1. Introduction
Forests have an essential functional role in maintaining biogeocenosis in a stable (unshakable) condition. After all, forests are the leading producers and accumulators of biomass. Moreover, they lead in regulating the atmosphere’s composition and oxygen production. At the same time, forests are the most oppressed and transformed by the anthropogenic activity elements of the biosphere. In the example of forests, the reaction of the biogeocenosis to centuries-old human expansion can be traced. As is known, anthropogenic-derived forest cenoses are characterized by low stability and the inability, in most cases, to develop normally and fully perform biospheric functions without constant human care [
1].
For Ukraine, the most striking example of such a fatal degradation of forest biocenoses due to anthropogenic impact is oak forests, which occupy over 38% of forest areas. For more than 100 years, there has been accelerated dieback of the main forest-forming species—the common oak. Researchers [
2,
3] have identified three periods of worsening of the pathological condition of oak: 1892–1911, 1927–1946, and 1964–1983. Periodically, in certain regions, and even within the entire range, the rate of the accelerated dieback of oak trees has become catastrophic. The total area of the dieback of common oak in the forests of Ukraine is 86,000 ha (according to official data from the State Forest Resources Agency of Ukraine [
4]). Compared to the annual indicator of 2010, the area of dieback for the first half of 2020 increased by 50% [
4]. Currently, the actual state of oak forests in Ukraine is such that it is worth talking about their degradation since oak stands are significantly weakened by synoptic and climatic anomalies (in particular, hydrothermal stress) [
5,
6], invasive species [
7,
8,
9], and infectious agents [
10,
11,
12,
13].
The pathologies of oak, which are caused by the activity of phytopathogenic bacteria, are particularly dangerous and the least researched. Such bacteria are an integral component of the accompanying microbiota of the plant organism. They are also causative agents of bacteriosis, which weaken the plant and often lead to its total dieback, sometimes reaching the extent of epiphytotic. It is necessary to establish the etiology of a disease of bacterial origin reliably and to carry out a bacteriological test to determine the morphological, cultural, and biochemical properties. Therefore, even though, today, the main symptomatic signs of bacteriosis are known, the study of the properties of phytopathogenic bacteria associated with the pathology of common oak is a valid research direction. To date, researchers have experimentally confirmed the involvement of more than 15 types of pathogens in the dieback of oak stands [
2,
3,
12]. However, the most common in Ukraine are: “soft bacterial rot” of acorns, bacterial dropsy (wetwood), white bacteriosis or the trunk form, “drip disease” of acorns, “dry rot” of branches and trunks, and canker disease.
For more than 30 years, typical symptoms of soft or wet rot of oak acorns have been recorded in the forests of Ukraine [
2]. The connection of aerobic bacteria (particularly
Brenneria goodwinii,
Gibbsiella quercinecans, and
Rahnella victoriana, with the phenomenon of “Oak decline” in Italy) was studied, and typical symptoms were noted [
14]. In addition, there is some information about the determination of the pathogenicity determinants of the pathogen by transposon mutagenesis [
15], the identification and characterization of the virulence of the protein that induces
Brenneria goodwinii,
Gibbsiella quercinecans, and
Rahnella victoriana [
16], and bacteriophages have been identified [
17].
“Drip disease” of acorns [
18] is also spreading worldwide, although it has not been detected in Ukraine. Currently, there are separate notes from Spain [
19] and Colorado [
20]. There is a well-known study on the potential of arthropods, namely, the role of insects in the spread of the pathogen of the “drip disease” of acorns from three orders (
Coleoptera,
Hemiptera, and
Hymenoptera) and eight families (
Buprestidae,
Coccinellidae,
Dermestidae,
Coreidae,
Pentatomidae and/or
Miridae,
Apidae, and
Vespidae) [
21]. Four subspecies of
Lonsdalea quercina (
L. quercina subsp.
quercina,
L. quercina subsp.
britannica,
L. quercina subsp.
iberica, and
L. quercina subsp.
populi) were studied using average nucleotide identity (ANI) [
22]. Extraction of the phytopathogenic bacterium
Lonsdalea quercina from the little bat in the Moravian Karst, Czech Republic [
23] was carried out, suggesting the methods of spreading the infection.
Bacterial dropsy (wetwood) is one of the most common and dangerous diseases in Ukraine of bacterial origin within the growth area of the common oak [
12]. The research on the morphological, cultural, and biochemical properties of the causative agent of bacterial wetwood of oak,
Lelliottia nimipressuralis, has been confirmed by bacteriological studies of oak wood with typical signs of damage [
24,
25,
26]. Selected information on identifying the bacteria
L. nimipressuralis was determined based on whole-genome sequencing [
26]. The authors also presented other phytopathogenic bacteria, including
Micrococcus dendroporthos,
Pseudomonas syringae,
Erwinia valachica,
Erwinia valachica f.
onaca,
Erwinia gueieicola, etc.—causative agents of “brown mucus” from oak—that are involved in the deep pathology of common oak in Ukraine [
12].
