Study on the Combustion Characteristics of Mountain Forest Vegetation
Abstract
:1. Introduction
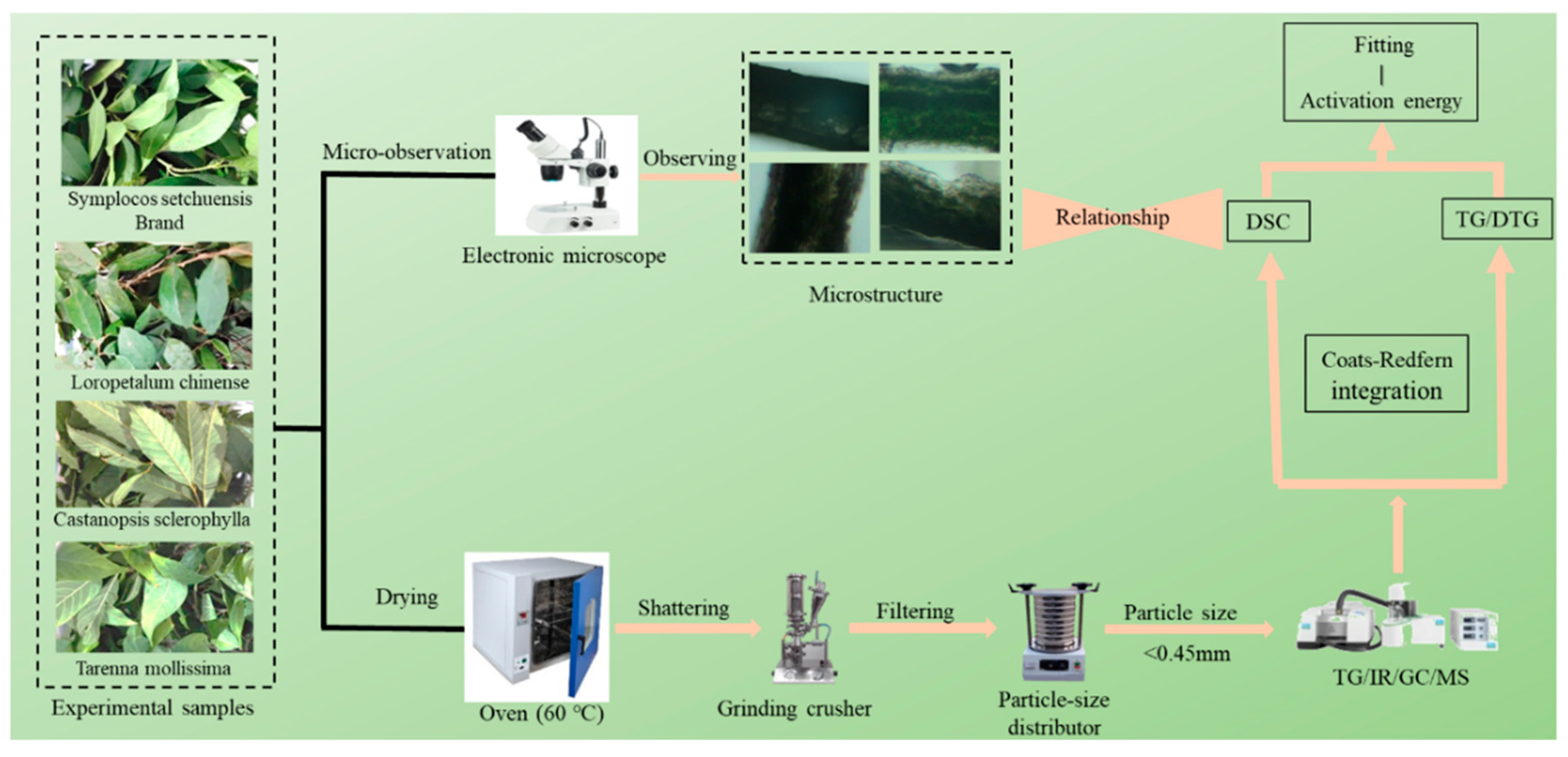
2. Materials and Methods
2.1. Study Site
2.2. Samples Preparation
2.3. Laboratory Experiments
2.3.1. Microstructure
2.3.2. DTG–DSC
2.4. Pyrolysis Reaction Kinetic Model
3. Results and Discussion
3.1. Microstructure
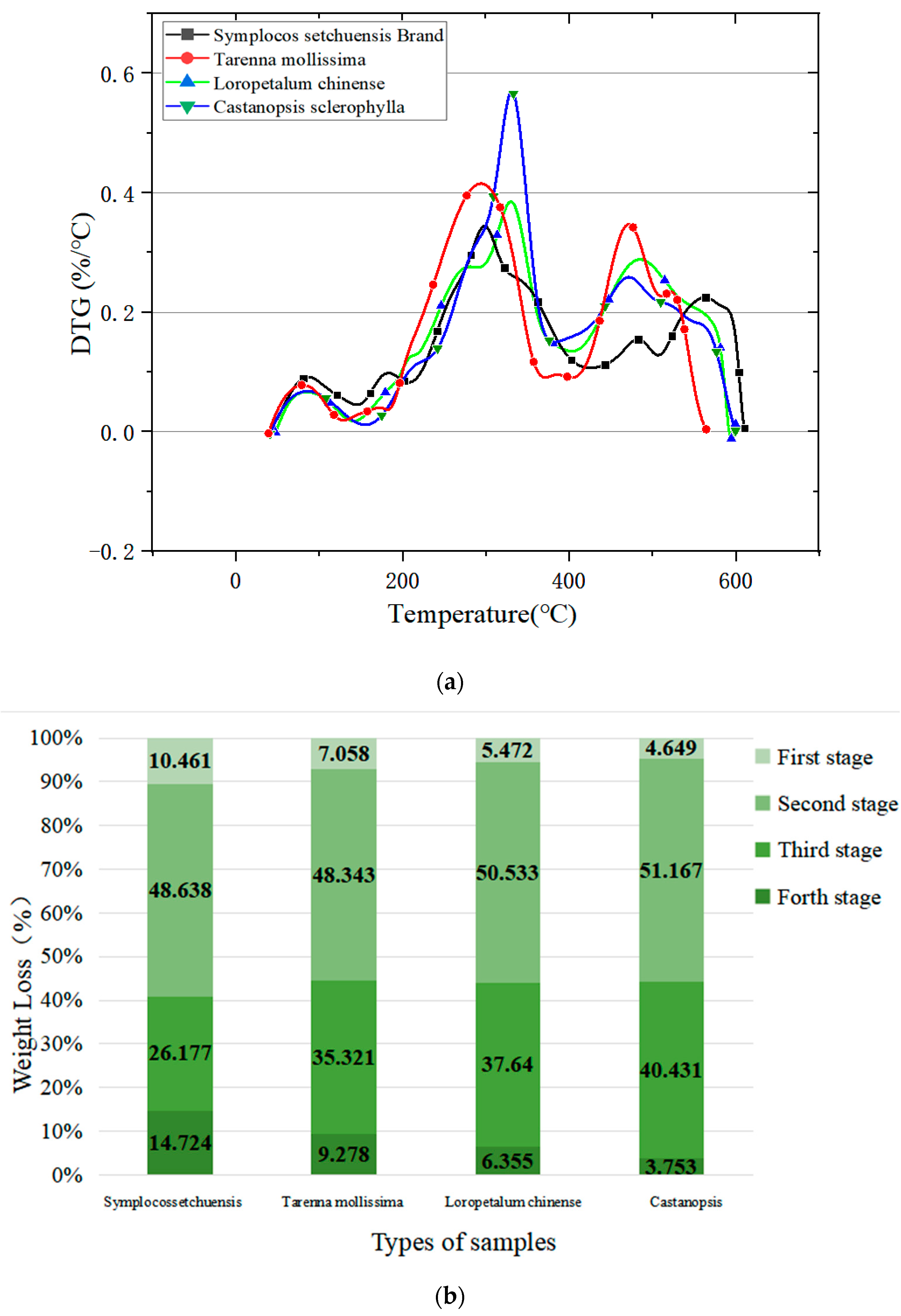
3.2. Results of TG–DTG
3.2.1. Analysis of TG
3.2.2. Analysis of DTG
3.2.3. Analysis on Pyrolysis Kinetics of TGA–DTG
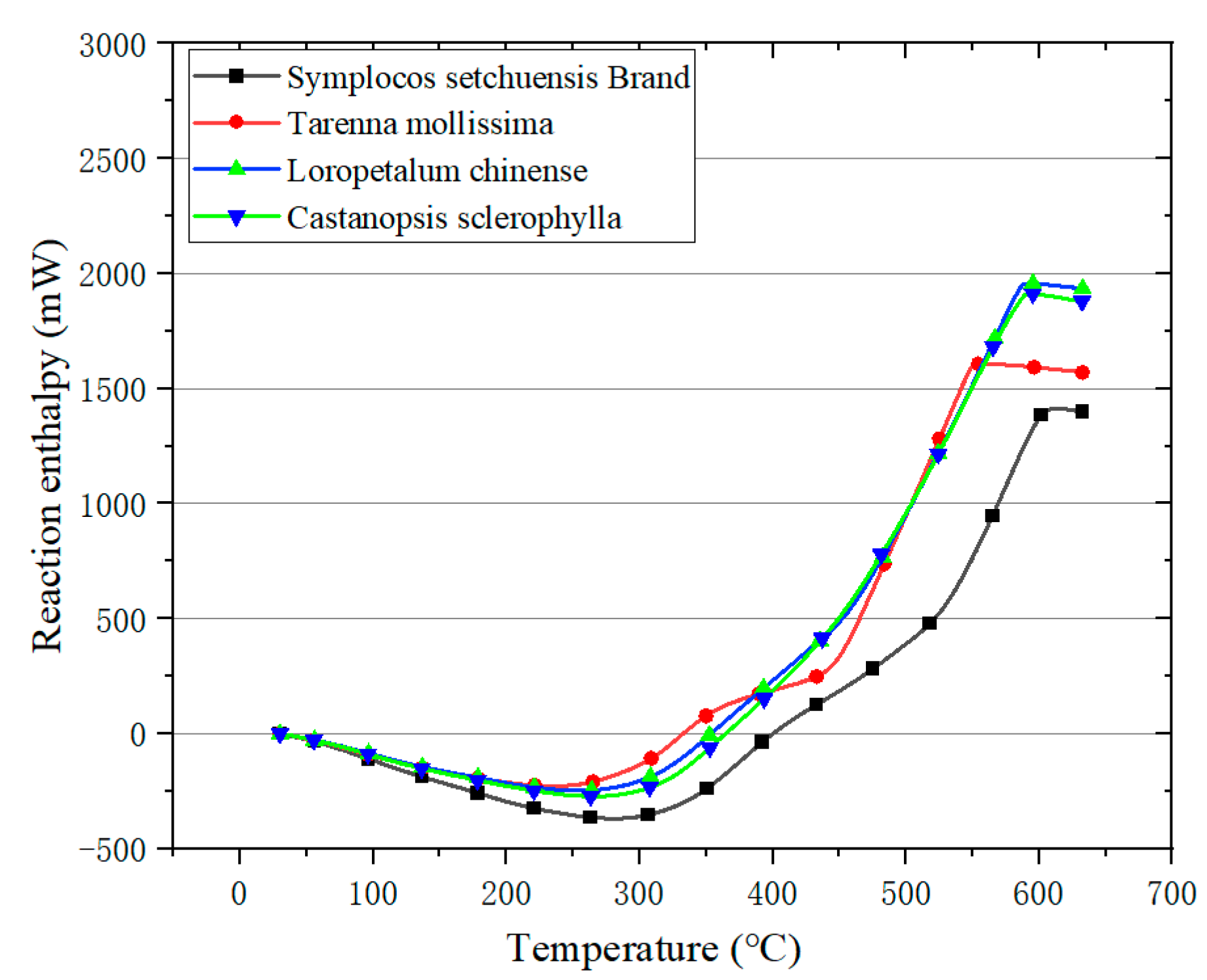
3.3. Results of DSC
3.3.1. Analysis on DSC
3.3.2. Reaction Enthalpy Analysis
3.3.3. Analysis on Pyrolysis Kinetics of DSC
3.4. Regression Analysis and Correlation Test
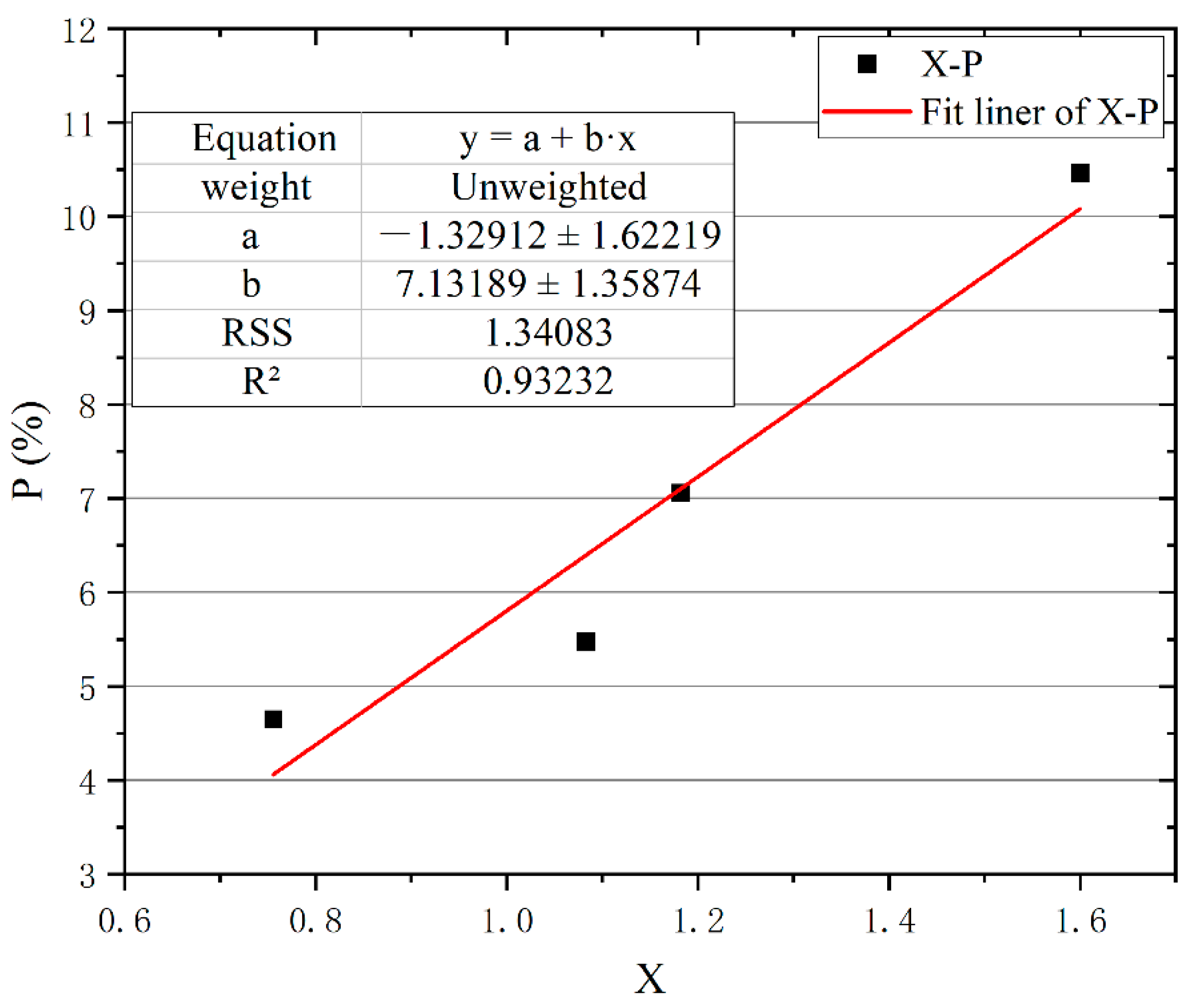
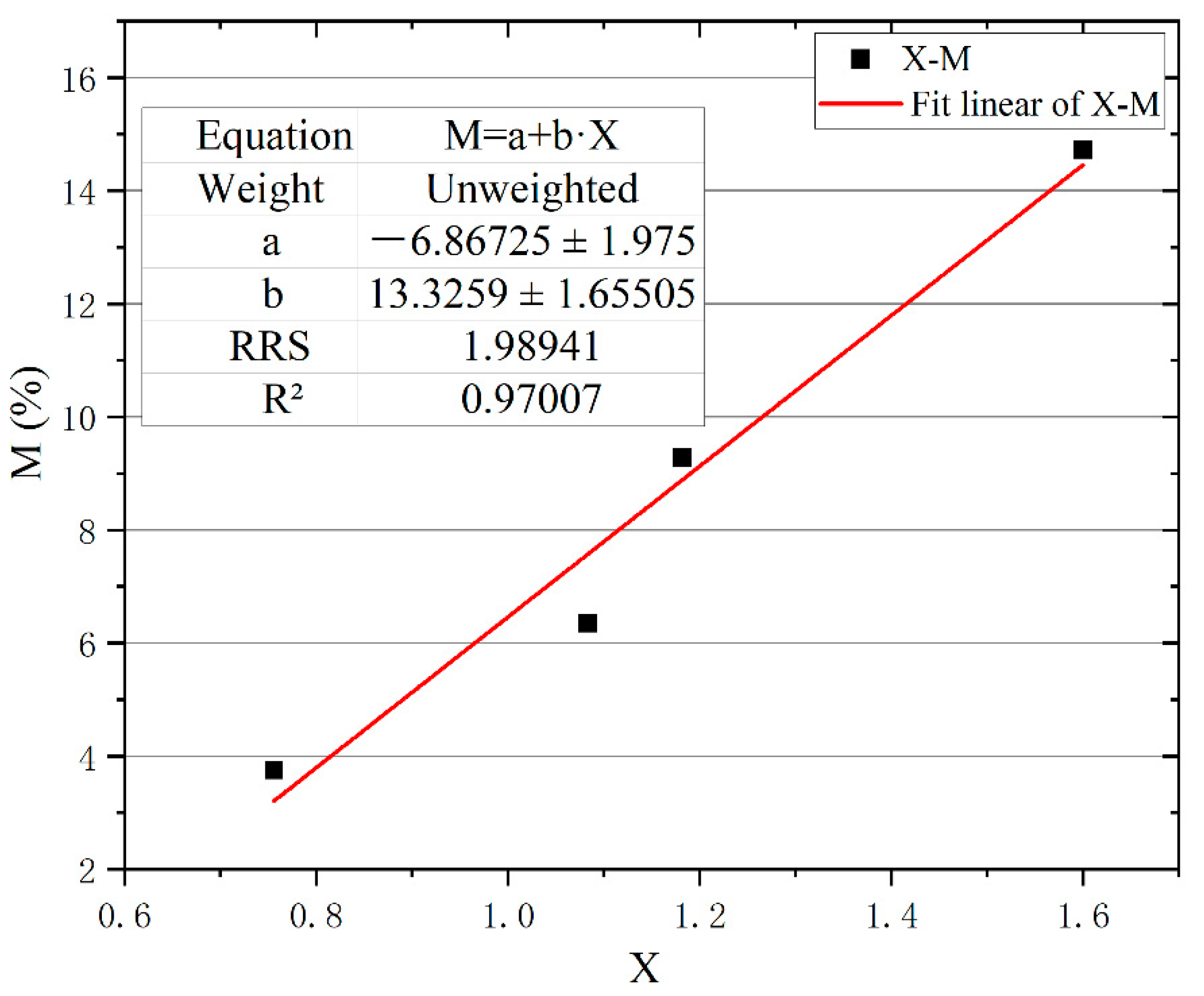
3.4.1. Microstructure Parameters and Pyrolysis Water Loss (P)
3.4.2. Microstructure and Residual Ash (M)
3.4.3. Microstructure and Fire Hazard (Y)
4. Conclusions
- According to the results of the enthalpy of the reaction and the DSC fitting equation, the activation energies calculated were similar (32 kJ/mol), implying that the fire hazard of different vegetations can be directly determined.
- The presented TG–DTG and DSC analysis showed a consistent result, namely, CS had the highest fire hazard, followed by LC, TM, and SSB.
- After the regression analysis and correlation test of the microstructure parameters, a higher percentage of spongy tissue can result in a larger transport speed of water. Moreover, this contributes to lower fire risk of samples.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wei, X.; Liang, C.; Chen, W. Exploring Current Status and Evolutionary Trends on the Paid Use of State-Owned Forest Resources in China: A Bibliometric Perspective. Sustainability 2022, 14, 5516. [Google Scholar] [CrossRef]
