Abstract
Soil ammonia-oxidizing microorganisms play important roles in nitrogen (N) cycling in cold ecosystems, but how changes in snow cover will affect their distribution and associated functional characteristics remains unclear. A snow manipulation experiment was conducted to explore the effects of snow exclusion on soil ammonia-oxidizing archaea (AOA) and ammonia-oxidizing bacteria (AOB) communities and functional characteristics in a spruce forest in the eastern Tibet Plateau. Results showed that the amoA gene abundance and community composition of AOA and AOB did not differ between snow regimes but varied among winter periods. AOA and AOB gene abundances showed a decreasing trend during the snow cover melting period. During the deep snow cover period, Thaumarchaeota and Crenarchaeota in the AOA community decreased significantly, while Proteobacteria and Nitrosospira in the AOB community increased significantly. The main factors affecting the changes in AOA and AOB community diversity and composition were soil MBN, nitrate nitrogen, and temperature, while AOA and AOB community diversity and composition were also significantly correlated with soil enzyme activities related to N cycling. These results recommend that the season-driven variations strongly affected soil ammonia-oxidizing community and functional characteristics more than momentary snow cover change. Such findings offer new insights into how soil N-cycling processes would respond to reduced snowfall in high-altitude regions.
1. Introduction
Seasonal snow cover is an essential factor affecting vegetation composition and biogeochemical cycles in cold ecosystems [1,2,3]. Many high latitudes and altitudes of snowy regions have experienced dramatic climate change in recent decades [4]. In these regions, rising temperatures have a profound impact on winter conditions, such as precipitation in winter being dominated by rain rather than snow [5], resulting in a dramatic reduction in winter snowpack [6]. The decline or absence of insulation of snow cover alters the soil micro-environment [7,8,9,10], resulting in higher root and microbial mortality [11], which are important available nitrogen (N) sources and play a critical role in soil N-cycling processes [10,12]. Therefore, the lack of snow cover in cold ecosystems may have complex and strong impacts on soil ecological processes, especially on soil microbiological processes related to N cycling.
As the first and rate-limiting step of the nitrification process, ammonia oxidation plays a vital role in the global N cycle [13]. In most terrestrial ecosystems, it is largely performed by ammonia-oxidizing archaea (AOA) and ammonia-oxidizing bacteria (AOB) [14,15,16]. Soil ammonia-oxidizing microbial communities involved in N cycling play an increasingly important role in N-limited subalpine ecosystems [17,18]. Therefore, understanding how ammonia-oxidizing microbial communities in this region respond to snow cover change will be critical. Studies have shown that snowpack thermal insulation prevents the soil temperature from falling well below freezing [19]. The decline or absence of snow cover can lead to lower soil temperature and deep freezing of soil, while also altering soil moisture content and freeze–thaw cycles [10,20,21], further influencing soil AOA and AOB abundances and function [22,23]. In addition, it has been reported that changes in snow cover can also affect the composition of soil microbial communities [7]. However, there are few related studies on the response of soil ammonia-oxidizing microbial community composition and diversity to altered snow cover. Therefore, exploring the effects of snow cover change on the ammonium-oxidizing microbial community is very essential to understanding N-associated ecological processes in cold soils.
In the Tibetan Plateau region, winter snowfall has decreased by 0.6 mm a−1 over the past few decades, and this trend is expected to continue due to strong winter warming [5,24,25]. Compared with high latitudes, the seasonal snow cover in this region has the unique characteristics of short duration and shallow depth [10]. Furthermore, winter soil temperatures are close to the physical melting point and are sensitive to changes in snow cover [26]. In particular, soil nitrifiers have critical roles in soil nitrification and nitrate (-N) availability [22,27]. They are also known to be sensitive to environmental changes [22]. Therefore, soil N-cycling processes in this system may be more significant than in other ecosystems under warming conditions [28]. Previous studies have found that the reduction of seasonal snow cover increased soil net N mineralization and N leaching [29], resulting in increased soil N availability [10]. However, how soil N-cycle-related microorganisms respond to snow cover changes in this region is still unclear. Therefore, we conducted a snow-manipulation experiment in a spruce forest on the eastern Tibetan Plateau to investigate the effects of snow exclusion on soil AOA and AOB communities across critical winter periods. Specifically, we hypothesized that (1) snow cover change affects AOA and AOB communities and function; (2) the AOA and AOB abundances should be lower and community composition should be more variable at the snow exclusion treatments compared to controls; (3) AOA and AOB communities will differ across sampling periods due to contrasting climatic conditions during winter.
2. Materials and Methods
2.1. Site Description and Experimental Design
This manipulation experiment was carried out in a dragon spruce (Picea asperata) forest at the Long-term Research Station of Alpine Forest Ecosystems of Sichuan Agricultural University on the eastern Tibetan Plateau of China (31°15′10″ N, 102°53′29″, 3021 m a.s.l.). The mean annual precipitation is about 850 mm, and the annual average temperature is about 3.0 °C. Snow cover accumulation and melting generally occur in late November of the first year and late March of the following year, respectively. The soil is classified as Cambic Umbrisols [30].
Winter snowfall was excluded using shelters [10]. Toward the beginning of November 2015, six wooden roofs (2 m in height, 3 m × 3 m ground area) were laid in the spruce forest to prevent snow accumulation. A control plot was established near each wooden roof to allow snow input. The snow control started in late November and finished in late March the next year [10]. Add the accumulated snow on each roof to the ground before the snow melts to guarantee a water balance between the with and without snow plots.
2.2. Microclimate Monitoring
Soil temperature (5 cm depth) and moisture (v/v) were measured by Thermochron iButton DS1923-F5 Recorders (Maxim Dallas Semiconductor Corp., Dallas, TX, USA) and Theta probe, respectively. Snow depth in control plots was measured approximately every 2 weeks. The maximum snow depth was 23 cm toward the beginning of March (Figure S1a). Snow exclusion decreased soil temperature (Figure S1a) but had no significant effect on soil moisture (Figure S1b).
2.3. Soil Sampling and Biochemical Analyses
After two years of snow treatment, soil samples (~15 cm) were collected in the early snow cover (ESC, early December 2016), deep snow cover (DSC, mid-February 2017), and snow cover melting (SCM, early April 2017), respectively. Soil samples were randomly collected from three snow exclusion plots and their corresponding control plots. During each sampling period, three soil cores (5 cm diameter, 15 cm deep) were randomly selected from each plot and thoroughly mixed. After removing any visible live plant material, the well-mixed soil was passed through a 2 mm sieve. The soil was used for biochemical analysis.
The soil pH of the moist field soil was determined using a pH meter (PHS-25CW, BANTE Instruments Limited, Shanghai, China) in a 1:2.5 (M/V) soil suspension. Soil ammonium nitrogen and nitrate nitrogen were extracted with 2 M KCl, followed by colorimetric determination of ammonium and nitrate ions in the extract [31]. Total dissolved N (TDN) was measured using a total C and N analyzer (TOC-VcPH + TNM-1, Shimazu Inc., Kyoto, Japan). Dissolved organic N (DON) was extracted by the method of Jones and Willett [32]. Soil microbial biomass nitrogen (MBN) and microbial biomass carbon (MBC) were measured by the fumigation–extraction method [33]. Soil nitrate and nitrite reductase activities were measured as previously described [34].
2.4. Real-Time PCR
The amoA gene clone and the method to create standard curves were as previously described [18]. For real-time PCR, the primers Arch-amoAF/Arch-amoAR and amoA-1F/amoA-2R were used for AOA and AOB, respectively [35,36]. Real-time PCR amplification was performed using the CFX96 system (Bio-Rad, Hercules, CA, USA) in 20 μL reactions containing 10 μL SYBR® Premix Ex TaqTM (TaKaRa, Dalian, China), 0.4 μL of BSA, 0.8 μL of each primer (5 μM), and 1 μL of diluted or undiluted purified soil DNA (1–10 ng) as a template. Three analytical replicates were performed for each soil sample [18].
2.5. Illumina MiSeq Sequencing and Data Processing
The PCR amplification was performed on an ABI GeneAmp® 9700 PCR thermocycler (ABI, CA, USA). The primers Arch-amoAF/Arch-amoAR and amoA-1F/amoA-2R were used for AOA and AOB, respectively [35,36]. The resulted PCR products were extracted from a 2% agarose gel and further purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA) and quantified using QuantiFluor™-ST (Promega, Madison, WI, USA). Purified amplicons were pooled in equimolar and paired-end sequenced (2 × 300) on an Illumina MiSeq platform (Illumina, San Diego, CA, USA) according to the standard protocols by Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China). The raw reads were deposited into the NCBI Sequence Read Archive (SRA) database (accession numbers: SRP159724 and SRP159764).