Dangerous bacterial diseases, especially in weakened oak stands, are “dry rot” of branches and trunks of common oak, the causative agent of which is
Erwinia rhapontici [
2,
27], and canker pathologies, which usually have a mixed infection. Quantitative determination of the genus
Erwinia has been carried out and related genera have been studied on the surface of the leaves and branches of
Quercus robur [
28,
29].
The phenomenon of acute oak decline (AOD) in the modern literature is often associated with phytopathogenic bacteria
Gibbsiella quercinecans,
Brenneria goodwinii, and
Rahnella victoriana [
10,
30,
31].
Oak tissue necrosis and mucus secretion from the trunk are caused by a combined effect of bacteria from different families. However, bacterial strains of the
Enterobacteriaceae family are numerically predominant, as evidenced by an isolation study of the oak microbiome, which is affected by AOD [
32].
Oak leaves are also susceptible to bacterial diseases; in particular, bacterial leaf scorch of oak caused by the bacterium
Xylella fastidiosa [
33] has been reported [
34,
35].
Thus, it has become clear that bacterioses are a widespread and hazardous group of infectious diseases of forest woody plants, namely common oak. The causative agents of bacteriosis—phytopathogenic bacteria—require thorough experimental studies and observations to clarify the complex relationships of the representatives of various systematic and functional groups of microorganisms in infectious pathology and to find effective and rational methods of protecting forests.
The aim of this study was to investigate the bacterial diseases of common oak in Ukraine. The study included establishing the specificity and key factors of their pathogenesis; comparison of the morphological, cultural, and biochemical properties of phytopathogenic bacteria controlling the symptoms of pathological processes; accordance of the biological properties of causative agents of bacteriosis with the key factors of pathogenesis.
2. Materials and Methods
2.1. Region of Research
Experimental research was carried out on the conditions of state forestry enterprises of the Right Bank Forest-Steppe, namely in the following state enterprises: “Zhytomyr forestry”, “Vinnitske forestry”, “Khmelnitske forestry”, and “Chortkivske forestry”, as well as on the southern border of the Polissia region of Ukraine, in particular in the state enterprises “Baranivske forestry” and the Boyarska Forest Research Station.
2.2. Experimental Plots, Selected Samples
Forest stands of different stand compositions, ages, and planting completeness and under different forest conditions were studied. A total of 34 trial plots were established. The age limits of the examined trees ranged from 20 to 119 years old. These stands were pure and mixed oak forests, occurring on different forest site types (dry, fresh, wet), with a high stand quality class (I, Ia), and an average stand density of 0.8. A total of 140 samples were taken for microbiological research; 62 bacterial isolates were isolated, including 37 strains of phytopathogenic bacteria, which were used for further analysis (
Table 1).
The general scheme of this study on bacterial diseases of common oak and their pathogens included the following stages: forest pathological surveys according to the generally accepted silvicultural, forest inventory, and phytopathological methods; selection of affected organs and tissues; isolation of microorganisms in pure culture; verification of pathogenic properties of isolates and their identification.
2.3. Cultivation and Identification of Microorganisms
For the bacteriological analysis, samples of wood (branch, trunk), seeds (acorn), and common oak leaves with typical signs of bacterial diseases were used. The bacteriological analysis was carried out by homogenizing the plant material, followed by inoculation in Petri dishes on agar nutrient media and growth under thermostatic conditions at 28 °C for 4–5 days. Bacteria colonies were selected for analysis and sifted onto agar nutrient medium in test tubes.
The microorganisms, depending on functional and other characteristics, were tested for their growth numbers on special nutrient media (potato agar, meat-peptone agar, meat-peptone broth, malt extract of agar, Chapek’s medium, etc.). From the total number of isolates under laboratory conditions, bacteria with signs of phytopathogens were selected for further research. Signs included the presence of pectolytic and cellulolytic activities, the ability to cause a hypersensitivity reaction when inoculated in the leaves of plants.
