The Soil Environment of Abandoned Charcoal Kiln Platforms in a Low-Altitude Central European Forest
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Field Survey
2.3. Laboratory Analysis
2.4. Data Processing
3. Results
3.1. Soil Stratification
3.2. Soil Chemical Properties
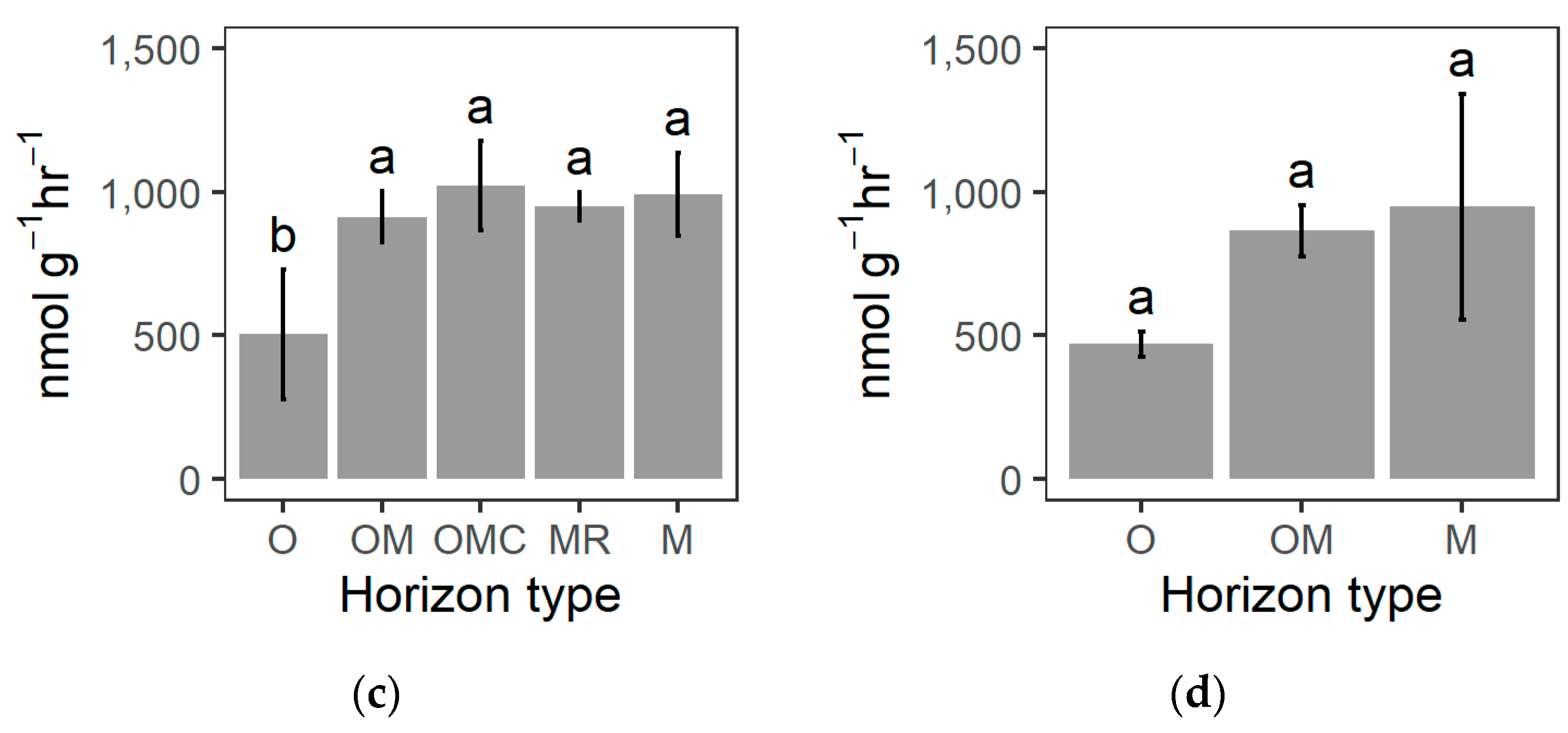
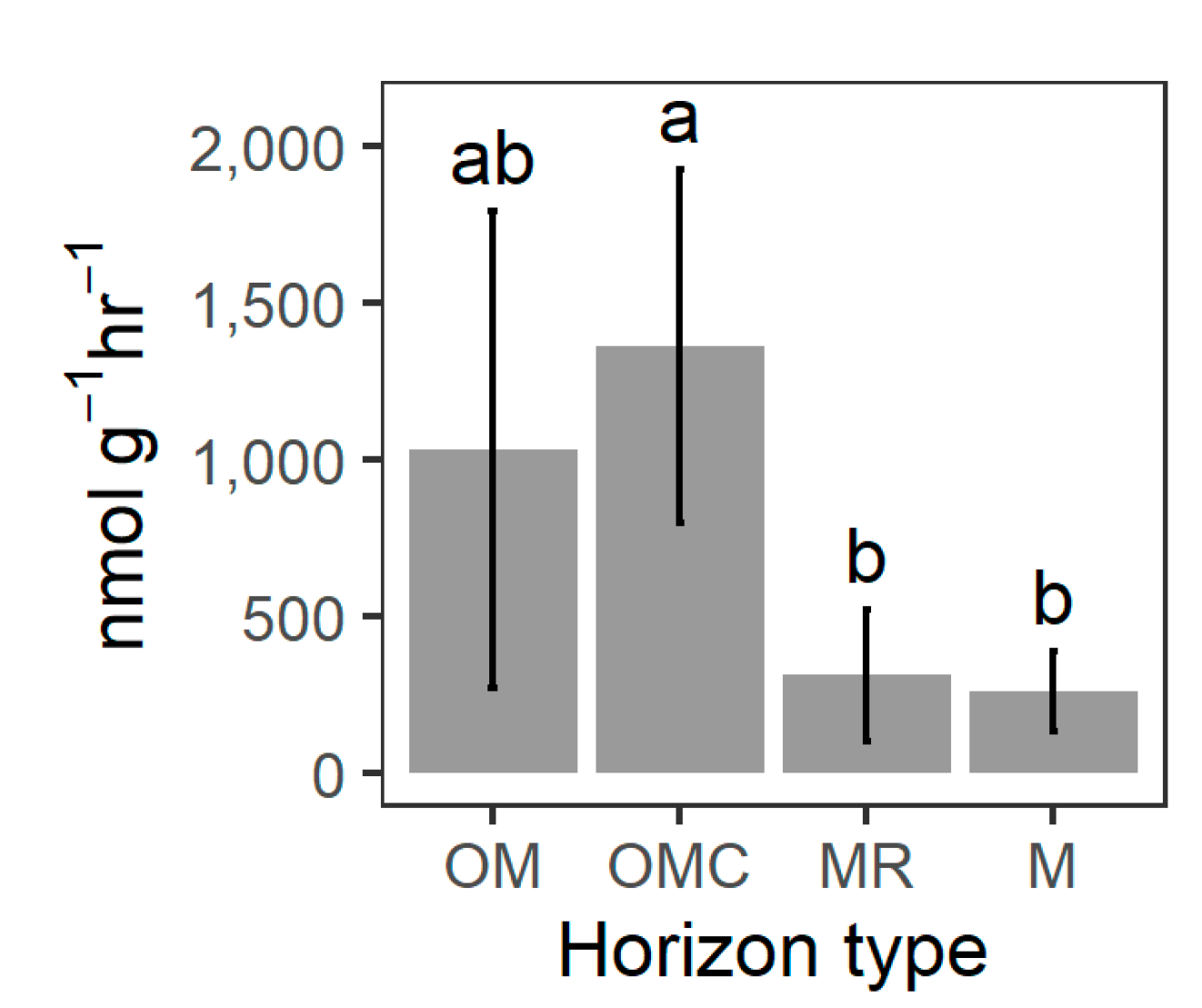
3.3. Soil Biochemical Properties
3.4. Utilizable Water Capacity and Nutrient Sums
3.5. Multivariate Interrelationships
4. Discussion
4.1. Topsoil Formation, Stratigraphy, and Base Characteristics
4.2. Rooting and Nutrient Content in the Charred Layers
4.3. Charcoal-Rich Layer Biochemistry
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raab, A.; Bonhage, A.; Schneider, A.; Raab, T.; Rösler, H.; Heußner, K.U.; Hirsch, F. Spatial Distribution of Relict Charcoal Hearths in the Former Royal Forest District Tauer (SE Brandenburg, Germany). Quat. Int. 2019, 511, 153–165. [Google Scholar] [CrossRef]
- Jabiol, B.; Jévy, G.; Bonneau, M.; Brêthes, A. Comprendre Les Sols Pour Mieux Gérer Les Forêts; AgroParisTech ENGREF: Nancy Cedex, 2009; ISBN 978-2-85710-081-2. [Google Scholar]
- Binkley, D. Forest Nutrition Management; John Wiley & Sons: Hoboken, NJ, USA, 1986; ISBN 0-471-81883-6. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Gimmi, U.; Bürgi, M.; Stuber, M. Reconstructing Anthropogenic Disturbance Regimes in Forest Ecosystems: A Case Study from the Swiss Rhone Valley. Ecosystems 2008, 11, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Agbeshie, A.A.; Abugre, S.; Atta-Darkwa, T.; Awuah, R. A Review of the Effects of Forest Fire on Soil Properties. J. For. Res. 2022, 33, 1419–1441. [Google Scholar] [CrossRef]
- Borchard, N.; Ladd, B.; Eschemann, S.; Hegenberg, D.; Möseler, B.M.; Amelung, W. Black Carbon and Soil Properties at Historical Charcoal Production Sites in Germany. Geoderma 2014, 232–234, 236–242. [Google Scholar] [CrossRef]
- Schmidt, M.; Mölder, A.; Schönfelder, E.; Engel, F.; Fortmann-Valtink, W. Charcoal Kiln Sites, Associated Landscape Attributes and Historic Forest Conditions: DTM-Based Investigations in Hesse (Germany). Ecosyst 2016, 3, 8. [Google Scholar] [CrossRef] [Green Version]
- Young, M.J.; Johnson, J.E.; Abrams, M.D. Vegetative and Edaphic Characteristics on Relic Charcoal Hearths in the Appalachian Mountains. Vegetatio 1996, 125, 43–50. [Google Scholar] [CrossRef]
- Mikan, C.J.; Abrams, M.D. Altered Forest Composition and Soil Properties of Historic Charcoal Hearths in Southeastern Pennsylvania. Can. J. For. Res. 1995, 25, 687–696. [Google Scholar] [CrossRef]
- Carrari, E.; Ampoorter, E.; Bottalico, F.; Chirici, G.; Coppi, A.; Travaglini, D.; Verheyen, K.; Selvi, F. The Old Charcoal Kiln Sites in Central Italian Forest Landscapes. Quat. Int. 2017, 458, 214–223. [Google Scholar] [CrossRef]
- Hardy, B.; Cornelis, J.T.; Houben, D.; Lambert, R.; Dufey, J.E. The Effect of Pre-Industrial Charcoal Kilns on Chemical Properties of Forest Soil of Wallonia, Belgium. Eur. J. Soil Sci. 2016, 67, 206–216. [Google Scholar] [CrossRef]
