Dynamics of Forage and Management Implications for Large Herbivore Habitat in Seasonally Dry Forest of Southeast Asia
Abstract
:1. Introduction
2. Materials and Methods
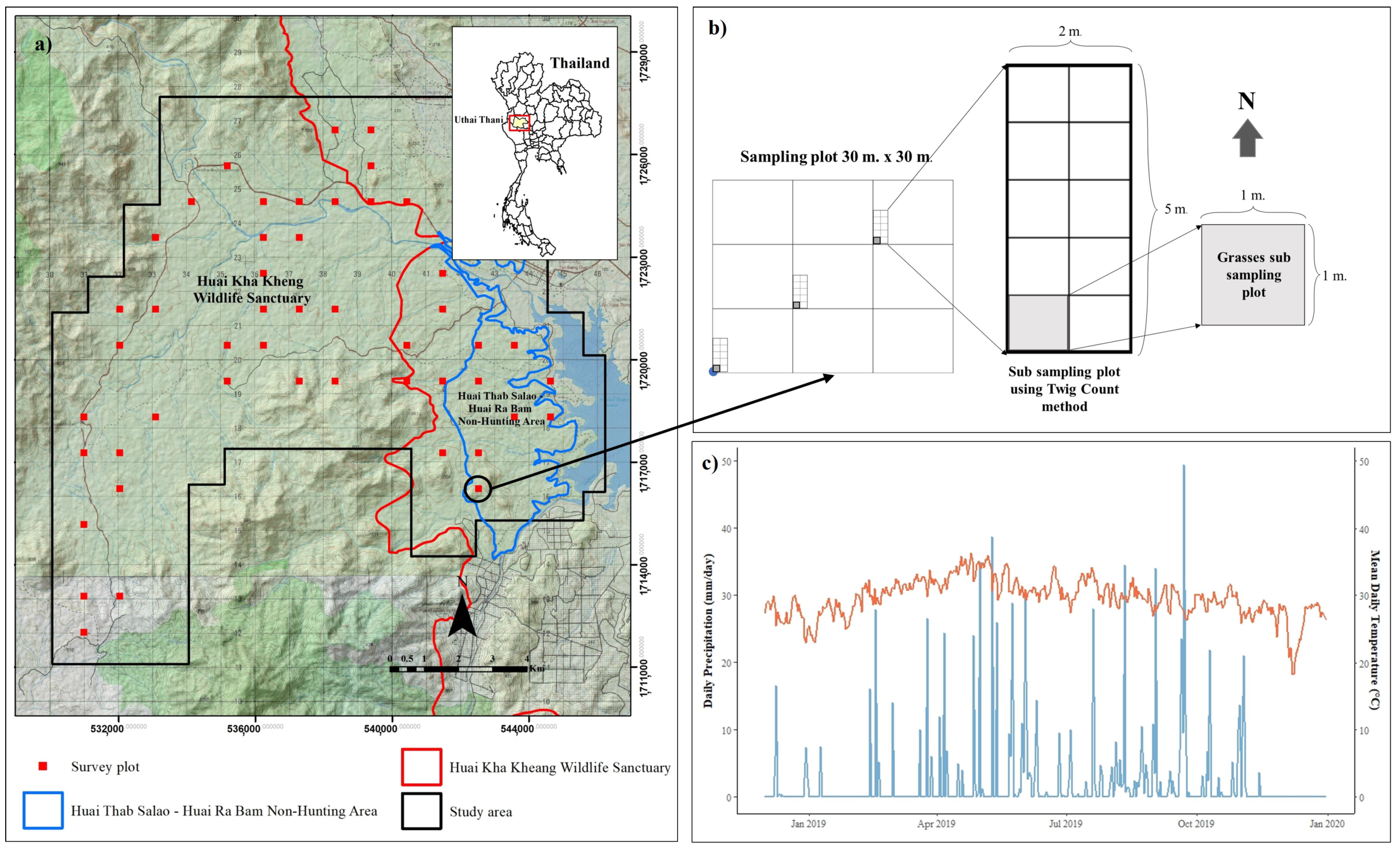
2.1. Study Site
2.2. Data Collection
2.2.1. Sampling Plot Design
2.2.2. Forage Availability
2.2.3. Environmental Factors
2.3. Data Analyses
3. Results
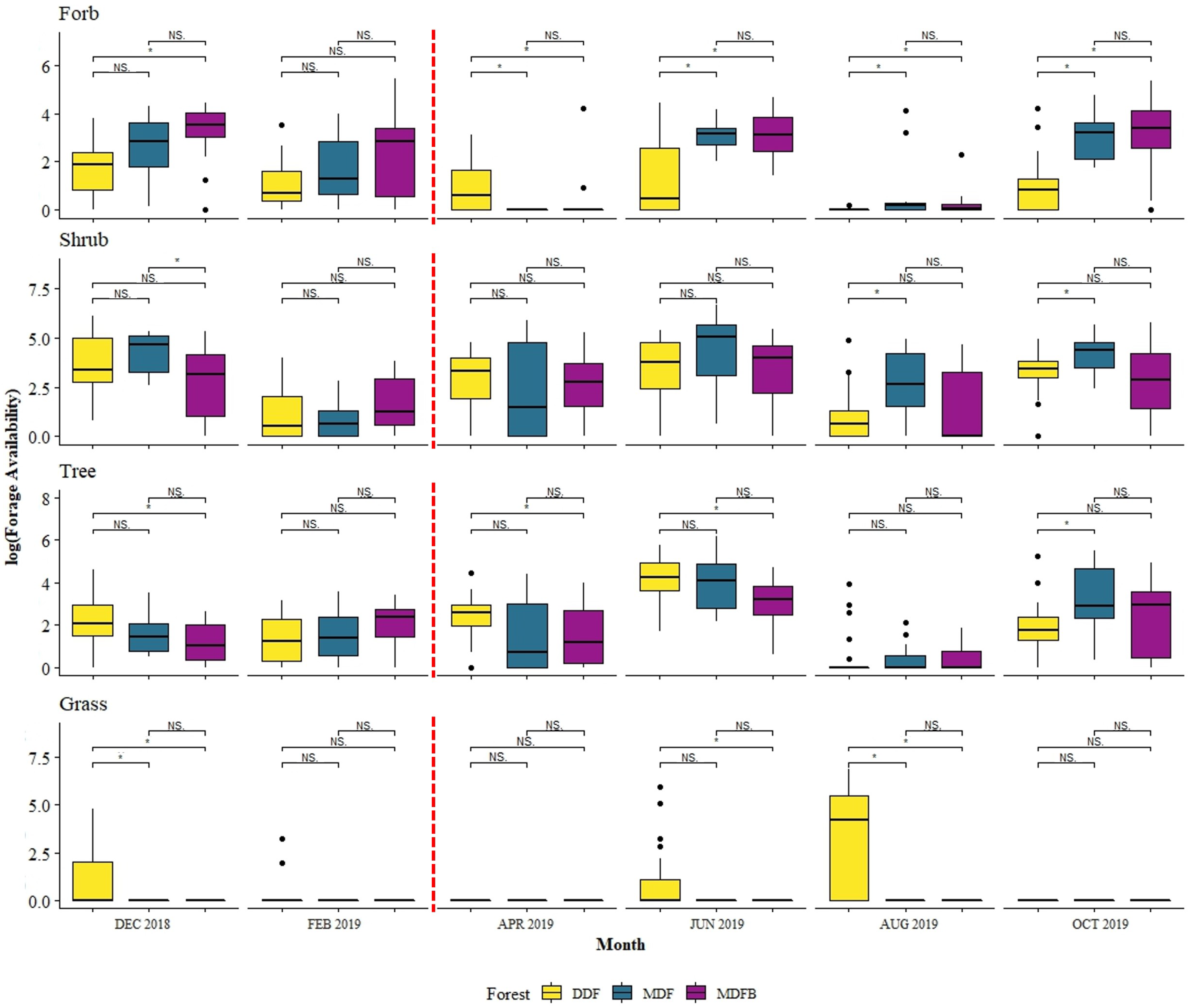
3.1. Temporal Variation of Forage Availability
3.2. Forage Availability by Forest Types
3.3. Forage Availability and Environmental Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allen, E.B.; Rincón, E.; Allen, M.F.; Pérez-Jimenez, A.; Huante, P. Disturbance and Seasonal Dynamics of Mycorrhizae in a Tropical Deciduous Forest in Mexico. Biotropica 1998, 30, 261–274. [Google Scholar] [CrossRef]
- Baker, P.J.; Bunyavejchewin, S.; Oliver, C.D.; Ashton, P.S. Disturbance History and Historical Stand Dynamics of a Seasonal Tropical Forest in Western Thailand. Ecol. Monogr. 2005, 75, 317–343. [Google Scholar] [CrossRef]
- McShea, W.J.; Davies, S.J. Seasonally dry forests of tropical Asia: An ecosystem adapted to seasonal drought, frequent fire, and human activity. In The Ecology and Conservation of Seasonally Dry Forests in Asia; McShea, W.J., Davies, S.J., Bhumpakphan, N., Eds.; Smithsonian Institution Scholarly Press: Washington, DC, USA, 2011. [Google Scholar]
- Williams, L.J.; Bunyavejchewin, S.; Baker, P.J. Deciduousness in a Seasonal Tropical Forest in Western Thailand: Interannual and Intraspecific Variation in Timing, Duration and Environmental Cues. Oecologia 2008, 155, 571–582. [Google Scholar] [CrossRef]
- Baker, P.J.; Bunyavejchewin, S.; Robinson, A.P. The Impact of Large-Scale, Low-Intensity Fires on the Forest of Continental South- East Asia. Int. J. Wildland Fire 2008, 17, 782–792. [Google Scholar] [CrossRef]