- Farooq, T.H.; Shakoor, A.; Wu, X.; Li, Y.; Rashid, M.H.U.; Zhang, X.; Gilani, M.M.; Kumar, U.; Chen, X.; Yan, W. Perspectives of plantation forests in the sustainable forest development of China. Iforest—Biogeosci. For. 2021, 14, 166–174. [Google Scholar] [CrossRef]
- Doerr, S.H.; Santin, C. Global trends in wildfire and its impacts: Perceptions versus realities in a changing world. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150345. [Google Scholar] [CrossRef] [PubMed]
- Si, L.; Shu, L.; Wang, M.; Zhao, F.; Chen, F.; Li, W.; Li, W. Study on forest fire danger prediction in plateau mountainous forest area. Nat. Hazards Res. 2022, 2, 25–32. [Google Scholar] [CrossRef]
- Tyukavina, A.; Potapov, P.; Hansen, M.C.; Pickens, A.H.; Stehman, S.V.; Turubanova, S.; Parker, D.; Zalles, V.; Lima, A.; Kommareddy, I.; et al. Global Trends of Forest Loss Due to Fire From 2001 to 2019. Front. Remote Sens. 2022, 3, 825190. [Google Scholar] [CrossRef]
- Wang, J.; Xin, L.; Tan, M.; Wang, Y. Spatial Heterogeneity in Chinese Forest Area Change in the Early 21st Century. Forests 2016, 7, 232. [Google Scholar] [CrossRef]
- Salmona, J.; Dixon, K.M.; Banks, S.C. The effects of fire history on hollow-bearing tree abundance in montane and subalpine eucalypt forests in southeastern Australia. For. Ecol. Manag. 2018, 428, 93–103. [Google Scholar] [CrossRef]
- Dos Reis, M.; Graca, P.; Yanai, A.M.; Ramos, C.J.P.; Fearnside, P.M. Forest fires and deforestation in the central Amazon: Effects of landscape and climate on spatial and temporal dynamics. J. Environ. Manag. 2021, 288, 112310. [Google Scholar] [CrossRef]
