Effects of Biological and Chemical Degradation on the Properties of Scots Pine—Part II: Wood-Moisture Relations and Viscoelastic Behaviour
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Sample Preparation
2.2.2. Dynamic Mechanical Analysis
2.2.3. Moisture Sorption Analyses
2.2.4. Hydroxyls Accessibility Measurements
3. Results and Discussion
3.1. Moisture Properties of Sound and Degraded Pine
3.2. Viscoelastic Behaviour of Sound and Degraded Pine Wood
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Tahira, A.; Howard, W.; Pennington, E.R.; Kennedy, A. Mechanical strength studies on degraded waterlogged wood treated with sugars. Stud. Conserv. 2016, 62, 223–228. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, L.; Zhang, B.; Hu, Y. A comparative study of reinforcement materials for waterlogged wood relics in laboratory. J. Cult. Heritage 2018, 36, 94–102. [Google Scholar] [CrossRef]
- Kennedy, A.; Pennington, E.R. Conservation of chemically degraded waterlogged wood with sugars. Stud. Conserv. 2014, 59, 194–201. [Google Scholar] [CrossRef]
- Broda, M.; Popescu, C.-M.; Curling, S.F.; Timpu, D.I.; Ormondroyd, G.A. Effects of Biological and Chemical Degradation on the Properties of Scots Pine Wood—Part I: Chemical Composition and Microstructure of the Cell Wall. Materials 2022, 15, 2348. [Google Scholar] [CrossRef] [PubMed]
- Brischke, C.; Alfredsen, G. Wood-water relationships and their role for wood susceptibility to fungal decay. Appl. Microbiol. Biotechnol. 2020, 104, 3781–3795. [Google Scholar] [CrossRef] [PubMed]
- Obataya, E.; Norimoto, M.; Gril, J. The effects of adsorbed water on dynamic mechanical properties of wood. Polymer 1998, 39, 3059–3064. [Google Scholar] [CrossRef]
- Trechsel, H.; Sherwood, G. Chapter 5—Moisture-Related Properties of Wood and the Effect of Moisture on Wood and Wood Products. Moisture Control. Build. 2008, 72–83. [Google Scholar] [CrossRef]
- Glass, S.; Zelinka, S. Moisture Relations and Physical Properties of Wood. Chapter 4 in Wood Handbook: Wood as an Engineering Material, General Technical Report FPL-GTR-282; Forest Products Laboratory: Madison, WI, USA, 2021; pp. 4–22. [Google Scholar]
- Fredriksson, M.; Thybring, E.E. On sorption hysteresis in wood: Separating hysteresis in cell wall water and capillary water in the full moisture range. PLoS ONE 2019, 14, e0225111. [Google Scholar] [CrossRef] [PubMed]
