Drought Exerted a Stronger Controlling Effect on Soil Carbon Release than Moisturizing in a Global Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection and Selection
2.2. Statistical Analyses
3. Results
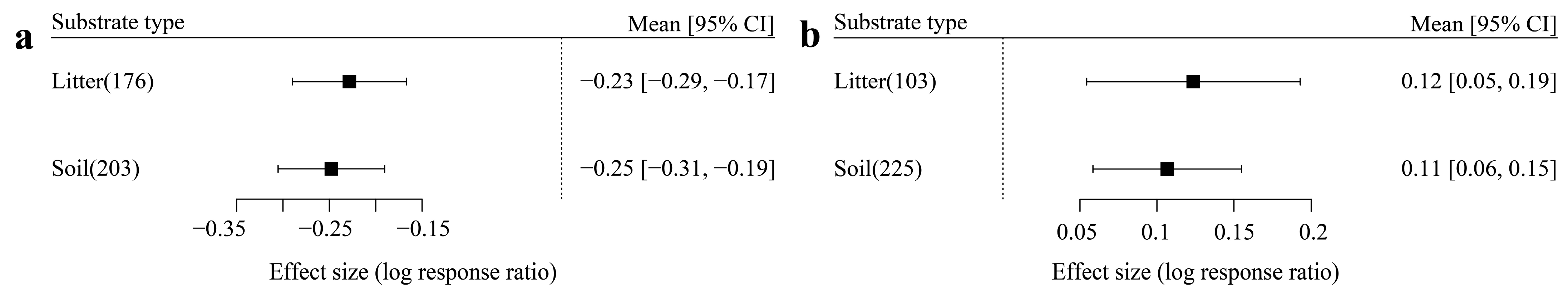
3.1. The Influence of Categorical Variables on Effect Size
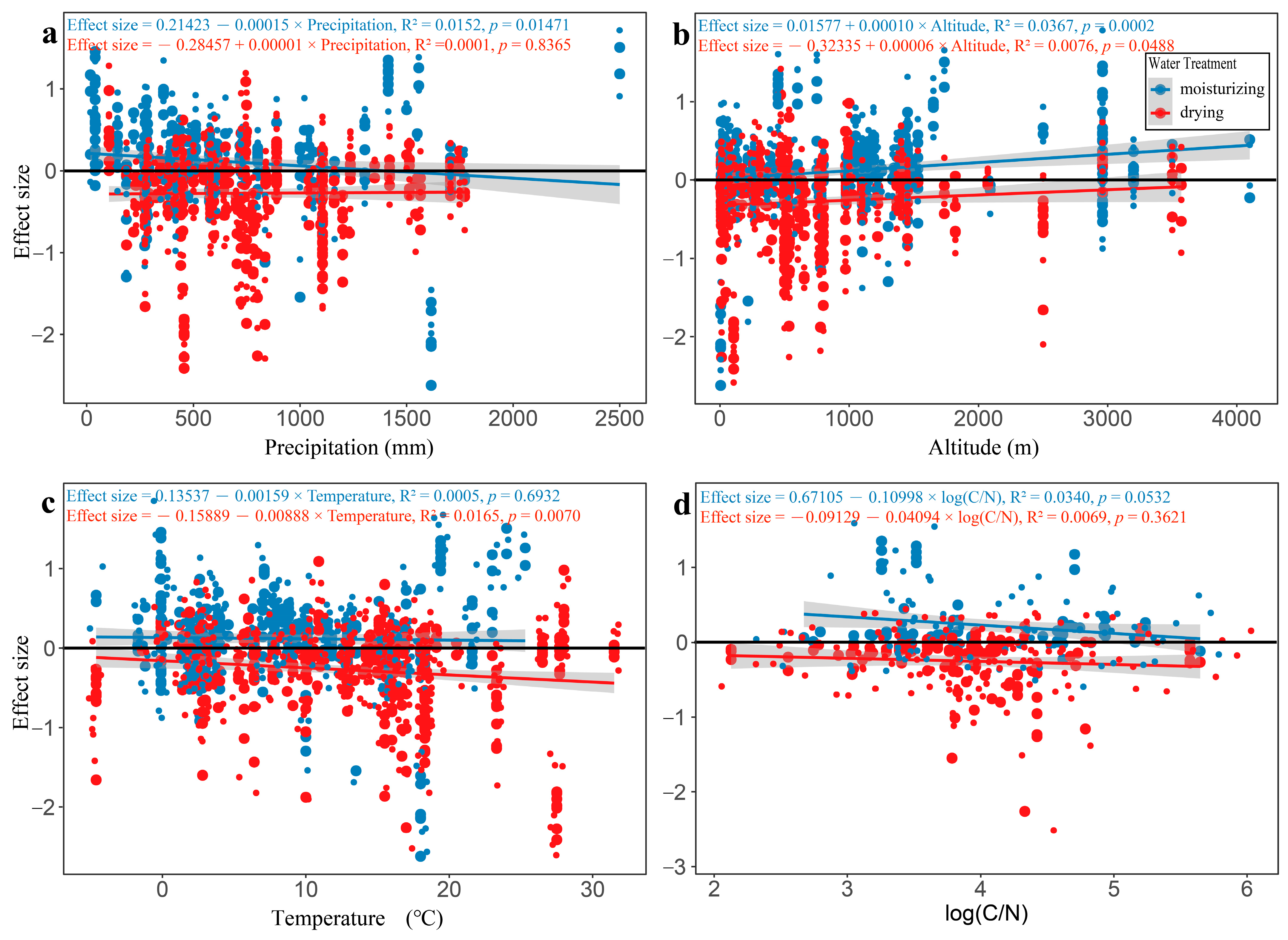
3.2. The Influence of Continuous Variables on Effect Size
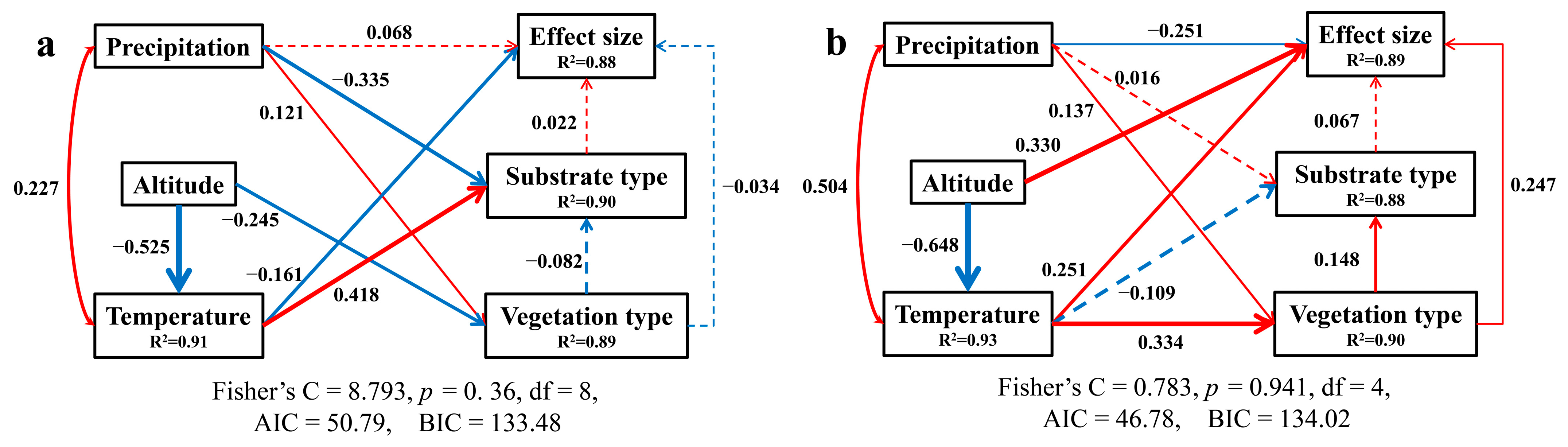
3.3. Structural Equation Model Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, R.; Zhang, J.Q.; Guo, E.L.; Zhao, C.L.; Cao, T.H. Spatial and temporal variations of precipitation concentration and their relationships with large-scale atmospheric circulations across Northeast China. Atmos. Res. 2019, 222, 62–73. [Google Scholar] [CrossRef]
- Fu, S.J.; Zhang, H.L.; Zhong, Q.; Chen, Q.G.; Liu, A.; Yang, J.; Pang, J.Z. Spatiotemporal variations of precipitation concentration influenced by large-scale climatic factors and potential links to flood-drought events across China 1958–2019. Atmos. Res. 2023, 282, 106507. [Google Scholar] [CrossRef]
- Rahman, M.S.; Senkbeil, J.C.; Keellings, D.J. Spatial and temporal variability of extreme precipitation events in the Southeastern United States. Atmosphere 2023, 14, 1301. [Google Scholar] [CrossRef]
- Deng, L.; Peng, C.H.; Kim, D.G.; Li, J.W.; Liu, Y.L.; Hai, X.Y.; Liu, Q.Y.; Huang, C.B.; Shangguan, Z.P.; Kuzyakov, Y. Drought effects on soil carbon and nitrogen dynamics in global natural ecosystems. Earth Sci. Rev. 2021, 214, 103501. [Google Scholar] [CrossRef]
- Allan, R.P.; Soden, B.J. Atmospheric warming and the amplification of precipitation extremes. Science 2008, 321, 1481–1484. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Working Group I Contribution of to the IPCC Fifth Assessment Report, Climate Change in 2013: The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Beillouin, D.; Corbeels, M.; Demenois, J.; Berre, D.; Boyer, A.; Fallot, A.; Feder, F.; Cardinael, R. A global meta-analysis of soil organic carbon in the Anthropocene. Nat. Commun. 2023, 14, 3700. [Google Scholar] [CrossRef]
- Tao, F.; Huang, Y.Y.; Hungate, B.A.; Manzoni, S.; Frey, S.D.; Schmidt, M.W.I.; Reichstein, M.; Carvalhais, N.; Ciais, P.; Jiang, L.F.; et al. Microbial carbon use efficiency promotes global soil carbon storage. Nature 2023, 618, 981–985. [Google Scholar] [CrossRef]
- Davidson, E.A.; Belk, E.; Boone, R.D. Soil water content and temperature as independent or confounded factors controlling soil respiration in a temperate mixed hardwood forest. Glob. Chang. Biol. 1998, 4, 217–227. [Google Scholar] [CrossRef]
- Marschner, P.; Zheng, B. Direction and magnitude of the change in water content between two periods influence soil respiration, microbial biomass and nutrient availability which can be modified by intermittent air-drying. Soil Biol. Biochem. 2022, 166, 108559. [Google Scholar] [CrossRef]