- Wanthongchai, K.; Goldammer, J.G. Fire management in South and Southeast Asia seasonally dry Forests: Colonial approaches, current problems, and perspectives. In The Ecology and Conservation of Seasonally Dry Forests in Asia; McShea, W.J., Davies, S.J., Bhumpakphan, N., Eds.; Smithsonian Institution Scholarly Press: Washington, DC, USA, 2011. [Google Scholar]
- Trisurat, Y.; Pattanavibool, A.; Gale, G.A.; Reed, D.H. Improving the viability of large-mammal populations by using habitat and landscape models to focus conservation planning. Wildl. Res. 2010, 37, 401–412. [Google Scholar] [CrossRef]
- Ariyaphithak, C.; Pongpattananurak, N.; Pattanavibool, A.; Podchong, S.; Chankhao, A. Applying SMART Patrol Database for Creating Species Distribution Model of Panthera tigris corbetti in Western Forest Complex, Thailand. J. Environ. Manag. 2020, 16, 42–59. [Google Scholar] [CrossRef]
- IUCN-World-Heritage. IUCN World Heritage Outlook 2: A Conservation Assessment of All Natural World Heritage Sites; IUCN: Gland, Switzerland, 2017. [Google Scholar] [CrossRef]
- Maxwell, J.F. A Synopsis of the Vegetation of Thailand. Nat. Hist. J. Chulalongkorn Univ. 2004, 4, 19–29. [Google Scholar]
- Roques, K.; O’Connor, T.; Watkinson, A. Dynamics of shrub encroachment in an African savanna: Relative influences of fire, herbivory, rainfall and density dependence. J. Appl. Ecol. 2001, 38, 268–280. [Google Scholar] [CrossRef]
- McComb, B.C. Wildlife Habitat Management Concepts and Applications in Forestry; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- FAO. Human-Wildlife Conflict in Africa, Causes, Consequences and Management Strategies; International Foundation for the Conservation of Wildlife (Foundation IGF): Paris, France, 2009. [Google Scholar]