The raw FASTQ files were de-multiplexed and quality filtered by Trimmomatic and merged by FLASH according to previous research [37]. The raw dataset included only high-quality sequences after the removal of short and low-quality reads. Clustering of operational taxonomic units (OTUs) using UPARSE (version 7.1 http://drive5.com/uparse/ (accessed on 29 October 2021)) with a 97% similarity cut-off. UCHIME was used to identify and delete chimeric sequences [38]. The classification of each amoA gene sequence was analyzed by the RDP Classifier algorithm (http://rdp.cme.msu.edu/ (accessed on 29 October 2021)) against the Fgr amoA database. Rarefaction analysis (abundance-based Sobs) was revealed by Mothur [39].
2.6. Statistical Analysis
One-way ANOVA with Tukey post hoc tests were performed to test the effects of the sampling period on AOA and AOB gene abundances and community alpha diversity under the same treatments. For an individual sampling date, Student’s t-test was used to compare the influence of snow exclusion on the AOA and AOB abundances. Differences of p < 0.05 were considered significant. Statistical analyses were performed using IBM SPSS Statistics, version 20.0 (Armonk, NY, USA).
The Alpha diversity metrics, including richness (Ace index) and diversity (Shannon–Wiener index), were calculated to determine the “richness” and “diversity” functions of the AOA and AOB communities. The permutational multivariate analysis of variance (PERMANOVA) was performed to test the effects of snow treatment and sampling period on the AOA and AOB community structure at the phylum level. Principal coordinates analysis (PCoA) was performed to examine differences in soil AOA and AOB community structure across the different snow regimes and sampling periods. The first axis score of PCoA (PC1) was extracted as one of the explanatory variables for enzyme activities in the subsequent analysis. The analysis of similarity (ANOSIM) was conducted to determine the significant dissimilarities in AOA and AOB communities across the different snow regimes and sampling periods, based on 999 permutations [40]. The variance inflation factors (VIFs) were calculated to check for collinearity among environmental variables. Environmental variables were excluded when VIF was >10. Spearman correlation analysis was used to analyze the correlation between soil variables (ammonium nitrogen, nitrate nitrogen, DON, MBN, MBC, moisture, temperature, and pH) and AOA or AOB community factors (gene abundance, community diversity, and community composition). Mantel test was performed to examine the linkage between AOA or AOB community factors and N-cycle enzymes (nitrate reductase and nitrite reductase). Spearman correlation analysis was performed to examine the autocorrelation among AOA or AOB community factors. Statistical analysis using the VEGAN package in R software [41,42].
3. Results
3.1. Soil N Pools, Microbial Biomass, and Enzyme Activities
Snow exclusion significantly increased soil nitrate nitrogen and MBN concentrations during the deep snow cover period but decreased MBN concentration during the early snow cover period (Table 1). Additionally, snow exclusion reduced soil enzyme activities involved in N cycling and reached a significant difference in the early snow cover period (Table 1). Meanwhile, the sampling period also had a significant impact on soil nitrate nitrogen, MBN, and enzyme activities (Table 1).

Table 1.
Effect of snow exclusion on soil N pools, microbial biomass, and enzyme activities.
3.2. AOA and AOB Abundance in Forest Soils
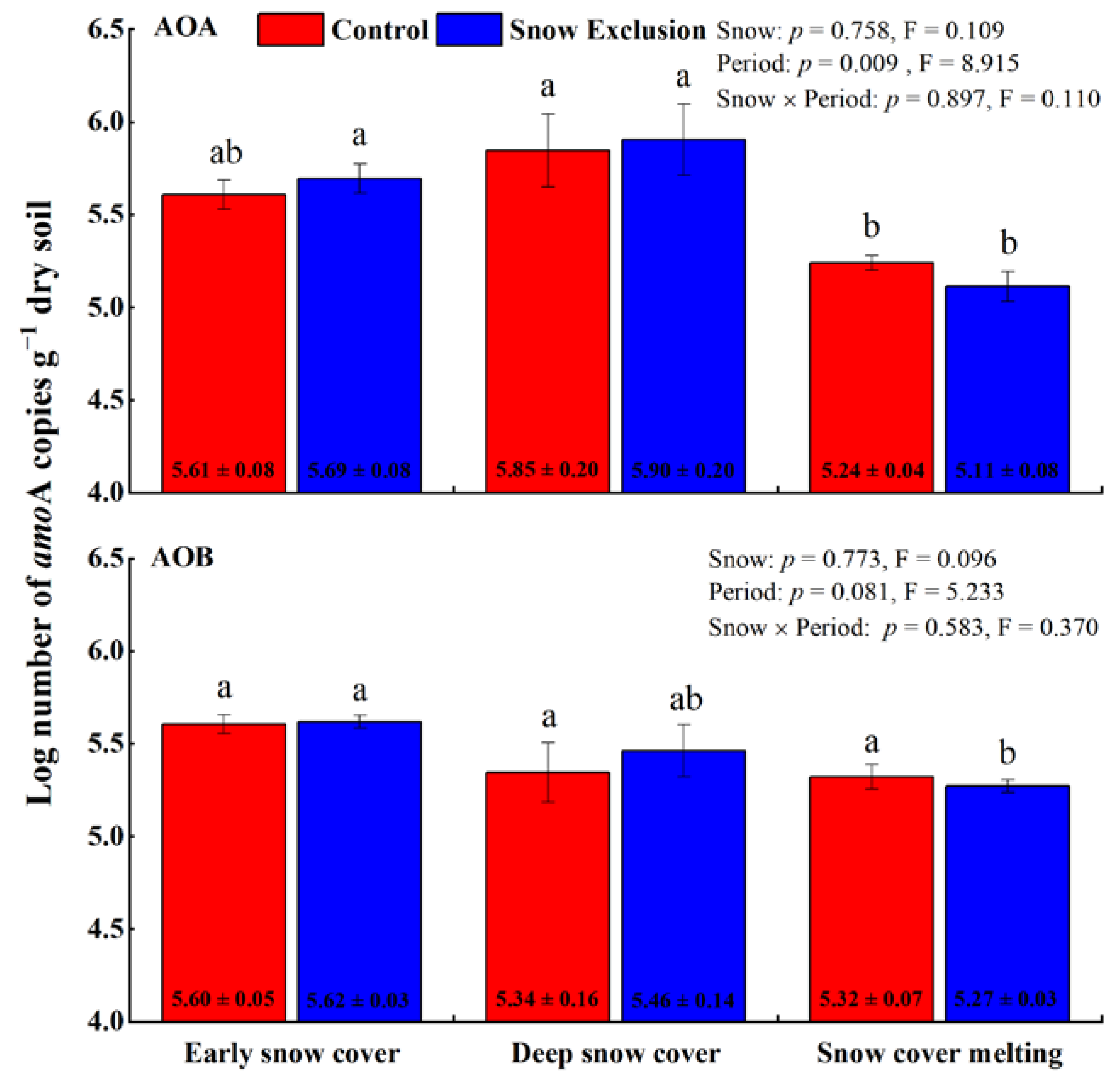
The amoA gene copy numbers of AOA and AOB ranged from 9.53 × 104 to 1.16 × 106 per gram of dry soils and 1.17 × 105 to 5.08 × 105 per gram of dry soils, respectively. Snow exclusion did not affect the AOA-amoA and AOB-amoA abundances (Figure 1). However, the AOA-amoA abundance varied with the sampling period in both treatments and decreased during the snow cover melting period (Figure 1). The abundance of AOB-amoA only changed significantly during snow exclusion and was lowest during the snow cover melting period (Figure 1).
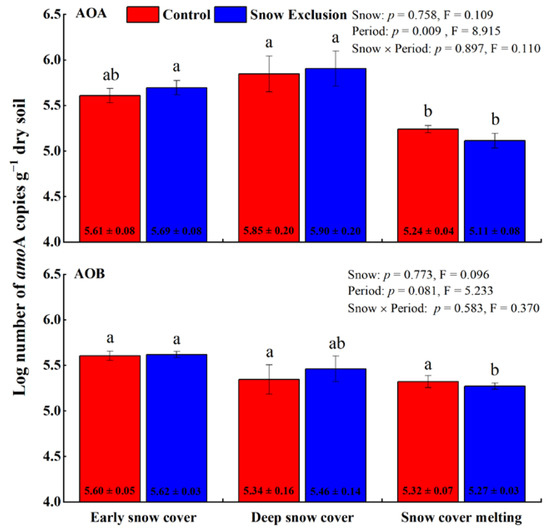
Figure 1.
The abundances of soil AOA and AOB amoA gene copy numbers (log10 transformed) in winter control and snow exclusion plots. Error bars represent the standard deviation (n = 3). Data shown are mean ± s.e. Different lowercase letters indicate significant differences between sampling periods under the same snow regime. p-Values for snow treatment (Snow), sampling period (Period), and their interactions from repeated measures ANOVA are inserted in the figure.
