1. Introduction
Intraspecific genetic diversity is important to maintain the fitness and evolutionary potential of species in changing environments [
1,
2,
3]. Forest practices can alter natural species composition, spatial distribution, and intraspecific genetic diversity patterns by changing mating dynamics [
4,
5,
6,
7]. The impact of forest management can be especially severe on resprouting species, which are able to regenerate both sexually and asexually [
8,
9].
After natural perturbations (e.g., herbivory, fire, floods, hurricanes, landslides, etc.), resprouting ability represents a valuable feature contributing to ecosystem resilience, being advantageous in environments where competitiveness is high [
10]. Forest management of resprouting woody species has been used for centuries to provide renewal and valuable forest products such as firewood, charcoal, and woody pastures [
11,
12]. Long-lasting coppicing affects natural genetic diversity patterns differently depending on the species and type of management [
12]. Examples of either reductions of genetic diversity (along with fragmentation in [
13]) or maintaining similar levels to unmanaged sites [
9,
14,
15] or even increasing genotype diversity [
16,
17] can be found. Due to the variability of the results, characterization of asexual and sexual reproduction rates should be considered in silvicultural practices to ensure that species genetic diversity and evolutionary processes are preserved [
7].
Spatial genetic structure is also sensitive to management [
9,
18]. Nevertheless, evidence for the impact of long-lasting forest practices on genetic patterns of resprouting tree species is still scarce, despite its relevant implications for nature conservation plans [
19,
20].
English holly (
Ilex aquifolium L.) occurs in Western Europe, from the Iberian Peninsula to western Scandinavia, North-West Africa in the Atlas Mountains, western Balkan Peninsula, North Turkey up to the Caucasus, in Atlantic and sub-Atlantic climates. In the sub-Mediterranean climate, this species is present at higher elevations, characterized by mild winter temperatures, relatively high summer precipitation and limited temperature ranges [
21]. Its distribution range is changing in Europe, because of increased winter temperatures in northern regions and drought in the south [
22]. Regarding light tolerance, it is a semi-shade species, while in Mediterranean climates it is an obligate shade plant [
23]. Throughout the northern part of the Iberian Peninsula (Atlantic biogeographic region), English holly is mainly present in discontinuous monospecific populations and becomes scarcer further south (Mediterranean biogeographic region), where it is mainly located in wet montane habitats under other species canopy such as beech (
Fagus sylvatica), oak (
Quercus pyrenaica or
Quercus petraea) or
Pinus sp. [
24].
English holly is a dioecious species with an outcrossing mating system that also reproduces asexually by stem and root suckering and layering. Due to its vegetative reproduction, holly trees have been exploited since the Neolithic period to obtain wood and food for livestock [
21,
25], becoming an economically important species as a provider of fodder in winter and shade and shelter in summer. Most of current formations of English holly in Spain are mainly linked to past human management for livestock uses [
24,
25] and are, therefore, highly vulnerable to abandonment of the traditional practices which sustained them, particularly if coupled with a predicted increase in temperature and decrease in precipitation [
23].
The ecological importance of this species lies on the presence of fruits in winter, which is a time of scarce resources, becoming a food source for birds [
26,
27,
28], rodents and ungulates [
21,
29,
30]. By the end of last century, the species was protected under regional laws in Spain (repealed afterwards in northern regions), and traditional uses were restricted in most areas, which, along with land use change and rural exodus, decreased human pressure. In northern Spain, ecological studies have shown that habitat fragmentation does not negatively affect regeneration dynamics [
31], whereas further south, summer drought and population isolation threatens species conservation [
23,
32], so protection laws still consider this species as being vulnerable to habitat changes. In the central area of the Iberian Peninsula, the species is distributed in patches of different size and the mosaic landscape might limit the recruitment potential and reduce the capacity for seed regeneration [
33]. Furthermore, present clonal diversity and the long-lasting coppice effect on the reproduction mode and genetic structure are still unknown in English holly.
A past management effect was evaluated in a mixed oak-beech forest in Central Spain, which represents the history of traditional forest uses and its recent abandonment in Mediterranean Europe. Documents from the XVI century already highlighted the economic value of English holly in the region and the necessity of protection in common woodlands [
34]. By the second half of the 20th century, after centuries of human pressure, traditional uses were progressively neglected, and livestock exploitation was finally restricted in 1974 in the study site, when the forest was protected [
35]. Since then, the former wood pasture has been transformed into a densely regenerated forest, where the effect of tree competition differs over subzones, species, and tree ages. In addition to high density, mortality and lack of vigor have been noted for English holly [
36].
Under the generalized situation of abandonment of traditional forest uses and the state of decay for English holly in a population located near the southern range of the species distribution, we aimed to determine the influence of past management in genetic diversity patterns and reproduction modes of this species in a coppiced plot compared to an area of wood pasture. Stronger clonal structure and lower genetic diversity levels are expected in the coppiced area compared to the wood pasture area, caused by past management. Furthermore, as a parting hypothesis we expect a different balance between sexual and asexual reproduction in both areas. Information on how the species reproduce in the present can serve as guidance for managing other Mediterranean forests which were subjected to coppicing.