- Thybring, E.E.; Thygesen, L.G.; Burgert, I. Hydroxyl accessibility in wood cell walls as affected by drying and re-wetting procedures. Cellulose 2017, 24, 2375–2384. [Google Scholar] [CrossRef]
- Broda, M.; Curling, S.F.; Spear, M.J.; Hill, C.A.S. Effect of methyltrimethoxysilane impregnation on the cell wall porosity and water vapour sorption of archaeological waterlogged oak. Wood Sci. Technol. 2019, 53, 703–726. [Google Scholar] [CrossRef] [Green Version]
- Han, L.; Guo, J.; Wang, K.; Grönquist, P.; Li, R.; Tian, X.; Yin, Y. Hygroscopicity of Waterlogged Archaeological Wood from Xiaobaijiao No.1 Shipwreck Related to Its Deterioration State. Polymers 2020, 12, 834. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhou, H.; Stevanic, J.S.; Dong, M.; Yu, M.; Salmén, L.; Yin, Y. Effects of ageing on the cell wall and its hygroscopicity of wood in ancient timber construction. Wood Sci. Technol. 2017, 52, 131–147. [Google Scholar] [CrossRef]
- Pizzo, B.; Pecoraro, E.; Lazzeri, S. Dynamic mechanical analysis (DMA) of waterlogged archaeological wood at room temperature. Holzforschung 2018, 72, 421–431. [Google Scholar] [CrossRef]
- Pizzo, B.; Pecoraro, E.; Sozzi, L.; Salvini, A. Collapsed and re-swollen archaeological wood: Efficiency and effects on the chemical and viscoelastic characteristics of wood. J. Cult. Heritage 2021, 51, 79–88. [Google Scholar] [CrossRef]
- Wu, M.; Han, X.; Qin, Z.; Zhang, Z.; Xi, G.; Han, L. A Quasi-Nondestructive Evaluation Method for Physical-Mechanical Properties of Fragile Archaeological Wood with TMA: A Case Study of an 800-Year-Old Shipwreck. Forests 2022, 13, 38. [Google Scholar] [CrossRef]
- Broda, M.; Curling, S.F.; Frankowski, M. The effect of the drying method on the cell wall structure and sorption properties of waterlogged archaeological wood. Wood Sci. Technol. 2021, 55, 971–989. [Google Scholar] [CrossRef]
- Glass, S.; Boardman, C.; Zelinka, S.L. Short hold times in dynamic vapor sorption measurements mischaracterize the equilibrium moisture content of wood. Wood Sci. Technol. 2016, 51, 243–260. [Google Scholar] [CrossRef]
- Kyyrö, S.; Altgen, M.; Seppäläinen, H.; Belt, T.; Rautkari, L. Effect of drying on the hydroxyl accessibility and sorption properties of pressurized hot water extracted wood. Wood Sci. Technol. 2021, 55, 1203–1220. [Google Scholar] [CrossRef]