- Mastrolonardo, G.; Francioso, O.; Certini, G. Relic Charcoal Hearth Soils: A Neglected Carbon Reservoir. Case Study at Marsiliana Forest, Central Italy. Geoderma 2018, 315, 88–95. [Google Scholar] [CrossRef]
- Ponge, J.-F. Humus Forms in Terrestrial Ecosystems: A Framework to Biodiversity. Soil Biol. Biochem. 2003, 35, 935–945. [Google Scholar] [CrossRef] [Green Version]
- Tolpeshta, I.I.; Sokolova, T.A. Aluminum Compounds in Soil Solutions and Their Migration in Podzolic Soils on Two-Layered Deposits. Eurasian Soil Sci. 2009, 42, 24–35. [Google Scholar] [CrossRef]
- Mládková, L.; Borůvka, L.; Drábek, O. Soil Properties and Selected Aluminium Forms in Acid Forest Soils as Influenced by the Type of Stand Factors. Soil Sci. Plant Nutr. 2005, 51, 741–744. [Google Scholar] [CrossRef]
- Huntington, T.G. Available Water Capacity and Soil Organic Matter. In Encyclopedia of Soil Science; United States Geological Survey: Augusta, ME, USA, 2007; pp. 139–143. [Google Scholar]
- Pirastru, M.; Castellini, M.; Giadrossich, F.; Niedda, M. Comparing the Hydraulic Properties of Forested and Grassed Soils on an Experimental Hillslope in a Mediterranean Environment. Procedia Environ. Sci. 2013, 19, 341–350. [Google Scholar] [CrossRef] [Green Version]
- Ponge, J.-F.; Chevalier, R.; Loussot, P. Humus Index: An Integrated Tool for the Assessment of Forest Floor and Topsoil Properties. Soil Sci. Soc. Am. J. 2002, 66, 1996. [Google Scholar] [CrossRef] [Green Version]
- Lal, R. Soil Carbon Sequestration Impacts on Global Climate Change and Food Security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef] [Green Version]
- Post, W.M.; Emanuel, W.R.; Zinke, P.J.; Stangenberger, A.G. Soil Carbon Pools and World Life Zones. Nature 1982, 298, 156–159. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of Soil Organic Matter as an Ecosystem Property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.W.I.; Noack, A.G.; Osmond, G. Black Carbon in Soils and Sediments: Analysis, Distribution, Implications, and Current Challenges. Glob. Biogeochem. Cycles 2000, 14, 777–793. [Google Scholar] [CrossRef]
- Andersson, M.; Kjøller, A.; Struwe, S. Microbial Enzyme Activities in Leaf Litter, Humus and Mineral Soil Layers of European Forests. Soil Biol. Biochem. 2004, 36, 1527–1537. [Google Scholar] [CrossRef]
- Gobat, J.-M.; Aragno, M.; Matthey, W. Le Sol Vivant, Bases de Pédologie—Biologie Des Sols, 3rd ed.; Presses Polytechniques et Universitaires Romandes: Lousanne, Switzerland, 2010; ISBN 978-2-88074-718-3. [Google Scholar]
- Lasota, J.; Błońska, E.; Babiak, T.; Piaszczyk, W.; Stępniewska, H.; Jankowiak, R.; Boroń, P.; Lenart-Boroń, A. Effect of Charcoal on the Properties, Enzyme Activities and Microbial Diversity of Temperate Pine Forest Soils. Forests 2021, 12, 1488. [Google Scholar] [CrossRef]
- Kutílek, M.; Nielsen, D.R. Soil Hydrology; Catena-Verlag: Cremlingen-Destedt, Germany, 1994; ISBN 9783923381265. [Google Scholar]
- Burt, R. (Ed.) Soil Survey Laboratory Methods Manual; USDA/NRCS: Lincoln, NE, USA; p. 2004.
