Species Composition and Diversity of Plants along Human-Induced Disturbances in Tropical Moist Sal Forests of Eastern Ghats, India
Abstract
:1. Introduction
2. Materials and Methods
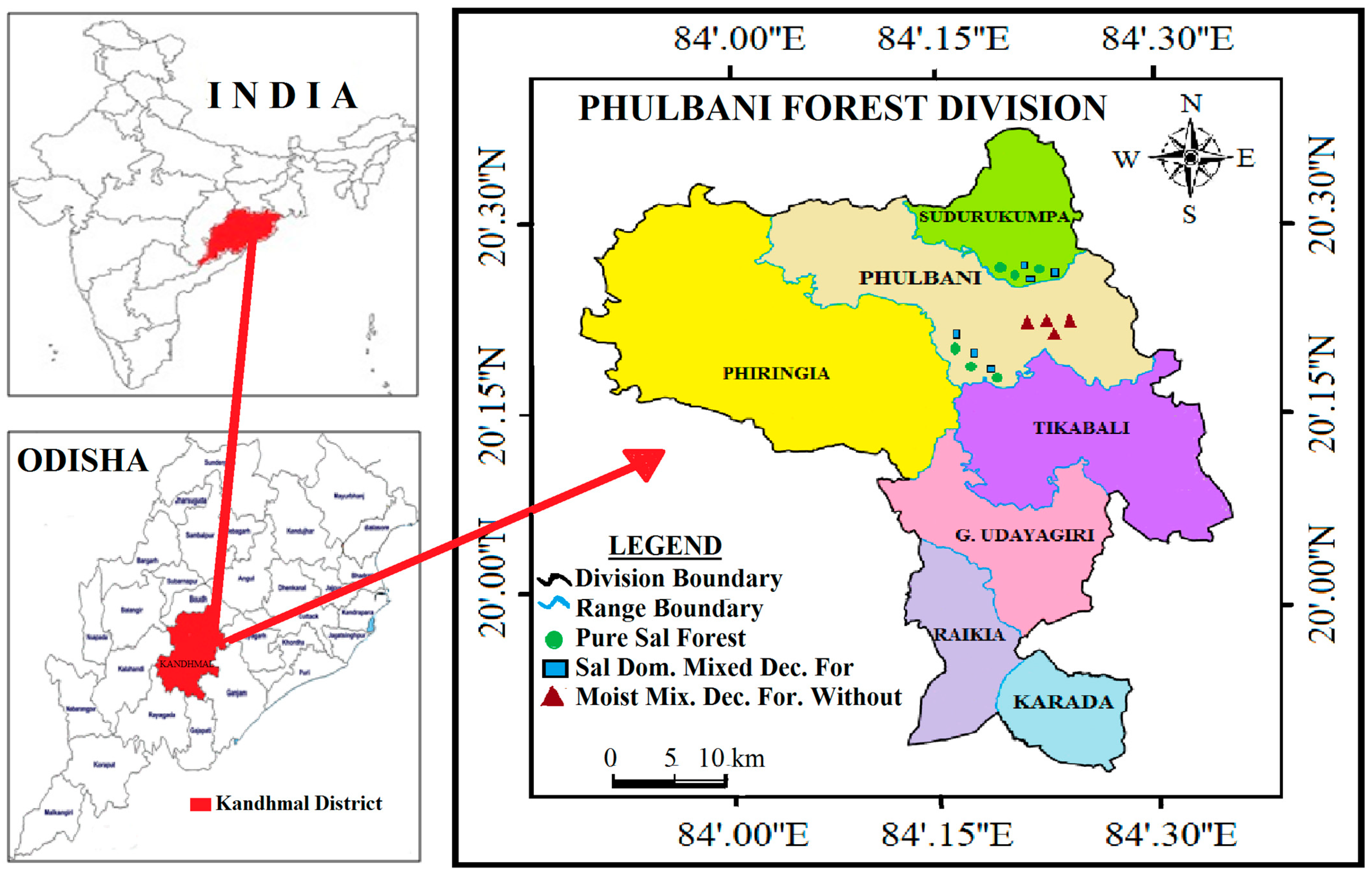
2.1. Study Area
2.2. Vegetation Sampling
2.3. Statistical Analysis of Data
3. Results
3.1. Floristic Composition
3.2. Distribution Pattern and Rarity of Species
3.3. Regeneration Status of Tree Species
3.4. Population Structure of Tree Species
4. Discussion
4.1. Species Composition
4.2. Community Structure
4.3. Distribution Patterns
4.4. Regeneration
4.5. Study Limitations
5. Conclusions
- -
- The species composition varied with respect to forest types: S. robusta, M. latifolia, C. collinus, and B. lanzan in the SDMDF, and T. alata, B. retulsa, D. melanxylon, and A. latifolia in the MDFWS were identified as the dominant tree species.
- -
- The magnitude of human-induced forest disturbance had a significant impact on the species composition, diversity, and richness of the species at sites.
- -
- Species diversity on undisturbed and low-disturbed sites was significantly higher than the mildly disturbed sites in all forest types.
- -
- Tree density and the basal area decreased with the increased level of disturbance; the regeneration density of S. robusta was higher in undisturbed sites compared to low- and moderately disturbed sites.
- -
- Forest disturbance was found affecting the population structure/regeneration of several economically important tree species, viz. P. marsupium, T. belerica, T. chebula, M. longifolia, S. anacardium, etc.
- -
- The increased number of rare species in the order of Moderately disturbed > Low-disturbed > Undisturbed sites signifies the conservation priorities of the species.