- Wei, S.L.B.; Li, X.; Wang, Z.; Wu, Z.; Luo, S.; Luo, Y.; Tao, Y.; Zhong, Y.; Li, Q. Analysis and Enlightenment of Casualties in “3.30” Forest Fire Fighting in Liangshan Prefecture. For. Fire Prev. 2020, 2, 10–13. (In Chinese) [Google Scholar]
- Colin, C.; Hardy, C.E.H. Fire danger rating in the United States of America: An evolution since 1916. Int. J. Wildland Fire 2007, 16, 217–231. [Google Scholar]
- Fujioka, F.M.; Weise, D.R.; Chen, S.-C.; Kim, S.H.; Kafatos, M.C. Reaction intensity partitioning: A new perspective of the National Fire Danger Rating System Energy Release Component. Int. J. Wildland Fire 2021, 30, 351. [Google Scholar] [CrossRef]
- Fan, W.H.D.; Wang, Q.; Sheng, H. Mechanism and Countermeasures of Forest Fire. Disaster Reduct. 1991, 1, 42–44. (In Chinese) [Google Scholar]
- Tracy, B.; Gartner, Z.G.C. Decomposition dynamics in mixed-species leaf litter. Oikos 2004, 104, 230–246. [Google Scholar]
- Sariyildiz, T. Interactions between litter quality, decomposition and soil fertility: A laboratory study. Soil Biol. Biochem. 2003, 35, 391–399. [Google Scholar] [CrossRef]
- Peter, M.; Vitousek, D.R.T. Litter Decomposition on the Mauna Loa Environmental Matrix Hawai’i: Patterns, Mechanisms, and Models. Ecology 1994, 75, 418–429. [Google Scholar]
- Liao, L.G.H.; Wang, S.; Ma, Y.; Huang, Z.; Yu, X. The effect of nitrogen addition on soil nutrient leaching and the decomposition of Chinese fir leaf litter. Acta Phytoecol. Sin. 2000, 24, 34–39. (In Chinese) [Google Scholar]
- Wang, W.G.J. Seasonal dynamics of environmental factors and decomposition rate of litter in Puccinellia tenuiflora community of Songnen grassland of China. Chin. J. Appl. Ecol. 2001, 6, 841–844. (In Chinese) [Google Scholar]