- Gan, H.J.; Roper, W.R.; Groffman, P.M.; Morris, T.F.; Guillard, K. Automated sensor-based quantification of soil water retention and microbial respiration across drying conditions. Soil Biol. Biochem. 2023, 180, 108987. [Google Scholar] [CrossRef]
- Luo, Z.K.; Feng, W.T.; Luo, Y.Q.; Baldock, J.; Wang, E. Soil organic carbon dynamics jointly controlled by climate, carbon inputs, soil properties and soil carbon fractions. Glob. Chang. Biol. 2017, 23, 4430–4439. [Google Scholar] [CrossRef] [PubMed]
- Ray, R.L.; Griffin, R.W.; Fares, A.; Elhassan, A.; Awal, R.; Woldesenbet, S.; Risch, E. Soil CO2 emission in response to organic amendments, temperature, and rainfall. Sci. Rep. 2020, 10, 5849. [Google Scholar] [CrossRef] [PubMed]
- Austin, A.T.; Vitousek, P.M. Precipitation, decomposition and litter decomposability of Metrosideros polymorpha in native forests on Hawai’i. J. Ecol. 2000, 88, 129–138. [Google Scholar] [CrossRef]
- Li, X.F.; Han, S.J.; Zhang, Y. Indirect effects of precipitation on litter decomposition of Quercus mongolica. Chin. J. Appl. Ecol. 2007, 18, 261–266. Available online: http://www.cjae.net/CN/abstract/abstract166.shtml (accessed on 9 July 2023).
- Huo, L.X.; Hong, M.; Zhao, B.; Gao, H.Y.; Ye, H. Effects of increased nitrogen deposition and changing rainfall patterns on litter decomposition in a desert grassland. Acta Ecol. Sin. 2019, 39, 2139–2146. [Google Scholar] [CrossRef]
- Cavelier, J.; Peñuela, M.C. Soil respiration in the cloud forest and dry deciduous forest of Serrania de Macuira, Colombia. Biotropica 1990, 22, 346–352. [Google Scholar] [CrossRef]
- Davidson, E.A.; Verchot, L.V.; Cattânio, J.H.; Ackerman, I.L.; Carvalho, J.E.M. Effects of soil water content on soil respiration in forests and cattle pastures of eastern Amazonia. Biogeochemistry 2000, 48, 53–69. [Google Scholar] [CrossRef]
- Gomez, E.J.; Delgado, J.A.; Gonzalez, J.M. Environmental factors affect the response of microbial extracellular enzyme activity in soils when determined as a function of water availability and temperature. Ecol. Evol. 2020, 10, 10105–10115. [Google Scholar] [CrossRef]
- Bian, H.F.; Li, C.; Zhu, J.X.; Xu, L.; Li, M.X.; Zheng, S.; He, N.P. Soil moisture affects the rapid response of microbes to labile organic C addition. Front. Ecol. Evol. 2022, 10, 857185. [Google Scholar] [CrossRef]
- Jansson, J.K.; Hofmockel, K.S. Soil microbiomes and climate change. Nat. Rev. Microbiol. 2020, 18, 35–46. [Google Scholar] [CrossRef]
- Bogati, K.; Walczak, M. The impact of drought stress on soil microbial community, enzyme activities and plants. Agronomy 2022, 12, 189. [Google Scholar] [CrossRef]