- -
- The study recommends the development of proper conservation plans with the help of local communities to enhance the regeneration potential of the trees, restore biodiversity, and promote ecosystem services to the local communities.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magurran, A.E. Ecological Diversity and Its Measurement; Princeton University Press: Princeton, NJ, USA, 1988. [Google Scholar]
- Huang, X.; Lang, X.; Li, S.; Liu, W.; Su, J. Indicator selection and driving factors of ecosystem multifunctionality: Research status and Perspectives. Biodivers. Sci. 2021, 29, 1673. [Google Scholar] [CrossRef]
- May, R.M.; Stumpf, M.P. Species-area relations in tropical forests. Science 2000, 290, 2084–2086. [Google Scholar] [CrossRef] [PubMed]
- Lalfakawma; Sahoo, U.K.; Roy, S.; Vanalalhriatpuia, K.; Vanlalhluna, P.C. Community composition and tree population structure in undisturbed and disturbed tropical semievergreen forest stands of northeast India. Appl. Ecol. Environ. Res. 2009, 7, 303–318. [Google Scholar] [CrossRef]
- Tripathi, O.P.; Tripathi, R.S. Community composition, structure and management of subtropical vegetation of forests in Meghalaya State, northeast India. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2010, 6, 157–163. [Google Scholar] [CrossRef]
- Sagar, R.; Devy, M.S. The Impact of Anthropogenic Disturbance to the Canopy Microclimate of Tropical Forests in the Southern Western Ghats, India. Front. For. Glob. Chang. 2022, 5, 734448. [Google Scholar] [CrossRef]
- Barlow, J.; Lennox, G.D.; Ferreira, J.; Berenguer, E.; Lees, A.C.; Nally, R.M.; Thomson, J.R.; de Barros Ferraz, S.F.; Louzada, J.; Gardner, T.A. Anthropogenic disturbance in tropical forests can double biodiversity loss from deforestation. Nature 2016, 535, 144–147. [Google Scholar] [CrossRef]
- Singh, S. Low to Moderate Level Forest Disturbance Effects on Plant Functional Traits and Associated Soil Microbial Diversity in Western Himalaya. Front. For. Glob. Chang. 2021, 4, 710658. [Google Scholar] [CrossRef]
- Chapagain, U.; Chapagain, B.P.; Nepal, S.; Manthey, M. Impact of disturbances on species diversity and regeneration of Nepalese Sal (Shorea robusta) forests managed under different management regimes. Earth 2021, 2, 826–844. [Google Scholar] [CrossRef]
- Dufour-Dror, J.M. Influence of cattle grazing on the density of oak seedlings and saplings in a Tabor oak forest in Israel. Acta Oecologica 2007, 31, 223–228. [Google Scholar] [CrossRef]
- Thakur, U.; Sahoo, U.K.; Bisth, N.S.; Kumar, A.; Kumar, M. Regeneration potential of forest vegetation of Churdhar Wildlife Sanctuary of India: Implications for forest management. Air Water Soil Pollut. 2021, 232, 373. [Google Scholar] [CrossRef]
- Wassenaar, T.; Gerber, P.; Verburg, P.H.; Rosales, M.; Ibrahim, M.; Steinfeld, H. Projecting land use changes in the Neotropics: The geography of pasture expansion into forest. Glob. Environ. Chang. 2007, 17, 86–104. [Google Scholar] [CrossRef]
- Morris, R.J. Anthropogenic impacts on tropical forest biodiversity: A network structure and ecosystem functioning perspective. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 3709–3718. [Google Scholar] [CrossRef] [PubMed]
- Caviedes, J.; Ibarra, J.T. Influence of anthropogenic disturbances on stand structural complexity in Andean temperate forests: Implications for managing key habitat for biodiversity. PLoS ONE 2017, 12, e0169450. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, A.; Sahoo, U.K. Impact of anthropogenic disturbance on species diversity and vegetation structure of a lowland tropical rainforest of eastern Himalaya, India. J. Mt. Sci. 2018, 15, 2453–2465. [Google Scholar] [CrossRef]
- Bradshaw, C.J.; Sodhi, N.S.; Brook, B.W. Tropical turmoil: A biodiversity tragedy in progress. Front. Ecol. Environ. 2009, 7, 79–87. [Google Scholar] [CrossRef]
- Jara-Guerrero, A.; González-Sánchez, D.; Escudero, A.; Espinosa, C.I. Chronic disturbance in a tropical dry forest: Disentangling direct and indirect pathways behind the loss of plant richness. Front. For. Glob. Chang. 2021, 4, 723985. [Google Scholar] [CrossRef]
- Sfair, J.C.; de Bello, F.; de França, T.Q.; Baldauf, C.; Tabarelli, M. Chronic human disturbance affects plant trait distribution in a seasonally dry tropical forest. Environ. Res. Lett. 2018, 13, 025005. [Google Scholar] [CrossRef]
- Cielo-Filho, R.; Gneri, M.A.; Martins, F.R. Position on slope, disturbance, and tree species coexistence in a seasonal semi-deciduous forest in SE-Brazil. Plant Ecol. 2007, 190, 189–203. [Google Scholar] [CrossRef]