- Popescu, C.-M.; Hill, C.A. The water vapour adsorption–desorption behaviour of naturally aged Tilia cordata Mill. wood. Polym. Degrad. Stab. 2013, 98, 1804–1813. [Google Scholar] [CrossRef]
- Hill, C.A.S.; Ramsay, J.; Keating, B.; Laine, K.; Rautkari, L.; Hughes, M.; Constant, B. The water vapour sorption properties of thermally modified and densified wood. J. Mater. Sci. 2011, 47, 3191–3197. [Google Scholar] [CrossRef]
- Brischke, C.; Stricker, S.; Meyer-Veltrup, L.; Emmerich, L. Changes in sorption and electrical properties of wood caused by fungal decay. Holzforschung 2018, 73, 445–455. [Google Scholar] [CrossRef]
- Zabel, R.A.; Morrell, J.J. Wood Microbiology: Decay and Its Prevention; Academic Press: Cambridge, MA, USA, 1992. [Google Scholar]
- Cowling, E.B. Comparative Biochemistry of the Decay of Sweetgum Sapwood by White-Rot and Brown-Rot Fungi; US Department of Agriculture: Washington, DC, USA, 1961.
- Thybring, E.E. Water relations in untreated and modified wood under brown-rot and white-rot decay. Int. Biodeterior. Biodegradation 2017, 118, 134–142. [Google Scholar] [CrossRef]
- Irbe, I.; Andersons, B.; Chirkova, J.; Kallavus, U.; Andersone, I.; Faix, O. On the changes of pinewood (Pinus sylvestris L.) Chemical composition and ultrastructure during the attack by brown-rot fungi Postia placenta and Coniophora puteana. Int. Biodeterior. Biodegrad. 2006, 57, 99–106. [Google Scholar] [CrossRef]
- Elder, T.; Houtman, C. Time-domain NMR study of the drying of hemicellulose extracted aspen (Populus tremuloides Michx.). Holzforschung 2013, 67, 405–411. [Google Scholar] [CrossRef]
- Yang, T.; Ma, E.; Cao, J. Effects of lignin in wood on moisture sorption and hygroexpansion tested under dynamic conditions. Holzforschung 2018, 72, 943–950. [Google Scholar] [CrossRef]
- Geng, X.; Henderson, W.A. Pretreatment of corn stover by combining ionic liquid dissolution with alkali extraction. Biotechnol. Bioeng. 2011, 109, 84–91. [Google Scholar] [CrossRef]
- Barman, D.N.; Haque, A.; Hossain, M.; Paul, S.K.; Yun, H.D. Deconstruction of Pine Wood (Pinus sylvestris) Recalcitrant Structure Using Alkali Treatment for Enhancing Enzymatic Saccharification Evaluated by Congo Red. Waste Biomass-Valorization 2018, 11, 1755–1764. [Google Scholar] [CrossRef]
- Ishikura, Y.; Nakano, T. Adsorption Properties and Structural Features of Alkali Treated Wood. J. Jpn. Wood Res. Soc. (Japan) 2005, 51, 364–371. [Google Scholar] [CrossRef]