- Rodríguez, A.; Durán, J.; Yuste, J.C.; Valladares, F.; Rey, A. The effect of tree decline over soil water content largely controls soil respiration dynamics in a Mediterranean woodland. Agric. For. Meteorol. 2023, 333, 109398. [Google Scholar] [CrossRef]
- Li, Y.B.; Cui, D.Z.; Sui, X.X.; Huang, C.; Huang, C.Y.; Fan, Q.Q.; Chu, X.S. Autophagic survival precedes programmed cell death in wheat seedlings exposed to drought stress. Int. J. Mol. Sci. 2019, 20, 5777. [Google Scholar] [CrossRef] [PubMed]
- Santos-Medellín, C.; Liechty, Z.; Edwards, J.; Nguyen, B.; Huang, B.; Weimer, B.C.; Sundaresan, V. Prolonged drought imparts lasting compositional changes to the rice root microbiome. Nat. Plants 2021, 7, 1065–1077. [Google Scholar] [CrossRef]
- Li, W.T.; Pacheco-Labrador, J.; Migliavacca, M.; Miralles, D.; van Dijke, A.H.; Reichstein, M.; Forkel, M.; Zhang, W.; Frankenberg, C.; Panwar, A.; et al. Widespread and complex drought effects on vegetation physiology inferred from space. Nat. Commun. 2023, 14, 4640. [Google Scholar] [CrossRef]
- Schlaepfer, D.R.; Bradford, J.B.; Lauenroth, W.K.; Munson, S.M.; Tietjen, B.; Hall, S.A.; Wilson, S.D.; Duniway, M.C.; Jia, G.; Pyke, D.A.; et al. Climate change reduces extent of temperate drylands and intensifies drought in deep soils. Nat. Commun. 2017, 8, 14196. [Google Scholar] [CrossRef]
- Almagro, M.; López, J.; Querejeta, J.I.; Martínez-Mena, M. Temperature dependence of soil CO2 efflux is strongly modulated by seasonal patterns of moisture availability in a Mediterranean ecosystem. Soil Biol. Biochem. 2009, 41, 594–605. [Google Scholar] [CrossRef]
- Yu, S.Q.; Mo, Q.F.; Li, Y.W.; Li, Y.X.; Zou, B.; Xia, H.P.; Li, Z.A.; Wang, F.M. Changes in seasonal precipitation distribution but not annual amount affect litter decomposition in a secondary tropical forest. Ecol. Evol. 2019, 9, 11344–11352. [Google Scholar] [CrossRef]
- Nahdia, Y.T.; Paembonan, S.A. Cacao leaf litter decomposition under different moisture and pH: Characteristic of soil C mineralization (NH4+ and NO3–) and greenhouse gas CO2, CH4, N2O flux emission. IOP Conf. Ser. Earth Environ. Sci. 2020, 473, 012096. [Google Scholar] [CrossRef]
- Patel, K.F.; Fansler, S.J.; Campbell, T.P.; Bond-Lamberty, B.; Smith, A.P.; RoyChowdhury, T.; McCue, L.A.; Varga, T.; Bailey, V.L. Soil texture and environmental conditions influence the biogeochemical responses of soils to drought and flooding. Commun. Earth Environ. 2021, 2, 127. [Google Scholar] [CrossRef]