- Sachro, L.; Strong, W.; Gates, C. Prescribed burning effects on summer elk forage availability in the subalpine zone, Banff National Park, Canada. J. Environ. Manag. 2005, 77, 183–193. [Google Scholar] [CrossRef]
- Masters, R.E.; Wilson, C.W.; Bukenhofer, G.A.; Payton, M.E. Effects of Pine-Grassland Restoration for Red-Cockaded Woodpeckers on White-Tailed Deer Forage Production. Wildl. Soc. Bull. 1996, 24, 77–84. [Google Scholar]
- Baker, P.J.; Bunyavejchewin, S. Fire behavior and fire effects across the forest landscape of continental Southeast Asia. In Tropical Fire Ecology: Climate Change, Land Use, and Ecosystem Dynamics; Cochrane, M.A., Ed.; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Bunyavejchewin, S.; Baker, P.J.; Davies, S.J. Seasonally dry tropical forests in continental Southeast Asia: Structure, composition and dynamics. In The Ecology and Conservation of Seasonally Dry Forests in Asia; McShea, W.J., Davies, S.J., Bhumpakphan, N., Eds.; Smithsonian Institution Scholarly Press: Washington, DC, USA, 2011. [Google Scholar]
- Belsky, A.J.; Canham, C.D. Forest Gaps and Isolated Savanna Trees. BioScience 1994, 44, 77–84. [Google Scholar] [CrossRef]
- Litvaitis, J.A. Importance of Early Successional Habitats to Mammals in Eastern Forests. Wildl. Soc. Bull. 2001, 29, 466–473. [Google Scholar]
- Donalty, S.; Henke, S.E.; Kerr, C.L. Use of Winter Food Plots by Nongame Wildlife Species. Wildl. Soc. Bull. 2003, 31, 774–778. [Google Scholar]
- Putman, R.J.; Staines, B.W. Supplementary winter feeding of wild red deer Cervus elaphus in Europe and North America: Justifications, feeding practice and effectiveness. Mammal Rev. 2004, 34, 285–306. [Google Scholar] [CrossRef]
- Månsson, J.; Roberge, J.M.; Edenius, L.; Bergström, R.; Nilsson, L.; Lidberg, M.; Komstedt, K.; Ericsson, G. Food plots as a habitat management tool: Forage production and ungulate browsing in adjacent forest. Wildl. Biol. 2015, 21, 246–253. [Google Scholar] [CrossRef]