2. Materials and Methods
2.1. Study Area and Sampling
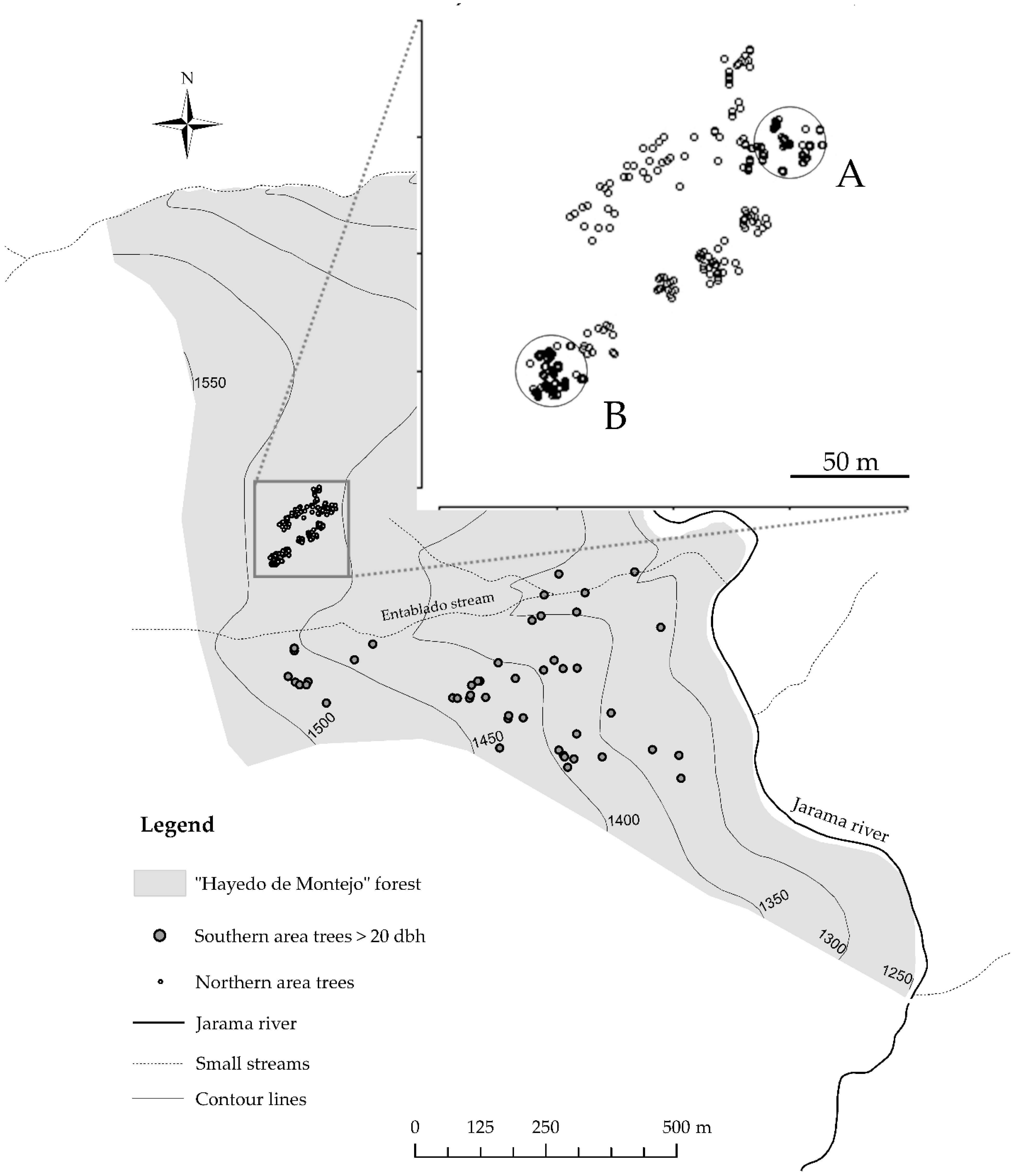
The study was carried out in a Biosphere Reserve sub-Mediterranean mixed forest in Central Spain (UTM ETRS89 458911 4550354) named “Hayedo de Montejo” (located next to Montejo village), mainly inhabited by melojo oak (
Quercus pyrenaica), European beech (
Fagus sylvatica), sessile oak (
Quercus petraea) and English holly (
I. aquifolium). The forest was used since the Middle Ages as a wood pasture to provide food (forage and seeds) and shelter for livestock, and firewood and other forest products for the Montejo neighbors. After the abandonment of traditional uses, tree species unevenly regenerated in different areas according to deep soil and exposure. Data collected in local periodical forest inventories allow for the differentiation of vegetation subareas in “Hayedo de Montejo” according to species composition and structure (
Figure S1, Supplementary Materials) [
34].