3.3. Richness and Diversity of AOA and AOB
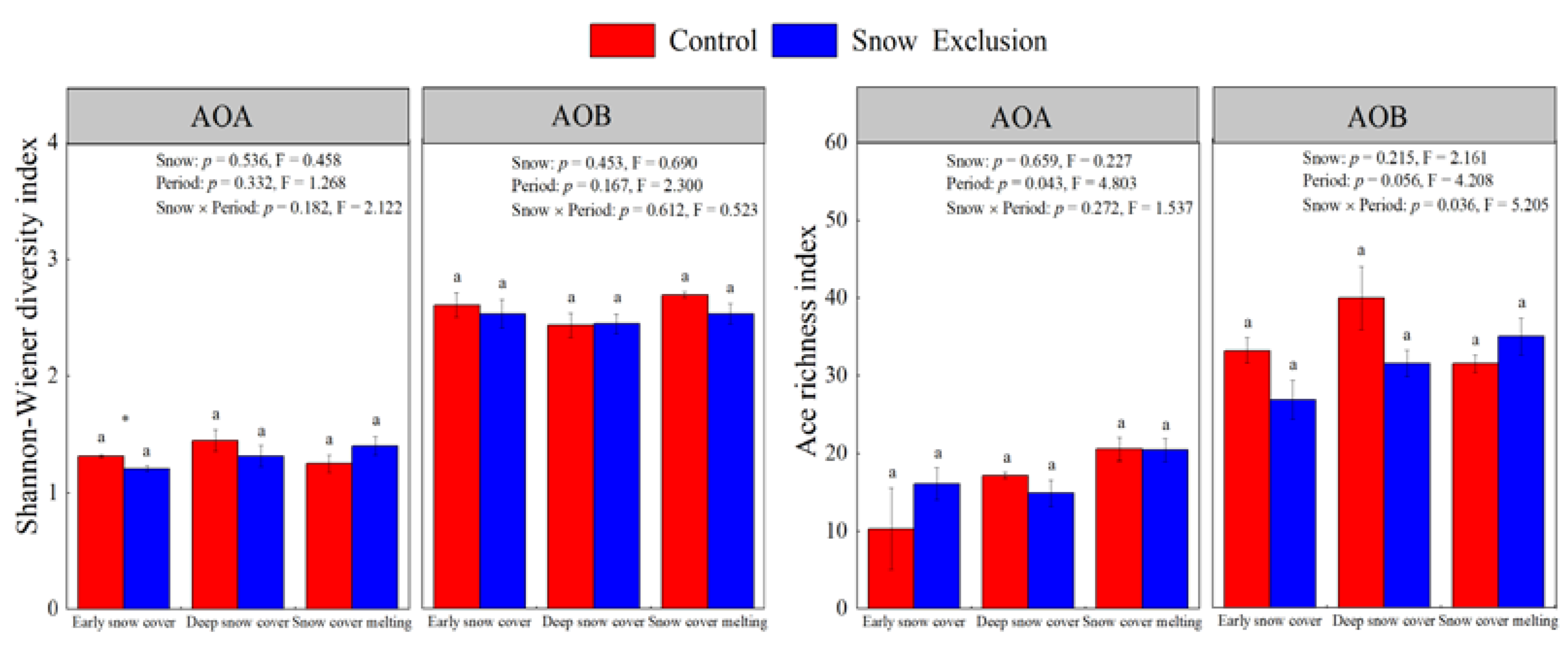
A total of 246,500 and 382,179 high-quality AOA and AOB sequences were identified in all the samples, respectively. After normalization, each library had 148,518 and 168,012 reads, and AOA and AOB sequences were clustered into 12 to 22 and 22 to 33 OTUs, respectively (Table S1). The AOA and AOB OTU numbers remained at a stable level among all treatments (Table S1, Figure S2). All rarefaction curves tended to approach the saturation plateau, indicating that the data volumes of sequenced reads were reasonable (Figure S2). Snow exclusion had little effect on the richness and diversity of AOA and AOB communities, only decreasing the diversity of AOA communities in the early snow cover period (Figure 2). The sampling period did not affect the richness and diversity of AOA and AOB communities (Figure 2).
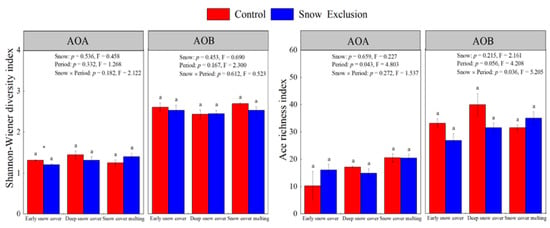
Figure 2.
The Ace richness index and Shannon diversity index of soil AOA and AOB communities in the control and snow exclusion plots during the winter. Data shown are mean ± s.e. Different lowercase letters indicate significant differences between sampling periods under the same snow regime. p-Values for snow treatment (Snow), sampling period (Period), and their interactions from repeated measures ANOVA are inserted in the figure.
3.4. Variation of AOA and AOB in Community Composition
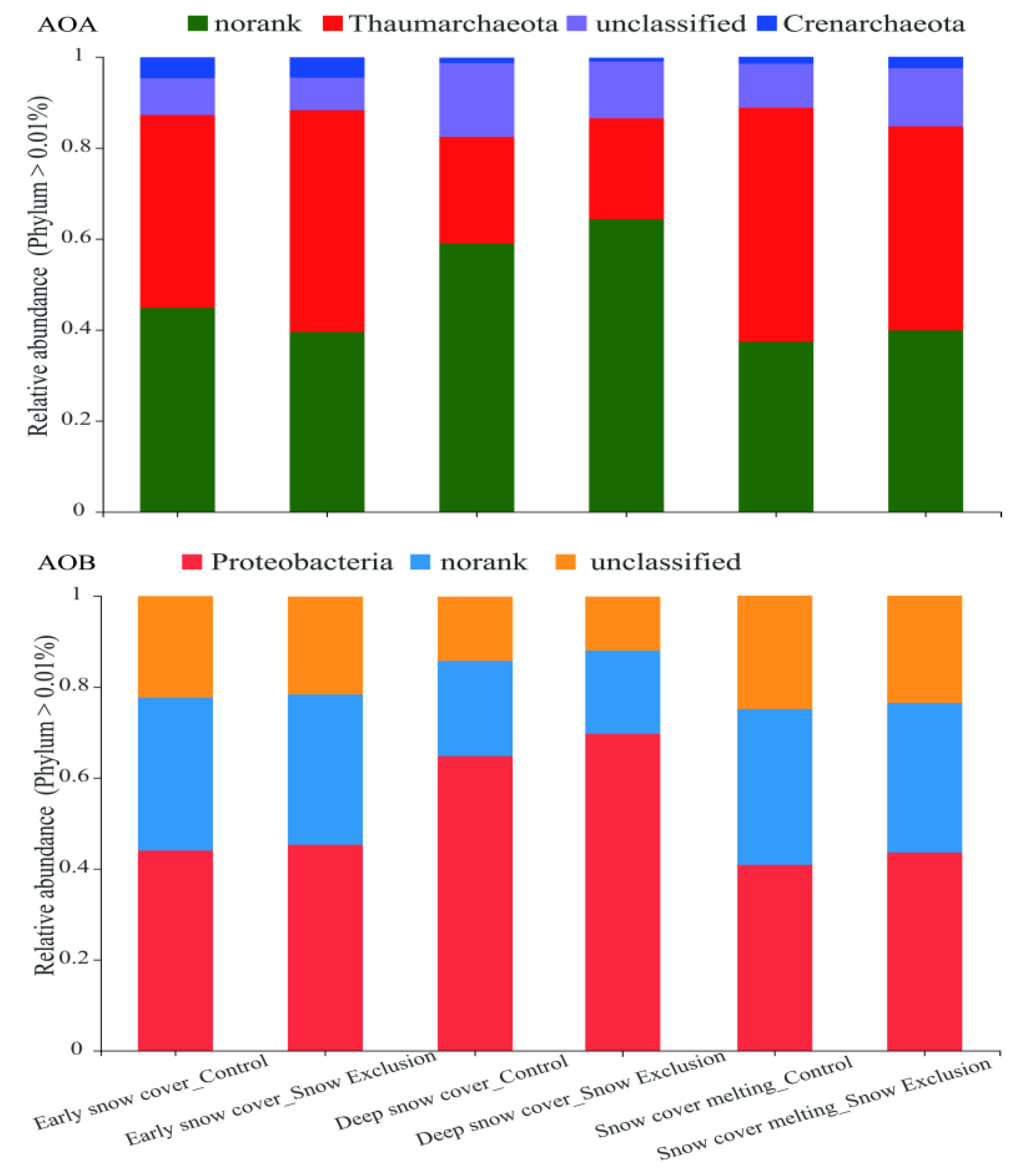
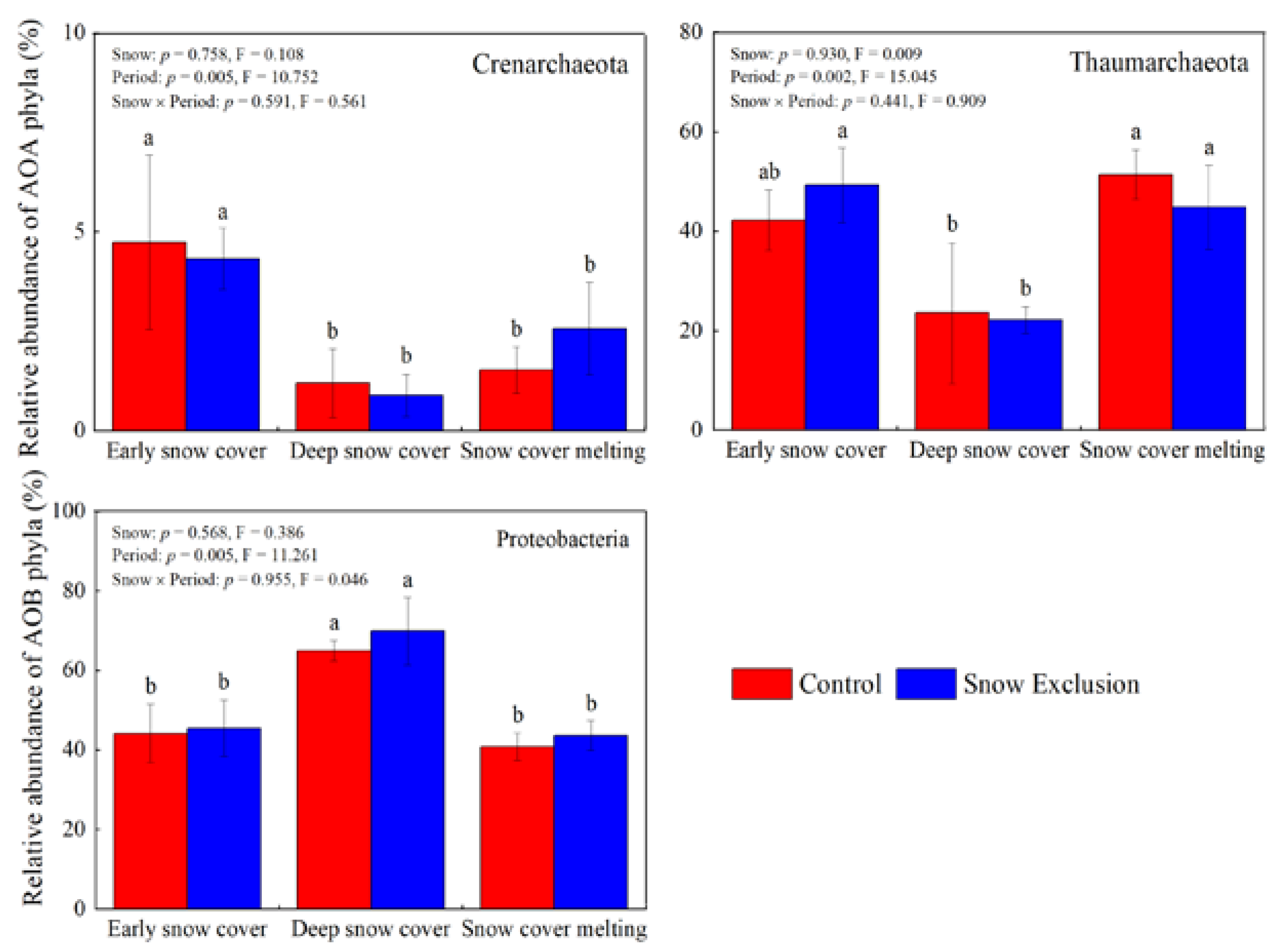