- Suksawat, L.; Sukmasuang, R.; Trisurat, Y. Foraging Preferences and Ecological Carrying Capacity of Banteng (Bos javanicus) and sambar deer (Rusa unicolor) in Huai Kha Khaeng Wildlife Sanctuary, Thailand. J. Trop. For. Res. 2018, 2, 69–81. [Google Scholar]
- McShea, W.J.; Sukmasuang, R.; Erickson, D.L.; Herrmann, V.; Ngoprasert, D.; Bhumpakphan, N.; Davies, S.J. Metabarcoding reveals diet diversity in an ungulate community in Thailand. Biotropica 2019, 51, 923–937. [Google Scholar] [CrossRef]
- Thai-Meteorological-Department. Annual Climate Summary. 2019. Available online: https://www.tmd.go.th/programs/uploads/yearlySummary/yearly_2562_th.pdf (accessed on 20 January 2022).
- Shafer, E.L., Jr. The Twig-Count Method for Measuring Hardwood Deer Browse. J. Wildl. Manag. 1963, 27, 428–437. [Google Scholar] [CrossRef]
- Parker, G.; Morton, L. The estimation of winter forage and its use by moose on clearcuts in northcentral Newfoundland. J. Range Manag. 1978, 31, 300–304. [Google Scholar] [CrossRef]
- United States Department of Agriculture. Growth Habits Codes and Definitions. Available online: https://plants.usda.gov/growth_habits_def.html (accessed on 31 December 2021).
- Wallis de Vries, M. Estimating forage intake and quality in grazing cattle: A reconsideration of the hand-plucking method. J. Range Manag. 1995, 48, 370–375. [Google Scholar] [CrossRef]
- Raynor, E.; Joern, A.; Briggs, J. Bison foraging responds to fire frequency in nutritionally heterogeneous grassland. Ecology 2015, 96, 1586–1597. [Google Scholar] [CrossRef]
- NASA; JPL. Nasa Shuttle Radar Topography Mission Global 1 Arc Second; NASA EOSDIS Land Processes DAAC; NASA: Washington, DC, USA, 2013.