- Cardelús, C.L.; Woods, C.L.; Mekonnen, A.B.; Dexter, S.; Scull, P.; Tsegay, B.A. Human disturbance impacts the integrity of sacred church forests, Ethiopia. PLoS ONE 2019, 14, e0212430. [Google Scholar] [CrossRef]
- Silva, J.L.S.; Cruz-Neto, O.; Rito, K.F.; Arnan, X.; Leal, I.R.; Peres, C.A.; Tabarelli, M.; Lopes, A.V. Divergent responses of plant reproductive strategies to chronic anthropogenic disturbance and aridity in the Caatinga dry forest. Sci. Total. Environ. 2020, 704, 135240. [Google Scholar] [CrossRef]
- Walker, L.R. The Biology of Disturbed Habitats; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Malik, Z.A.; Bhatt, A.B. Regeneration status of tree species and survival of their seedlings in Kedarnath Wildlife Sanctuary and its adjoining areas in Western Himalaya, India. Trop. Ecol. 2016, 57, 677–690. [Google Scholar]
- Shekhar Silori, C. Status and distribution of anthropogenic pressure in the buffer zone of Nanda Devi Biosphere Reserve in western Himalaya, India. Biodivers. Conserv. 2001, 10, 1113–1130. [Google Scholar] [CrossRef]
- Pokhriyal, P.; Chauhan, D.S.; Todaria, N.P. Effect of altitude and disturbance on structure and species diversity of forest vegetation in a watershed of central Himalaya. Trop. Ecol. 2012, 53, 307–315. [Google Scholar]
- Uniyal, P.; Pokhriyal, P.; Dasgupta, S.; Bhatt, D.; Todaria, N.P. Plant diversity in two forest types along the disturbance gradient in Dewalgarh Watershed, Garhwal Himalaya. Curr. Sci. 2010, 98, 938–943. [Google Scholar]
- Kumar, A.; Ram, J. Anthropogenic disturbances and plant biodiversity in forests of Uttaranchal, central Himalaya. Biodivers. Conserv. 2005, 14, 309–331. [Google Scholar] [CrossRef]
- Majumdar, K.; Datta, B.K. Vegetation types, dominant compositions, woody plant diversity and stand structure in Trishna wildlife sanctuary of northeast India. J. Environ. Biol. 2015, 36, 409–418. [Google Scholar]
- Dutta, G.; Devi, A. Plant diversity, population structure, and regeneration status in disturbed tropical forests in Assam, northeast India. J. For. Res. 2013, 24, 715–720. [Google Scholar] [CrossRef]
- Bhuyan, P.; Khan, M.L.; Tripathi, R.S. Tree diversity and population structure in undisturbed and human-impacted stands of tropical wet evergreen forest in Arunachal Pradesh, Eastern Himalayas, India. Biodivers. Conserv. 2003, 12, 1753–1773. [Google Scholar] [CrossRef]
- Gautam, M.K.; Manhas, R.K.; Tripathi, A.K. Patterns of diversity and regeneration in unmanaged moist deciduous forests in response to disturbance in Shiwalik Himalayas, India. J. Asia-Pac. Biodivers. 2016, 9, 144–151. [Google Scholar] [CrossRef]
- Kala, C.P. Forest structure and anthropogenic pressures in the Pachmarhi biosphere reserve of India. J. For. Res. 2015, 26, 867–874. [Google Scholar] [CrossRef]
- Kala, C.P.; Dubey, Y. Anthropogenic disturbances and status of forest and wildlife in the dry deciduous forests of Chhattisgarh state in India. J. For. Res. 2012, 23, 45–52. [Google Scholar] [CrossRef]
- Anitha, K.; Joseph, S.; Ramasamy, E.V.; Prasad, S.N. Changes in structural attributes of plant communities along disturbance gradients in a dry deciduous forest of Western Ghats, India. Environ. Monit. Assess. 2009, 155, 393–405. [Google Scholar] [CrossRef]
- Daniels, R.J.R.; Gadgil, M.; Joshi, N.V. Impact of human extraction on tropical humid forests in the Western Ghats Uttara Kannada, South India. J. Appl. Ecol. 1995, 32, 866–874. [Google Scholar] [CrossRef]
- Chittibabu, C.V.; Parthasarathy, N. Attenuated tree species diversity in human-impacted tropical evergreen forest sites at Kolli hills, Eastern Ghats, India. Biodivers. Conserv. 2000, 9, 1493–1519. [Google Scholar] [CrossRef]
- Sahoo, T.; Acharya, L.; Panda, P.C. Plant diversity along disturbance gradients in Tropical Moist deciduous forests of Eastern Ghats of India. Plant Arch. 2020, 20, 2007–20017. [Google Scholar]
- Sahoo, T.; Acharya, L.; Panda, P.C. Structure and composition of tree species in tropical moist deciduous forests of Eastern Ghats of Odisha, India, in response to human-induced disturbances. Environ. Sustain. 2020, 3, 69–82. [Google Scholar] [CrossRef]
- Champion, H.G.; Seth, S.K. A Revised Survey of the Forest Types of India; Natraj Publications: Dehra Dun, India, 1968. [Google Scholar]
- Ministry of Environment, Forest and Climate Change, Government of India, New Delhi. India State of Forest Report 2019. Available online: https://fsi.nic.in/forest-report-2021-details (accessed on 12 June 2023).