- Adams, W.A.; Evans, G.M. Effects of Lime Applications to Parts of an Upland Catchment on Soil Properties and the Chemistry of Drainage Waters. J. Soil Sci. 1989, 40, 585–597. [Google Scholar] [CrossRef]
- Zbíral, J.; Tieffová, P.; Plhalová, Š.; Urbánková, E.; Niedobová, E.; Srnková, J.; Strížová, I. Analýza Půd II. Jednotné Pracovní Postupy (in Czech); ÚKZÚZ (Central Institute for Supervising and Testing in Agriculture): Brno, Czech Republic, 2011; ISBN 978-80-7401-040-8. [Google Scholar]
- Jönsson, U.; Rosengren, U.; Nihlgård, B.; Thelin, G. A Comparative Study of Two Methods for Determination of PH, Exchangeable Base Cations, and Aluminum. Commun. Soil Sci. Plant Anal. 2002, 33, 3809–3824. [Google Scholar] [CrossRef]
- Zbíral, J.; Honsa, I.; Malý, S.; Čižmár, D. Analýza Půd III. Jednotné Pracovní Postupy (in Czech); ÚKZÚZ Brno: Brno, Czech Republic, 2004; ISBN 80-86548-60-0. [Google Scholar]
- Bremner, J.M. Determination of Nitrogen in Soil by the Kjeldahl Method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Bárta, J.; Šlajsová, P.; Tahovská, K.; Picek, T.; Šantrůčková, H. Different Temperature Sensitivity and Kinetics of Soil Enzymes Indicate Seasonal Shifts in C, N and P Nutrient Stoichiometry in Acid Forest Soil. Biogeochemistry 2014, 117, 525–537. [Google Scholar] [CrossRef]
- Weil, R.R.; Islam, K.R.; Stine, M.A.; Gruver, J.B.; Samson-Liebig, S.E. Estimating Active Carbon for Soil Quality Assessment: A Simplified Method for Laboratory and Field Use. Am. J. Altern. Agric. 2003, 18, 3–17. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.; Blanchet, G.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Community Ecology Package “Vegan”, Version 2.6-2. 2022. Available online: Https://Github.Com/Vegandevs/Vegan (accessed on 11 October 2022).
- Wickham, H.; Lionel, H.; Pedersen, T.L.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D. Create Elegant Data Visualisations Using the Grammar of Graphics, Package Ggplot2 Version 3.3.0 for R Software for Statistical Computing. 2020. Available online: Https://Ggplot2.Tidyverse.Org/ (accessed on 21 September 2020).
- Panda, S.K.; Baluska, F.; Matsumoto, H. Aluminium Stress Signaling in Plants. Plant Signal. Behav. 2009, 4, 592–597. [Google Scholar] [CrossRef] [Green Version]
- Raczuk, J.; Deska, J. Buffer Properties of Forest Soils in Selected Protected Areas. Ecol. Chem. Eng. A 2012, 19, 231–237. [Google Scholar] [CrossRef]
- Hirsch, F.; Raab, T.; Ouimet, W.; Dethier, D.; Schneider, A.; Raab, A. Soils on Historic Charcoal Hearths: Terminology and Chemical Properties. Soil Sci. Soc. Am. J. 2017, 81, 1427–1435. [Google Scholar] [CrossRef] [Green Version]
- Oulehle, F.; Hofmeister, J.; Hruska, J. Modeling of the Long-Term Effect of Tree Species (Norway Spruce and European Beech) on Soil Acidification in the Ore Mountains. Ecol. Model. 2007, 204, 359–371. [Google Scholar] [CrossRef]