- Hill, C.A.; Keating, B.A.; Jalaludin, Z.; Mahrdt, E. A rheological description of the water vapour sorption kinetics behaviour of wood invoking a model using a canonical assembly of Kelvin-Voigt elements and a possible link with sorption hysteresis. Holzforschung 2012, 66, 35–47. [Google Scholar] [CrossRef]
- Engelund, E.T.; Thygesen, L.G.; Svensson, S.; Hill, C.A.S. A critical discussion of the physics of wood–water interactions. Wood Sci. Technol. 2012, 47, 141–161. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Avramidis, S. Water sorption hysteresis in wood: I review and experimental patterns—Geometric characteristics of scanning curves. Holzforschung 2017, 71, 307–316. [Google Scholar] [CrossRef]
- Lillqvist, K.; Källbom, S.; Altgen, M.; Belt, T.; Rautkari, L. Water vapour sorption properties of thermally modified and pressurised hot-water-extracted wood powder. Holzforschung 2019, 73, 1059–1068. [Google Scholar] [CrossRef]
- Endo, K.; Obataya, E.; Zeniya, N.; Matsuo, M. Effects of heating humidity on the physical properties of hydrothermally treated spruce wood. Wood Sci. Technol. 2016, 50, 1161–1179. [Google Scholar] [CrossRef]
- Salin, J.-G. Drying of Liquid Water in Wood as Influenced by the Capillary Fiber Network. Dry. Technol. 2008, 26, 560–567. [Google Scholar] [CrossRef]
- Chen, M.; Coasne, B.; Guyer, R.; Derome, D.; Carmeliet, J. Role of hydrogen bonding in hysteresis observed in sorption-induced swelling of soft nanoporous polymers. Nat. Commun. 2018, 9, 3507. [Google Scholar] [CrossRef]
- Esteban, L.; de Palacios, P.; Fernandez, F.G.; García-Amorena, I. Effects of burial of Quercus spp. wood aged 5910±250BP on sorption and thermodynamic properties. Int. Biodeterior. Biodegradation 2010, 64, 371–377. [Google Scholar] [CrossRef]
- Guo, J.; Xiao, L.; Han, L.; Wu, H.; Yang, T.; Wu, S.; Yin, Y. Deterioration of the cell wall in waterlogged wooden archeological artifacts, 2400 years old. IAWA J. 2019, 40, 820–844. [Google Scholar] [CrossRef]
- Jakob, M.; Mahendran, A.R.; Gindl-Altmutter, W.; Bliem, P.; Konnerth, J.; Müller, U.; Veigel, S. The strength and stiffness of oriented wood and cellulose-fibre materials: A review. Prog. Mater. Sci. 2021, 125, 100916. [Google Scholar] [CrossRef]
- Spear, M.; Broda, M. Comparison of Contemporary Elm (Ulmus spp.) and Degraded Archaeological Elm: The Use of Dynamic Mechanical Analysis Under Ambient Moisture Conditions. Materials 2020, 13, 5026. [Google Scholar] [CrossRef]