- Grootemaat, S.; Wright, I.J.; Bodegom, P.M.; Cornelissen, J.H.C.; Cornwell, W.K.; Schweitzer, J. Burn or rot: Leaf traits explain why flammability and decomposability are decoupled across species. Funct. Ecol. 2015, 29, 1486–1497. [Google Scholar] [CrossRef]
- Daniel, W.; Krix, B.R.M. Landscape variation in plant leaf flammability is driven by leaf traits responding to environmental gradients. Ecosphere 2018, 9, e02093. [Google Scholar]
- Guerrero, F.; Carmona, C.; Hernández, C.; Toledo, M.; Arriagada, A.; Espinoza, L.; Bergmann, J.; Taborga, L.; Yañez, K.; Carrasco, Y.; et al. Drivers of Flammability of Eucalyptus globulus Labill Leaves: Terpenes, Essential Oils, and Moisture Content. Forests 2022, 13, 908. [Google Scholar] [CrossRef]
- Dimitrakopoulos, A.; Papaioannou, K.K. Flammability Assessment of Mediterranean Forest Fuels. Fire Technol. 2001, 37, 143–152. [Google Scholar] [CrossRef]
- Liodakis, S.; Bakirtzis, D.; Dimitrakopoulos, A. Dimitrakopoulos. Ignition characteristics of forest species in relation to thermal analysis data. Thermochim. Acta 2002, 390, 83–91. [Google Scholar] [CrossRef]
- Singh, R.K.; Pandey, D.; Patil, T.; Sawarkar, A.N. Pyrolysis of banana leaves biomass: Physico-chemical characterization, thermal decomposition behavior, kinetic and thermodynamic analyses. Bioresour. Technol. 2020, 310, 123464. [Google Scholar] [CrossRef] [PubMed]
- Liodakis, S.; Kakardakis, T. Thermal analysis of forest species dominating wildland/urban interface in Athens. For. Ecol. Manag. 2006, 234, S138. [Google Scholar] [CrossRef]
- Dimitrakopoulos, A.P. Thermogravimetric analysis of Mediterranean plant species. J. Anal. Appl. Pyrolysis 2001, 60, 123–130. [Google Scholar] [CrossRef]