The OTUs of the AOA and AOB communities were classified into four and three phyla, respectively (Figure 3). Sequences that cannot be classified into any known group are designated as unclassified (Figure 3). In the AOA communities, Thaumarchaeota and Crenarchaeota were two known dominant phyla, and Nitrososphaera was the known genus. Proteobacteria and Nitrosospira were the known dominant phylum and genus, respectively, in the AOB communities (Figure 3 and Figure S3).
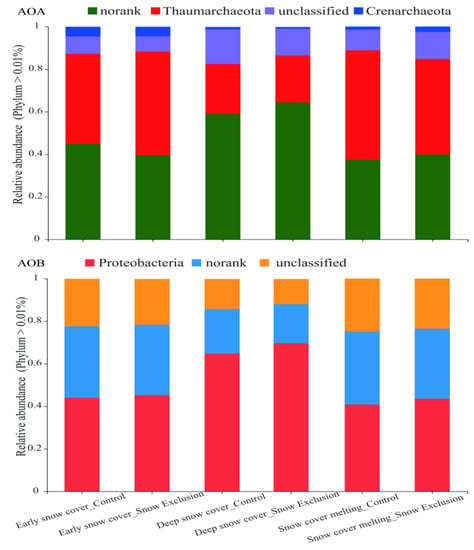
Figure 3.
Relative abundance of different AOA and AOB phyla in the control and snow exclusion plots during the winter.
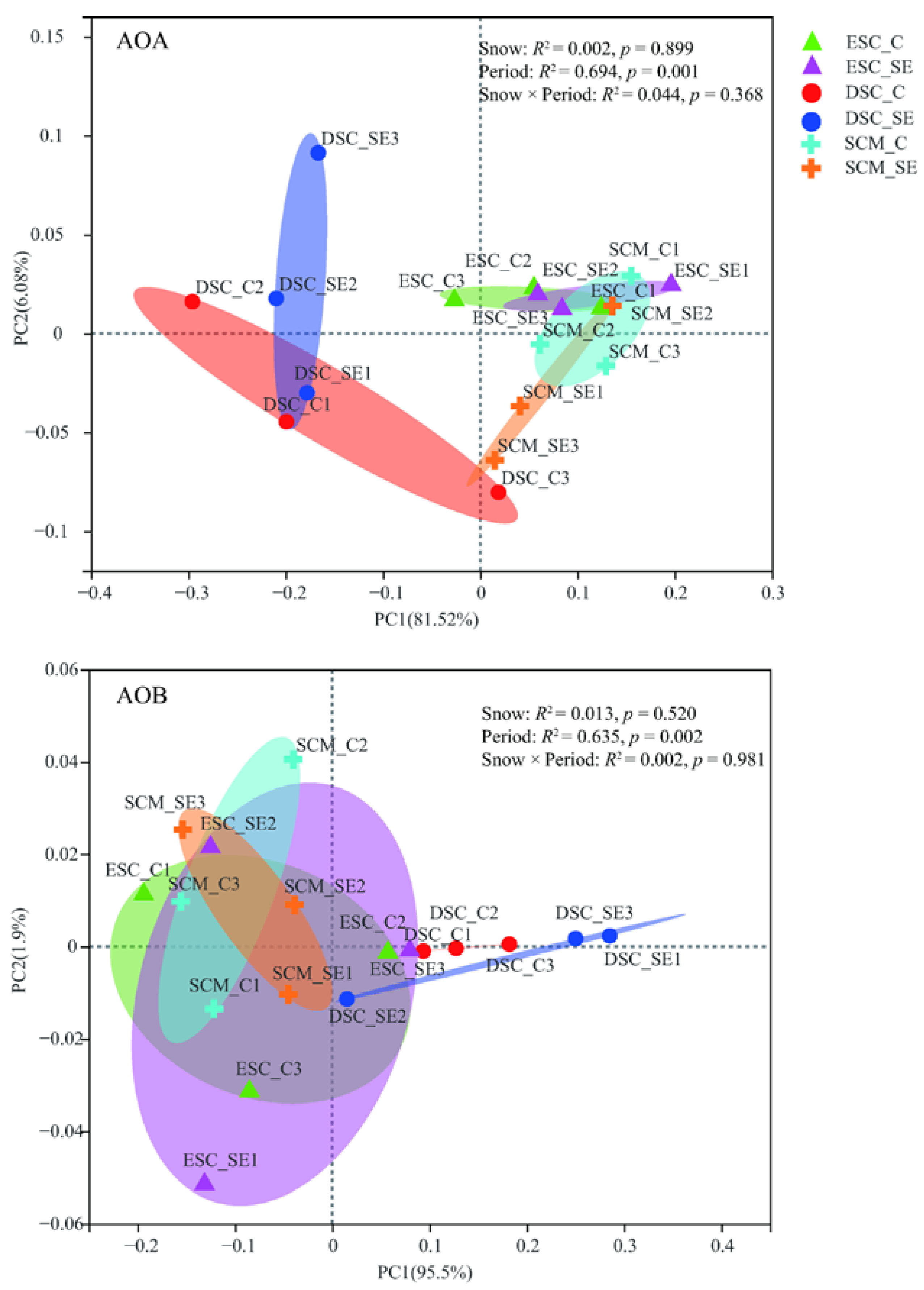
Snow exclusion did not affect the community compositions of AOA and AOB (Figure 4 and Figure S4). The PERMANOVA showed that the sampling period significantly influenced the AOA (p = 0.001) and AOB (p = 0.002) community structure at the phylum level, and they explained 69.4% and 63.5% of the total variance, respectively (Figure 4). The relative abundance of Crenarchaeota in the early snow cover period was much higher than that in the deep snow cover and snow cover melting periods. The relative abundance of Thaumarchaeota was much lower in the deep snow cover period than that in the early snow cover and snow cover melting periods (Figure 5). However, the relative abundance of Proteobacteria and Nitrosospira in the deep snow cover period was much higher than that in the early snow cover and snow cover melting periods (Figure 5 and Figure S4).