In present times, English holly grows in different wood structures (
Figure 1) in “Hayedo de Montejo”. In the northern area (zone 1) the species forms a dense subcanopy of spatially aggregated groups of stems of variated density and structures, covered mainly by beech and sessile oak. The last revision of the forest inventory in 2015 showed that in this subarea, English holly had reached a plateau in growth rate and number of individuals, and high mortality was detected [
36,
37]. In this highly dense zone, we sampled patches in a 2.5 ha area to assess genetic diversity, clonal extension, and spatial genetic structure. Coordinates and dasometric measures of two individuals from each spatially aggregated clump of holly trees, and all the isolated stems, were collected (up to a total of 125 individuals). Furthermore, aiming to detect fine-scale clonal structure, two plots of 15 m radii (A and B) located at the limits of the former 2.5 ha area, were exhaustively sampled up to a total of 262 individuals. Diameter at breast height (dbh) in the northern area ranged from 1.3 to 47.75 cm.
The southern area of “Hayedo de Montejo” (zone 2) is dominated by centenary oaks (mainly
Quercus pyrenaica) at low densities accompanied mainly by scattered holly trees of greater diameters, either isolated or in patches, aggregated or not in clumps. Furthermore, holly trees of smaller diameter can be found scattered in the area. In this low-density area, covering 42 ha, we sampled 46 individuals of diameter greater than 20 cm (
Figure 1). Diameters (dbh) in the southern area ranged from 20.7 to 71.3 cm dbh.
A total of 433 trees of different diameters were registered. Stem diameter, spatial coordinates, and sex (when possible) were recorded, and leaves were collected with the prescriptive permission and preserved in silica gel until lab analyses.
2.2. DNA Extraction and Microsatellite Analysis
In the northern area (zone 1), all individuals in the patches (N = 125) were included in the genetic analysis. Moreover, from the two intensively sampled plots in the extremes (A and B), all the isolated stems and the two stems of larger dbh from each aggregation of trees were selected for genetic analyses (N = 94). In total, N = 219 samples were analyzed in zone 1. In the southern area (zone 2), all the great-diameter individuals sampled (N = 46) were genetically analyzed. Overall, DNA extractions were performed in 265 stems using 3 to 5 mg of ground dried leaf tissue and the commercial kit Invisorb
® Spin Plant Mini Kit (Invitek, Berlin, Germany) following the manufacturer’s protocol. Six simple sequence repeat (SSR) nuclear DNA markers [
38], which were successfully transferred to
I. aquifolium from
Ilex leucoclada by [
39] (ILE03-38, ILE04-10, ILE01-47, ILE04-04, ILE03-01, ILE04-17), were used for the analysis. Two more SSR (ILE 04-02, ILE04-06) were tested but ILE04-02 did not amplify properly, so we used the remaining seven markers.
Each locus was amplified in separate 10 µL reaction volumes containing 1 µL MgCl2-free Buffer, 0.3 µL MgCl2 (50 mM) for most loci (except 0.5 µL for ILE04-10 and 0.4 µL for ILE04-17), 0.2 µL dNTPs 10 mM, 0.3 µL of each primer 5µM (forward and reverse), 0.4 µL Taq polymerase 1 U/µL, 0.1 µL BSA (Bovine Serum Albumin) 2 mg/mL PierceTM (Thermo Fisher Scientific, Rockford, IL, USA) only for four loci (ILE01-47, ILE04-04, ILE03-01 and ILE04-17) and 5 ng/µL of DNA template. Polymerase Chain Reaction (PCR) amplification was performed using either an Applied Biosystems 2720 Thermal Cycler (Applied Biosystems, Foster City, CA, USA) or an Applied Biosystems GeneAmp PCR System 9700 (Applied Biosystems, Foster City, CA, USA) set for the following program: a first step of 5 min at 94 °C, then 45 s at 94 °C, followed by 20 to 30 cycles at annealing temperatures between 56 and 56.5 °C (cycles and annealing temperature varied for each locus), 45 s at 72 °C, and a final step of 5 min at 72 °C. PCR products were separated using electrophoresis in acrylamide gels loaded in a LiCor 4300 DNA Analyzer (LI-COR, Lincoln, NE, USA) and allele sizes identified by using SequaMarkTM DNA Size Marker (Invitrogen, Carlsbad, CA, USA). The scoring of alleles was performed independently by three people with the use of SAGAMX (LI-COR, Lincoln, NE, USA) software in order to minimize scoring errors during the processing of the information.