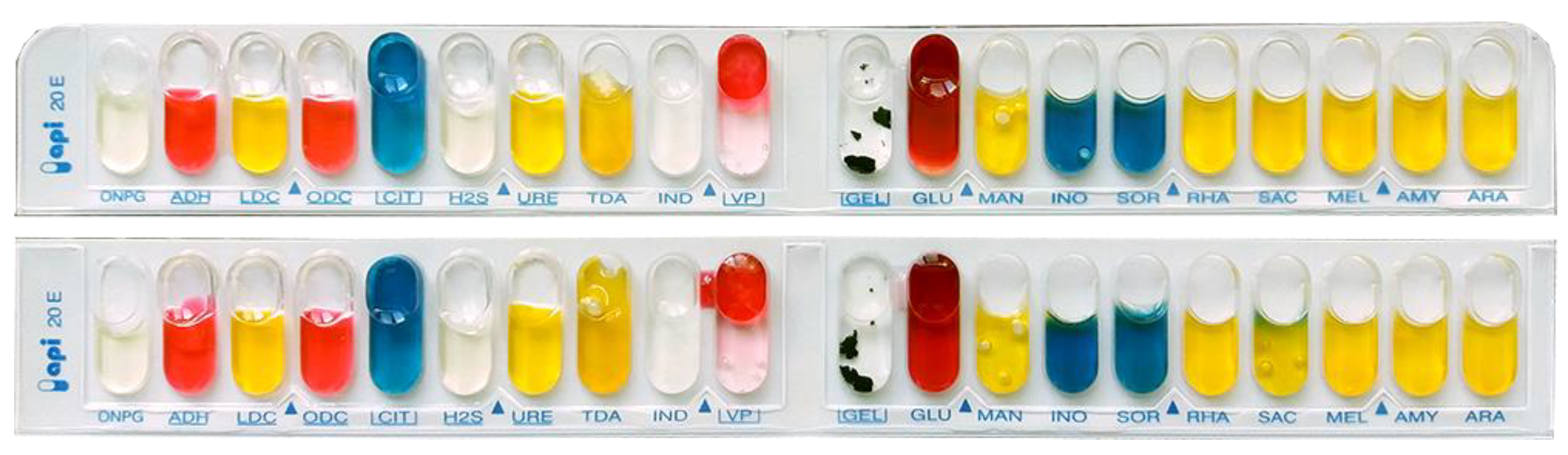
Anatomical–morphological and physiological–biochemical characteristics of the selected strains (oxidative/fermentation glucose test, Kovac’s oxidase test, dilution of gelatin, acid formation from carbohydrates, growth in 5% NaCl, formation of reducing sugars from sucrose, etc.) were determined by the standard protocols and according to the methods [
36] using an API 20E test system and a NEFERMtest24 MikroLaTEST
® test system, Erba Lachema, Brno, Czech Republic.
The species names of bacterial and fungal isolates are given according to identifiers [
37] and other specialized literature [
36]. The Latin names of the species of higher plants are given according to The Plant List [
38], and microbiota are named according to the List of Prokaryotic Names with Standing in Nomenclature [
33].
2.4. Phytopathogenicity Test
The study of the pathogenicity of isolated bacteria was carried out adhering to the provisions of the Henle–Koch triad. Each isolated species of phytopathogenic bacteria was subject to testing (in particular, Erwinia carotovora subsp. carotovora, Lelliottia nimipressuralis, Lonsdalea quercina, Erwinia rhapontici and Pseudomonas fluorescens). Additionally, collection strains of similar bacterial species from the Department of Phytopathogenic Bacteria of the Institute of Microbiology and Virology were used for control (testing pathogenicity). The experiment was carried out in 3-fold repetition and depending on the characteristics of the pathogenesis of the studied type of bacteriosis. The inoculation of a bacterial suspension of a daily culture of microorganisms (8.6–9.9 million colony-forming units/mL) was carried out in cuttings, acorn cotyledons, test plants Quercus robur L., and indicator plants (Phaseolus vulgaris L., Nicotiana tabacum L., Kalanchoe laciniata L., Sorghum sudanense (Piper.) Stapf., Raphanus sativus L. (various varieties) and plants of the genus Triticum L., etc.) in vivo and in vitro conditions. Artificial inoculation was carried out in two approaches during the growing season of 2020–2021: infection 9 October 2020, registration 11 April 2021; II infection 2 May 2021, registration 14 September 2021, taking into account weather conditions and circadian rhythms. Positive (collection strains) and negative (sterile tap water) controls were carried out. In total, 90 manipulations of artificial infection were performed.
2.5. Analytical Profile Index (API) Test
Due to further identification of the studied isolates, in particular, using the API 20E diagnostic test system, the following physiological and biochemical features were established and tested: the ability to synthesize β-galactosidase, arginine dihydrolase, lysine decarboxylase, urease, the ability to form hydrogen sulfide, indole, acetone, ferment glucose, mannitol, sorbitol, inositol, rhamnose, sucrose, melibiose, amygdalin and arabinose.
3. Results and Discussion
3.1. Soft Bacterial Rot of Acorns of Common Oak
Key factors in the pathogenesis of bacterial diseases are controlled by the specialized biochemical and genetic properties of phytopathogenic bacteria and the relevant mechanisms produced during the interaction of the host plant and phytopathogenic bacteria. This is an evolutionary adaptation in phylogenesis and an ontogenetic adjustment of the pathogens’ morphological, biochemical, and genetic properties [
3].