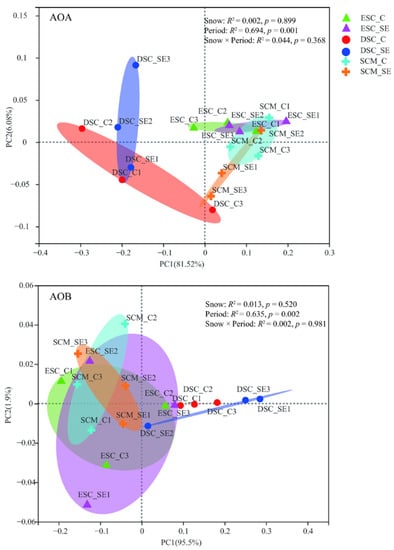
Figure 4.
Principal coordinates analysis (PCoA) of soil AOA and AOB community composition (Bray–Curis distance) at phylum level between snow treatments and sampling periods. Different sampling periods are represented by different symbols. The diamond, circle, and cruciform represent the early snow cover (ESC), deep snow cover (DSC), and snow cover melting (SCM), respectively. The red represents Control (C), and the blue represents snow exclusion (SE).
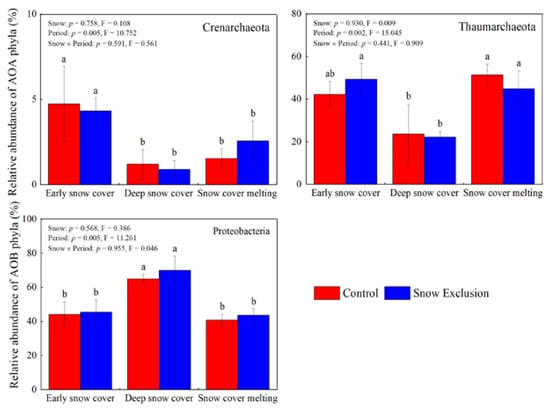
Figure 5.
Relative abundance of the known group of AOA and AOB communities at the phylum level in winter. Different lowercase letters indicate significant differences between sampling periods under the same snow regime. The p-values for snow treatment (Snow), sampling period (Period), and their interactions from repeated measures ANOVA are inserted in the figure.
3.5. Correlation between Soil Variables and AOA and AOB
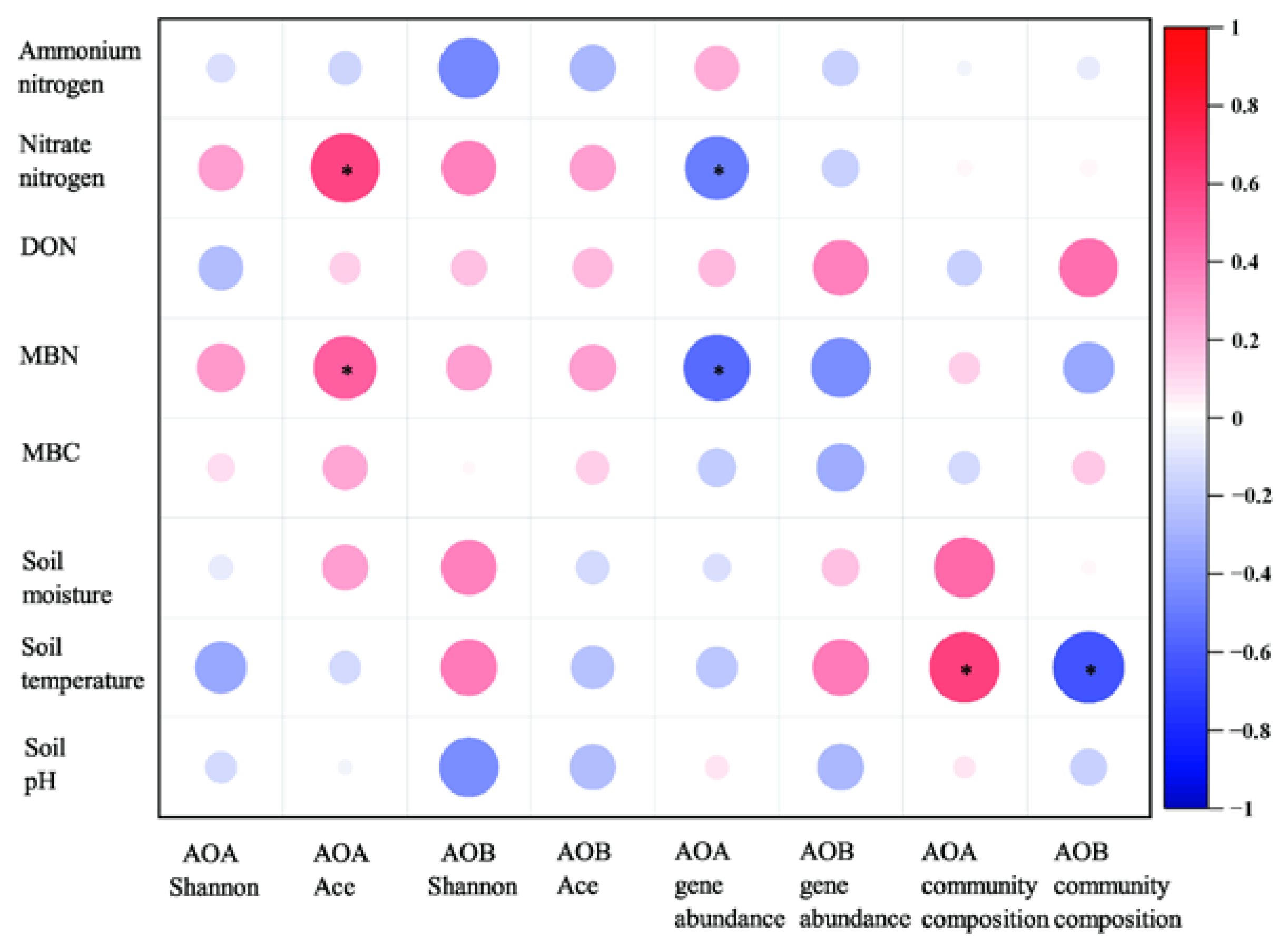
A Spearman correlation analysis was performed to assess the relationships among soil properties and AOA and AOB gene abundance, community diversity, and community composition (Figure 6). The analysis revealed that increasing soil MBN and nitrate nitrogen concentration increased AOA richness (Ace) but decreased AOA-amoA gene abundance (Figure 6). Meanwhile, soil temperature has a significant positive correlation with AOA and a negative correlation with AOB community composition (Figure 6).
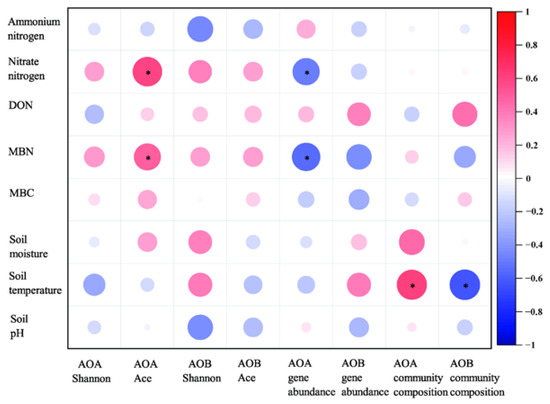
Figure 6.
Correlation analysis between soil variables and AOA or AOB gene abundance, community diversity, and community composition. TDN: total dissolved nitrogen; DON: dissolved organic nitrogen; MBN: microbial biomass nitrogen; MBC: microbial biomass carbon. * p < 0.05.