2.3. Data Analysis
2.3.1. Genotypic and Genetic Diversity
Genet (defined as an individual that develops from one original zygote, and that produces ramets vegetatively during growth [
40]) assignment was assessed for all samples using
genclone v. 2.0 [
41]. We calculated
Pgen, the probability of a given multilocus genotype (MLG) occurring in a population, which mainly depends on allele frequencies (f
i) and the number of markers analyzed:
and
Psex, the probability that stems with the same MLG (i.e., repeated genotypes) originated from distinct sexual reproductive events:
which depends on
Pgen and number of stems sharing each MLG. Both estimates were performed taking account of Hardy-Weinberg equilibrium departures, so the slightly modified
Pgen (fis) and
Psex (fis) were used for the analysis [
41,
42,
43]. We considered a
Psex (fis) value of 0.05, a common threshold in literature [
44,
45,
46] below which two stems sharing MLG can be considered to have the same reproductive origin (i.e., are part of the same clone). The
p-value for testing the significance of
Psex (fis) for each MLG was estimated by performing 1000 permutations using the software RClone [
47]. Furthermore, to evaluate the reliability of the clonal assignment, we considered the stem sex and the spatial distribution of stems sharing the same MLG.
Furthermore, due to possible scoring errors and somatic mutations, MLGs differing in only a few alleles may be part of the same clone forming a named multilocus lineage (MLL). We looked for a peak in frequency distribution at very low genetic distances among pairs of MLGs [
42,
48] to evaluate the occurrence of both processes. To estimate a threshold for MLL assignment we also used RClone software [
47,
49]. Furthermore, we manually explored each pair of MLGs differing in one repeat motif (two base pairs) in just one allele (from the 14 analyzed by the seven SSRs) [
42], considering different sex and physical distance over the maximum range detected for stems of the same clone to discard clonal membership (results not shown).
After genet assignment, we considered one copy per genet to estimate genetic diversity parameters to avoid bias in allele frequencies derived from many individuals sharing the same genotype. Number of alleles (NA), effective number of alleles (NA
e), expected heterozygosity (He), observed heterozygosity (Ho) and inbreeding (Fis), expressing the departure from Hardy-Weinberg genotypic proportions, were calculated using the software SPAGeDI 1.5 [
50]. Furthermore, the frequency of null alleles was assessed [
51] using the Brookfield2 estimator in Micro-Checker v.2.2.3 [
52] and the program INEst v.2.2 [
53] was also used to calculate the parameters Ho and He corrected for the presence of null alleles.
2.3.2. Clonal Diversity and Structure
Using
genclone v. 2.0 software [
41] genotypic richness was calculated as the proportion of distinct genets (G) present in the sample relative to the number of sampling units, R = (G − 1)/(N − 1) [
54]. Clonal heterogeneity was calculated as an adapted estimate of the Simpson’s complement index (D* = 1 – D) ranging from ‘0′ when all trees of a population are a single clone to ‘1′ when they are all unique genotypes. Finally, clonal evenness was used to describe the equitability of the distribution of ramets among genets using the Simpson evenness (ED*) estimate, which ranges from ‘0′ when there is dominance of one or a few genets to ‘1′ when ramets are distributed equally among genets [
42]. In addition, the frequency of unique genotypes was evaluated to estimate the ratio of sexual to asexual reproduction.
Clonal structure at the fine scale was determined by means of clone size using all the individuals in the two intensively sampled plots A and B, including the ones which were not genetically analyzed (N = 168). Clone size was described by number of stems, average distance among stems, maximum distance among stems and their spatial distribution. Moreover, clonal identity probability (i.e., the probability of finding two identical genotypes) against different distance classes visually reflects clonal size. Furthermore, stems that were not genetically analyzed that were located between ramets of the same clone were included in the analysis to estimate clonal size more accurately.
2.3.3. Spatial Genetic Structure
Spatial genetic structure was assessed using the kinship coefficient (Fij) [
55], which was calculated using one stem and the central coordinate from each genet. The FSGS analyses were performed independently for northern zone 1 (including all genets from the plots A and B, and sampled patches between them (N = 96 genets) and southern zone 2 of “Hayedo de Montejo” forest (including the greater diameter individuals (>20 cm dbh; N = 46). Mean values of Fij kinship coefficient were obtained for eight distance intervals of 25 m covering 200 m in zone 1, and eight distance intervals more of 100 m at zone 2 covering up to 1 km. The statistical significance of the autocorrelation analyses was tested with a permutation method (10,000 permutations) using SPAGeDI 1.5 software. Furthermore, Sp statistics were calculated for the whole forest, and independently for the northern and southern areas, according to the formula Sp = −b/(1 −
) proposed by [
56] to quantify FSGS, where b is the slope of the regression of Fij represented against distance intervals and
is the mean Fij between individuals belonging to the first distance interval that should include all pairs of neighbors. Historical gene dispersal sigma (σ), understood as the square root of half the mean square parent-offspring distance [
56], was evaluated for the whole population and independently for northern and southern areas using an estimation of effective population density (De), which typically ranges from one half to one-tenth the density of adults in natural plant populations. Neighborhood size (Nb) represents the relation between local genetic drift and gene dispersal [
56].