“Soft” bacterial rot of acorns of common oak (causative agent
Pectobacterium carotovorum subsp.
carotovorum [
39,
40]; other scientific name:
Erwinia carotovora subsp.
carotovora [
39,
41]) is shown in
Figure 1. A characteristic symptom of the disease is a change in the typical color of the acorn cotyledons to brown, brown-gray, or dirty brown-gray (the lesion can be continuous, or these spots are located specifically on the cotyledon). At room temperature, the cotyledons are hard, but in a humid chamber, they completely lose their hardness after 5–7 days with the formation of soft brown rot. At the same time, exudate is quite intensively released. Cotyledons affected by the “soft” rot pathogen can lead to massive damage to acorns by contact if the storage conditions are violated.
The cells of P. carotovorum subsp. carotovorum are Gram-negative rods 0.5–0.8 × 1.4–2.1 µm in size, mobile, with a peritrichous arrangement of flagella, the length of which is 3–4 and sometimes 5 times the length of the bacterial cell. On the potato agar, the colonies were round, 1–2.5 mm in size, and semi-translucent. With light passing through them, they were “amber”, with weakly visible radial rays. The surface of the colonies was gradually raised, smooth, and shiny; a groove ran along the periphery. The edge was wavy and serrated. On the slant potato agar, growth was plentiful. However, the culture did not spread over the surface but remained within the culture. On the meat-peptone agar, the colonies were round with dense edges, shiny, whitish-gray, and translucent. Bacteria grew well in the meat-peptone broth. On the King A media, the colonies were a dirty milky white, and on the King B media, they were milky white and shiny, and their growth was plentiful and intensive.
All strains were quite active on media-containing carbohydrates. The isolates of glucose, sucrose, lactose, mannitol, sorbitol, ribose, arabinose, mannose, and trehalose were used in a day with the release of acid and gas. On media with cellobiose, inositol, xylose, rhamnose, and glycerol, the bacteria formed only acid. Aesculin, raffinose, salicin, and melibiose were used to form acid, or acid and gas. At the same time, all strains absorbed glycerol, inositol (on the 4th day of culturing), rhamnose, xylose, and cellobiose (on the 7th–8th day) much more slowly. The strains did not absorb galactitol and maltose. On Hiss’ medium, on the second day, the isolates first formed acid and gas on all carbohydrates used, and on the 4th–7th day, the medium became alkaline with galactitol, lactose, mannose, glucose, and rhamnose. On Omelyansky’s medium (with a nitrogen source), the isolates alkalized asparagine, glutamic, and aspartic on the 4th day, and γ-aminobutyric acids on the 8th day. The bacteria also produced ketoglutaric, formic, and citric acids on Omelyansky’s medium. Thus, the isolates used significantly more amino acids for nitrogen nutrition than that for carbon. The bacteria liquefied gelatin and reduced nitrates. Milk was quickly coagulated, but not peptonized. The bacteria emitted hydrogen sulfide and much less ammonia, but not indole. Litmus serum was first acidified and then alkalized. Starches were not hydrolyzed. The oxidase tests were negative. Amylase, urease, and lipase were not found, but pectin-degrading enzymes were. Based on these features,
P. carotovorum can be assigned to
P. carotovorum subsp.
carotovorum. Its properties do not differ from those described in
Bergey’s Manual of Determinative Bacteriology [
37] and, in particular, the study of Patyka et al. [
36].
All strains of P. carotovorum subsp. carotovorum (isolated from common oak) used inositol and did not digest maltose. Such a precise characterization of strains isolated from oak acorns according to these features can be explained by the specific conditions for the existence of bacteria, because if the ecological niche of P. carotovorum strains was reflected even in their antigenic composition, then such an effect on other properties of bacteria should also be expected.
In modern conditions, no great systematic importance is attached to the gas formation on a medium with sugars (as a rule, it is noted only on glucose). Gas formation for the isolates of P. carotovorum subsp. carotovorum depends on the strain. All strains isolated from oak produce gas.
3.2. Bacterial Dropsy (Wetwood) of the Common Oak
Among the main habitual manifestations of bacterial dropsy (wetwood) of common oak (causative agent
Lelliottia nimipressuralis [
42,
43]; other scientific name:
Enterobacter nimipressuralis [
42,
44]), it should be noted that the affected trees usually form small lesions and are characterized by excessive crown “openwork”, as well as defoliation and dieback of 1–2-year-old branches. On the trunks, cracking of the bark is noted with the outflow of foaming liquid and dark-colored mucus with a typical smell of butyric fermentation. In some areas, the cracks turn into inflamed necrotic wet wounds. A wet bast is visualized under the periderm layer, sometimes deep into the cambium. In the latter case, a canker forms on the trunk. There are also “water shoots” on the trunks, which is additional direct evidence of bacteriosis damage to oak trees (
Figure 2).