- Vesterdal, L.; Schmidt, I.; Callesen, I.; Nilsson, L.; Gundersen, P. Carbon and Nitrogen in Forest Floor and Mineral Soil under Six Common European Tree Species. For. Ecol. Manag. 2008, 255, 35–48. [Google Scholar] [CrossRef]
- Rybníček, M.; Kyncl, T.; Vavrčík, H.; Kolář, T. Dendrochronology Improves Understanding of the Charcoal Production History. Dendrochronologia 2022, 75, 125994. [Google Scholar] [CrossRef]
- Zapletal, M. Atmospheric Deposition of Nitrogen and Sulphur Compounds in the Czech Republic. TheScientificWorldJournal 2001, 1 (Suppl. 2), 294–303. [Google Scholar] [CrossRef] [Green Version]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. A Review of Biochar and Its Use and Function in Soil. Adv. Agron. 2010, 105, 47–82. [Google Scholar] [CrossRef]
- Vaccari, F.P.; Baronti, S.; Lugato, E.; Genesio, L.; Castaldi, S.; Fornasier, F.; Miglietta, F. Biochar as a Strategy to Sequester Carbon and Increase Yield in Durum Wheat. Eur. J. Agron. 2011, 34, 231–238. [Google Scholar] [CrossRef]
- Nelissen, V.; Rütting, T.; Huygens, D.; Staelens, J.; Ruysschaert, G.; Boeckx, P. Maize Biochars Accelerate Short-Term Soil Nitrogen Dynamics in a Loamy Sand Soil. Soil Biol. Biochem. 2012, 55, 20–27. [Google Scholar] [CrossRef]
- Cools, N.; Vesterdal, L.; De Vos, B.; Vanguelova, E.; Hansen, K. Tree Species Is the Major Factor Explaining C: N Ratios in European Forest Soils. For. Ecol. Manag. 2014, 311, 3–16. [Google Scholar] [CrossRef]
- Hu, Y.; Schmidhalter, U. Drought and Salinity: A Comparison of their Effect on Mineral Nutrition of Plants. J. Plant. Nutr. Soil Sci. 2005, 168, 541–549. [Google Scholar] [CrossRef]
- Silva, D.D.; Kane, M.E.; Beeson, R.C. Changes in Root and Shoot Growth and Biomass Partition Resulting from Different Irrigation Intervals for Ligustrum Japonicum Thunb. HortScience 2012, 47, 1634–1640. [Google Scholar] [CrossRef]
- Aroca, R.; Porcel, R.; Ruiz-Lozano, J.M. Regulation of Root Water Uptake under Abiotic Stress Conditions. J. Exp. Bot. 2012, 63, 43–57. [Google Scholar] [CrossRef]
- Karhu, K.; Mattila, T.; Bergström, I.; Regina, K. Biochar Addition to Agricultural Soil Increased CH4 Uptake and Water Holding Capacity—Results from a Short-Term Pilot Field Study. Agric. Ecosyst. Environ. 2011, 140, 309–313. [Google Scholar] [CrossRef]
- Yu, O.Y.; Raichle, B.; Sink, S. Impact of Biochar on the Water Holding Capacity of Loamy Sand Soil. Int. J. Energy Environ. Eng. 2013, 4, 44. [Google Scholar] [CrossRef] [Green Version]
- Ogundele, A.T.; Eludoyin, O.S.; Oladapo, O.S. Assessment of Impacts of Charcoal Production on Soil Properties in the Derived Savanna, Oyo State, Nigeria. J. Soil Sci. Environ. Manag. 2011, 2, 142–146. [Google Scholar]
- Sombroek, W.G.; Nachtergaele, F.O.; Hebel, A. Amounts, Dynamics and Sequestering of Carbon in Tropical and Subtropical Soils. Ambio 1993, 22, 417–426. [Google Scholar]