- Government of India, Ministry of Home Affairs. Census of India 2011. Population Census of India; Government of India, Ministry of Home Affairs: New Delhi, India, 2011. [Google Scholar]
- Behera, M. Non timber forest products and tribal livelihood: A case study from Kandhamal district of Orissa. Indian For. 2009, 135, 1127–1134. [Google Scholar]
- Chown, S.L. Temporal biodiversity change in transformed landscapes: A southern African perspective. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 3729–3742. [Google Scholar] [CrossRef]
- Ishii, H.T.; Tanabe, S.I.; Hiura, T. Exploring the relationships among canopy structure, stand productivity, and biodiversity of temperate forest ecosystems. For. Sci. 2004, 50, 342–355. [Google Scholar]
- Clark, J.A.; Covey, K.R. Tree species richness and the logging of natural forests: A meta-analysis. For. Ecol. Manag. 2012, 276, 146–153. [Google Scholar] [CrossRef]
- Rao, P.; Barik, S.K.; Pandey, H.N.; Tripathi, R.S. Community composition and tree population structure in a sub-tropical broad-leaved forest along a disturbance gradient. Vegetation 1990, 88, 151–162. [Google Scholar] [CrossRef]
- Curtis, J.T.; Cottam, G. Plant Ecology Workbook: A Laboratory, Field and Reference Manual; Burgess Publication Company: Minneapolis, MN, USA, 1956; p. 193. [Google Scholar]
- Sundriyal, R.C.; Sharma, E. Anthropogenic pressure on tree structure and biomass in the temperate forest of Mamlay watershed in Sikkim. For. Ecol. Manag. 1996, 81, 113–134. [Google Scholar] [CrossRef]
- Shankar, U. A case of high tree diversity in a sal (Shorea robusta)-dominated lowland forest of Eastern Himalaya: Floristic composition, regeneration and conservation. Curr. Sci. 2001, 81, 776–786. [Google Scholar]
- Misra, R. Ecology Work Book; Oxford and IBH Publishing Company: New Delhi, India, 1968. [Google Scholar]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1963; p. 117. [Google Scholar]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Pielou, E.C. Species-diversity and pattern-diversity in the study of ecological succession. J. Theor. Biol. 1966, 10, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Margalef, R. Perspectives in Ecological Theory; University of Chicago Press: Chicago, IL, USA, 1968; p. 111. [Google Scholar]
- Sorensen, T. A method of establishing groups of equal amplitude in plant sociology based on similarity of species content and its application to analyses of the vegetation on Danish commons. Biol. Skr. 1948, 5, 1–34. [Google Scholar]
- Kadavul, K.; Parthasarathy, N. Plant biodiversity and conservation of tropical semi-evergreen forest in the Shervarayan hills of Eastern Ghats, India. Biodivers. Conserv. 1999, 8, 419–437. [Google Scholar] [CrossRef]
- Saxena, H.O.; Brahmam, M. The Flora of Orissa; Orissa Forest Development Corporation Ltd: Bhubaneswar, India, 1996; Volume I–IV. [Google Scholar]
- Mishra, R.K.; Upadhyay, V.P.; Nayak, P.K.; Pattanaik, S.; Mohanty, R.C. Composition and stand structure of Tropical moist deciduous forest of Similipal biosphere reserve, Orissa, India. In Forest Ecosystems—More Than Just Trees; Blanco, J.A., Ed.; Intech: London, UK, 2012; pp. 106–136. ISBN 978-953-51-0202-1. [Google Scholar]
- Statistical Package for Social Sciences, Version 20.0; IBM SPSS Statistics: Belmont, CA, USA; Wadsworth, Australia, 2021.
- Anderson, M.J. Permutational Multivariate Analysis of Variance; Wiley: Hoboken, NJ, USA, 2017; pp. 1–15. [Google Scholar] [CrossRef]
- Magurran, A.E.; McGill, B.J. (Eds.) Biological Diversity: Frontiers in Measurement and Assessment; OUP Oxford: Oxford, UK, 2010. [Google Scholar]
- Sahoo, T.; Panda, P.C. Comparative assessment of structure, composition and diversity of tree species of tropical moist deciduous forests in three forest ranges of Nayagarh Forest Division, Odisha, India. Plant Sci. Res. 2015, 37, 39–48. [Google Scholar]
- Sahu, S.C.; Dhal, N.K.; Reddy, C.S.; Pattanaik, C.; Brahmam, M. Phytosociological study of tropical dry deciduous forest of Boudh district, Orissa, India. Res. J. For. 2007, 1, 66–72. [Google Scholar] [CrossRef]
- Sarmah, R. Non-timber forest products: Extraction and impact on plant community structure in and around Namdapha National Park of Arunachal Pradesh, India. Indian J. Plant Sci. 2012, 1, 192–207. [Google Scholar]
- Borah, N.; Athokpam, F.D.; Garkoti, S.C.; Das, A.K.; Hore, D.K. Structural and compositional variations in undisturbed and disturbed tropical forests of Bhuban hills in south Assam, India. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2014, 10, 9–19. [Google Scholar] [CrossRef]