- Kulasinski, K.; Salmén, L.; Derome, D.; Carmeliet, J. Moisture adsorption of glucomannan and xylan hemicelluloses. Cellulose 2016, 23, 1629–1637. [Google Scholar] [CrossRef]
- Navi, P.; Stanzl-Tschegg, S. Micromechanics of creep and relaxation of wood. A review COST Action E35 2004–2008: Wood machining–micromechanics and fracture. Holzforschung 2009, 63, 186–195. [Google Scholar] [CrossRef]
- Ashaduzzaman; Hale, M.D.; Ormondroyd, G.A.; Spear, M.J. Dynamic mechanical analysis of Scots pine and three tropical hardwoods. Int. Wood Prod. J. 2020, 11, 189–203. [Google Scholar] [CrossRef]
- Broda, M.; Spear, M.J.; Curling, S.F.; Ormondroyd, G.A. The Viscoelastic Behaviour of Waterlogged Archaeological Wood Treated with Methyltrimethoxysilane. Materials 2021, 14, 5150. [Google Scholar] [CrossRef]
- Obataya, E. Mechanical and Dielectric Relaxations of Wood in a Low Temperature Range. I. Relaxations Due to Methylol Groups and Adsorbed Water. Mokuzai Gakkaishi 1996, 42, 243–249. [Google Scholar]
- Obataya, E.; Norimoto, M.; Tomita, B. Mechanical relaxation processes of wood in the low-temperature range. J. Appl. Polym. Sci. 2001, 81, 3338–3347. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, J.; Lyu, J. Moisture-dependent orthotropic viscoelastic properties of Chinese fir wood during quenching in the temperature range of 20 to −120 °C. Holzforschung 2019, 74, 10–19. [Google Scholar] [CrossRef]
- Backman, A.C.; Lindberg, K.A.H. Differences in wood material responses for radial and tangential direction as measured by dynamic mechanical thermal analysis. J. Mater. Sci. 2001, 36, 3777–3783. [Google Scholar] [CrossRef]
- Havimo, M. A literature-based study on the loss tangent of wood in connection with mechanical pulping. Wood Sci. Technol. 2009, 43, 627–642. [Google Scholar] [CrossRef]
- Kelley, S.S.; Rials, T.G.; Glasser, W.G. Relaxation behaviour of the amorphous components of wood. J. Mater. Sci. 1987, 22, 617–624. [Google Scholar] [CrossRef]
- Jiang, J.; Lu, J.; Yan, H. Dynamic Viscoelastic Properties of Wood Treated by Three Drying Methods Measured at High-Temperature Range. Wood Fiber Sci. 2008, 40, 72–79. [Google Scholar]
- Einfeldt, J.; Meißner, D.; Kwasniewski, A. Polymerdynamics of cellulose and other polysaccharides in solid state-secondary dielectric relaxation processes. Prog. Polym. Sci. 2001, 26, 1419–1472. [Google Scholar] [CrossRef]