- Smith, N.J.H. Anthrosols and Human Carrying Capacity in Amazonia. Ann. Assoc. Am. Geogr. 1980, 70, 553–566. [Google Scholar] [CrossRef]
- Lasota, J.; Babiak, T.; Błońska, E. C:N:P Stoichiometry Associated with Biochar in Forest Soils at Historical Charcoal Production Sites in Poland. Geoderma Reg. 2022, 28, e00482. [Google Scholar] [CrossRef]
- Heitkötter, J.; Marschner, B. Interactive Effects of Biochar Ageing in Soils Related to Feedstock, Pyrolysis Temperature, and Historic Charcoal Production. Geoderma 2015, 245–246, 56–64. [Google Scholar] [CrossRef]
- Gómez-Luna, B.E.; Ruiz-Aguilar, G.M.d.l.L.; Vázquez-Marrufo, G.; Dendooven, L.; Olalde-Portugal, V. Enzyme Activities and Metabolic Profiles of Soil Microorganisms at KILN Sites in Quercus Spp. Temperate Forests of Central Mexico. Appl. Soil Ecol. 2012, 52, 48–55. [Google Scholar] [CrossRef]
- Eivazi, F.; Bayan, M.R. Effects of Long-Term Prescribed Burning on the Activity of Select Soil Enzymes in an Oak-Hickory Forest. Can. J. For. Res. 1996, 26, 1799–1804. [Google Scholar] [CrossRef]
- Li, Q.; Song, X.; Yrjälä, K.; Lv, J.; Li, Y.; Wu, J.; Qin, H. Biochar Mitigates the Effect of Nitrogen Deposition on Soil Bacterial Community Composition and Enzyme Activities in a Torreya Grandis Orchard. For. Ecol. Manag. 2020, 457, 117717. [Google Scholar] [CrossRef]
- Coomes, O.T.; Miltner, B.C. Indigenous Charcoal and Biochar Production: Potential for Soil Improvement under Shifting Cultivation Systems. Land Degrad. Dev. 2017, 28, 811–821. [Google Scholar] [CrossRef]
- Xu, G.; Sun, J.N.; Shao, H.B.; Chang, S.X. Biochar Had Effects on Phosphorus Sorption and Desorption in Three Soils with Differing Acidity. Ecol. Eng. 2014, 62, 54–60. [Google Scholar] [CrossRef]
- Pietikäinen, J.; Kiikkilä, O.; Fritze, H. Charcoal as a Habitat for Microbes and Its Effect on the Microbial Community of the Underlying Humus. Oikos 2000, 89, 231–242. [Google Scholar] [CrossRef]
- Warnock, D.D.; Lehmann, J.; Kuyper, T.W.; Rillig, M.C. Mycorrhizal Responses to Biochar in Soil—Concepts and Mechanisms. Plant Soil 2007, 300, 9–20. [Google Scholar] [CrossRef]
- Wallstedt, A.; Coughlan, A.; Munson, A.D.; Nilsson, M.C.; Margolis, H.A. Mechanisms of Interaction between Kalmia Angustifolia Cover and Picea Mariana Seedlings. Can. J. For. Res. 2002, 32, 2022–2031. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, L.; Zheng, B. Biochar to Improve Soil Fertility. A Review. Agron. Sustain. Dev. 2016, 36, 36. [Google Scholar] [CrossRef] [Green Version]
- Hardy, B.; Sleutel, S.; Dufey, J.E.; Cornelis, J.T. The Long-Term Effect of Biochar on Soil Microbial Abundance, Activity and Community Structure Is Overwritten by Land Management. Front. Environ. Sci. 2019, 7, 110. [Google Scholar] [CrossRef] [Green Version]