- Dutta, G.; Devi, A. Impact of lopping on tree species of tropical Indian forests. Trop. Plant Res. 2015, 2, 1–4. [Google Scholar]
- Sagar, R.; Raghubanshi, A.S.; Singh, J.S. Tree species composition, dispersion and diversity along a disturbance gradient in a dry tropical forest region of India. For. Ecol. Manag. 2003, 186, 61–71. [Google Scholar] [CrossRef]
- Zenner, E.K.; Kabrick, J.M.; Jensen, R.G.; Peck, J.E.; Grabner, J.K. Responses of ground flora to a gradient of harvest intensity in the Missouri Ozarks. For. Ecol. Manag. 2006, 222, 326–334. [Google Scholar] [CrossRef]
- Jackson, S.W.; Harper, C.A.; Buckley, D.S.; Miller, B.F. Short-term effects of silvicultural treatments on microsite heterogeneity and plant diversity in mature Tennessee oak-hickory forests. North. J. Appl. For. 2006, 23, 197–203. [Google Scholar] [CrossRef]
- Raizada, A.; Joshi, S.P.; Srivastava, M.M. Composition and vegetational diversity in an alpine grassland in the Garhwal Himalayas. Trop. Ecol. 1998, 39, 133–141. [Google Scholar]
- Sagar, R.; Singh, J.S. Predominant phenotypic traits of disturbed tropical dry deciduous forest vegetation in northern India. Community Ecol. 2003, 4, 63–71. [Google Scholar] [CrossRef]
- Sunil, C.; Somashekar, R.K.; Nagaraja, B.C. Impact of anthropogenic disturbances on riparian forest ecology and ecosystem services in Southern India. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2011, 7, 273–282. [Google Scholar] [CrossRef]
- Ngoc Le, D.T.; Van Thinh, N.; Mitlöhner, R. Effect of disturbance regimes on spatial patterns of tree species in three sites in a tropical evergreen forest in Vietnam. Int. J. For. Res. 2016, 2016, 4903749. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Adhikari, D.; Gudasalamani, R.; Saikia, P.; Khan, M.L. Ecological niche modeling for assessing potential distribution of Pterocarpus marsupium Roxb. In Ranchi, eastern India. Ecol. Res. 2020, 35, 1095–1105. [Google Scholar] [CrossRef]
- Khanal, S.; Timilsina, R.; Behroozian, M.; Peterson, A.T.; Poudel, M.; Alwar, M.S.S.; Wijewickrama, T.; Osorio-Olvera, L. Potential impact of climate change on the distribution and conservation status of Pterocarpus marsupium, a Near Threatened South Asian medicinal tree species. Ecol. Inform. 2022, 70, 101722. [Google Scholar] [CrossRef]
- Buragohain, M.K.; Dar, A.A.; Babu, K.N.; Parthasarathy, N. Tree community structure, carbon stocks and regeneration status of disturbed lowland tropical rain forests of Assam, India. Trees For. People 2023, 11, 100371. [Google Scholar] [CrossRef]
- Sharma, A.; Patel, S.K.; Singh, G.S. Variation in Species Composition, Structural Diversity, and Regeneration Along Disturbances in Tropical Dry Forest of Northern India. J. Asia-Pac. Biodivers. 2023, 16, 83–95. [Google Scholar] [CrossRef]
- Nayak, S.; Sahoo, U.K. Tree diversity and ecological status of Madhuaca latifolia (Roxb.) JF Macbr. in forests of Odisha. Ind. J. Ecol. 2020, 47, 138–149. [Google Scholar]
- Sahoo, U.K.; Lalfakawma. Population dynamics of Schima wallichii in an undisturbed vs. disturbed forest stand of northeast India. Int. J. Ecol. Environ. Sci. 2010, 36, 157–165. [Google Scholar]
- Joshi, V.C.; Bisht, D.; Sundriyal, R.C.; Pant, H. Species richness, diversity, structure, and distribution patterns across dominating forest communities of low and mid-hills in the Central Himalaya. Geol. Ecol. Landsc. 2022, 10, 1–11. [Google Scholar] [CrossRef]
- Nath, P.C.; Arunachalam, A.; Khan, M.L.; Arunachalam, K.; Barbhuiya, A.R. Vegetation analysis and tree population structure of tropical wet evergreen forests in and around Namdapha National Park, northeast India. Biodivers. Conserv. 2005, 14, 2109–2135. [Google Scholar] [CrossRef]
- Sahoo, T.; Panda, P.C.; Acharya, L. Structure, composition and diversity of tree species in tropical moist deciduous forests of Eastern India: A case study of Nayagarh Forest Division, Odisha. J. For. Res. 2017, 28, 1219–1230. [Google Scholar] [CrossRef]
- Opuni-Frimpong, E.; Gabienu, E.; Adusu, D.; Opuni-Frimpong, N.Y.; Damptey, F.G. Plant diversity, conservation significance, and community structure of two protected areas under different governance. Trees For. People 2021, 4, 100082. [Google Scholar] [CrossRef]
- Lolila, N.J.; Shirima, D.D.; Mauya, E.W. Tree species composition along environmental and disturbance gradients in tropical sub-montane forests, Tanzania. PLoS ONE 2023, 18, e0282528. [Google Scholar] [CrossRef]