- Heidrich, L.; Bae, S.; Levick, S.; Seibold, S.; Weisser, W.; Krzystek, P.; Magdon, P.; Nauss, T.; Schall, P.; Serebryanyk, A.; et al. Heterogeneity–diversity relationships differ between and within trophic levels in temperate forests. Nat. Ecol. Evol. 2020, 4, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Deák, B.; Kovács, B.; Rádai, Z.; Apostolova, I.; Kelemen, A.; Kiss, R.; Lukács, K.; Palpurina, S.; Sopotlieva, D.; Báthori, F.; et al. Linking environmental heterogeneity and plant diversity: The ecological role of small natural features in homogeneous landscapes. Sci. Total Environ. 2021, 763, 144199. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.L.; Huo, T.C.; Zhang, Y.W.; Guo, T.T.; Liang, J.Y. Response of soil organic carbon decomposition to intensified water variability co-determined by the microbial community and aggregate changes in a temperate grassland soil of northern China. Soil Biol. Biochem. 2023, 176, 108875. [Google Scholar] [CrossRef]
- Ladau, J.; Eloe-Fadrosh, E.A. Spatial, temporal, and phylogenetic scales of microbial ecology. Trends Microbiol. 2019, 27, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Burda, B.U.; O’Connor, E.A.; Webber, E.M.; Redmond, N.; Perdue, L.A. Estimating data from figures with a web-based program: Considerations for a systematic review. Res. Synth. Methods 2017, 8, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.Y.; Bian, J.J.; Ge, Q.S.; Yin, Y.H. The climate regionalization in China for 1951-1980 and 1981-2010. Geogr. Res. 2013, 32, 987–997. [Google Scholar] [CrossRef]
- Zhang, B.W.; Li, W.J.; Chen, S.P.; Tan, X.R.; Wang, S.S.; Chen, M.L.; Ren, T.T.; Xia, J.Y.; Huang, J.H.; Han, X.G. Changing precipitation exerts greater influence on soil heterotrophic than autotrophic respiration in a semiarid steppe. Agric. For. Meteorol. 2019, 271, 413–421. [Google Scholar] [CrossRef]
- Hedges, L.V.; Gurevitch, J.G.; Curtis, P.S. The meta-analysis of response ratios in experimental ecology. Ecology 1999, 80, 1150–1156. [Google Scholar] [CrossRef]
- Liao, C.Z.; Peng, R.H.; Luo, Y.Q.; Zhou, X.H.; Wu, X.W.; Fang, C.M.; Chen, J.K.; Li, B. Altered ecosystem carbon and nitrogen cycles by plant invasion: A meta-analysis. N. Phytol. 2008, 177, 706–714. [Google Scholar] [CrossRef]
- Feng, J.F.; Wang, J.S.; Ding, L.B.; Yao, P.P.; Qiao, M.P.; Yao, S.C. Meta-analyses of the effects of major global change drivers on soil respiration across China. Atmos. Environ. 2017, 150, 181–186. [Google Scholar] [CrossRef]
- Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Wickham, H.; Chang, W. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Calcagno, V.; Mazancourt, C.D. glmulti: An R package for easy automated model selection with (generalized) linear models. J. Stat. Softw. 2010, 34, 1–29. [Google Scholar] [CrossRef]
- Lefcheck, J.S. piecewiseSEM: Piecewise structural equation modeling in R for ecology, evolution, and systematics. Methods Ecol. Evol. 2016, 7, 573–579. [Google Scholar] [CrossRef]