The cells of L. nimipressuralis are Gram-negative, elliptical rods 0.6–0.8 × 0.7–1.6 µm in size. The cells are motile, with peritrichous flagella. In smears from the broth and agar cultures, the cells were placed singly, in pairs, or less often, in short chains. Spores and capsules did not form. On the potato agar, the colonies were round, 4–5 mm in diameter, gray, gray-white, sometimes with a creamy tint, translucent, convex, and shiny. The edge of the colonies was slightly wavy and less often even. A corrugated strip ran along the periphery, and radial rays and translucent circles were visible in the light.
On the meat-peptone agar, the colonies were more minor, gray, translucent, shiny, smooth, slightly convex, and granular. The edge was ridge-like and weakly radially outlined. Bacteria grew well in the meat-peptone broth, forming even opacity, pellicle, and precipitate. On the King A and B media, the colonies were dirty milky white or milky white, and growth is plentiful. A common feature of the isolated strains was the absence of growth on a nutrient medium with galactitol. The bacteria grew well on the Ushinsky, Eijkman, Liske, and Fermi media with asparagine (they formed strongly pronounced or moderate turbidity, pellicle, and sediment). All strains formed acid and gas on mineral media with arabinose, glucose, xylose, galactose, fructose, sucrose, maltose, lactose, mannose, raffinose, mannitol, sorbitol, and salicin. Some amino acids and amides were used as a carbon source. No changes were noted in the medium with cystine, cysteine, leucine, tryptophan, and tyrosine. All strains did not use tartaric and oxalic acids. The mineral media with sodium salts of ketoglutaric, citric, formic, acetic, malic, succinic, fumaric, and lactic acids were intensively alkalized during the day.
The bacteria quickly coagulated milk, the gelatin was not diluted, and the formation of oxidase and protopectinase was not noted. The bacteria formed catalase and urease and reduce nitrates. The habitats of bacteria affect some of their properties [
42,
45,
46]. According to their morphological, cultural, and biochemical properties (
Figure 3), these bacteria were assigned to
L. nimipressuralis, the causative agent of bacterial dropsy (wetwood) of coniferous and deciduous forest woody plants. This pathogen belongs to polybiotrophs.
3.3. White Bacteriosis or Trunk Form of “Drip Disease” of Common Oak
White bacteriosis is the trunk form of “drip disease” of common oak (causative agent—
Lonsdalea quercina [
47] (other scientific names:
Erwinia quercina [
48,
49];
Erwinia quercina sp.
nova [
48];
Erwinia amylovora var.
quercina ([
45,
48];
Brenneria quercina ([
40,
48]).
At the beginning of summer, and in some years, in the middle of summer, intense exudate release is observed in the cracks of the bark on the trunks of the affected oak. It is white, frothy, and may become slightly pink over time but does not darken even at the end of the active period of the disease. Even a dried pellicle of exudate remains white. Infected wood, bark, and bast are waterlogged. If the bark is removed, the bast breaks up into individual fibers and separates along the fibers, between which there is an exudate, easily separated from the bark. Between the bark and wood, a large amount of exudate accumulates in the form of a slimy mass, which usually accumulates between the fibers of the bast. The exudate is released very strongly with many air bubbles (gas) and flows down the trunk to the ground (
Figure 4).
During the period of intensive development of the disease, about 70–100 mL of exudate flows from the site of the lesion within an hour. The bacteria form a gas, resulting in an intensive exfoliation of the bark from the wood with the formation of large narrow ellipsoidal necrotic formations 15–20 × 35–70 cm in size, and the bark cracks in places of its most negligible thickness. The released exudate has a characteristic fermentation odor, the so-called “sour” odor, which, with the intensive development of the disease, can be sensed by olfactory organs from a distance of up to 15–20 m. The exudate attracts many species of insects who use it for food. It is possible that they can be carriers of the disease (ceteris paribus). Sometimes, up to five lesions are formed on one tree, but more often one to three. It should be noted that oaks with primary lesions visually have no signs of weakening and belong to category I of the condition. The research in subsequent years has shown a gradual weakening of oak, which experience dieback six years after the detection of the primary infection. Category I of the disease was found on 80–100-year-old oaks in the Kyiv region (Boyarska Forest Research Station). Later, single manifestations of the disease (massive oak damage has not been found to date) were found in the oak forests of Ukraine, as well as forest belts and oak stands of urbanized environments, in particular, Kyiv. The causative agent was isolated from exudate, affected wood, and bast and identified as
Erwinia quercina. This bacterium was first isolated and described by Hildebrand and Schroth [
48] as the causative agent of acorns’ “drop disease” [
2]. Because of the symptoms of the disease, the most prevalent of which is the release of white foamy exudate on the trunks, we propose calling the disease white bacteriosis of common oak.