- Sugiyama, M.; Obataya, E.; Norimoto, M. Viscoelastic properties of the matrix substance of chemically treated wood. J. Mater. Sci. 1998, 33, 3505–3510. [Google Scholar] [CrossRef]
- Heijboer, J. Secondary Loss Peaks in Glassy Amorphous Polymers. Int. J. Polym. Mater. Polym. Biomater. 1977, 6, 11–37. [Google Scholar] [CrossRef]
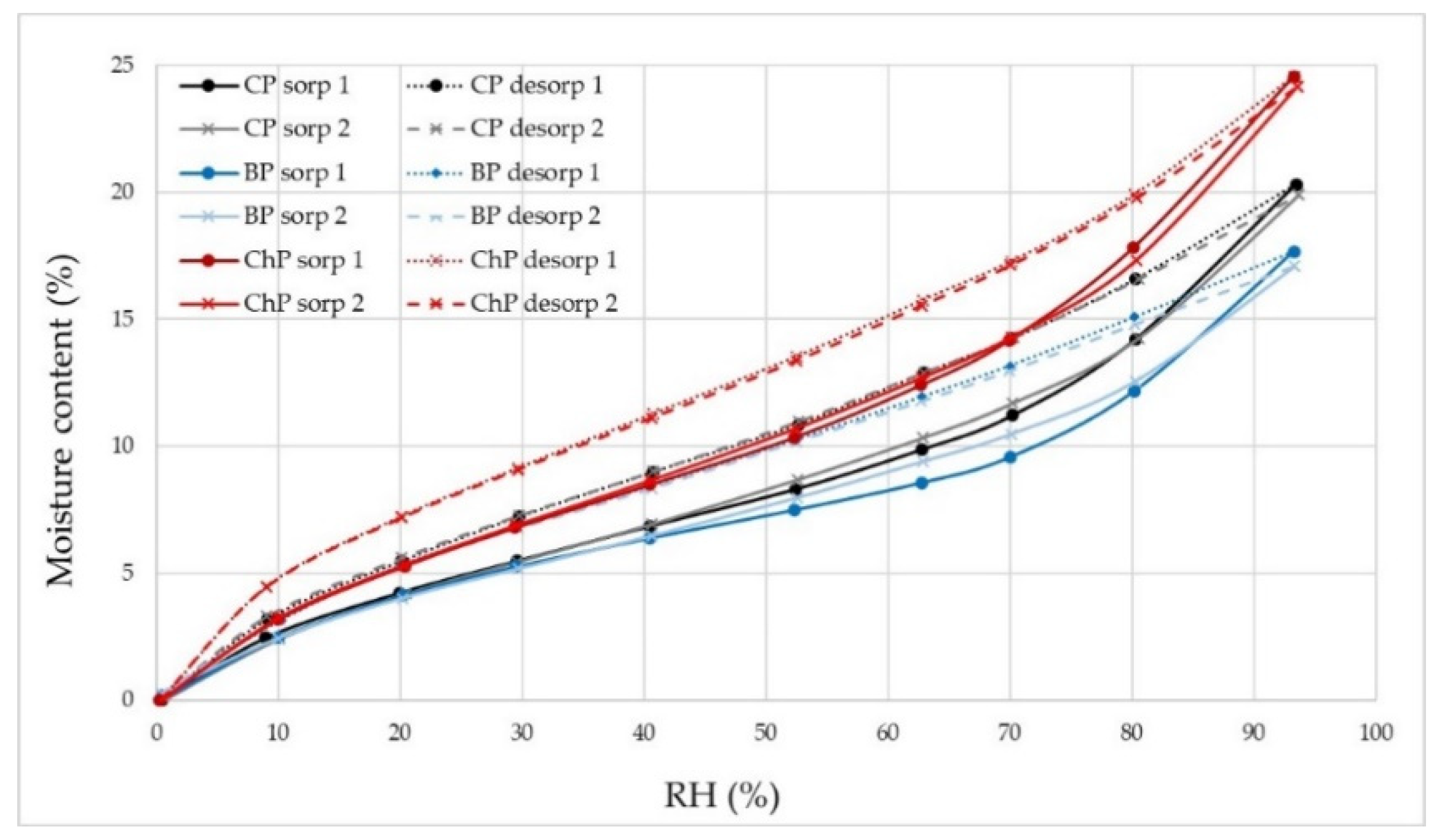
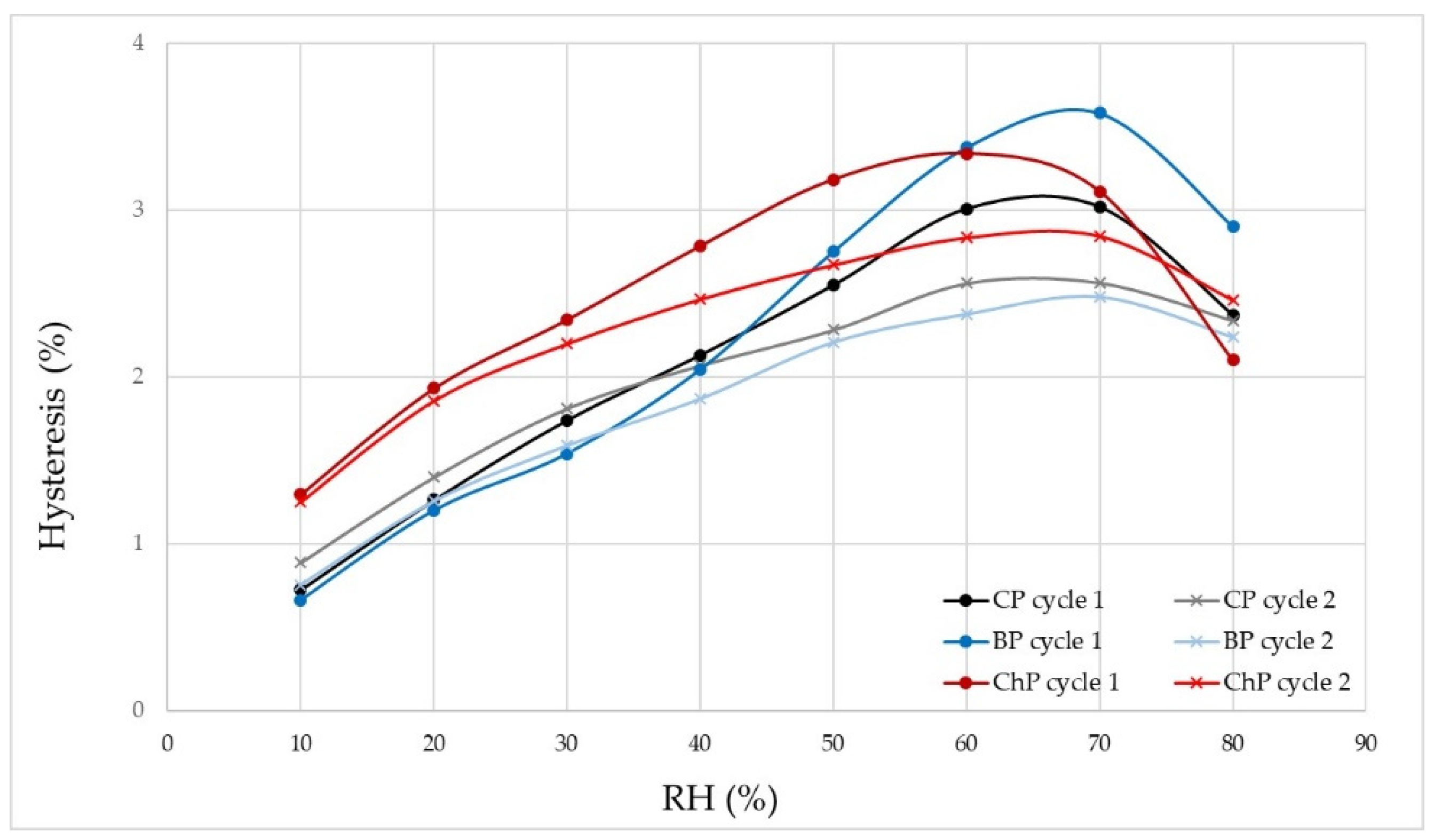


| Wood ID | Hydroxyls [mmol g−1] | H1max [%] | H2max [%] | ΔHmax [%] | EMC1 [%] | EMC2 [%] | ΔEMC [%] |
|---|---|---|---|---|---|---|---|
| CP | 7.90 ± 0.14 | 3.02 | 2.57 | 0.45 | 20.3 | 19.9 | 0.4 |
| BP | 8.04 ± 0.99 | 3.58 | 2.48 | 1.1 | 17.7 | 17.1 | 0.6 |
| ChP | 11.32 ± 0.34 | 3.34 | 2.85 | 0.49 | 24.6 | 24.2 | 0.4 |
| Wood ID | MC [%] | ρ [g cm−3] | Tan δ Response [°C] | E′ at 25 °C [MPa] | E″ at 25 °C [MPa] | Tan δ at 25 °C | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| γ | βwet | β3 | β2 | β1 | α | ||||||
| CP | 6.3 ± 0.1 | 0.44 ± 0.02 | −90 ± 5 | −65 ± 3 | 12 ± 1 | 92.8 | 105.4 | - | 95.4 ± 14.1 | 3.5 ± 0.4 | 0.0367 ± 0.0012 |
| BP | 6.2 ± 0.2 | 0.44 ± 0.03 | −86.6 | (−34) | - | 30 ± 6 | 76.7 | 101.4 | 52.3 ± 10.8 | 2.2 ± 0.3 | 0.0429 ± 0.0038 |
| ChP | 6.9 ± 0.1 | 0.65 ± 0.02 | −79.9 | - | (−7) | 48.6 | 83.1 | 90.8 ± 17.0 | 4.2 ± 1.0 | 0.0475 ± 0.0116 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Broda, M.; Spear, M.J.; Curling, S.F.; Dimitriou, A. Effects of Biological and Chemical Degradation on the Properties of Scots Pine—Part II: Wood-Moisture Relations and Viscoelastic Behaviour. Forests 2022, 13, 1390. https://doi.org/10.3390/f13091390
Broda M, Spear MJ, Curling SF, Dimitriou A. Effects of Biological and Chemical Degradation on the Properties of Scots Pine—Part II: Wood-Moisture Relations and Viscoelastic Behaviour. Forests. 2022; 13(9):1390. https://doi.org/10.3390/f13091390
Chicago/Turabian StyleBroda, Magdalena, Morwenna J. Spear, Simon F. Curling, and Athanasios Dimitriou. 2022. "Effects of Biological and Chemical Degradation on the Properties of Scots Pine—Part II: Wood-Moisture Relations and Viscoelastic Behaviour" Forests 13, no. 9: 1390. https://doi.org/10.3390/f13091390
APA StyleBroda, M., Spear, M. J., Curling, S. F., & Dimitriou, A. (2022). Effects of Biological and Chemical Degradation on the Properties of Scots Pine—Part II: Wood-Moisture Relations and Viscoelastic Behaviour. Forests, 13(9), 1390. https://doi.org/10.3390/f13091390