3. Results
3.1. Genetic Diversity
A total of 23 alleles were detected with seven microsatellite loci. Locus ILE04-10 is the most diverse loci, with seven different alleles, although four of them constitute rare alleles (frequency lower than 0.05). The number of alleles per locus and over loci, allele frequencies and heterozygosity values are presented in
Table 1. The expected heterozygosity ranged from 0.2655 to 0.706 among loci, with an average of 0.523. Mean observed heterozygosity was very similar to mean expected heterozygosity, so Fis was not significantly different from zero. The estimated frequency of null alleles was only slightly significant for locus ILE04-04 (frequency null = 0.073), and average frequency null for the other six loci was 0.014. Thus, allele frequencies were adjusted to calculate diversity values (cHe in
Table 1).
There was a slight difference in genetic diversity between the northern area (He = 0.540) and the southern area (He = 0.481), despite the contrasting number of genets analyzed (MLGs = 96 vs. MLGs = 45). Overall, genetic diversity reached He = 0.525 in the “Hayedo de Montejo” population, and no deviation from HW proportions was found. In the northern area, the expected heterozygosity was higher for unique genotypes (He = 0.555) than for genotypes with more than one ramet, i.e., clones (He = 0.529), but a small significant deviation from HW proportion was found in the first case (Fis = 0.096). Considering diametric size, smaller genets were more diverse (He = 0.549) than bigger ones (He = 0.517), especially when considering small and unique genotypes (He 0.571; N = 29 which showed significant inbreeding coefficient (Fis = 0.130) versus big clones (He = 0.524; N = 23).
3.2. Clonal Assignment
Initially, a total of 141 multilocus genotypes (MLGs) were identified among the 265 analyzed stems: 85 MLGs were represented by single stems, thus being unique genotypes, while 56 MLGs were formed by a different number of stems. Values of Pgen (fis) for all MLGs ranged from 0 to 0.00099 with an average value of 0.00014, providing high precision for clonal assignment. Psex (fis) for repeated MLGs ranged from 0 to 0.194 with an average value of 0.016. Eight clonal MLGs (14.3%) had Psex (fis) values higher than the threshold of 0.05, but the stems were separated at distances from 1.3 to 6.3 m, which are within the estimated clonal range in this study (see next section), so we could not discount them from being clones. Furthermore, two MLGs with Psex (fis) values under the 0.05 threshold showed very high distances between stems, suggesting they originated from different reproductive events. The first, which had the highest Pgen (fis) value of the sample, had three stems in a northern intermediate patch (separated 4, 5 and 9 m) and two more in the southern area (both groups separated more than 250 m and the southern two stems separated 64.5 m). The second one had six stems in two different patches of the northern area separated more than 45 m. Considering the spatial distribution information, 143 genets were considered, 86 of which were unique genotypes and 57 which were clones.
Regarding somaclonal mutations, after inspecting the histogram of pairwise comparisons, we observed no peak in the lower genetic distances (
Figure S2, Supplementary Materials). Furthermore, after using RClone to observe the superposition of simulated and actual frequency distributions, little difference was found (data not presented), showing that the number of MLGs accurately reflects the number of genets. However, we still individually evaluated each pair of MLGs differing in less than two base pairs (in one allele) and concluded that we did not have enough proof to determine the existence of MLL.
3.3. Clonal Diversity and Structure
Overall clonal richness was estimated to be R = 0.54, clonal heterogeneity D* = 0.99 and evenness ED* = 0.975. In the northern area, out of the 219 stems analyzed, 96 MLGs were identified, so clonal richness was 0.44. In the southern area, we considered only one repeated MLG (45–1/46–1 = 0.98; i.e., clonal richness is almost equal to one), according to sampling design and forest structure.
Specifically, in plots A and B (
Figure 2 and
Figure 3) we detected 35 MLGs among 94 stems sampled (R = 0.37), among which 20 were clones (57%) and 15 were unique genotypes (43%).
Clones were mainly composed of two or three stems (70%) while the rest (30%) had between four and 11 stems (the greater clone, number 5, was found in plot B; see
Figure 3) and the average was 4.16 stems per clone. Clones had their stems grouped together in dense aggregations without intermingling. Average distance between stems of the same clone was 2.76 m and average maximum distance was 3.74 m. The maximum distance detected was 10.78 m. The probability of two stems being identical (corresponding to the same clone) reached 75% if they were separated less than 2 m, decreased to 68% in a 2–3 m range and was below 0.5% in the range of 10 to 12 m (
Figure 4). The average diameter of ramets (12.53 cm) was not significantly different from unique genotypes (12.25 cm). Dead trees were located mainly in aggregations and rarely isolated (
Figure 2 and
Figure 3), being evenly distributed in diameter classes (
Figure 5).