- Zhou, G.Z.Y.; Yu, S.; Bai, S.; Lu, F. Combustibility of Litters of Three Woody Species with Different Water Contents by Cone Calorimeter. Sci. Silvae Sin. 2008, 44, 96–101. (In Chinese) [Google Scholar]
- Rodríguez-Añón, J.A.; Proupín-Castiñeiras, J.; Villanueva-López, M.; Mouriño-Carballido, B. Development of an experimental procedure for energy evaluation of forest communities by calorimetry and thermal analysis. Thermochim. Acta 2007, 458, 5–10. [Google Scholar] [CrossRef]
- Kumar Mohalik, N.; Mandal, S.; Kumar Ray, S.; Mobin Khan, A.; Mishra, D.; Krishna Pandey, J. TGA/DSC study to characterise and classify coal seams conforming to susceptibility towards spontaneous combustion. Int. J. Min. Sci. Technol. 2022, 32, 75–88. [Google Scholar] [CrossRef]
- Zhang, K.L.Y.; Tan, S.; Zhu, X. Vegetation Succession Trend in Yuelu Mountain, Hunan, China. Life Sci. Res. 2009, 13, 313–319. (In Chinese) [Google Scholar] [CrossRef]
- Dariga Altynbaeva, A.A.; Tabakaev, R. Kinetics of biomass low-temperature pyrolysis by coats–redfern method. MATEC Web Conf. 2018, 194, 01058. [Google Scholar] [CrossRef]
- Sack, L.; Cowan, P.D.; Jaikumar, N.; Holbrook, N.M. The ‘hydrology’ of leaves: Co-ordination of structure and function in temperate woody species. Plant Cell Environ. 2003, 26, 1343–1356. [Google Scholar] [CrossRef]
- Muir, C.D.; Hangarter, R.P.; Moyle, L.C.; Davis, P.A. Morphological and anatomical determinants of mesophyll conductance in wild relatives of tomato (Solanum sect. Lycopersicon, sect. Lycopersicoides; Solanaceae). Plant Cell Environ. 2014, 37, 1415–1426. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.Z.S.; Zhu, J.; Shen, Z.; Cao, K. Impact of leaf morphological and anatomical traits on mesophyll conductance and leaf hydraulic conductance in mangrove plants. Plant Sci. J. 2016, 34, 909–919. (In Chinese) [Google Scholar]