- Back, R. Soil Analysis Handbook of Reference Methods; CRC Press: Boca Raton, FL, USA, 1999; p. 247. [Google Scholar]
- Fornacca, D.; Ren, G.; Xiao, W. Evaluating the Best Spectral Indices for the Detection of Burn Scars at Several Post-Fire Dates in a Mountainous Region of Northwest Yunnan, China. Remote. Sens. 2018, 10, 1196. [Google Scholar] [CrossRef]
- Peterson, D.W.; Reich, P.B. Prescribed Fire in Oak Savanna: Fire frequency effects on stand structure and dynamics. Ecol. Appl. 2001, 11, 914–927. [Google Scholar] [CrossRef]
- Heisler, J.L.; Briggs, J.M.; Knapp, A.; Blair, J.M.; Seery, A. Direct and Indirect Effects of Fire on Shrub Density and Aboveground Productivity in a Mesic Grassland. Ecology 2004, 85, 2245–2257. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S; Springer: New York, NY, USA, 2002. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer Science+Business Media: New York, NY, USA, 2009. [Google Scholar]
- R-Core-Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Callaway, R.M.; Walker, L.R. Competition and Facilitation: A Synthetic Approach to Interactions in Plant Communities. Ecology 1997, 78, 1958–1965. [Google Scholar] [CrossRef]
- Duncan, R.S.; Chapman, C.A. Tree–Shrub Interactions During Early Secondary Forest Succession in Uganda. Restor. Ecol. 2003, 11, 198–207. [Google Scholar] [CrossRef]
- Li, X.; Wilson, S.D.; Song, Y. Secondary succession in two subtropical forests. Plant Ecol. 1999, 143, 13–21. [Google Scholar] [CrossRef]
- Peterson, D.W.; Reich, P.B.; Wrage, K.J. Plant functional group responses to fire frequency and tree canopy cover gradients in oak savannas and woodlands. J. Veg. Sci. 2007, 18, 3–12. [Google Scholar] [CrossRef]
- Watson, P.J.; Bradstock, R.A.; Morris, E.C. Fire frequency influences composition and structure of the shrub layer in an Australian subcoastal temperate grassy woodland. Austral Ecol. 2009, 34, 218–232. [Google Scholar] [CrossRef]
- Hill, S.J.; French, K. Potential impacts of fire and grazing in an endangered ecological community Plant composition and shrub and eucalypt regeneration in Cumberland Plain Woodland. Aust. J. Bot. 2004, 52, 23–29. [Google Scholar] [CrossRef]
- Vlok, J.H.J.; Yeaton, R.I. Competitive interactions between overstorey Proteas and sprouting understorey species in South African mountain fynbos. Divers. Distrib. 2000, 6, 273–281. [Google Scholar] [CrossRef]
- Zhu, L.; Cooper, D.J.; Yang, J.; Zhang, X.; Wang, X. Rapid warming induces the contrasting growth of Yezo spruce (Picea jezoensis var. microsperma) at two elevation gradient sites of northeast China. Dendrochronologia 2018, 50, 52–63. [Google Scholar] [CrossRef]
- Wood, G.W. Effects of Prescribed Fire on Deer Forage and Nutrients. Wildl. Soc. Bull. 1988, 16, 180–186. [Google Scholar]
- Pabian, S.E.; Rummel, S.M.; Sharpe, W.E.; Brittingham, M.C. Terrestrial Liming as a Restoration Technique for Acidified Forest Ecosystems. Int. J. For. Res. 2012, 2012, 976809. [Google Scholar] [CrossRef]
- Bailey, S.; Horsley, S.; Long, R. Thirty Years of Change in Forest Soils of the Allegheny Plateau, Pennsylvania. Soil Sci. Soc. Am. J. 2005, 69, 681–690. [Google Scholar] [CrossRef] [Green Version]
- Malhotra, H.; Vandana; Sharma, S.; Pandey, R. Phosphorus nutrition: Plant growth in response to deficiency and excess. In Plant Nutrients and Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B., Eds.; Springer Nature: Singapore, 2018. [Google Scholar]
- Meyer, G.; Bell, M.J.; Doolette, C.L.; Brunetti, G.; Zhang, Y.; Lombi, E.; Kopittke, P.M. Plant-Available Phosphorus in Highly Concentrated Fertilizer Bands: Effects of Soil Type, Phosphorus Form, and Coapplied Potassium. J. Agric. Food Chem. 2020, 687, 7571–7580. [Google Scholar] [CrossRef]