In the course of the observations of acorns during the growing season, a “drop disease” in the classical form was not detected, which can be explained by the growing conditions, some individual characteristics, and possibly by the varieties of common oak. In the experiment, the bacterium caused spotting on the leaves and dieback of the branches. The spots were light brown, the transition to healthy tissue was gradual, and brown-affected vessels were visible on the longitudinal section of the inoculated branches without a clear boundary.
The cells of L. quercina are Gram-negative, small, straight, non-spore-bearing rods 0.6–1.1 × 1.0–2.5 µm in size, (0.5–1.5 × 1.0–3.0 µm) with peritrichous placed flagella. On the potato agar, the colonies were about 3 mm in diameter, white, with a smooth edge and a cone-shaped raised, smooth, shiny surface. The colonies on the meat-peptone agar were small, whitish, and shiny, with a smooth, convex, and almost opaque edge. In the meat-peptone broth, they formed uniform turbidity and a slight precipitate. The bacteria grew well on the rare media of Eijkman, Ushinsky, and Fermi. No growth was observed on Koch’s medium. On the King A and B media, growth was plentiful. Pigmentation was not detected on all media used. The optimum growth temperature was 27–32 °C. L. quercina is an optional aerobe.
Bacteria have a limited set of enzymes, which is especially clearly observed in the study of carbon nutrition. On the mineral medium of Omelyansky, on the second day, the isolates fermented glucose, sucrose, glycerol, salicin, ribose, mannose, esculin, and mannitol with the formation of acid without gas, and slowly fermented (on days 4–5) fructose and galactose. They did not grow on rhamnose, lactose, xylose, maltose, raffinose, inositol, galactitol, melibiose, or cellobiose. Variable properties were obtained on arabinose and sorbitol. On the Omelyansky medium with glucose (without nitrogen), the bacteria acidified cystine, cysteine, leucine, tyrosine, and α-aminobutyric acid on the fourth day. In contrast, aspargin, arginine, glutamine, glutamic, and aspartic acids became alkaline. The isolates also alkalized the mineral medium of Omelyansky with ketoglutaric, formic, lactic, and malonic acids. Growth in milk was not observed.
The bacteria did not liquefy gelatin or alkalinize litmus serum with subsequent reduction. Nitrates were not reduced, and indole, hydrogen sulfide, and ammonia were not formed. According to the biochemical properties, L. quercina should be attributed to the Amylovora group. However, the absence of pectinases tested on a medium with pectate should not be considered absolute. Since bacteria are capable of causing the decay of acorns and some succulent parts of vegetable crops, that is, they contain pectinases, they should be attributed to the Carotovora group. So far, the general opinion is that this bacterium should be eliminated as an independent species.
3.4. Dry Rot of Branches and Trunks of Common Oak
The disease of dry rot of branches and trunks of common oak (the causative agent—
Erwinia rhapontici [
50,
51] (other scientific name
Pectobacterium rhapontici [
49,
50,
52]) is ubiquitous, especially in pre-matured, mature, and overmature trees. In places experiencing a mass dieback of trees, dry rot was found on a third of plants aged 20–30 years. Bacteria infect thin and thick branches, and sometimes trunks with a smooth (primary) bark. The causative agent of the disease forms local necrosis; however, the branch (trunk) is ringed due to their large number, which leads to their rapid dieback. Affected wood is separated, often connected to the bark with a narrow strip through several summer tree rings. The lesion size is small, penetrating to a depth of up to half of the branch, and not limited to annual tree rings. Over time, the affected wood changes color and becomes lighter (
Figure 5).
The cells of E. rhapontici are typical of the genus Erwinia. They are small, non-spore-bearing, Gram-negative, motile, thin, or elliptical, polymorphic rods with peritrichous flagella. In smears from meat-peptone broth and potato agar, the cells were mainly single, and less often in pairs or short chains. They grew in different nutrient media in aerobic and anaerobic conditions. On days 5–10, the bacteria formed a pink pigment diffusing into the agar. The same dye was formed in Omelyansky’s liquid mineral medium with galactitol and sorbitol. The pigment’s formation and intensity were not related to the virulence of the strains.
On the potato agar, the colonies were round, 3–4 mm in diameter, gray, with a blue or yellowish-cream tint, translucent, and sometimes with darker inclusions in the form of gas bubbles. The surface was shiny and slightly radially dissected. The center was gradually raised, and the edge was corrugated. On the meat-peptone agar, the colonies had a smooth edge and a convex, shiny surface. In the meat-peptone broth, the growth is uniform throughout the entire thickness of the broth, with the formation of a film and loss of sediment. Cultures grew well on the Ushinsky and Fermi media. No bacterial growth was found on the Liske medium.