- Carter, Z.W.; Sullivan, B.W.; Qualls, R.G.; Blank, R.R.; Schmidt, C.A.; Verburg, P.S.J. Charcoal Increases Microbial Activity in Eastern Sierra Nevada Forest Soils. Forests 2018, 9, 93. [Google Scholar] [CrossRef] [Green Version]
- Bonhage, A.; Hirsch, F.; Schneider, A.; Raab, A.; Raab, T.; Donovan, S. Long Term Anthropogenic Enrichment of Soil Organic Matter Stocks in Forest Soils—Detecting a Legacy of Historical Charcoal Production. For. Ecol. Manag. 2020, 459, 117814. [Google Scholar] [CrossRef]
- DeLuca, T.H.; Nilsson, M.C.; Zackrisson, O. Nitrogen Mineralization and Phenol Accumulation along a Fire Chronosequence in Northern Sweden. Oecologia 2002, 133, 206–214. [Google Scholar] [CrossRef]
- Berglund, L.M.; DeLuca, T.H.; Zackrisson, O. Activated Carbon Amendments to Soil Alters Nitrification Rates in Scots Pine Forests. Soil Biol. Biochem. 2004, 36, 2067–2073. [Google Scholar] [CrossRef]
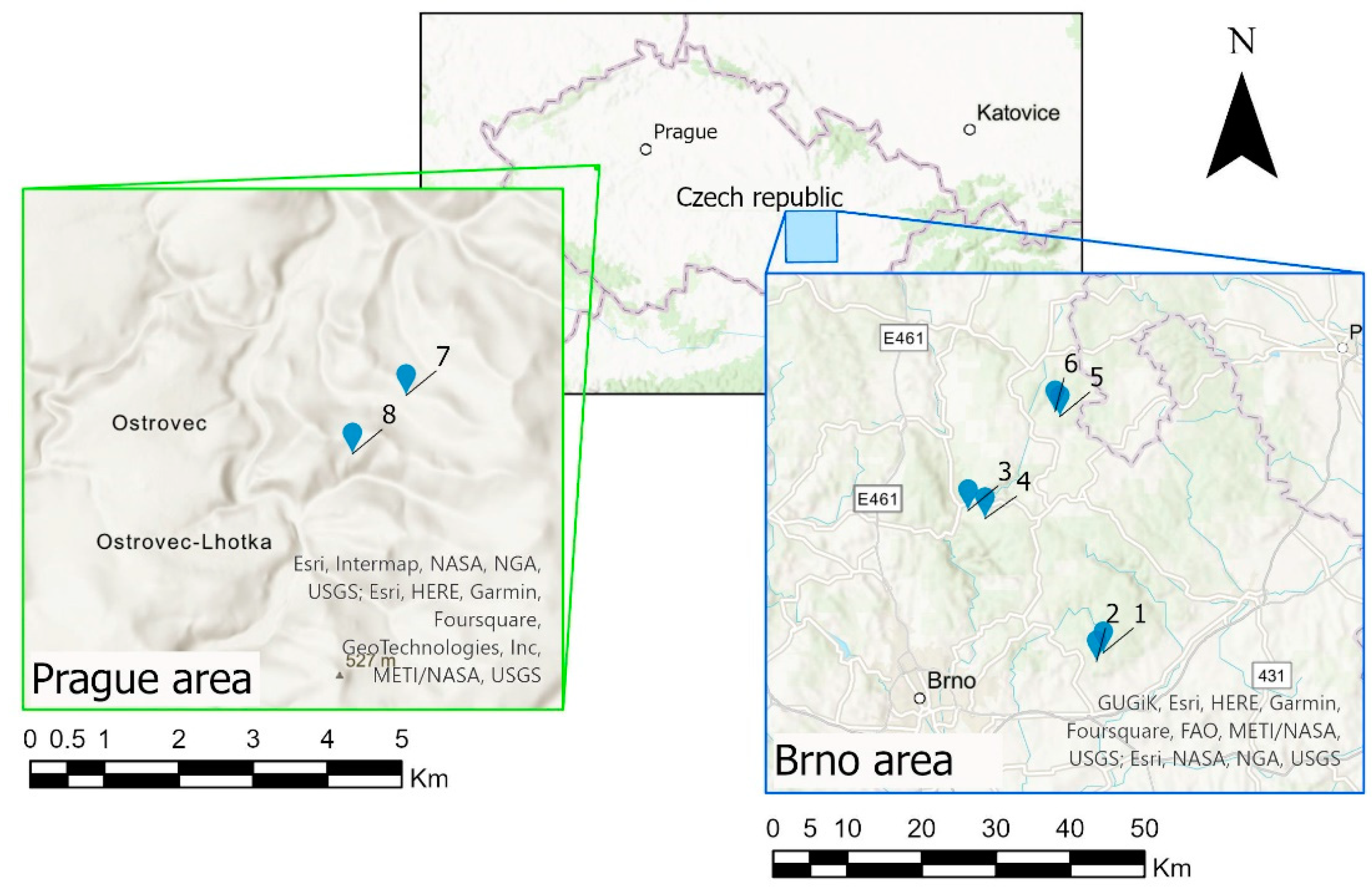
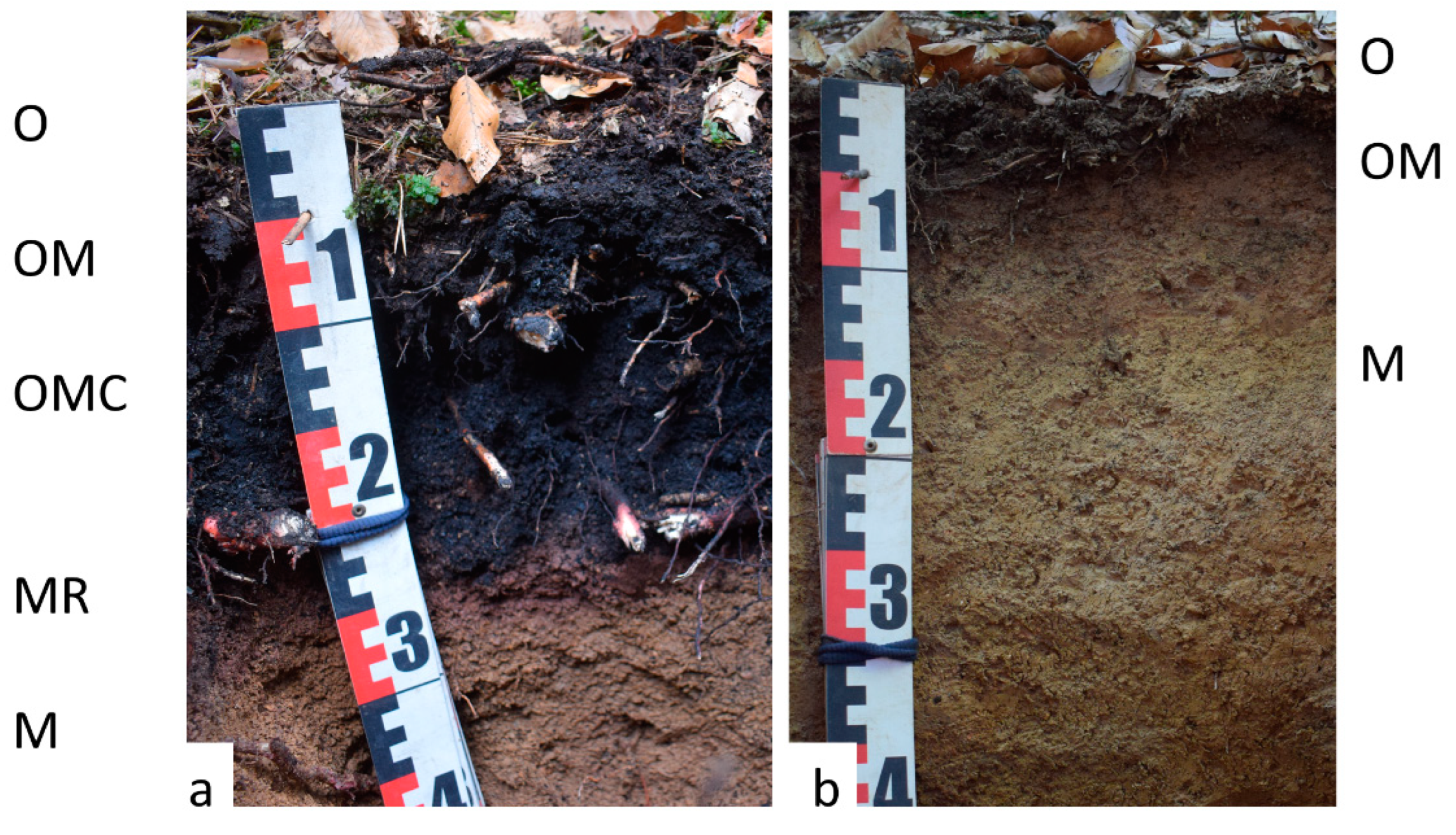
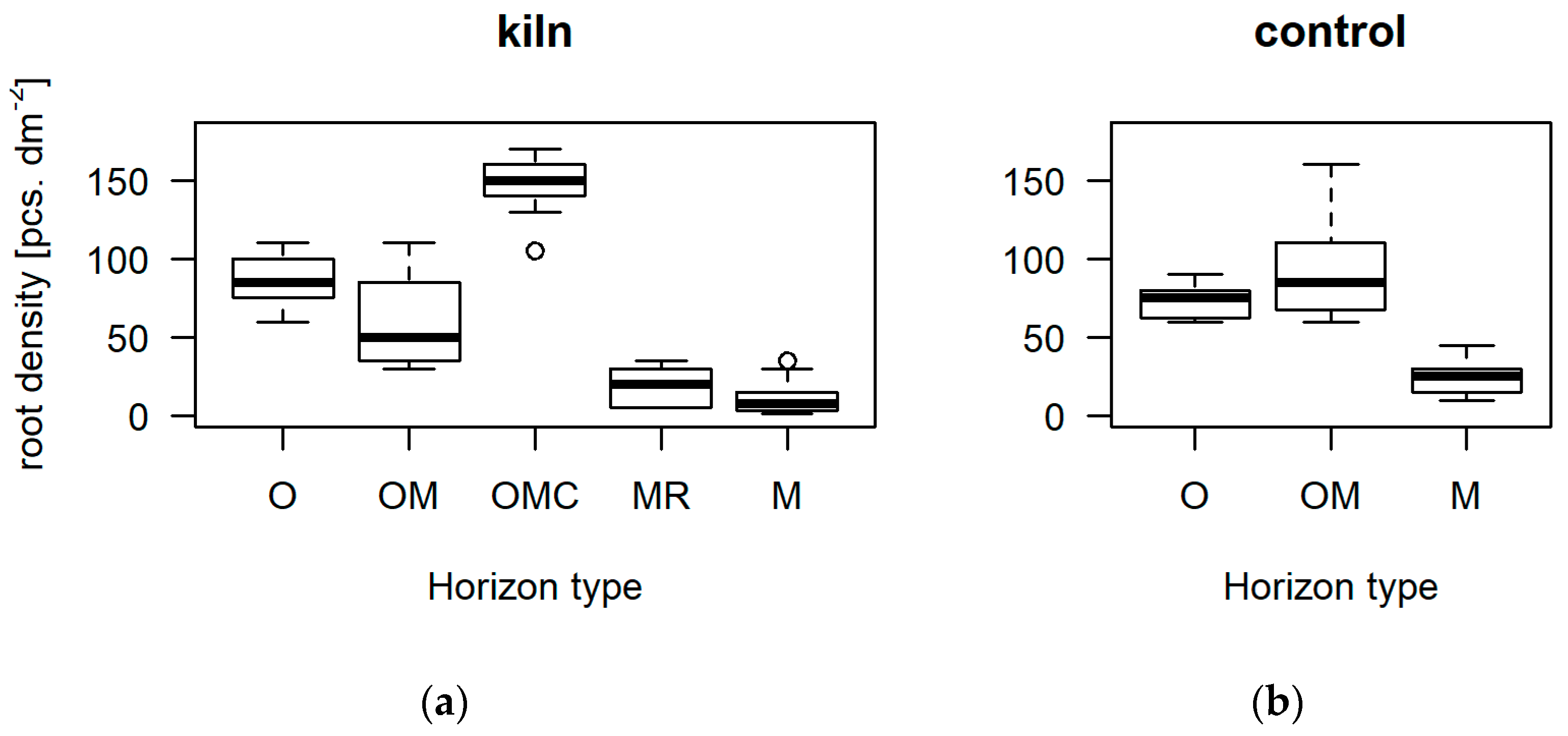


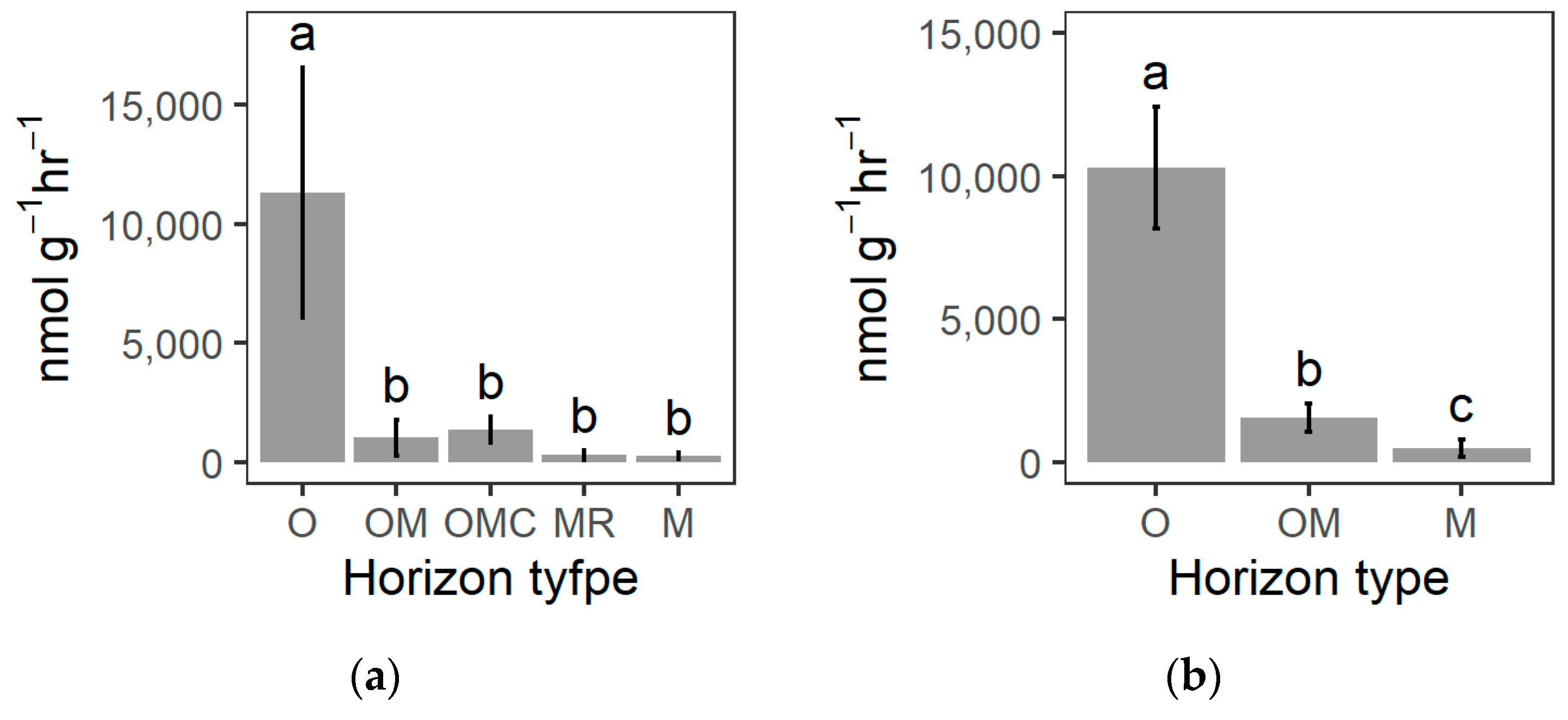

| Kiln Site | Study Area | GPS Coordinates | Altitude (m a.s.l.) | Size - Fall Line (m) | Size-Contour Line (m) | Slope (°) | Current Forest Type |
|---|---|---|---|---|---|---|---|
| 1 | Brno | N 49°14′0.67″ E 16°49′33.13″ | 446 | 4 | 6 | 8.8 | mixed |
| 2 | Brno | N 49°13′34.81″ E 16°49′3.73″ | 390 | 6.5 | 8.5 | 19.6 | mixed |
| 3 | Brno | N 49°20′44.54″ E 16°39′44.17″ | 346 | 10 | 12.5 | 9.3 | mixed |
| 4 | Brno | N 49°20′22.15″ E 16°40′58.34″ | 446 | 10 | 12 | 9 | mixed |
| 5 | Brno | N 49°25′11.13″ E 16°46′24.38″ | 549 | 10.5 | 13 | 8.6 | mixed |
| 6 | Brno | N 49°25′24.82″ E 16°46′3.29″ | 586 | 12 | 12 | 7.7 | mixed |
| 7 | Prague | N 49°55′8.609″ E 13°45′15.592″ | 475 | 10 | 11 | 16 | mixed |
| 8 | Prague | N 49°54′55.7″ E 13°44′52.2″ | 489 | 11 | 10 | 15 | mixed |
| Kiln | Control | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Units | O | OM | OMC | MR | M | O | OM | M | |
| Root density | pcs. dm−2 | x¯ | 86.7 | 60.0 | 147.9 | 19.3 | 11.5 | 72.9 | 90.3 | 24.4 |
| sd | 15.7 | 35.6 | 16.1 | 11.2 | 12.0 | 11.5 | 27.6 | 11.5 | ||
| ρd | g cm−3 | x¯ | 0.41 | 0.85 | 0.71 | 1.31 | 1.58 | 0.64 | 0.91 | 1.43 |
| sd | 0.12 | 0.25 | 0.05 | 0.28 | 0.13 | 0.02 | 0.32 | 0.19 | ||
| ΘRWC | % vol. | x¯ | 48.10 | 31.92 | 34.91 | 29.11 | 27.08 | 62.67 | 28.12 | 32.06 |
| sd | 17.68 | 2.58 | 4.23 | 5.09 | 3.40 | 4.77 | 13.04 | 12.27 | ||
| ΘPWP | % vol. | x¯ | 8.73 | 25.30 | 19.98 | 12.99 | 14.66 | 6.46 | 8.08 | 13.89 |
| sd | 1.16 | 3.55 | 2.70 | 6.13 | 8.26 | 1.03 | 2.87 | 8.96 | ||
| AMCC | % vol. | x¯ | 3.67 | 6.40 | 9.61 | 4.78 | 3.17 | 0.99 | 4.98 | 3.33 |
| sd | 3.24 | 2.02 | 2.96 | 2.11 | 1.67 | 0.21 | 2.02 | 1.84 | ||
| pH/KCl | - | x¯ | 4.03 | 3.67 | 3.59 | 3.89 | 3.74 | 3.70 | 3.44 | 3.75 |
| sd | 0.54 | 0.64 | 0.27 | 0.62 | 0.24 | 0.77 | 0.45 | 0.35 | ||
| CEC | mmol kg−1 | x¯ | 249.74 | 112.65 | 159.48 | 72.81 | 72.79 | 191.46 | 101.73 | 76.60 |
