier, J. New insights into the regulation of plant immunity by amino acid metabolic pathways. Plant Cell Env. 2013, 36, 2085–2103. [Google Scholar] [CrossRef] [PubMed]
- Sønderby, I.E.; Geu-Flores, F.; Halkier, B.A. Biosynthesis of glucosinolates–gene discovery and beyond. Trends Plant Sci. 2010, 15, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Killiny, N.; Hijaz, F. Amino acids implicated in plant defense are higher in Candidatus Liberibacter asiaticus-tolerant citrus varieties. Plant Signal Behav. 2016, 11, e1171449. [Google Scholar] [CrossRef]
- Iven, T.; König, S.; Singh, S.; Braus-Stromeyer, S.A.; Bischoff, M.; Tietze, L.F.; Braus, G.H.; Lipka, V.; Feussner, I.; Dröge-Laser, W. Transcriptional Activation and Production of Tryptophan-Derived Secondary Metabolites in Arabidopsis Roots Contributes to the Defense against the Fungal Vascular Pathogen Verticillium longisporum. Mol. Plant 2012, 5, 1389–1402. [Google Scholar] [CrossRef] [Green Version]
- Gill, R.I.S.; Ellis, B.E. Over-expression of Tryptophan Decarboxylase Gene in Poplar and its Possible Role in Resistance Against Malacosoma disstria. New For. 2006, 31, 195–209. [Google Scholar] [CrossRef]
- Harborne, J.B. Introduction to Ecological Biochemistry; Academic Press: New York, NY, USA, 2014. [Google Scholar]
- Thawabteh, A.; Juma, S.; Bader, M.; Karaman, D.; Scrano, L.; Bufo, S.A.; Karaman, R. The Biological Activity of Natural Alkaloids against Herbivores, Cancerous Cells and Pathogens. Toxins 2019, 11, 656. [Google Scholar] [CrossRef] [Green Version]
- Divekar, P.; Kumar, P.; Suby, S. Screening of maize germplasm through antiobiosis mechanism of resistance against Chilo partellus (Swinhoe). J. Entomol. Zool. Stud. 2019, 7, 1115–1119. [Google Scholar]



| Raw Material (%) | Active Ingredient Content (%) | Group Name | Concentration (ppm) | Fertilizer Rate (mL) |
|---|---|---|---|---|
| CH4N2O (46%) | (N) 21.47% | N1 | 200 | Each plant 167 mL |
| N2 | 400 | Each plant 167 mL | ||
| N3 | 600 | Each plant 167 mL | ||
| KH2PO4 (52%) | (P) 11.84% | P1 | 200 | Each plant 167 mL |
| P2 | 400 | Each plant 167 mL | ||
| P3 | 600 | Each plant 167 mL | ||
| K2SO4 (95%) | (K) 42.63% | K1 | 200 | Each plant 167 mL |
| K2 | 400 | Each plant 167 mL | ||
| K3 | 600 | Each plant 167 mL | ||
| Ca (NO3)2 4H2O (25%) | (Ca) 18% | Ca1 | 200 | Each plant 167 mL |
| Ca2 | 400 | Each plant 167 mL | ||
| Ca3 | 600 | Each plant 167 mL | ||
| HZ (special fertilizer for pepper; 72.7%) | (N) 12.2% (P) 14.7% (K) 19.1% (Ca) 13.6% (Mg) 2.4% (S) 8.8% (B) 1.9% | HZ1 | 300 | Each plant 167 mL |