- Baggaley, R.G.; Hornsby, P.R.; Yahya, R.; Cusack, P.A.; Monk, A.W. The Influence of Novel Zinc Hydroxystannate-Coated Fillers on the Fire Properties of Flexible PVC. Fire Mater. 1997, 21, 179–185. [Google Scholar] [CrossRef]
- Hostikka, S.; Matala, A. Pyrolysis Model for Predicting the Heat Release Rate of Birch Wood. Combust. Sci. Technol. 2017, 189, 1373–1393. [Google Scholar] [CrossRef]
- Marek Wesołowski, P.K.N. Thermal decomposition and elemental composition of medicinal plant materials—Leaves and flowers Principal component analysis of the results. Thermochim. Acta 2003, 397, 171–180. [Google Scholar] [CrossRef]
- Liu, N.; Fan, W.C. Modelling the Thermal Decompositions of Wood and Leaves Under a Nitrogen Atmosphere. Fire Mater. 1998, 22, 103–108. [Google Scholar] [CrossRef]
- Liu, N.; Fan, W.; Dobashi, R.; Huang, L. Kinetic modeling of thermal decomposition of natural cellulosic materials in air atmosphere. J. Anal. Appl. Pyrolysis 2002, 63, 303–325. [Google Scholar] [CrossRef]
- Zhang, C.-W.; Wang, C.-Z.; Tao, R. Thermal Decomposition Kinetics ofGinkgo bilobaLeaves Polyprenol in Nitrogen Atmosphere. Int. J. Chem. Kinet. 2016, 48, 671–678. [Google Scholar] [CrossRef]
- Yan, Y.; Meng, Y.; Tang, L.; Kostas, E.T.; Lester, E.; Wu, T.; Pang, C.H. Ignition and Kinetic Studies: The Influence of Lignin on Biomass Combustion. Energy Fuels 2019, 33, 6463–6472. [Google Scholar] [CrossRef]
- Tian, X.; Shu, L.; Zhang, X. Study on the influence of microstructure of tree’s twig and leaf on combustibility. Sci. Silvae Sin. 2003, 39, 84–88. (In Chinese) [Google Scholar]