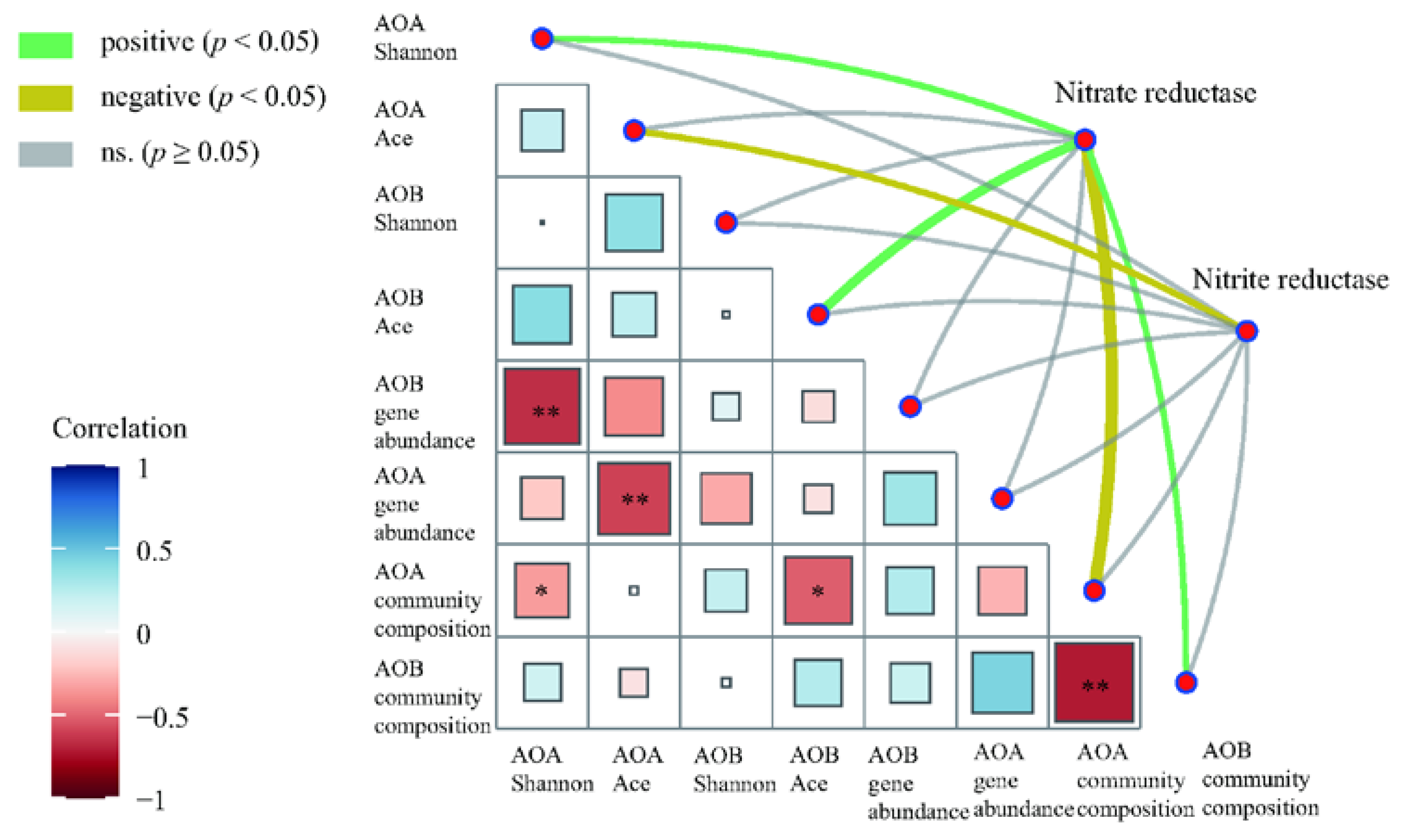
Mantel test was performed to assess the correlation between enzyme activities associated with N cycling and AOA and AOB community factors (Figure 7). Among them, nitrate reductase activity has a significant positive correlation with the AOA Shannon index, AOB Ace index, and AOB community composition but a significant negative correlation with AOA community composition (Figure 7). Nitrite reductase activity has a significant negative correlation with the AOA Ace index (Figure 7).
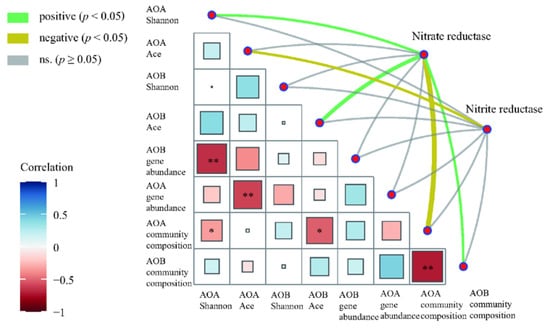
Figure 7.
Correlation of N-cycle enzymes with AOA and AOB microbial community. Pairwise comparisons of AOA and AOB gene abundance, community diversity, and community composition with a color gradient denoting the Spearman’s correlation coefficients. Enzymes were related to each microbial community factor by Mantel test. The width of edges corresponds to the Mantel’s statistic for the corresponding distance correlations. The color of the edges represents the positive and negative values of r. * p < 0.05, ** p < 0.01.
4. Discussion
Global climate change is leading to a reduction in the depth and duration of winter snow cover in snow-covered ecosystems, especially the Tibetan Plateau [5,10]. Yet, the impact of reduced winter snow on soil N-related microbial communities and related functions is unknown. Studies have shown that the impact of changes in environmental conditions on soil microbes may be assessed by measuring changes in nitrifier gene abundance and community composition [16]. Therefore, we utilized a snow-absence experiment to test the effect of snow exclusion on the soil AOA and AOB gene abundance and community structure in a subalpine spruce forest on the eastern Tibetan Plateau. The findings partially support our hypothesis that snow cover changes do affect AOA and AOB communities and their associated functions. This effect was primarily due to environmental differences between sampling periods, rather than snow exclusion treatments. This indicates that soil AOA and AOB communities were not sensitive to short-term snow cover absence.
Previous studies have found that reduced winter snow cover increased AOA gene abundance but decreased AOB gene abundance [22]. However, our study showed that the lack of snow cover did not affect AOA and AOB gene abundances (Figure 1). This may be due to the fact that the 2016–2017 winter saw the highest mean air temperature compared to the last seven winters [43], resulting in a milder winter. Compared with the 2015–2016 winter, the deepest snow cover was decreased by about 17 cm [10], making the snow cover weaker insulation. Therefore, the differences in soil microenvironment between snow cover and exclusion treatments are likely not severe enough to cause lasting changes in the AOA and AOB gene abundances. At the same time, we found that AOA and AOB gene abundances changed with the sampling period. They were higher in the early snow cover and deep snow cover periods. This is consistent with the findings in subalpine grasslands that soil AOA and AOB gene abundances were higher in mid-winter [22]. The accumulated snow cover in the middle of winter may provide a stable microenvironment and available substrate for the proliferation of microorganisms [44,45,46].
The results showed that the AOA community comprised the known phylum and genus of Thaumarchaeota, Crenarchaeota, and Nitrososphaera, while the known phylum and genus identified in the AOB community were Proteobacteria and Nitrosospira. These results were consistent with other ecosystems, such as grassland [16], agriculture [47], and sediment [48], indicating that AOA and AOB communities exhibit a wide range of adaptations.
Consistent with previous studies, snow exclusion had limited effects on soil microbial diversity and community composition [49,50]. Previous findings on soil bacteria and fungi in this region have also shown similar results [37,51]. It may be due to the long-term low-temperature environment helping microorganisms to develop some unique adaptation strategies to extreme cold conditions [52,53]. As a result, short-term mild soil freezing is insufficient to alter the composition of AOA and AOB communities in alpine forest soils. AOA and AOB community composition significantly varied across the sampling period (Figure 4 Figure 5 and Figure S4), although no significant treatment effects were detected. The deep snow cover period inhibited the relative abundance of Crenarchaeota and Thaumarchaeota and enhanced the relative abundance of Proteobacteria and Nitrosospira. It may be that the deep snow cover period provided a stable environment for the growth of Proteobacteria and Nitrosospira. Freeze–thaw cycles in early snow cover and snow cover melting periods may promote the coexistence of additional, cold-adapted taxa [7], suggesting that AOA exhibits stronger adaptability to soil freeze–thaw cycles through community shift. In addition, the present study found that the richness and diversity of AOB were higher than that of AOA in both snow exclusion and control treatments, which was different from the study in sediments [47,54].
Environmental factors, such as temperature, pH, moisture, C/N ratio, nitrate concentration, and total nitrogen, were considered as driving forces affecting the AOA or AOB gene abundance [22,23]. Previous studies demonstrated that soil pH was closely correlated with AOA and AOB gene abundances [16,55]. However, there was no significant correlation between the abundance of AOA and AOB genes and soil pH in this study. This may be attributed to the small changes in soil pH (5.35–5.58) between different treatments, indicating that AOA and AOB are not sensitive to the variation of soil pH in the tested soil. At the same time, the study found that soil MBN and nitrate nitrogen concentrations were the main influencing factors of AOA gene abundance, while no noticeable environmental factors were identified for affecting AOB gene abundance. Soil pH and ammonium concentration were reported to have beneficial effects on ammonia-oxidizing microbial diversity [56]. However, the study did not reach similar conclusions. This study found that the AOA richness was related to soil nitrate nitrogen and MBN, which was consistent with previous research [57]. Meanwhile, we found that soil temperature was the main factor affecting the AOA and AOB community composition in this region [57]. The temperature was positively correlated with AOA community composition but negatively correlated with AOB community composition, which was consistent with previous studies [47]. This may be due to changes in soil temperature within the study area affecting physical conditions, water, and nutrient availability [18] and further affecting AOA and AOB community composition. In addition, changes in AOA and AOB diversity and community composition were associated with enzyme activities related to soil N cycling. Soil nitrate reductase was significantly associated with the community abundance of AOA and AOB, as was studied in sediments [54]. The results of this study showed that nitrate reductase had a positive relationship with the AOB community composition, but there was a significant negative correlation with the AOA community composition. This indicates that soil enzymes can partially reflect the composition and structure of soil microbial communities. In addition, the functional characteristics of soil microbial communities may vary with their composition.