| HZ2 | 500 | Each plant 167 mL | ||
| HZ3 | 1200 | Each plant 167 mL | ||
| CK (control group) | 0% | CK | 0 | Each plant 167 mL |
| Treatment | 28 d | 56 d | 84 d | 112 d |
|---|---|---|---|---|
| Height/cm | Height/cm | Height/cm | Height/cm | |
| N1 | 12.46 ± 1.99 a | 23.23 ± 3.14 a | 41.66 ± 1.73 a | 72.73 ± 2.02 a |
| N2 | 7.78 ± 2.13 bc | 19.34 ± 1.83 a | 39.31 ± 3.36 a | 71.29 ± 4.38 a |
| N3 | 7.34 ± 4.40 c | 18.11 ± 5.65 a | 38.84 ± 6.11 a | 69.70 ± 3.45 a |
| CK0 | 12.01 ± 1.13 ab | 19.19 ± 2.07 a | 32.82 ± 2.78 b | 56.32 ± 3.65 b |
| P1 | 14.24 ± 1.77 a | 26.97 ± 3.27 a | 48.87 ± 2.55 a | 87.30 ± 2.09 a |
| P2 | 13.41 ± 2.04 a | 25.25 ± 3.43 a | 40.50 ± 7.59 b | 65.89 ± 7.72 b |
| P3 | 10.78 ± 3.98 a | 20.54 ± 3.15 b | 36.86 ± 4.83 bc | 64.98 ± 6.75 b |
| CK0 | 12.01 ± 1.13 a | 19.19 ± 2.07 b | 32.82 ± 2.78 c | 56.32 ± 3.65 c |
| K1 | 12.86 ± 2.41 a | 22.27 ± 2.33 a | 45.21 ± 1.96 a | 82.16 ± 3.15 a |
| K2 | 11.25 ± 2.29 a | 21.73 ± 3.31 a | 44.18 ± 7.02 a | 78.77 ± 8.53 ab |
| K3 | 10.98 ± 1.76 a | 20.79 ± 2.70 a | 42.09 ± 4.49 a | 74.21 ± 5.76 b |
| CK0 | 12.01 ± 1.13 a | 19.19 ± 2.07 a | 32.82 ± 2.78 b | 56.32 ± 3.65 c |
| Ca1 | 14.03 ± 2.90 a | 27.68 ± 2.33 a | 53.22 ± 3.02 a | 80.60 ± 4.79 a |
| Ca2 | 13.29 ± 1.01 a | 23.79 ± 1.74 b | 39.69 ± 1.66 b | 66.49 ± 2.83 b |
| Ca3 | 12.89 ± 1.33 a | 20.30 ± 1.98 c | 37.40 ± 6.93 bc | 63.79 ± 8.71 b |
| CK0 | 12.01 ± 1.13 a | 19.19 ± 2.07 c | 32.82 ± 2.78 c | 56.32 ± 3.65 c |
| HZ1 | 14.59 ± 1.76 a | 24.53 ± 1.96 a | 40.40 ± 2.31 a | 68.06 ± 1.66 a |
| HZ2 | 12.04 ± 3.59 a | 23.97 ± 2.76 a | 38.90 ± 2.39 ab | 67.63 ± 3.24 a |
| HZ3 | 11.46 ± 1.96 a | 21.20 ± 2.85 ab | 36.24 ± 3.62 bc | 65.29 ± 3.11 a |
| CK0 | 12.01 ± 1.13 a | 19.19 ± 2.07 b | 32.82 ± 2.78 c | 56.32 ± 3.65 b |
| Treatment | 28 d | 56 d | 84 d | 112 d |
|---|---|---|---|---|
| Diameter/mm | Diameter/mm | Diameter/mm | Diameter/mm | |
| N1 | 3.40 ± 0.16 a | 3.85 ± 0.14 a | 5.21 ± 0.11 a | 7.05 ± 0.25 a |
| N2 | 2.64 ± 0.26 bc | 3.19 ± 0.30 b | 4.85 ± 0.34 a | 6.84 ± 0.24 a |
| N3 | 2.39 ± 0.88 c | 3.13 ± 0.49 b | 4.72 ± 0.57 a | 6.73 ± 0.31 a |
| CK0 | 3.20 ± 0.10 ab | 3.63 ± 0.15 a | 5.02 ± 0.30 a | 6.02 ± 0.47 b |
| P1 | 3.13 ± 0.20 a | 4.01 ± 0.30 a | 5.77 ± 0.27 a | 8.04 ± 0.34 a |
| P2 | 3.11 ± 0.28 a | 3.58 ± 0.26 b | 4.88 ± 0.43 b | 6.26 ± 0.47 b |
| P3 | 2.96 ± 0.22 a | 3.43 ± 0.19 b | 4.86 ± 0.35 b | 6.22 ± 0.70 b |
| CK0 | 3.20 ± 0.10 a | 3.63 ± 0.15 b | 5.02 ± 0.30 b | 6.02 ± 0.47 b |
| K1 | 3.15 ± 0.31 a | 3.64 ± 0.26 a | 5.47 ± 0.32 a | 7.66 ± 0.26 a |