- Pande, R.; Bargali, K.; Pande, N. Impacts of disturbance on the population structure and regeneration status of tree species in a Central Himalayan Mixed-Oak Forest, India. Taiwan J. For. Sci. 2014, 29, 179–192. [Google Scholar]
- Ben-Said, M. Spatial point-pattern analysis as a powerful tool in identifying pattern-process relationships in plant ecology: An updated review. Ecol. Process. 2021, 10, 56. [Google Scholar] [CrossRef]
- Odum, E.P. Fundamentals of Ecology; W. B. Saunders: Philadelphia, PA, USA, 1971; p. 574. [Google Scholar]
- Devi, L.S.; Yadava, P.S. Floristic diversity assessment and vegetation analysis of tropical semievergreen forest of Manipur, northeast India. Trop. Ecol. 2006, 47, 89–98. [Google Scholar]
- Malik, Z.A.; Pandey, R.; Bhatt, A.B. Anthropogenic disturbances and their impact on vegetation in Western Himalaya, India. J. Mt. Sci. 2016, 13, 69–82. [Google Scholar] [CrossRef]
- Khaine, I.; Woo, S.Y.; Kwak, M.; Lee, S.H.; Je, S.M.; You, H.; Lee, T.; Jang, J.; Lee, H.K.; Cheng, H.C.; et al. Factors affecting natural regeneration of tropical forests across a precipitation gradient in Myanmar. Forests 2018, 9, 143. [Google Scholar] [CrossRef]
- Hérault, B.; Piponiot, C. Key drivers of ecosystem recovery after disturbance in a neotropical forest. For. Ecosyst. 2018, 5, 2. [Google Scholar] [CrossRef]
- Sapkota, I.P.; Tigabu, M.; Odén, P.C. Spatial distribution, advanced regeneration and stand structure of Nepalese Sal (Shorea robusta) forests subject to disturbances of different intensities. For. Ecol. Manag. 2009, 257, 1966–1975. [Google Scholar] [CrossRef]
- Pandey, S.K.; Shukla, R.P. Regeneration strategy and plant diversity status in degraded sal forests. Curr. Sci. 2001, 81, 95–102. [Google Scholar]
- Davidar, P.; Munoz, F.; Puyravaud, J.P.; Mohandass, D.; Ramachandran, V.S. Multiple facets of rarity among rain forest trees in the Western Ghats of India. Biol. Conserv. 2018, 228, 110–119. [Google Scholar] [CrossRef]
- Hubbell, S.P.; Foster, R.B. Diversity of canopy trees in a neotropical forest and implications for conservation. In The Tropical Rain Forest: Ecology and Management; Sutton, S.L., Whitmore, T.C., Chadwick, A., Eds.; Blackwell Scientific: Oxford, UK, 1983; pp. 25–41. [Google Scholar]





| Disturbance Source | Relative Score (%) | Impact on Different Forest Types | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PSF | SDMDF | MDFWS | |||||||
| MD | LD | UD | MD | LD | UD | MD | UD | ||
| Forest road | ≤1 road—1, 2–4 road—2, ≥5 roads—3 | 2 | 3 | 1 | 3 | 3 | 0 | 3 | 1 |
| Distance from human settlement | ≤3.0 km—3, 4–7 km—2, ≥8.0 km—1 | 3 | 2 | 1 | 3 | 2 | 1 | 3 | 1 |
| Forest fire (previous year) | Surface fire—1, Ground fire—2, crown fire—3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Grazing intensity | ≤50 cattle units/day—1, 51–100 cattle units/day—2, ≥101 cattle units/day—3 | 3 | 2 | 0 | 3 | 2 | 0 | 3 | 0 |
| NTFP collection | ≤5 items—1, 6–9 items—2, ≥10 items—3 | 2 | 1 | 1 | 2 | 1 | 1 | 2 | 1 |
| Fuel wood collection | 2–3 months—1, 4–5 months—2, >5 months—3 | 3 | 2 | 1 | 3 | 2 | 0 | 3 | 0 |
| Wild animal | ≤5 spots—1, 6–9 spots—2, ≥10 spots—3 | 1 | 1 | 3 | 1 | 0 | 2 | 1 | 2 |
| Canopy cover (densitometer-based) | ≥70%—1, 40–70%—2, <40%—3 | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 1 |
| Tree stump-based disturbance factor | SDI | 46.5 | 22.25 | 0 | 43.0 | 24.25 | 2 | 49.75 | 1 |
| Total disturbance factor | 63.5 | 36.25 | 9.0 | 61.00 | 37.25 | 8.0 | 67.75 | 8.0 | |
| Family | Pure Sal Forest | Sal-Dominated Moist Deciduous Forest | Moist Deciduous Forest without Sal | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UD | LD | MD | UD | LD | MD | UD | MD | |||||||||
| G(S) | D | G(S) | D | G(S) | D | G(S) | D | G(S) | D | G(S) | D | G(S) | D | G(S) | D | |
| Anacardiaceae | 2(2) | 23 | 3(3) | 33 | 2(2) | 20 | 3(3) | 70 | 3(3) | 60 | 4(4) | 55 | 4(4) | 53 | 3(3) | 88 |
| Bignoniaceae | - | - | - | - | - | - | - | - | - | - | 1(1) | 3 | 1(1) | 8 | ||
| Burseraceae | 1(1) | 23 | - | - | - | - | 1(1) | 13 | - | - | - | 1(1) | 38 | 1(1) | 20 | |
| Combretaceae | 1(1) | 3 | 1(1) | 30 | 1(1) | 8 | 2(3) | 55 | 2(2) | 58 | 2(2) | 33 | 3(5) | 134 | 2(4) | 149 |
| Dipterocarpaceae | 1(1) | 418 | 1(1) | 413 | 2(2) | 443 | 2(2) | 256 | 2(2) | 278 | 2(2) | 160 | 1(1) | 10 | 1(1) | 13 |
| Ebenaceae | 1(1) | 15 | 1(1) | 5 | - | - | 1(1) | 21 | 2(2) | 25 | - | 1(2) | 45 | 1(1) | 35 | |
| Fabaceae | 2(2) | 40 | 2(2) | 25 | - | - | 2(3) | 56 | 3(3) | 48 | - | - | 6(8) | 129 | 6(6) | 112 |