As no intermingling of clones was observed, no genetically analyzed individuals in the two plots could be considered as part of a certain clone if they were located between genotyped ramets of that clone (see reassignment in
Figure 2 and
Figure 3). In some cases (18%), one clump had two different MLGs, some of them even merging in the base. As a result of the reassignment, the average number of stems per clone increased from 4.16 to 6.16 and the maximum number of stems rose from 11 to 18. Furthermore, the average distance between stems of the same clone decreased from 2.76 to 2.55 m, while maximum distances did not change as reassignment only included the stems in between.
Unique genotypes were evenly distributed in diameter classes (
Figure 5) and in space, located within clonal aggregates, either isolated or established next to other species (e.g.,
Crataegus monogyna). Considering reassignment criteria, unique genotypes included in an aggregation of with not-analyzed trees could not be discarded from being clones. As a result, the number of unique genotypes could decrease from 15 to 6 and their average diameter from 14.5 to 9.7 cm.
3.4. Spatial Genetic Structure among Genets
For the whole forest, significant patterns of FSGS were found for the first and the third distance classes of 25 and 75 m, where the kinship coefficient Fij reach values of 0.020 and 0.009, respectively (
Figure 6a). Analyzed separately, in the northern area, Fij reached an average value of 0.017 for the first distance class of 25 m, accounting for the decreasing pattern observed when considering the whole population (
Figure 6b). On the contrary, the southern area, where holly trees grew scattered, did not show any significant FSGS pattern, and average Fij values raised from −0.010 to 0.025 from the first to the second distance classes, showing an erratic pattern until 1000 m distance (
Figure 6c). The northern area pattern of FSGS in the first distance class was also found for the independent analysis of individuals greater and smaller than 20 cm dbh (in both areas), reaching higher values for the greater diameter trees than the smaller ones (Fij = 0.034 and Fij = 0.018, respectively).
The values for statistic Sp differed among the whole forest, the northern coppiced area, and the scattered southern area, according to the different patterns of FSGS found in each zone, reaching values of Sp = 0.0057, Sp = 0.0137, and Sp = −0.0003, respectively.
The values of sigma (σ) and the neighborhood size (Nb) also differed among analyzed zones. For the southern scattered area, σ and Nb did not converge to any values at any chosen De value (0.1; 0.25; 0.5), whereas for the northern coppiced area or when considered all MLGs in the whole forest, σ and Nb reached values from 168.82 to 87.86 individuals and 8.82 and 4.98 m, respectively (
Table 2).
4. Discussion
In this study on I. aquifolium clonal diversity and structure, we aimed at elucidating the extent to which forest management affected these traits, as well as spatial genetic structure and the contribution of clonality to species reproduction. Despite the low polymorphism of molecular markers, clonal assignment was feasible, and the genetic diversity levels found do not explain the mortality and lack of vigor of the species in the northern area. Moreover, we could evaluate how management influenced forest structures and clonal propagation, conditioning current patterns of spatial genetic structure and reproduction modes in this forest, as described in the paragraphs below.
4.1. Genetic Diversity and Clonal Assignment
Genetic diversity in terms of number of alleles per loci was low (A = 3.5) compared to the species for which they were developed (
I. leucoclada; A = 18) [
38]. Compared to previous studies using similar molecular markers, the number of alleles was similar to
Ilex perado ssp. (A = 2.1–3.6) [
57] and lower than
Ilex canariensis (A = 6.3) in Canary Islands [
57] and
I. aquifolium in Denmark (A = 5.5–6.7) [
39]. Nevertheless, all these studies were performed in multiple populations, so they gathered the genetic diversity of broader distribution scales and thus obtained a higher number of alleles. A low number of alleles might lead to an underestimation of genetic diversity, but using a large sample size, we ensured that all of the genetic diversity was represented. We found a slightly significant frequency (0.073) of null alleles for one locus (ILE04-04), a feature often observed when primers are transferred to a species for which they were not developed [
52,
58]. Despite the low number of alleles (A = 2), this locus was not excluded from the rest of the analyses, and heterozygosity levels were adjusted (
Table 1). Notwithstanding, no evidence of inbreeding (
Table 1) was found in this study, and the average heterozygosity level (He = 0.549) was among values reported for Macaronesian species (
Ilex perado ssp.; He = 0.263–0.575 and
Ilex canariensis; He = 0.410–0.667) with seven microsatellites (ILE04-10, ILE03-38, ILE03-01 and ILE04-06 among them) [
57], and only slightly lower than for wild populations of
I. aquifolium in Denmark (He = 0.554–0.610) with six microsatellites used in the present study (ILE04-10, ILE03-38, ILE03-01, ILE04-06, ILE04-17 and ILE01-47) [
39]. Therefore, despite climatic changes and human intervention on “Hayedo de Montejo”, we found no evidence of lack of heterozygotes or inbreeding in the population.