| sd | 87.27 | 38.49 | 65.35 | 23.86 | 26.02 | 16.29 | 26.94 | 31.27 | ||
| BS | % | x¯ | 84.7 | 49.0 | 42.8 | 61.7 | 72.5 | 71.8 | 39.9 | 45.5 |
| sd | 19.6 | 30.9 | 19.8 | 28.0 | 22.5 | 20.3 | 20.7 | 24.3 | ||
| TOC | % | x¯ | 27.56 | 8.07 | 13.85 | 1.68 | 0.70 | 28.06 | 5.51 | 1.06 |
| sd | 10.10 | 5.34 | 4.20 | 1.12 | 0.28 | 6.38 | 2.77 | 0.76 | ||
| TN | % | x¯ | 1.18 | 0.32 | 0.47 | 0.11 | 0.08 | 1.40 | 0.34 | 0.09 |
| sd | 0.35 | 0.17 | 0.11 | 0.04 | 0.02 | 0.15 | 0.17 | 0.03 | ||
| C/N | - | x¯ | 23.00 | 23.33 | 30.50 | 15.00 | 9.40 | 20.40 | 16.00 | 11.33 |
| sd | 3.27 | 5.03 | 8.04 | 5.24 | 3.58 | 4.62 | 2.39 | 4.92 | ||
| Alo/Alt | - | x¯ | 0.078 | 0.048 | 0.100 | 0.017 | 0.011 | 0.063 | 0.030 | 0.023 |
| sd | 0.036 | 0.031 | 0.013 | 0.009 | 0.003 | 0.018 | 0.010 | 0.009 | ||
| P | mg kg−1 | x¯ | 44.29 | 13.00 | 24.50 | 13.75 | 12.20 | 82.60 | 15.50 | 14.93 |
| sd | 20.70 | 4.36 | 27.97 | 8.99 | 6.14 | 43.42 | 13.16 | 17.56 | ||
| Mg | mg kg−1 | x¯ | 258.9 | 112.0 | 91.6 | 123.6 | 208.2 | 246.8 | 120.6 | 143.7 |
| sd | 90.07 | 11.53 | 46.71 | 75.62 | 120.44 | 21.26 | 57.53 | 141.22 | ||
| Ca | mg kg−1 | x¯ | 2674.1 | 648.3 | 978.0 | 570.9 | 684.0 | 1329.4 | 492.8 | 450.8 |
| sd | 1440.8 | 159.3 | 776.9 | 351.2 | 427.8 | 565.2 | 403.1 | 513.2 | ||
| K | mg kg−1 | x¯ | 299.6 | 111.0 | 70.3 | 126.0 | 121.8 | 502.0 | 141.1 | 73.3 |
| sd | 69.3 | 44.4 | 24.8 | 55.1 | 44.0 | 204.0 | 77.8 | 29.3 | ||
| Parameter | Units | Kiln | Control | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| O | OM | OMC | MR | M | O | OM | M | |||
| β-glucosidase | nmol g−1 hr−1 | x¯ | 3507.0 | 452.0 | 350.6 | 86.7 | 59.2 | 4886.5 | 544.5 | 162.4 |
| sd | 1999.1 | 373.3 | 161.7 | 81.4 | 46.8 | 2945.0 | 256.3 | 156.0 | ||
| Chitinase | nmol g−1 hr−1 | x¯ | 929.2 | 55.6 | 98.8 | 38.7 | 88.7 | 642.2 | 145.4 | 102.4 |
| sd | 421.6 | 37.7 | 52.3 | 31.3 | 31.7 | 66.9 | 96.9 | 91.5 | ||
| Leucine-aminopeptidase | nmol g−1 hr−1 | x¯ | 105.3 | 20.6 | 32.2 | 11.4 | 4.3 | 70.3 | 57.5 | 19.3 |
| sd | 90.5 | 13.4 | 30.5 | 11.0 | 3.0 | 93.1 | 67.2 | 30.0 | ||
| Phosphatase | nmol g−1 hr−1 | x¯ | 6607.7 | 476.0 | 841.1 | 164.3 | 95.5 | 4599.1 | 762.3 | 187.3 |
| sd | 3021.9 | 354.0 | 413.9 | 119.1 | 39.8 | 1191.3 | 323.4 | 107.9 | ||
| Arylsulphatase | nmol g−1 hr−1 | x¯ | 147.9 | 27.2 | 38.9 | 10.2 | 10.4 | 98.9 | 49.1 | 23.9 |
| sd | 52.6 | 35.4 | 34.8 | 8.3 | 9.7 | 60.7 | 39.9 | 27.2 | ||
| Depolymerization enzymes | nmol g−1 hr−1 | ∑ | 11,297.0 | 1031.0 | 1362.0 | 311.0 | 258.0 | 10,297.0 | 1559.0 | 495.0 |
| sd | 5248.0 | 759.0 | 562.0 | 209.0 | 128.0 | 2117.0 | 478.0 | 309.0 | ||
| x¯ | 2259.4 | 206.2 | 272.4 | 62.2 | 51.6 | 2059.4 | 311.8 | 99.0 | ||
| Peroxidase | nmol g−1 hr−1 | x¯ | 362.4 | 832.7 | 848.5 | 867.2 | 874.0 | 374.3 | 770.1 | 847.7 |
| sd | 206.2 | 96.2 | 132.2 | 66.1 | 102.4 | 47.3 | 111.2 | 355.6 | ||