The colonies were a dirty milky white on the King A medium and bluish gray on the King B medium. Starches were not hydrolyzed, and the ratio to nitrates was not constant and depended on the strain, but most isolates did not restore them. Indole and hydrogen sulfide did not form, and not all strains weakly emitted ammonia.
E. rhapontici absorbed large amounts of carbohydrates, alcohols, organic acids, and amino acids. The bacteria formed acid without gas on arabinose, rhamnose, xylose, glucose, galactose, maltose, fructose, sucrose, raffinose, glycerol, inositol, sorbitol, mannitol, galactitol, and salicin. On Omelyansky’s medium with glucose, the bacteria were first acidified, and after 4–6 days, asparagine, glutamic, and aspartic acids were alkalized. Cystine, cysteine, leucine, arginine, glutamine, tryptophan, tyrosine, and α-aminobutyric acid bacteria were acidified in a day without subsequent alkalization. The isolates alkalized the medium with ketoglutaric, formic, lactic, and malic acids. The bacteria did not use tartaric acid. The cultures did not liquefy gelatin, their activity on milk was very weak (most strains left it unchanged), and they were oxidase-negative. They did not form pectin-degrading enzymes on pieces of potatoes, carrots, beets, or pumpkins. The bacteria first acidified the litmus serum and then alkalinized with subsequent reduction.
E. rhapontici reducing nitrates and forming ammonia in meat-peptone broth is a strain trait, and according to Bergey’s Manual [
37], it is a species trait. In addition, the ability of the bacteria to use lactose was noted by the mentioned authors. The strains isolated from common oak and those isolated from other woody plants, mainly beech, did not possess these properties. However, the storage of strains in the collection for several years resulted in the ability of some strains to use lactose [
46], which may be associated with the plasmid.
3.5. Canker Disease of Common Oak
Canker disease of common oak (causative agent
Pseudomonas fluorescens [
49] and
Pseudomonas sp.) is primarily distributed in the oak forests of Ukraine. Bacteria infect mainly one- to two-year-old branches and trunks with young (smooth) bark. Wet, odorless spots form at the branches base, dormancy buds, and mechanical damage. Over time, the bark becomes darker with a characteristic purple hue. In humid weather, a colorless exudate is observed. In places of damage, the bark of branches and trunks dries up over time. Due to the dying off of the tissue, it sinks, and a narrow, elongated crack forms in it, from which dieback wood can be observed. The affected bast is visible, brown, brownish-brown, and almost black in the lower area of the diseased part. The disease spreads more intensively along the trunk or branch. With the intensive development of the disease, several zones of damage are formed on the affected branches or trunks, leading to their rapid dieback. Adjacent infected areas are usually interconnected by brown, dark brown, and sometimes almost black vessels. The leaves on the affected branches turn brown, curl, and do not fall off for a long time.
The bacteria of the genus Pseudomonas were isolated from canker disease of branches and young trunks of common oak during different periods of the year. The most highly active strains were isolated from mid-May to early June. The cells of P. fluorescens are Gram-negative, 0.4–0.8 × 1.2–3.0 µm in size, non-spore-bearing, of unequal thickness, and motile (due to one or more polar flagella) rods. The ends of the sticks are smoothly rounded. All strains are physiologically very close. On the potato agar, the colonies were 5–8 mm in diameter, gray-blue, sometimes with a creamy tint, and wavy along the periphery, with jagged edges and a hilly surface. The center was raised, white-gray, smooth, and shiny. In the meat-peptone broth, turbidity began at the top. Subsequently, a film, a slight residue, and a yellow-green, fluorescent pigment were formed throughout the thickness. The bacteria grew well on the Fermi and Eijkman media (with film formation). Slower growth was observed on the Kohn and Lisk media. On the King A and B media, the bacteria formed a yellow-green pigment diffusing into the agar. P. fluorescens is a distinct aerobe (it does not ferment glucose under mineral oil). The bacteria did not hydrolyze starch and did not form hydrogen sulfide or indole. They produced very little ammonia. Instead, they had oxidase, catalase, and urease activity. They did not form levansucrase or lecithinase. Milk was peptonized but not coagulated. The gelatin was liquefied. The litmus serum was alkalized, followed by reduction; nitrates were also reduced. The strains assimilated many carbohydrates, alcohols, organic and amino acids well. On the mineral medium of Omelyansky, arabinose, xylose, glucose, galactose, mannose, fructose, inositol, sorbitol, and mannitol were used to form acid without gas. None of the strains digested lactose, maltose, sucrose, galactitol, salicin, or esculin. As a source of carbohydrates, arginine, aspargin, alanine, aspartic, glutamic, and γ-aminobutyric acids, ketoglutaric, formic, malonic, and malic acids were used in the alkalization of the medium. The bacteria utilized leucine and cystine with the acidification of the medium but did not use tartaric acid.