| Sample | The Percentage of Micro-Tissues of Leaves (%) | Spongy/Palisade | |||
|---|---|---|---|---|---|
| Cuticle | Palisade Tissue | Spongy Tissue | Interspace | ||
| Symplocos setchuensis Brand (SSB) | 14 | 30 | 48 | 8 | 1.600 |
| Tarenna mollissima (TM) | 18 | 33 | 39 | 10 | 1.182 |
| Loropetalum chinense (LC) | 10 | 36 | 39 | 15 | 1.083 |
| Castanopsis sclerophylla (CS) | 12 | 45 | 34 | 9 | 0.756 |
| Sample | Initial Weight (g) | Residual Weight (g) | Pyrolysis Kinetic Equation | Temperature Range (°C) | Reaction Order | R2 | a | b | E (kJ·mol−1) | A (min−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| SSB | 20.929 | 3.082 | Y = −10.5646 − 582.5/T | 198–444 | 1.0 | 0.9441 | −10.5646 | −582.5 | 4.842648 | 0.301 |
| Y = −7.8849 − 2085.8/T | 444–590 | 1.0 | 0.7110 | −7.8849 | −2085.8 | 17.34109 | 15.701 | |||
| TM | 20.111 | 2.912 | Y = −10.4085 − 545.3/T | 202–403 | 1.0 | 0.9449 | −10.4085 | −545.3 | 4.53317 | 0.329 |
| Y = −7.5605 − 1951.8/T | 403–611 | 1.0 | 0.8555 | −7.5605 | −1951.8 | 16.22707 | 20.323 | |||
| LC | 21.300 | 1.976 | Y = −11.0089 − 431.5/T | 182–406 | 1.0 | 0.9715 | −11.0089 | −431.5 | 3.58728 | 0.143 |
| Y = −8.1659 − 1790.7/T | 406–615 | 1.0 | 0.7615 | −8.1659 | −1790.7 | 14.88789 | 10.178 | |||
| CS | 22.664 | 1.44 | Y = −10.6653 − 545.7/T | 185–413 | 1.0 | 0.9281 | −10.6653 | −545.7 | 4.53707 | 0.255 |
| Y = −8.4854 − 1606.3/T | 413–601 | 1.0 | 0.7227 | −8.4854 | −1606.3 | 13.35454 | 6.632 |
| Sample | Initial Weight (g) | Residual Weight (g) | Temperature Range (°C) | H0 (mW) | Fitting Equation | a | b | E (kJ/mol) | A (min−1) | Reaction Order | R2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SSB | 20.929 | 3.082 | 371–632 | 5787.9 | Y = −5.9599 − 3781.8/T | −5.9599 | −3781.8 | 31.442 | 195.2 | 0.8 | 0.895 |