Furthermore, genetic diversity was high enough to identify clonal assemblies in I. aquifolium for the first time, with overall high probabilities. From the 56 multi-stem MLGs detected, 48 (85.7%) presented Psex (fis) values lower than the commonly used threshold of 0.05, i.e., they had a very low probability of their stems sharing a common MLG as a result of independent sexual reproductive events. In the case of the other eight clones, their higher Psex (fis) values (from 0.052 to 0.194 with an average of 0.093) are probably due to the low number of ramets they are composed of rather than high Pgen (fis) values. They were considered clones also due to the distances separating them, which were lower than the maximum distance identified for stems belonging to the same clone (10.78 m), and may correspond to root sprouting.
4.2. Clonal Diversity
In the northern area of “Hayedo de Montejo” forest, clonal richness for English holly (R = 0.436) is in accordance with the average genotypic diversity of 77 clonal species reviewed by [
59] for SSR markers. High clonal richness (R = 0.77) found in a natural population of
I. leucoclada can be explained by its foundation over multiple genetically different genets [
60] or by a management and reproduction of the species that favored genetic diversity. However, in “Hayedo de Montejo” forest, the lower richness values are probably driven by the intensive past use of the species, especially in the dense areas where clear signs of coppicing and pruning are still apparent. Therefore, vegetative propagation contributed to the historical regeneration of this species, although clonal clumps do not occupy very extensive areas (maximum distance between ramets is around 10 m).
Currently, the northern area is in a process of recovery, both through vegetative reproduction and seed recruitment. This seed recruitment seems to be recent, as after the reassignment, the average diameter of unique genotypes was small (9.7 cm). Consequently, the species seems to be recovering from intensive use, and recruitment in the last decades accounts mainly from asexually originating individuals, but also from recent seed originated ones located under the canopy of bigger trees of different species, such as
Sorbus aria and
Crataegus monogyna, where birds usually perch [
28].
The Simpson’s diversity index (D = 0.987) obtained is similar to
I. leucoclada [
60] (D = 0.99), and higher to the average value for clonal species (D = 0.62) found by [
61] and to the diversity index (D = 0.85 ± 0.01) reported for clonal species by [
59]. The difference in this last case might be associated with the type of marker used. Studies performed with microsatellites, such as those carried out in our investigation, usually report higher clonal diversity compared to those using allozymes [
42].
The high value of evenness (ED* = 0.975) indicates a relatively homogeneous distribution of stems among genets, with an equilibrium in the abundance of the different clone sizes. Furthermore, the presence of unique genotypes of different diameter classes indicates that over time there is a stable incorporation of individuals originated from seed. Homogeneity might indicate that management equally affected all genets independently of size, which could lead to the similar distribution of mortality among different clones (
Figure 5). As we could not detect an effect of intraspecific competence (between stems of the same clone), mortality might be related to light availability [
23], as there are other species in “Hayedo de Montejo” that grow above
I. aquifolium trees.
In the southern area, due to the sampling method and the forest structure, only two MLGs had more than one ramet, and due to the high distance separating the ramets, one of them was discarded as a clone. Consequently, the clonality of the area is very low, but stem resprouting motivated by wild animals browsing and cattle that still has access to the area can be observed.
4.3. Management and Clonal Structure
In the northern area, reassignment provides a more accurate distribution of ramets given the absence of intermingling among clones. Furthermore, reassignment shows how densely aggregated the groups of stems of the same clones are as a response to stem resprouting. Furthermore, different aggregations of the same clone are usually not far from one another (average maximum distance of 3.74 m) but can be distanced up to 10 m. This feature shows the ability of the species to resprout not only from stem, but also from the root systems, even at long distances. Longstanding traditional management could have enhanced this trait, promoted by human cuttings and cattle browsing performed in “Hayedo de Montejo” since the Middle Ages.
In
I. leucoclada, a Japanese holly described in natural conditions [
60], the probability of finding ramets from the same clone for a range of 3 m (55%) was lower than in
I. aquifolium (68%), indicating that English holly has stronger clonal structures. Furthermore, this probability decreases more steadily in English holly than in
I. leucoclada, where it descends to a 20% for a range of 4 m, which points to greater size clones in English holly, with a similar mosaic pattern of clonal expansion. Japanese holly has been shown to form a more intermingled structure, which is highly influenced by heavy snow in an area with steep slopes that keeps stems pressed to the ground, and results in a downhill distribution [
44]. In the present study, although we cannot discount this phenomenon, clones do not present a clear directional pattern, so we think that the more common round-shaped clones are probably a consequence of stem and root resprouting triggered by past management.
Stems located in the external area of clones might be ramets of already described clones or genotypes not detected in the present study (such as those around genotypes 3–55, 57–77, 43–61, 47–51 in
Figure 3 and genotypes 6–7, 48–50, 2–52, 89–90 in
Figure 4), which could mean an underestimation of clonal richness in this study.