The non-fermenting bacteria of the genus Pseudomonas, in contrast to P. fluorescens, on dense nutrient media were characterized by slow growth, smaller size, and creamy tint. On the meat-peptone agar, the colonies were small, 1–2 mm in diameter, spherical, with a smooth edge, gray-white, and more transparent. The bacteria were inert to all tested sources of carbohydrates on Omelyansky mineral medium. Growth in the meat-peptone broth varied in intensity, depending on the strain. First, a medium haze was formed, and then a film and a weak precipitate formed. The bacteria used some amino acids and salts of organic acids as a source of carbohydrates and were oxidase-negative. All strains had catalase activity, but pectin-degrading enzymes, amylase, and urease were not found. Milk was only peptonized. All strains alkalized litmus serum in a day. The optimum growth temperature was 25–30 °C, and they did not grow at 4 °C.
The non-fermenting group of the genus
Pseudomonas isolated from the cankerous lesions of common oak does not differ from
Pseudomonas syringae subsp.
Savastanoi [
53].
Comparing the morphological, cultural, and biochemical properties of the phytopathogenic bacteria species indicated as pathogens of common oak bacterioses demonstrated the ratio of the leading indicators of bacteria by which they can be classified (
Table 2).
A specific type of pathology (symptom, sign) corresponds to a particular biological organization of the pathogen that can cause this pathology. In infection, the role of pathogenesis (at the physiological and biochemical levels) is played by enzymes, toxins, and hormones produced by phytopathogenic bacteria. At the molecular genetic level, specialization is ensured by genotypic variability and gene mutations, including the transfer of genes of the host plant and the species of bacteria, fungi, and insects occupying the same ecological niche by a plasmid [
3]. In the pathogenesis of the studied bacterial diseases, it is possible to identify the key symptoms caused by the properties of pathogenic bacteria (
Table 3).
The synthesis of pectin-degrading enzymes (pectinase, protopectinase, and pectate lyase) by phytopathogenic bacteria leads to liquefaction of the median lamina and the destruction of the intercellular septa of plant cells, resulting in tissue maceration (soft rot: seeds, fruits, cones, acorns) and the outflow of cellular contents with exudate, mucus, and liquid leaks. It is a result of this biological characteristic of P. carotovorum subsp. carotovorum, and typical symptoms of soft bacterial rot of common oak acorns are formed.
Fermentation (with the release of acid and gas) in many carbohydrate media provides the ability of L. nimipressuralis to cause cracks in the affected branches and trunks with the rupture of xylem and phloem and to provoke the release of liquid and mucus, which, of course, characterizes the manifestation of bacterial dropsy (wetwood). Moisture saturation of the wood core and sapwood occurs because the causative agent of bacterial dropsy (wetwood) is a facultative anaerobe that survives in the inner layers of the xylem. The formation of wounds on branches and trunks and the necrotization and maceration of the bark, cambium, and wood are explained by the fact that L. nimipressuralis does not form pectolytic enzymes.
Symptoms of drip disease of oak acorns are provided by a biological characteristic of L. quercina such as the release of acid in many carbohydrate environments. Local necrosis on the branches and trunks of oak due to the defeat of E. rhapontici occurs because the bacterium does not form pectolytic enzymes and does not cause cell maceration. The canker disease of the common oak, caused by phytopathogenic bacteria P. fluorescens and Pseudomonas sp., is characterized by the dying off of the affected tissues and the formation of cracks in the affected organs due to the release of acid by the causative agent in some carbohydrate media.
So, the key factors of the harmfulness of phytopathogenic bacteria are the aggressiveness and pathogenicity of the pathogen, as well as the congeniality of the pathogen and the host plant. Aggressiveness, pathogenicity, and congeniality are determined by many morphological, physiological, biochemical, and molecular genetic properties of bacteria, their hosts, woody plants, and various environmental factors.
3.6. Checking the Pathogenic Properties of Bacteria
The first results after artificial infection of oak plants in the field were observed after 4 months and were characterized by a slow development of pathology. All manipulations using collection strains gave a positive result and some characterized by a high lesion score (7) and a strong (51%–75%) infectious class. All manipulations using sterile tap water had a negative result. The intensity of damage to test plants by isolated bacteria after artificial inoculation was different and is presented in
Table 4.
Thus, adhering to the Henle–Koch triad, based on the reisolated species of phytopathogenic bacteria, conclusions were drawn about the involvement or non-involvement of a particular bacterium in bacteriosis.