- Lin, L.F.; Chu, H.T.; Murad, M.H.; Hong, C.; Qu, Z.Y.; Cole, S.R.; Chen, Y. Empirical comparison of publication bias tests in meta-analysis. J. Gen. Intern. Med. 2018, 33, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Tóth, Z.; Táncsics, A.; Kriszt, B.; Kröel-Dulay, G.; Ónodi, G.; Hornung, E. Extreme effects of drought on composition of the soil bacterial community and decomposition of plant tissue. Eur. J. Soil Sci. 2017, 68, 504–513. [Google Scholar] [CrossRef]
- Ge, X.G.; Tong, R.; Cao, Y.H.; Zhou, B.Z.; Xiao, W.F.; Wang, X.M.; Lu, R.F. Effect of litterfall input on soil respiration and its temperature sensitivity in moso bamboo forest under simulated drought. Chin. J. Appl. Ecol. 2018, 29, 2233–2242. [Google Scholar] [CrossRef]
- Gao, H.Y.; Hong, M.; Huo, L.X.; Ye, H.; Zhao, B.Y.; De, H.S. Effects of exogenous nitrogen input and water change on litter decomposition in a desert grassland. Chin. J. Appl. Ecol. 2018, 29, 3167–3174. [Google Scholar] [CrossRef]
- Kuzyakov, Y. Sources of CO2 efflux from soil and review of partitioning methods. Soil Biol. Biochem. 2006, 38, 425–448. [Google Scholar] [CrossRef]
- Schindlbacher, A.; Zechmeister-Boltenstern, S.; Jandl, R. Carbon losses due to soil warming: Do autotrophic and heterotrophic soil respiration respond equally? Glob. Chang. Biol. 2009, 15, 901–913. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, G.F.; Yin, L.; Ma, L.; Xu, C.; Chen, H.L.; Ma, T.; Su, Y.H.; Zhu, Y.T.; He, L.Y.; et al. Optimal soil water content and temperature sensitivity differ among heterotrophic and autotrophic respiration from oasis agroecosystems. Geoderma 2022, 425, 116071. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Dong, X.J.; Xu, B.X.; Chen, Y.L.; Zhao, Y.; Gao, Y.H.; Hu, Y.G.; Huang, L. Soil respiration sensitivities to water and temperature in a revegetated desert. J. Geophys. Res. Biogeosci. 2015, 120, 773–787. [Google Scholar] [CrossRef]
- Allison, S.D. Microbial drought resistance may destabilize soil carbon. Trends Microbiol. 2023, 8, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Austin, A.T.; Vitousek, P.M. Nutrient dynamics on a precipitation gradient in Hawai’i. Oecologia 1998, 113, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Shang, Q.; Wang, L.; Tian, Y.; Ju, Y.X.; Gan, J.B. 2016. Responses of soil respiration to changing precipitation regimes in an oak forest at a climate transitional zone. Acta Ecol. Sin. 2016, 36, 8054–8061. [Google Scholar] [CrossRef]
- Zhou, S.X.; Huang, C.D.; Xiang, Y.B.; Han, B.H.; Xiao, Y.X.; Tang, J.D. Effects of nitrogen deposition and precipitation change on soil respiration in natural evergreen broadleaved forest in the rainy area of western China. J. Northwest A&F Univ. (Nat. Sci. Ed.) 2017, 45, 94–101+110. Available online: http://www.xnxbz.net/xbnlkjdxzr/ch/reader/view_abstract.aspx?file_no=20170414&flag=1 (accessed on 12 July 2023).