5. Conclusions
In summary, our findings suggest that soil ammonia-oxidizing communities are not sensitive to short-term snow cover change. The abundance and community composition of soil AOA and AOB only exhibited obvious dynamic variations across different critical periods of snow cover. This change is mainly affected by soil temperature, implying that snow-cover-associated changes in soil frost patterns may have more profound implications for these communities and the functions they provide. Therefore, we encourage future studies to focus more on snow-cover-related ecological processes in climate-change-sensitive areas.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f13091483/s1, Figure S1: Air and soil temperature, snow depth (a), and soil moisture (b) in the control and snow exclusion plots during the winter; Figure S2: Rarefaction curve of the OTU number at 97% similarity cutoff for AOA and AOB; Figure S3: Relative abundance of different AOA and AOB genera in the control and snow exclusion plots during the winter; Figure S4: Relative abundance of Nitrososphaera and Nitrosospira in winter control and snow exclusion plots; Table S1: Sequencing results and number of observed and estimated OTUs at the species level (70% amoA identity).
Author Contributions
Conceptualization, Z.X. and B.T.; methodology, C.Y. and B.T.; software, L.Z. and L.W.; validation, H.L. and C.Y.; formal analysis, L.Z.; writing—original draft preparation, L.Z.; writing—review and editing, S.L., L.W. and Z.X.; visualization, L.Z.; supervision, Z.X.; funding acquisition, L.Z., H.L., S.L. and Z.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (31901295, 32071745, and 32001165), the Program of Sichuan Applied Basic Research Foundation (2022NSFSC0083, 2022NSFSC0997, and 2022NSFSC1753), and the Program of Sichuan Excellent Youth Sci-Tech Foundation (2020JDJQ0052).
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful to the Long-term Research Station of Alpine Forest Ecosystems and the Collaborative Innovation Center of Ecological Security in the Upper Reaches of the Yangtze River.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Broadbent, A.A.D.; Bahn, M.; Pritchard, W.J.; Newbold, L.K.; Goodall, T.; Guinta, A.; Snell, H.S.K.; Cordero, I.; Michas, A.; Grant, H.K.; et al. Shrub expansion modulates belowground impacts of changing snow conditions in alpine grasslands. Ecol. Lett. 2022, 25, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Wubs, E.R.J.; Woodin, S.J.; Stutter, M.I.; Wipf, S.; Sommerkorn, M.; van der Wal, R. Two decades of altered snow cover does not affect soil microbial ability to catabolize carbon compounds in an oceanic alpine heath. Soil Biol. Biochem. 2018, 124, 101–104. [Google Scholar] [CrossRef]
- Zinger, L.; Shahnavaz, B.; Baptist, F.; Geremia, R.A.; Choler, P. Microbial diversity in alpine tundra soils correlates with snow cover dynamics. ISME J. 2009, 3, 850–859. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Summary for Policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Wang, J.; Zhang, M.; Wang, S.; Ren, Z.; Che, Y.; Qiang, F.; Qu, D. Decrease in snowfall/rainfall ratio in the Tibetan Plateau from 1961 to 2013. J. Geogr. Sci. 2016, 26, 1277–1288. [Google Scholar] [CrossRef]
- Broadbent, A.A.D.; Snell, H.S.K.; Michas, A.; Pritchard, W.J.; Newbold, L.; Cordero, I.; Goodall, T.; Schallhart, N.; Kaufmann, R.; Griffiths, R.I.; et al. Climate change alters temporal dynamics of alpine soil microbial functioning and biogeochemical cycling via earlier snowmelt. ISME J. 2021, 15, 2264–2275. [Google Scholar] [CrossRef]
- Aanderud, Z.T.; Jones, S.E.; Schoolmaster, D.R.; Fierer, N.; Lennon, J.T. Sensitivity of soil respiration and microbial communities to altered snowfall. Soil Biol. Biochem. 2013, 57, 217–227. [Google Scholar] [CrossRef]
- Kreyling, J.; Henry, H.A.L. Vanishing winters in Germany: Soil frost dynamics and snow cover trends, and ecological implications. Clim. Res. 2011, 46, 269–276. [Google Scholar] [CrossRef]
- Kreyling, J.; Haei, M.; Laudon, H. Absence of snow cover reduces understory plant cover and alters plant community composition in boreal forests. Oecologia 2012, 168, 577–587. [Google Scholar] [CrossRef]
- Li, Z.; Yang, W.; Yue, K.; Justine, M.F.; He, R.; Yang, K.; Zhuang, L.; Wu, F.; Tan, B.; Zhang, L.; et al. Effects of snow absence on winter soil nitrogen dynamics in a subalpine spruce forest of southwestern China. Geoderma 2017, 307, 107–113. [Google Scholar] [CrossRef]
- Walker, V.K.; Palmer, G.R.; Voordouw, G. Freeze-thaw tolerance and clues to the winter survival of a soil community. Appl. Environ. Microb. 2006, 72, 1784–1792. [Google Scholar] [CrossRef] [Green Version]
- Schimel, J.P.; Bilbrough, C.; Welker, J.M. Increased snow depth affects microbial activity and nitrogen mineralization in two Arctic tundra communities. Soil Biol. Biochem. 2004, 36, 217–227. [Google Scholar] [CrossRef]
- Prosser, J.I. Autotrophic nitrification in bacteria. Adv. Microb. Physiol. 1989, 30, 125–181. [Google Scholar] [CrossRef] [PubMed]
- Coca-Salazar, A.; Richaume, A.; Florio, A.; Carnol, M. Response of ammonia-oxidizing bacteria and archaea abundance and activity to land use changes in agricultural systems of the Central Andes. Eur. J. Soil Biol. 2021, 102, 103263. [Google Scholar] [CrossRef]
- Mushinski, R.M.; Gentry, T.J.; Dorosky, R.J.; Boutton, T.W. Forest harvest intensity and soil depth alter inorganic nitrogen pool sizes and ammonia oxidizer community composition. Soil Biol. Biochem. 2017, 112, 216–227. [Google Scholar] [CrossRef]
- Wang, F.; Li, Z.; Wei, Y.; Su, F.; Guo, H.; Guo, J.; Wang, Y.; Zhang, Y.; Hu, S. Responses of soil ammonia-oxidizing bacteria and archaea to short-term warming and nitrogen input in a semi-arid grassland on the Loess Plateau. Eur. J. Soil Biol. 2021, 102, 103267. [Google Scholar] [CrossRef]
- Robson, T.M.; Baptist, F.; Clément, J.; Lavorel, S. Land use in subalpine grasslands affects nitrogen cycling via changes in plant community and soil microbial uptake dynamics. J. Ecol. 2010, 98, 62–73. [Google Scholar] [CrossRef]
- Wang, A.; Wu, F.; Yang, W.; Wu, Z.; Wang, X.; Tan, B. Abundance and composition dynamics of soil ammonia-oxidizing archaea in an alpine fir forest on the eastern Tibetan Plateau of China. Can. J. Microbiol. 2012, 58, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.C.; Scalenghe, R.; Freppaz, M. Changes in the seasonal snow cover of alpine regions and its effect on soil processes: A review. Quatern. Int. 2007, 162–163, 172–181. [Google Scholar] [CrossRef]
- Sorensen, P.O.; Finzi, A.C.; Giasson, M.; Reinmann, A.B.; Sanders-DeMott, R.; Templer, P.H. Winter soil freeze-thaw cycles lead to reductions in soil microbial biomass and activity not compensated for by soil warming. Soil Biol. Biochem. 2018, 116, 39–47. [Google Scholar] [CrossRef]
- Wipf, S.; Sommerkorn, M.; Stutter, M.I.; Wubs, E.R.J.; van der Wal, R. Snow cover, freeze-thaw, and the retention of nutrients in an oceanic mountain ecosystem. Ecosphere 2015, 6, 207–216. [Google Scholar] [CrossRef]
- Jusselme, M.D.; Saccone, P.; Zinger, L.; Faure, M.; Roux, X.L.; Guillaumaud, N.; Bernard, L.; Clement, J.; Poly, F. Variations in snow depth modify N-related soil microbial abundances and functioning during winter in subalpine grassland. Soil Biol. Biochem. 2016, 92, 27–37. [Google Scholar] [CrossRef]