4.4. Spatial Genetic Structure
For the whole studied area, the fine-scale spatial genetic structure was significant in the first and the third distance classes of 25 and 75 m. Nevertheless, the two differently managed areas, the northern coppiced and the southern scattered zones, contributed with contrasting patterns of FSGS to the whole population. Whereas in the southern area holly trees did not show any significant pattern, in the northern area, Fij reached significant positive values only for the first distance class, being almost two-fold for bigger individuals (>20 cm dbh; Fij = 0.034) than for smaller ones (<20 cm dbh; and Fij = 0.018).
Past management techniques could explain the different genetic structures currently found in the “Hayedo de Montejo” forest. Holly trees located in the scattered southern area could be more than 130 years old [
62] and were in the past part of an open woody pasture, dispersed around suitable places, as the other tree species. Nevertheless, contrary to oaks and beech trees which also inhabit this forest [
63,
64,
65], big holly trees in the southern area are not familiarly related. Only in the dense northern area does the species show familiar structures up to 25 m, greater for bigger than for smaller individuals. These differences would be the result of past coppice management in the northern area, where recent regeneration forming a dense understory is reducing familiar structure.
When historical dispersal distances were estimated for the whole forest, the results obtained may indicate a limited gene dispersal (σ = 4.98–8.36), which is striking for an entomophilous and zoochorous species. In this sense, the behavior of dispersers could have contributed to coancestry levels and gene dispersal limitation derived from insects siring female individuals with pollen from a reduced number of male individual and birds codispersing half-sib and full-sib seeds (as argued by [
66]). High density of clonal individuals in the northern area could have exacerbated this behavior [
67].
Outcrossing species generally have low Sp, so higher Sp values mean strong family structures which can be due to limited dispersal abilities. The values in the present study (Sp = 0.0057 for the whole forest, or Sp = 0.0137 for the dense norther area) is among the ranges reported for other outcrossing, insect-pollinated and animal-dispersed tree species [
56]. For instance, another species which shares life form and mating systems with
I. aquifolium,
Brucea javanica, presented a similar Sp value (Sp = 0.0145) [
56,
68]. For
Symphonia globulifera, another tree species essentially outcrossing, animal-pollinated and animal-dispersed like English holly, presented a similar Sp value in its Neotropical populations (Sp = 0.011) [
69]. Furthermore, although the pollination and seed dispersal system are different, both
Quercus petraea and
Quercus pyrenaica showed similar values (Sp = 0.0145 and Sp = 0.0118, respectively) in the same area of study [
64]. Conversely, the Sp value we obtained is lower than the values reported for
I. leucoclada [
66] in two different environments, one an old-growth forest (Sp = 0.059) and the other a secondary beech forest (Sp = 0.080). However, both values are higher than the maximum range obtained by [
56] due to the presence of strong spatial genetic structure because of kin-structured seed dispersal.
5. Conclusions
The English holly population of “Hayedo de Montejo” forest has been traditionally managed for centuries, which derived in two forest types. In the former northern coppiced area, English holly had a predominance of asexual reproduction. Different distances and aggregation sizes implies a good capacity for stem and root resprouting. Clones mainly form round-shaped dense aggregations of two to 18 ramets with an average of six ramets per clone distanced less than 3 m (exceptional long inter-ramet distances over 10 m). The smaller size of English holly clones compared to other Ilex species may also be a result of traditional cattle browsing. Management abandonment affected genets of all sizes equally and interspecific competence might be promoting mortality, which is evenly distributed among them. Stronger clonal structure was found in the northern area compared to the southern area, but fine-scale genetic structure was only significant in the first distance class of study (25 m), being greater for trees (genotypes) over 20 cm dbh. This result could point towards a restriction of gene dispersal (supported by the lower heterozygosity in sexually originated unique genotypes), probably caused by stem distribution or limited seed dispersal, although this is not common in bird-dispersed trees.
In the southern scattered area, the former multispecific woody pasture, the absence of FSGS found is common for a bird-dispersed tree and the present forest regeneration dynamics could be similar to a natural regeneration of an accompanying species. The other dominant species in this area i.e.,
Quercus sp. [
63] and
F. sylvatica [
65], however, showed significant patterns of FSGS in centenary trees in the same forest for even greater distances.
The estimated genetic diversity (in terms of number of alleles and richness) is lower than the one determined for other Ilex species analyzed at the same loci. However, we found no evidence that management had affected heterozygosity or inbreeding in the population. Furthermore, recent sexual recruitment was also observed both in the northern and southern area.
In summary, it is concluded that management abandonment in this forest has promoted an increase in the number of trees of English holly and its genotypic diversity. Furthermore, genetic diversity was not negatively affected, and sexual reproduction is present in both areas of the forest. However, interspecific competence might compromise the dynamic of the northern area, and small coppicing-like interventions could be a management option in case cultural values are to be preserved.