| TM | 20.111 | 2.912 | 318–632 | 5690.5 | Y = −4.6187 − 3905.1/T | −4.6187 | −3905.1 | 32.467 | 770.5 | 0.8 | 0.870 |
| LC | 21.300 | 1.976 | 338–632 | 8017.9 | Y = −4.5678 − 3929.7/T | −4.5678 | −3929.7 | 32.671 | 815.9 | 0.8 | 0.842 |
| CS | 22.664 | 1.44 | 346–632 | 10,520 | Y = −5.5938 − 3922.6/T | −5.5938 | −3922.6 | 32.612 | 291.9 | 0.8 | 0.933 |
| Sample | X | P (%) | M (%) | H (mW/mg) | t (s) | Y (mW·mg−1·s−1) |
|---|---|---|---|---|---|---|
| SSB | 1.600 | 10.461 | 14.724 | 68.06 | 996 | 0.068 |
| TM | 1.182 | 7.058 | 9.278 | 120.35 | 615 | 0.196 |
| LC | 1.083 | 5.472 | 6.355 | 148.54 | 674 | 0.220 |
| CS | 0.756 | 4.649 | 3.753 | 192.15 | 707 | 0.272 |
| P | ||
|---|---|---|
| X | Pearson Correlation | 0.966 * |
| Sig. (single tail) | 0.017 | |
| M | ||
|---|---|---|
| X | Pearson Correlation | 0.985 ** |
| Sig. (single tail) | 0.008 | |
| Y | ||
|---|---|---|
| X | Pearson Correlation | −0.984 ** |
| Sig. (single tail) | 0.008 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, J.; Huang, R.; Wang, Y. Study on the Combustion Characteristics of Mountain Forest Vegetation. Forests 2022, 13, 1443. https://doi.org/10.3390/f13091443
Jia J, Huang R, Wang Y. Study on the Combustion Characteristics of Mountain Forest Vegetation. Forests. 2022; 13(9):1443. https://doi.org/10.3390/f13091443
Chicago/Turabian StyleJia, Jiacheng, Rui Huang, and Yi Wang. 2022. "Study on the Combustion Characteristics of Mountain Forest Vegetation" Forests 13, no. 9: 1443. https://doi.org/10.3390/f13091443
APA StyleJia, J., Huang, R., & Wang, Y. (2022). Study on the Combustion Characteristics of Mountain Forest Vegetation. Forests, 13(9), 1443. https://doi.org/10.3390/f13091443