- Brin, L.D.; Goyer, C.; Zebarth, B.J.; Burton, D.L.; Chantigny, M.H. Linking changes in snow cover with microbial nitrogen cycling functional gene abundance and expression in agricultural soil. FEMS Microbiol. Ecol. 2019, 95, fiz073. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhu, Q.; Peng, C.; Wu, N.; Wang, Y.; Fang, X.; Gao, Y.; Zhu, D.; Yang, G.; Tian, J.; et al. The impacts of climate change and human activities on biogeochemical cycles on the Qinghai-Tibetan Plateau. Glob. Chang. Biol. 2013, 19, 2940–2955. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Pepin, N.C.; Chen, Y. Changes of snowfall under warming in the Tibetan Plateau. J. Geophys. Res-Atmo. 2017, 122, 7323–7341. [Google Scholar] [CrossRef]
- Wang, L.F.; Cai, Y.J.; Xie, H.T. Relationships of soil physical and microbial properties with nitrous oxide emission under effects of freezing-thawing cycles. Chi. J. Appl. Ecol. 2007, 18, 2361–2366. [Google Scholar]
- Ribbons, R.R.; Levy-Booth, D.J.; Masse, J.; Grayston, S.J.; McDonald, M.A.; Vesterdal, L.; Prescott, C.E. Linking microbial communities, functional genes and nitrogen-cycling processes in forest floors under four tree species. Soil Biol. Biochem. 2016, 103, 181–191. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, Q.; Yin, H. Effects of temperature on soil net nitrogen mineralisation in two contrasting forests on the eastern Tibetan Plateau, China. Soil Res. 2014, 52, 562–567. [Google Scholar] [CrossRef]
- Yin, R.; Jiang, X.; Xu, Z.; Wu, F.; Yang, W.; Xiong, L.; Li, Z.; Wang, B.; Tang, S.; Ding, W. Effects of seasonal snow pack on winter soil nitrogen mineralization and leachate in Subalpine Abies faxoniana forest in western Sichuan province. J. Soil Water Conserv. 2013, 27, 138–143. [Google Scholar]
- IUSS Working Group. World Reference Base for Soil Resources 2006. First Update 2007; World Soil Resources Reports No. 103; FAO: Rome, Italy, 2007. [Google Scholar]
- Xu, Z.; Hu, R.; Xiong, P.; Wan, C.; Cao, G.; Liu, Q. Initial soil responses to experimental warming in two contrasting forest ecosystems, Eastern Tibetan Plateau, China: Nutrient availabilities, microbial properties and enzyme activities. Appl. Soil Ecol. 2010, 46, 291–299. [Google Scholar] [CrossRef]
- Jones, D.; Willett, V. Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biol. Biochem. 2006, 38, 991–999. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Xiong, L.; Xu, Z.F.; Wu, F.Z.; Yang, W.Q.; Yin, R.; Li, Z.P.; Gou, X.L.; Tang, S.S. Effects of snow pack on soil nitrogen transformation enzyme activities in a subalpine Abies faxioniana forest of western Sichuan, China. Chin. J. Appl. Ecol. 2014, 25, 1293–1299. [Google Scholar]
- Francis, C.A.; Roberts, K.J.; Beman, J.M.; Santoro, A.E.; Oakley, B.B. Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc. Natl. Acad. Sci. USA 2005, 102, 14683–14688. [Google Scholar] [CrossRef] [Green Version]
- Rotthauwe, J.H.; Witzel, K.P.; Liesack, W. The ammonia monooxygenase structural gene amoA as a functional marker: Molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl. Environ. Microb. 1997, 63, 4704–4712. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ren, Y.; Yang, K.; Li, Z.; Tan, B.; Liu, Y.; Li, H.; You, C.; Liu, S.; Wang, L.; et al. Immediate and legacy effects of snow exclusion on soil fungal diversity and community composition. For. Ecosyst. 2021, 8, 22. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial community. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Henry, M.; et al. Vegan: Community ecology package. R package version 2.0-10. Agric. Sci. 2013, 7, 6. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015; Available online: http://www.R-project.org/ (accessed on 29 October 2021).
- Yang, K.; Peng, C.; Peñuelas, J.; Kardol, P.; Li, Z.; Zhang, L.; Ni, X.; Yue, K.; Tan, B.; Yin, R.; et al. Immediate and carry-over effects of increased soil frost on soil respiration and microbial activity in a spruce forest. Soil Biol. Biochem. 2019, 135, 51–59. [Google Scholar] [CrossRef]
- Drotz, S.H.; Sparrman, T.; Nilsson, M.B.; Schleucher, J.; Öquist, M.G. Both catabolic and anabolic heterotrophic microbial activity proceed in frozen soils. Proc. Natl. Acad. Sci. USA 2010, 107, 21046–21051. [Google Scholar] [CrossRef] [PubMed]
- Lipson, D.A.; Schmidt, S.K. Seasonal changes in an alpine soil bacterial community in the Colorado Rocky Mountains. Appl. Environ. Microb. 2004, 70, 2867–2879. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Szele, Z.; Schilling, R.; Munch, J.C.; Schloter, M. Influence of freeze-thaw stress on the structure and function of microbial communities and denitrifying populations in soil. Appl. Environ. Microbiol. 2006, 72, 2148–2154. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.D.; Ren, Y.F.; Wang, X.Q.; Hu, Y.G.; Wang, Z.M.; Zeng, Z.H. Ammonia-oxidizing archaea and bacteria responding differently to fertilizer type and irrigation frequency as revealed by Illumina Miseq sequencing. J. Soil Sediment 2018, 18, 1029–1040. [Google Scholar] [CrossRef]
- He, H.; Zhen, Y.; Mi, T.; Fu, L.; Yu, Z. Ammonia-oxidizing archaea and bacteria differentially contribute to ammonia oxidation in sediments from adjacent waters of Rushan bay, China. Front. Microbiol. 2018, 9, 116. [Google Scholar] [CrossRef]
- Gavazov, K.; Ingrisch, J.; Hasibeder, R.; Mills, R.T.E.; Buttler, A.; Gleixner, G.; Pumpanen, J.; Bahn, M. Winter ecology of a subalpine grassland: Effects of snow removal on soil respiration, microbial structure and function. Sci. Total Environ. 2017, 590–591, 316–324. [Google Scholar] [CrossRef]
- Männistö, M.; Vuosku, J.; Stark, S.; Saravesi, K.; Suokas, M.; Markkola, A.; Martz, F.; Rautio, P. Bacterial and fungal communities in boreal forest soil are insensitive to changes in snow cover conditions. FEMS Microbiol. Ecol. 2018, 94, fiy123. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zhang, L.; Yang, K.; Li, Z.; Yin, R.; Tan, B.; Wang, L.; Liu, Y.; Li, H.; You, C.; et al. Short-term effects of snow cover manipulation on soil bacterial diversity and community composition. Sci. Total Environ. 2020, 741, 140454. [Google Scholar] [CrossRef]
- Gao, D.; Hagedorn, F.; Zhang, L.; Liu, J.; Qu, G.; Sun, J.; Peng, B.; Fan, Z.; Zheng, J.; Jiang, P.; et al. Small and transient response of winter soil respiration and microbial communities to altered snow depth in a mid-temperate forest. Appl. Soil Ecol. 2018, 130, 40–49. [Google Scholar] [CrossRef]
- Sorensen, P.O.; Templer, P.H.; Finzi, A.C. Contrasting effects of winter snowpack and soil frost on growing season microbial biomass and enzyme activity in two mixed-hardwood forests. Biogeochemistry 2016, 128, 141–154. [Google Scholar] [CrossRef]
- Wei, D.; Zeng, S.; Hou, D.; Zhou, R.; Xing, C.; Deng, X.; Yu, L.; Wang, H.; Deng, Z.; Weng, S.; et al. Community diversity and abundance of ammonia-oxidizing archaea and bacteria in shrimp pond sediment at different culture stages. J. Appl. Microbiol. 2021, 130, 1442–1455. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Xiao, X.; He, N.; Zhu, W.; Liu, M.; Xie, G. Effects of reducing chemical fertilizer combined with organic amendments on ammonia-oxidizing bacteria and archaea communities in a low-fertility red paddy field. Environ. Sci. Pollut. Res. Int. 2020, 27, 29422–29432. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, C.; Luo, Y. Meta-analysis of the impacts of global change factors on soil microbial diversity and functionality. Nat. Commun. 2020, 11, 3072. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Liu, C.; Yu, L.; Song, C.; Peng, L.; Li, X.; Tao, L.; Li, G. Organic Matter Regulates Ammonia-oxidizing bacterial and archaeal communities in the surface sediments of Ctenopharyngodon idellus aquaculture ponds. Front. Microbiol. 2018, 9, 2290. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).