| K2 | 3.12 ± 0.40 a | 3.61 ± 0.46 a | 5.35 ± 0.58 a | 7.13 ± 0.78 ab |
| K3 | 2.85 ± 0.12 a | 3.55 ± 0.21 a | 5.05 ± 0.63 a | 6.68 ± 1.03 bc |
| CK0 | 3.20 ± 0.10 a | 3.63 ± 0.15 a | 5.02 ± 0.30 a | 6.02 ± 0.47 c |
| Ca1 | 3.51 ± 0.26 a | 4.20 ± 0.24 a | 6.30 ± 0.26 a | 7.85 ± 0.30a |
| Ca2 | 2.87 ± 0.17 c | 3.56 ± 0.11 b | 4.92 ± 0.26 b | 6.43 ± 0.47 b |
| Ca3 | 2.99 ± 0.15 bc | 3.50 ± 0.12 b | 4.90 ± 0.29 b | 6.37 ± 0.67 b |
| CK0 | 3.20 ± 0.10 b | 3.63 ± 0.15 b | 5.02 ± 0.30 b | 6.02 ± 0.47 b |
| HZ1 | 3.38 ± 0.11 a | 3.82 ± 0.13 a | 5.46 ± 0.20 a | 7.02 ± 0.29 a |
| HZ2 | 3.05 ± 0.31 b | 3.55 ± 0.40 a | 5.13 ± 0.51 a | 6.98 ± 0.54 a |
| HZ3 | 3.02 ± 0.25 b | 3.53 ± 0.21 a | 5.06 ± 0.17 a | 6.87 ± 0.28 a |
| CK0 | 3.20 ± 0.10 ab | 3.63 ± 0.15 a | 5.02 ± 0.30 a | 6.02 ± 0.47 b |
| Treatment | N | P | K | Ca | HZ | CK | Average Number |
|---|---|---|---|---|---|---|---|
| Total Raw Reads (M) | 43,82 | 43.82 | 43.82 | 43.82 | 43.82 | 43.82 | 43.82 |
| Total Clean Reads (M) | 42.81 | 42.49 | 42.71 | 42.84 | 42.79 | 42.72 | 42.691 |
| Total Clean Bases (Gb) | 6.39 | 6.37 | 6.40 | 6.42 | 6.42 | 6.41 | 6.40 |
| Clean Reads Q20 (%) | 97.93 | 97.88 | 98.12 | 97.72 | 97.73 | 97.90 | 97.88 |
| Clean Reads Q30 (%) | 94.23 | 93.97 | 94.59 | 93.65 | 93.62 | 94.04 | 94.01 |
| Clean Reads Ratio (%) | 97.23 | 96.96 | 97.45 | 97.75 | 97.64 | 97.48 | 97.42 |
| Item | Number |
|---|---|
| Total expressed unigenes | 58,964 |
| Annotated genes | 54,863 |
| Novel genes | 4101 |
| Coding transcript | 27,533 |
| The mapped ratio of genome (%) | 75.28 |
| Total protein-coding genes | 65,566 |
| Annotated protein-coding genes | 61,379 |
| Novel protein-coding genes | 4187 |
| The mapped ratio of genes (%) | 60.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kassen, K.; Niyazbekova, Z.; Zhang, T.; Nasir, M.; Li, F.; Li, M. Effects of Nutrient Elements on Growth and Expression of Insect-Defense Response Genes in Zanthoxylum bungeanum Maxim. Forests 2022, 13, 1365. https://doi.org/10.3390/f13091365
Kassen K, Niyazbekova Z, Zhang T, Nasir M, Li F, Li M. Effects of Nutrient Elements on Growth and Expression of Insect-Defense Response Genes in Zanthoxylum bungeanum Maxim. Forests. 2022; 13(9):1365. https://doi.org/10.3390/f13091365
Chicago/Turabian StyleKassen, Kuanysh, Zhannur Niyazbekova, Tingting Zhang, Mubasher Nasir, Feifei Li, and Menglou Li. 2022. "Effects of Nutrient Elements on Growth and Expression of Insect-Defense Response Genes in Zanthoxylum bungeanum Maxim" Forests 13, no. 9: 1365. https://doi.org/10.3390/f13091365