| Lecythidaceae | 1(1) | 3 | 1(1) | 5 | - | - | 1(1) | 20 | 1(1) | 13 | 1(1) | 8 | 1(1) | 15 | 1(1) | 3 |
| Loganiaceae | - | - | - | - | - | - | - | - | - | - | 1(1) | 10 | 1(2) | 10 | ||
| Lythraceae | - | - | - | - | 1(1) | 8 | 1(1) | 11 | 1(1) | 28 | - | - | 1(2) | 21 | 1(1) | 10 |
| Malvaceae | - | - | - | - | - | - | 1(1) | 20 | - | 1(1) | 7 | 4(4) | 58 | 2(2) | 20 | |
| Moraceae | - | - | - | - | - | - | - | 1(1) | 15 | - | - | 1(1) | 5 | 1(1) | 8 | |
| Myrtaceae | 1(1) | 15 | - | - | - | - | 1(1) | 20 | - | 1(1) | 18 | 1(2) | 48 | - | - | |
| Oleaceae | - | - | - | - | - | - | 2(2) | 31 | - | - | - | 2(2) | 28 | 1(1) | 8 | |
| Phyllanthaceae | 2(2) | 50 | 1(1) | 10 | 1(1) | 8 | 3(3) | 83 | 3(3) | 70 | 3(3) | 68 | 3(3) | 126 | 2(2) | 68 |
| Rubiaceae | - | - | - | - | - | - | 4(4) | 34 | - | 1(1) | 25 | 2(2) | 43 | 3(3) | 56 | |
| Rutaceae | - | - | - | - | - | - | - | - | - | - | 1(1) | 3 | 2(2) | 8 | ||
| Sapindaceae | 1(1) | 5 | - | 1(1) | 13 | 1(1) | 18 | 1(1) | 38 | 1(1) | 33 | |||||
| Sapotaceae | 1(1) | 38 | - | - | - | - | 1(1) | 24 | 1(1) | 20 | 1(1) | 17 | 1(1) | 8 | 1(1) | 13 |
| Sterculiaceae | - | - | - | - | - | - | - | - | - | - | - | - | 1(1) | 15 | - | - |
| Dilleniaceae | - | - | - | - | - | - | 1(1) | 11 | - | - | 1(1) | 18 | - | - | 1(1) | 8 |
| Lamiaceae | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1(1) | 3 |
| Euphorbiaceae | - | - | - | - | - | - | - | - | - | - | 2(2) | 45 | - | - | - | - |
| Ulmaceae | - | - | - | - | - | - | - | - | - | - | 1(1) | 5 | - | - | - | - |
| Total | 13(13) | 628 | 10(10) | 521 | 8(8) | 492 | 26(28) | 725 | 20(20) | 628 | 21(21) | 477 | 37(44) | 830 | 33(36) | 673 |
| Vegetation Attributes | Growth Form | Pure Sal Forest | Sal-Dominated Moist Deciduous Forest | Moist Deciduous Forest without Sal | Total (All Sites) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| UD | LD | MD | UD | LD | MD | UD | MD | |||
| No. of Species | Tree | 13 | 10 | 8 | 28 | 20 | 21 | 44 | 36 | 61 |
| Shrub | 24 | 17 | 17 | 24 | 22 | 18 | 22 | 19 | 40 | |
| Herb | 25 | 27 | 34 | 25 | 33 | 35 | 45 | 49 | 60 | |
| No. of Family | Tree | 10 | 7 | 6 | 15 | 11 | 13 | 20 | 20 | 24 |
| Shrub | 16 | 15 | 14 | 18 | 17 | 13 | 17 | 14 | 23 | |
| Herb | 18 | 22 | 25 | 18 | 22 | 23 | 27 | 28 | 30 | |
| No. of Genera | Tree | 13 | 10 | 8 | 26 | 18 | 21 | 37 | 33 | 51 |
| Shrub | 22 | 17 | 17 | 23 | 22 | 17 | 22 | 18 | 35 | |
| Herb | 25 | 22 | 32 | 18 | 33 | 33 | 45 | 49 | 57 | |
| Density (Ind. ha−1) | Tree | |||||||||
| Mature | 625 | 520 | 490 | 756 | 600 | 473 | 825 | 663 | 619 | |
| Sapling | 1175 | 943 | 728 | 1070 | 963 | 730 | 748 | 615 | 872 | |
| Seedling | 184,375 | 98,125 | 59,375 | 204,375 | 102,500 | 62,500 | 159,375 | 112,500 | 122,891 | |
| Shrub | 7400 | 8400 | 12,100 | 6300 | 10,900 | 13,350 | 6450 | 12,200 | 9638 | |
| Herb | 92,500 | 112,500 | 235,000 | 75,000 | 115,000 | 223,125 | 112,500 | 185,000 | 143,828 | |
| Basal area (m−2 ha−1) | Tree | |||||||||
| Mature | 30.12 | 18.82 | 16.66 | 21.69 | 20.13 | 11.68 | 32.06 | 22.26 | 21.68 | |
| Sapling | 3.27 | 2.88 | 2.17 | 4.97 | 3.06 | 2.75 | 5.94 | 2.25 | 3.41 | |
| Shrub | 6.80 | 7.24 | 8.85 | 5.98 | 7.49 | 10.14 | 6.58 | 11.68 | 8.10 | |
| Margalef’s species richness index | Tree | 5.07 | 2.06 | 1.33 | 5.15 | 5.41 | 3.82 | 7.24 | 5.74 | 7.05 |
| Shrub | 4.75 | 4.09 | 2.92 | 4.55 | 3.90 | 2.86 | 4.53 | 4.02 | 3.47 | |
| Herb | 4.80 | 5.01 | 5.23 | 5.01 | 5.24 | 5.78 | 8.47 | 8.44 | 4.23 | |
| Shannon–Weiner diversity index | Tree | 1.55 | 1.06 | 0.71 | 2.67 | 2.62 | 2.37 | 3.63 | 3.30 | 2.92 |
| Shrub | 3.03 | 2.68 | 2.52 | 2.98 | 2.86 | 2.27 | 2.91 | 2.69 | 3.21 | |
| Herb | 2.99 | 3.20 | 3.42 | 2.98 | 3.34 | 3.41 | 3.73 | 3.76 | 3.87 | |
| Simpson’s dominance index | Tree | 0.40 | 0.59 | 0.71 | 0.15 | 0.18 | 0.12 | 0.03 | 0.04 | 0.15 |
| Shrub | 0.05 | 0.08 | 0.11 | 0.06 | 0.06 | 0.16 | 0.06 | 0.09 | 0.06 | |
| Herb | 0.06 | 0.04 | 0.03 | 0.06 | 0.03 | 0.37 | 0.02 | 0.03 | 0.03 | |
| Pielou’s evenness index | Tree | 0.46 | 0.43 | 0.34 | 0.76 | 0.86 | 0.87 | 0.97 | 0.94 | 0.71 |
| Shrub | 0.96 | 0.87 | 0.89 | 0.95 | 0.93 | 0.80 | 0.93 | 0.86 | 0.87 | |
| Herb | 0.93 | 0.97 | 0.98 | 0.98 | 0.97 | 0.95 | 0.98 | 0.96 | 0.94 | |
| Parameter | F | R2 | p | |
|---|---|---|---|---|
| Tree | Number of species | 30.754 | 0.872 | 0.001 |
| Basal area | 16.394 | 0.784 | 0.001 | |
| Density | 41.627 | 0.902 | 0.002 | |
| Margalef’s species richness index | 23.642 | 0.840 | 0.001 | |
| Shannon–Weiner diversity index | 22.241 | 0.832 | 0.001 | |
| Simpson’s dominance index | 26.671 | 0.856 | 0.001 | |