- Osono, T.; Takeda, H. Fungal decomposition of Abies needle and Betula leaf litter. Mycologia 2006, 98, 172–179. [Google Scholar] [CrossRef]
- Yuan, Z.L.; Chen, L.Q. The role of endophytic fungal individuals and communities in the decomposition of Pinus massionana needle litter. PLoS ONE 2014, 9, 105911. [Google Scholar] [CrossRef]
- Chen, Y.L.; Du, Z.L.; Weng, Z.; Sun, K.; Zhang, Y.Q.; Liu, Q.; Yang, Y.; Li, Y.; Wang, Z.B.; Luo, Y.; et al. Formation of soil organic carbon pool is regulated by the structure of dissolved organic matter and microbial carbon pump efficacy: A decadal study comparing different carbon management strategies. Glob. Chang. Biol. 2023, 29, 5445–5459. [Google Scholar] [CrossRef]
- Palta, J.A.; Nobel, P.S. Root respiration for Agave deserti: Influence of temperature, water status and root age on daily patterns. J. Exp. Bot. 1989, 40, 181–186. [Google Scholar] [CrossRef]
- Shi, W.Y.; Tateno, R.; Zhang, J.G.; Wang, Y.L.; Yamanaka, N.; Du, S. Response of soil respiration to precipitation during the dry season in two typical forest stands in the forest-grassland transition zone of the Loess Plateau. Agric. For. Meteorol. 2011, 151, 854–863. [Google Scholar] [CrossRef]
- Illeris, L.; Michelsen, A.; Jonasson, S. Soil plus root respiration and microbial biomass following water, nitrogen, and phosphorus application at a high arctic semi desert. Biogeochemistry 2003, 65, 15–29. [Google Scholar] [CrossRef]
- Zhang, X.; Xing, Y.J.; Yan, G.Y.; Wang, Q.G. Response of fine roots to precipitation change: A meta-analysis. Chin. J. Plant Ecol. 2018, 42, 164–172. Available online: https://www.plant-ecology.com/CN/10.17521/cjpe.2017.0203 (accessed on 16 July 2023).
- Yu, C.L.; Hui, D.F.; Deng, Q.; Dzantor, E.K.; Fay, P.A.; Shen, W.J.; Luo, Y.Q. Responses of switchgrass soil respiration and its components to precipitation gradient in a mesocosm study. Plant Soil 2017, 420, 105–117. [Google Scholar] [CrossRef]
- Wang, B.; Chen, Y.L.; Li, Y.; Zhang, H.; Yue, K.; Wang, X.C.; Ma, Y.D.; Chen, J.; Sun, M.; Chen, Z.; et al. Differential effects of altered precipitation regimes on soil carbon cycles in arid versus humid terrestrial ecosystems. Glob. Chang. Biol. 2021, 27, 6348–6362. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Liu, Y.X.; Wang, Y.J.; Fu, B.J. Greater increases in China’s dryland ecosystem vulnerability in drier conditions than in wetter conditions. J. Environ. Manage. 2021, 291, 112689. [Google Scholar] [CrossRef]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Zhou, X.H.; Zhou, L.Y.; Nie, Y.Y.; Fu, Y.L.; Du, Z.G.; Shao, J.J.; Zheng, Z.M.; Wang, X.H. Similar responses of soil carbon storage to drought and irrigation in terrestrial ecosystems but with contrasting mechanisms: A meta-analysis. Agric. Ecosyst. Environ. 2016, 228, 70–81. [Google Scholar] [CrossRef]
- Ren, C.J.; Zhao, F.Z.; Shi, Z.; Chen, J.; Han, X.H.; Yang, G.H.; Feng, Y.Z.; Ren, G.X. Differential responses of soil microbial biomass and carbon-degrading enzyme activities to altered precipitation. Soil Biol. Biochem. 2017, 115, 1–10. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef]
- Öquist, M.G.; Sparrman, T.; Klemedtsson, L.; Drotz, S.H.; Grip, H.; Schleucher, J.; Nilsson, M. Water availability controls microbial temperature responses in frozen soil CO2 production. Glob. Chang. Biol. 2009, 15, 2715–2722. [Google Scholar] [CrossRef]
- Li, C.B.; Peng, Y.F.; Nie, X.Q.; Yang, Y.H.; Yang, L.C.; Li, F.; Fang, K.; Xiao, Y.M.; Zhou, G.Y. Differential responses of heterotrophic and autotrophic respiration to nitrogen addition and precipitation changes in a Tibetan alpine steppe. Sci. Rep. 2018, 8, 16546. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.L.; Peng, Q.; Dong, Y.S.; He, Y.L.; Yan, Z.Q.; Guo, Y.; Qin, S.Q.; Qi, Y.C. Response of soil respiration to water and nitrogen addition and its influencing factors: A four-year field experiment in a temperate steppe. Plant Soil 2022, 471, 427–442. [Google Scholar] [CrossRef]
- Birch, H.F. The effect of soil drying on humus decomposition and nitrogen availability. Plant Soil 1958, 10, 9–31. [Google Scholar] [CrossRef]
- Norton, U.; Mosier, A.R.; Morgan, J.A.; Derner, J.D.; Ingram, L.J.; Stahl, P.D. Moisture pulses, trace gas emissions and soil C and N in cheatgrass and native grass-dominated sagebrush-steppe in Wyoming, USA. Soil Biol. Biochem. 2008, 40, 1421–1431. [Google Scholar] [CrossRef]