| Phenoloxidase | nmol g−1 hr−1 | x¯ | 142.2 | 80.5 | 174.6 | 83.1 | 117.8 | 94.8 | 94.1 | 100.0 |
| sd | 81.5 | 8.7 | 56.4 | 34.0 | 71.4 | 75.4 | 36.9 | 80.4 | ||
| Humification enzymes | nmol g−1 hr−1 | ∑ | 505.0 | 913.0 | 1023.0 | 950.0 | 992.0 | 469.0 | 864.0 | 948.0 |
| sd | 226.0 | 91.0 | 156.0 | 50.0 | 144.0 | 44.0 | 90.0 | 394.0 | ||
| x¯ | 252.5 | 456.5 | 511.5 | 475.0 | 496.0 | 234.5 | 432.0 | 474.0 | ||
| POxC | mg C kg−1 dry mass | x¯ | 2483.6 | 1353.1 | 1075.1 | 291.0 | 177.4 | 2841.4 | 1183.0 | 205.0 |
| sd | 686.1 | 257.8 | 383.4 | 149.9 | 98.8 | 18.4 | 497.8 | 149.1 | ||
| Parameter | Units | p-Value S–W Test | Kiln | Control | p-Value t-Test | ||
|---|---|---|---|---|---|---|---|
| Mean | Median | Mean | Median | ||||
| sum-UWC | mm | 0.646 | 54.3 | 53.7 | 57.2 | 57.1 | 0.6522 |
| sum-TOC | kg m−2 | 0.664 | 17.5 | 19.0 | 10.0 | 11.1 | 0.0331 |
| sum-TN | kg m−2 | 0.099 | 0.77 | 0.87 | 0.65 | 0.70 | 0.3409 |
| sum-P | g m−2 | 0.003 | 53.1 | 46.1 | 90.0 | 49.5 | 0.9497 |
| sum-Mg | g m−2 | 0.140 | 58.4 | 49.4 | 73.9 | 54.1 | 0.5404 |
| sum-Ca | g m−2 | 0.033 | 325.6 | 296.9 | 236.4 | 122.8 | 0.2284 |
| sum-K | g m−2 | 0.514 | 47.7 | 46.2 | 39.6 | 40.1 | 0.2284 |
| sum-Na | g m−2 | 0.014 | 19.0 | 17.5 | 27.6 | 19.4 | 0.9497 |
| Enzyme (y ~) | Site | Horizon | p-Value |
|---|---|---|---|
| Depolenz | K | O + OM + OMC + MR + M | *** |
| Depolenz | C | O + OM + M | *** |
| Humifenz | K | O + OM + OMC + MR + M | *** |
| Humifenz | C | O + OM + M | . |
| Depolenz | K | OM + OMC + MR + M | *** |
| Humifenz | K | OM + OMC + MR + M | ns |
| β-Glucosidase | K | OM + OMC + MR + M | *** |
| Chitinase | K | OM + OMC + MR + M | . |
| Leucine-aminopeptidase | K | OM + OMC + MR+M | *** |
| Phosphatase | K | OM + OMC + MR+M | *** |
| Arylsulphatase | K | OM + OMC + MR+M | * |
| Peroxidase | K | OM + OMC + MR+M | ns |
| Phenoloxidase | K | OM + OMC + MR+M | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kučera, A.; Holík, L.; Knott, R.; Adamec, Z.; Volánek, J.; Bajer, A. The Soil Environment of Abandoned Charcoal Kiln Platforms in a Low-Altitude Central European Forest. Forests 2023, 14, 29. https://doi.org/10.3390/f14010029
Kučera A, Holík L, Knott R, Adamec Z, Volánek J, Bajer A. The Soil Environment of Abandoned Charcoal Kiln Platforms in a Low-Altitude Central European Forest. Forests. 2023; 14(1):29. https://doi.org/10.3390/f14010029
Chicago/Turabian StyleKučera, Aleš, Ladislav Holík, Robert Knott, Zdeněk Adamec, Jiří Volánek, and Aleš Bajer. 2023. "The Soil Environment of Abandoned Charcoal Kiln Platforms in a Low-Altitude Central European Forest" Forests 14, no. 1: 29. https://doi.org/10.3390/f14010029
APA StyleKučera, A., Holík, L., Knott, R., Adamec, Z., Volánek, J., & Bajer, A. (2023). The Soil Environment of Abandoned Charcoal Kiln Platforms in a Low-Altitude Central European Forest. Forests, 14(1), 29. https://doi.org/10.3390/f14010029