| Pielou’s evenness index | 11.242 | 0.602 | 0.001 | |
| Shrub | Number of species | 20.703 | 0.663 | 0.001 |
| Basal area | 8.364 | 0.443 | 0.001 | |
| Density | 8.672 | 0.452 | 0.001 | |
| Margalef’s species richness index | 24.005 | 0.695 | 0.002 | |
| Shannon–Weiner diversity index | 27.945 | 0.727 | 0.001 | |
| Simpson’s dominance index | 35.749 | 0.773 | 0.005 | |
| Pielou’s evenness index | 36.667 | 0.778 | 0.001 | |
| Herb | Number of species | 97.125 | 0.956 | 0.001 |
| Density | 65.066 | 0.936 | 0.001 | |
| Margalef’s species richness index | 95.72 | 0.963 | 0.001 | |
| Shannon–Weiner diversity index | 53.34 | 0.971 | 0.001 | |
| Simpson’s dominance index | 17.418 | 0.794 | 0.001 | |
| Pielou’s evenness index | 16.414 | 0.785 | 0.008 |
| Forest Type | Disturbance Level | Tree | Shrub | Herb |
|---|---|---|---|---|
| Pure Sal Forest | UD vs. MD | 47.62 | 53.66 | 40.68 |
| LD vs. MD | 55.56 | 82.35 | 45.90 | |
| UD vs. LD | 78.26 | 58.54 | 53.85 | |
| Sal-Dominated Moist Deciduous Forest | UD vs. MD | 51.06 | 61.90 | 66.67 |
| LD vs. MD | 58.54 | 80.00 | 66.67 | |
| UD vs. LD | 60.87 | 78.26 | 58.62 | |
| Moist Deciduous Forest Without Sal | UD vs. MD | 80.00 | 78.05 | 78.72 |
| Species Category | Pure Sal Forest | Sal-Dominated Moist Deciduous Forest | Moist Deciduous Forest without Sal | |||||
|---|---|---|---|---|---|---|---|---|
| UD | LD | MD | UD | LD | MD | UD | MD | |
| Very Rare (<2 ind./ha) | - | - | - | - | - | - | - | - |
| Rare (2–10 ind./ha) | - | 1 | 5 | 2 | 3 | 6 | 3 | 13 |
| Common (10–20 ind./ha) | 8 | 7 | 1 | 11 | 8 | 11 | 21 | 8 |
| Dominant (20–50 ind./ha) | 4 | 1 | 1 | 13 | 8 | 3 | 20 | 15 |
| Pre-dominant (>50 ind./ha) | 1 | 1 | 1 | 2 | 1 | 1 | - | - |
| Total | 13 | 10 | 8 | 28 | 20 | 21 | 44 | 36 |
| Forest Type | Disturbance Level | Distribution Pattern | Contagious | Random | Regular |
|---|---|---|---|---|---|
| Pure Sal Forest | UD | Tree | 61.54 | 23.08 | 15.38 |
| Shrub | 70.83 | 25.00 | 4.17 | ||
| Herb | 64.00 | 36.00 | - | ||
| LD | Tree | 40.00 | 40.00 | 20.00 | |
| Shrub | 52.94 | 35.29 | 11.76 | ||
| Herb | 70.37 | 29.63 | 0.00 | ||
| MD | Tree | 12.50 | 37.50 | 50.00 | |
| Shrub | 47.06 | 17.65 | 35.29 | ||
| Herb | 58.82 | 41.18 | - | ||
| Sal-Dominated Moist Deciduous Forest | UD | Tree | 67.86 | 25.00 | 7.14 |
| Shrub | 70.83 | 29.17 | - | ||
| Herb | 66.67 | 30.95 | 2.38 | ||
| LD | Tree | 30.00 | 55.00 | 15.00 | |
| Shrub | 63.64 | 27.27 | 9.09 | ||
| Herb | 76.47 | 23.53 | - | ||
| MD | Tree | 19.05 | 52.38 | 28.57 | |
| Shrub | 50.00 | 44.44 | 5.56 | ||
| Herb | 81.82 | 18.18 | - | ||
| Moist Deciduous Forest Without Sal | UD | Tree | 90.91 | 9.9 | - |
| Shrub | 68.32 | 31.82 | - | ||
| Herb | 77.78 | 22.22 | - | ||
| MD | Tree | 77.78 | 13.89 | 8.33 | |
| Shrub | 53.33 | 43.33 | 3.34 | ||
| Herb | 73.47 | 24.49 | 2.04 |
| Regeneration Status | Pure Sal Forest | Sal-Dominated Moist Deciduous Forest | Moist Deciduous Forest without Sal | |||||
|---|---|---|---|---|---|---|---|---|
| UD | LD | MD | UD | LD | MD | UD | MD | |
| Good | 11 | 6 | 4 | 12 | 5 | 3 | 13 | 10 |
| Fair | 2 | 2 | 1 | 15 | 14 | 10 | 26 | 17 |
| Poor | 0 | 0 | 0 | 1 | 0 | 2 | 3 | 4 |
| None | 0 | 2 | 3 | 0 | 1 | 6 | 2 | 5 |
| New arrival | 27 | 18 | 21 | 15 | 10 | 23 | 10 | 17 |
| Total | 40 | 28 | 29 | 43 | 35 | 44 | 54 | 53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behera, M.C.; Sahoo, U.K.; Mohanty, T.L.; Prus, P.; Smuleac, L.; Pascalau, R. Species Composition and Diversity of Plants along Human-Induced Disturbances in Tropical Moist Sal Forests of Eastern Ghats, India. Forests 2023, 14, 1931. https://doi.org/10.3390/f14101931
Behera MC, Sahoo UK, Mohanty TL, Prus P, Smuleac L, Pascalau R. Species Composition and Diversity of Plants along Human-Induced Disturbances in Tropical Moist Sal Forests of Eastern Ghats, India. Forests. 2023; 14(10):1931. https://doi.org/10.3390/f14101931
Chicago/Turabian StyleBehera, Madhab Chandra, Uttam Kumar Sahoo, Tanmay Lalitendu Mohanty, Piotr Prus, Laura Smuleac, and Raul Pascalau. 2023. "Species Composition and Diversity of Plants along Human-Induced Disturbances in Tropical Moist Sal Forests of Eastern Ghats, India" Forests 14, no. 10: 1931. https://doi.org/10.3390/f14101931
APA StyleBehera, M. C., Sahoo, U. K., Mohanty, T. L., Prus, P., Smuleac, L., & Pascalau, R. (2023). Species Composition and Diversity of Plants along Human-Induced Disturbances in Tropical Moist Sal Forests of Eastern Ghats, India. Forests, 14(10), 1931. https://doi.org/10.3390/f14101931