- Meisner, A.; Bååth, E.; Rousk, J. Microbial growth responses upon rewetting soil dried for four days or one year. Soil Biol. Biochem. 2013, 66, 188–192. [Google Scholar] [CrossRef]
- Zhu, E.X.; Cao, Z.J.; Jia, J.; Liu, C.Z.; Zhang, Z.H.; Wang, H.; Dai, G.H.; He, J.S.; Feng, X.J. Inactive and inefficient: Warming and drought effect on microbial carbon processing in alpine grassland at depth. Glob. Chang. Biol. 2021, 27, 2241–2253. [Google Scholar] [CrossRef]
- Li, H.; Yang, S.; Semenov, M.V.; Yao, F.; Ye, J.; Bu, R.C.; Ma, R.A.; Lin, J.J.; Kurganova, I.; Wang, X.G.; et al. Temperature sensitivity of SOM decomposition is linked with a K-selected microbial community. Glob. Chang. Biol. 2021, 27, 2763–2779. [Google Scholar] [CrossRef]
- Allison, S.D.; Treseder, K.K. Warming and drying suppress microbial activity and carbon cycling in boreal forest soils. Glob. Chang. Biol. 2008, 14, 2898–2909. [Google Scholar] [CrossRef]
- Christiansen, C.T.; Haugwitz, M.S.; Priemé, A.; Nielsen, C.S.; Elberling, B.; Michelsen, A.; Grogan, P.; Blok, D. Enhanced summer warming reduces fungal decomposer diversity and litter mass loss more strongly in dry than in wet tundra. Glob. Chang. Biol. 2016, 23, 406–420. [Google Scholar] [CrossRef]
- Illeris, L.; Christensen, T.R.; Mastepanov, M. Moisture effects on temperature sensitivity of CO2 exchange in a subarctic heath ecosystem. Biogeochemistry 2004, 70, 315–330. [Google Scholar] [CrossRef]
- Welker, J.M.; Fahnestock, J.T.; Jones, M.H. Annual CO2 flux in dry and moist arctic tundra: Field responses to increases in summer temperatures and winter snow depth. Clim. Chang. 2000, 44, 139–150. [Google Scholar] [CrossRef]
- Atarashi-Andoh, M.; Koarashi, J.; Ishizuka, S.; Hirai, K. Seasonal patterns and control factors of CO2 effluxes from surface litter, soil organic carbon, and root-derived carbon estimated using radiocarbon signatures. Agric. For. Meteorol. 2012, 152, 149–158. [Google Scholar] [CrossRef]
- Deslippe, J.R.; Hartmann, M.; Simard, S.W.; Mohn, W.W. Long-term warming alters the composition of arctic soil microbial communities. FEMS Microbiol. Ecol. 2012, 82, 303–315. [Google Scholar] [CrossRef] [PubMed]
- DeAngelis, K.M.; Pold, G.; Topçuoğlu, B.D.; van Diepen, L.T.A.; Varney, R.M.; Blanchard, J.L.; Melillo, J.; Frey, S.D. Long-term forest soil warming alters microbial communities in temperate forest soils. Front. Microbiol. 2016, 6, 104. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.Z.; Zang, Z.H.; Xie, Z.Q.; Chen, Q.S.; Xu, W.T.; Zhao, C.M.; Shen, G.Z. Soil respiration of four forests along elevation gradient in northern subtropical China. Ecol. Evol. 2019, 9, 12846–12857. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Zhang, W.Q.; Feng, Y.J.; Mo, Q.F.; Su, Y.Q.; Njoroge, B.; Qu, C.; Gan, X.H.; Liu, X.D. Soil organic carbon primarily control the soil moisture characteristic during forest restoration in subtropical China. Front. Ecol. Evol. 2022, 10, 1003532. [Google Scholar] [CrossRef]
- Raich, J.W.; Tufekcioglu, A. Vegetation and soil respiration: Correlations and controls. Biogeochemistry 2000, 48, 71–90. [Google Scholar] [CrossRef]
- Grogan, P. Cold season respiration across a low arctic landscape: The influence of vegetation type, snow depth, and interannual climatic variation. Arct. Antarct. Alp. Res. 2012, 44, 446–456. [Google Scholar] [CrossRef]
- Han, G.X.; Xing, Q.H.; Luo, Y.Q.; Rafique, R.; Yu, J.B.; Mikle, N. Vegetation types alter soil respiration and its temperature sensitivity at the field scale in an estuary wetland. PLoS ONE 2014, 9, 91182. [Google Scholar] [CrossRef]
- Grand, S.; Rubin, A.; Verrecchia, E.P.; Vittoz, P. Variation in soil respiration across soil and vegetation types in an Alpine valley. PLoS ONE 2016, 11, 0163968. [Google Scholar] [CrossRef]
- Shedayi, A.A.; Xu, M.; Naseer, I.; Khan, B. Altitudinal gradients of soil and vegetation carbon and nitrogen in a high altitude nature reserve of Karakoram ranges. SpringerPlus 2016, 5, 320. [Google Scholar] [CrossRef]
- Huang, N.; Wang, L.; Song, X.P.; Andrew Black, T.; Jassal, R.S.; Myneni, R.B.; Wu, C.Y.; Wang, L.; Song, W.J.; Ji, D.B.; et al. Spatial and temporal variations in global soil respiration and their relationships with climate and land cover. Sci. Adv. 2020, 6, 8508. [Google Scholar] [CrossRef]