- French, C.E.; McEwen, L.C.; Magruder, N.D.; Ingram, R.H.; Swift, R.W. Nutrient Requirements for Growth and Antler Development in the White-Tailed Deer. J. Wildl. Manag. 1956, 20, 221–232. [Google Scholar] [CrossRef]
- Alldredge, M.W.; Peek, J.M.; Wall, W.A. Nutritional quality of forages used by elk in northern Idaho. J. Range Manag. 2002, 55, 253–259. [Google Scholar] [CrossRef]
- Grant, C.; Scholes, M. The importance of nutrient hot- spots in the conservation and management of large wild mammalian herbivores in semi-arid savannas. Biol. Conserv. 2006, 130, 426–437. [Google Scholar] [CrossRef]
- Hedtcke, J.; Posner, J.; Rosemeyer, M.; Albrecht, K. Browsing for conservation: Springtime forage value of midstory shrubs of degraded oak savannas in southern Wisconsin. Renew. Agric. Food Syst. 2009, 24, 293–299. [Google Scholar] [CrossRef]
- Hobbs, N.T.; Spowart, R.A. Effects of Prescribed Fire on Nutrition of Mountain Sheep and Mule Deer during Winter and Spring. J. Wildl. Manag. 1984, 48, 551–560. [Google Scholar] [CrossRef]
- Lopez, R.R.; Parker, I.D.; Morrison, M.L. Applied Wildlife Habitat Management; Texas A and M University Press: College Station, TX, USA, 2017. [Google Scholar]
- Haney, A.; Apfelbaum, S.I. Characterization of Midwestern Oak Savannas. In 1993 Proceedings of the Midwest Oak Savanna Conferences; University of Wisconsin Press: Madison, WI, USA, 1993. [Google Scholar]
- Sahunalu, P. Structure and Species Composition in the Long-term Dynamic Plots of Sakaerat Deciduous Dipterocarp Forest, Northeastern Thailand. J. For. Manag. 2009, 3, 1–20. [Google Scholar]
- Bunyavejchewin, S. Canopy Structure of the Dry Dipterocarp Forest in Thailand. Thai For. Bull. (Bot.) 1983, 14, 1–93. [Google Scholar]
- Harlow, R.F.; Lear, D.H.V. Silvicultural Effects on Wildlife Habitat in the South (an Annotated Bibliography) 1953–1979; Clemson University, College of Forest and Recreation Resources: Clemson, SC, USA, 1981. [Google Scholar]
- Murphy, A.; Ehrenreich, J.H. Effects of timber harvest and stand Improvement on forage production. J. Wildl. Manag. 1965, 29, 734–739. [Google Scholar] [CrossRef]
- Natural-Resources-Conservation-Service. Food Plots for Wildlife (Iowa Job Sheet). 2012. Available online: https://www.nrcs.usda.gov/Internet/FSE_DOCUMENTS/nrcs142p2_007210.pdf (accessed on 20 January 2022).




| Life Form | Diameter | Total Length | Fresh Weight | Dry Weight | Water Content |
|---|---|---|---|---|---|
| (n = Number of Samples) | (mm) | (mm) | (g) | (g) | (%) |
| Forbs (n = 68) | 1.8 ± 0.1 | 238.2 ± 60.6 | 1.9 ± 0.2 | 0.4 ± 0.05 | 73.9 ± 1.49 |
| Shrubs (n = 60) | 1.9 ± 0.1 | 197.8 ± 13.0 | 2.3 ± 0.3 | 0.8 ± 0.1 | 66.5 ± 0.98 |
| Tree seedlings (n = 92) | 2.0 ± 0.1 | 178.1 ± 9.0 | 2.7 ± 0.3 | 0.8 ± 0.1 | 66.4 ± 0.95 |
| Grasses (n = 9) | NA | 162.8 ± 59.3 | 10.0 ± 2.5 | 2.96 ± 6.71 | 63.7 ± 4.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chankhao, A.; Kraichak, E.; Phumsathan, S.; Pongpattananurak, N. Dynamics of Forage and Management Implications for Large Herbivore Habitat in Seasonally Dry Forest of Southeast Asia. Forests 2022, 13, 1463. https://doi.org/10.3390/f13091463
Chankhao A, Kraichak E, Phumsathan S, Pongpattananurak N. Dynamics of Forage and Management Implications for Large Herbivore Habitat in Seasonally Dry Forest of Southeast Asia. Forests. 2022; 13(9):1463. https://doi.org/10.3390/f13091463
Chicago/Turabian StyleChankhao, Andaman, Ekaphan Kraichak, Sangsan Phumsathan, and Nantachai Pongpattananurak. 2022. "Dynamics of Forage and Management Implications for Large Herbivore Habitat in Seasonally Dry Forest of Southeast Asia" Forests 13, no. 9: 1463. https://doi.org/10.3390/f13091463
APA StyleChankhao, A., Kraichak, E., Phumsathan, S., & Pongpattananurak, N. (2022). Dynamics of Forage and Management Implications for Large Herbivore Habitat in Seasonally Dry Forest of Southeast Asia. Forests, 13(9), 1463. https://doi.org/10.3390/f13091463