- De Deyn, G.B.; Cornelissen, J.H.C.; Bardgett, R.D. Plant functional traits and soil carbon sequestration in contrasting biomes. Ecol. Lett. 2008, 11, 516–531. [Google Scholar] [CrossRef]
- Moyes, A.B.; Bowling, D.R. Plant community composition and phenological stage drive soil carbon cycling along a tree-meadow ecotone. Plant Soil 2016, 401, 231–242. [Google Scholar] [CrossRef]
- Thomas, A.D.; Elliott, D.R.; Dougill, A.J.; Stringer, L.C.; Hoon, S.R.; Sen, R. The influence of trees, shrubs, and grasses on microclimate, soil carbon, nitrogen, and CO2 efflux: Potential implications of shrub encroachment for Kalahari rangelands. Land Degrad. Dev. 2018, 29, 1306–1316. [Google Scholar] [CrossRef]
- Bewley, D.; Essery, R.; Pomeroy, J.; Ménard, C. Measurements and modelling of snowmelt and turbulent heat fluxes over shrub tundra. Hydrol. Earth Syst. Sci. 2010, 14, 1331–1340. [Google Scholar] [CrossRef]
- Blok, D.; Heijmans, M.M.P.D.; Schaepman-Strub, G.; Konono, A.V.; Maximov, T.C.; Berendse, F. Shrub expansion may reduce summer permafrost thaw in Siberian tundra. Glob. Chang. Biol. 2010, 16, 1296–1305. [Google Scholar] [CrossRef]
- Parker, T.C.; Subke, J.; Wookey, P.A. Rapid carbon turnover beneath shrub and tree vegetation is associated with low soil carbon stocks at a subarctic treeline. Glob. Chang. Biol. 2015, 21, 2070–2081. [Google Scholar] [CrossRef] [PubMed]
- Wezel, A.; Rajot, J.L.; Herbrig, C. Influence of shrubs on soil characteristics and their function in sahelian agro-ecosystems in semi-arid Niger. J. Arid Environ. 2000, 44, 383–398. [Google Scholar] [CrossRef]
- Qu, W.L.; Yang, X.P.; Zhang, C.T.; Wei, B. Shrub-mediated “fertile island” effects in arid and semi-arid grassland. Acta Prataculturae Sin. 2015, 24, 201–207. [Google Scholar] [CrossRef]
- Sun, Q.; Meyer, W.S.; Koerber, G.R.; Marschner, P. Response of respiration and nutrient availability to drying and rewetting in soil from a semi-arid woodland depends on vegetation patch and a recent wildfire. Biogeosciences 2015, 12, 5093–5101. [Google Scholar] [CrossRef]
- Chen, Y.C.; Li, W.P.; You, Y.; Ye, C.; Shu, X.; Zhang, Q.F.; Zhang, K.R. Soil properties and substrate quality determine the priming of soil organic carbon during vegetation succession. Plant Soil 2022, 471, 559–575. [Google Scholar] [CrossRef]
- Hanson, P.J.; Edwards, N.T.; Garten, C.T.; Andrews, J.A. Separating root and soil microbial contributions to soil respiration: A review of methods and observations. Biogeochemistry 2000, 48, 115–146. [Google Scholar] [CrossRef]
- Wei, H.; Chen, X.M.; Xiao, G.L.; Guenet, B.; Vicca, S.; Shen, W.J. Are variations in heterotrophic soil respiration related to changes in substrate availability and microbial biomass carbon in the subtropical forests? Sci. Rep. 2015, 5, 18370. [Google Scholar] [CrossRef]
- Wang, H.; Button, T.W.; Xu, W.H.; Hu, G.Q.; Jiang, P.; Bai, E. Quality of fresh organic matter affects priming of soil organic matter and substrate utilization patterns of microbes. Sci. Rep. 2015, 5, 10102. [Google Scholar] [CrossRef]
- Jing, H.; Liu, Y.; Wang, G.L.; Liu, G.B. Effects of nitrogen addition on root respiration of trees and understory herbs at different temperatures in Pinus tabulaeformis forest. Plant Soil 2021, 463, 447–459. [Google Scholar] [CrossRef]
- Ataka, M.; Sun, L.; Nakaji, T.; Katayama, A.; Hiura, T. Five-year nitrogen addition affects fine root exudation and its correlation with root respiration in a dominant species, Quercus crispula, of a cool temperate forest, Japan. Tree Physiol. 2020, 40, 367–376. [Google Scholar] [CrossRef]
- He, H.; Liu, Y.X.; Hu, Y.; Zhang, M.Q.; Wang, G.D.; Shen, W.B. Soil microbial community and its interaction with soil carbon dynamics following a wetland drying process in Mu Us sandy land. Int. J. Environ. Res. Public Health 2020, 17, 4199–4217. [Google Scholar] [CrossRef]
- Huang, X.L.; Chen, J.Z.; Wang, D.; Deng, M.M.; Wu, M.Y.; Tong, B.L.; Liu, J.M. Simulated atmospheric nitrogen deposition inhibited the leaf litter decomposition of Cinnamomum migao H.W. Li in southwest China. Sci. Rep. 2021, 11, 1748. [Google Scholar] [CrossRef]
- Li, W.; Wang, J.L.; Li, X.L.; Wang, S.L.; Liu, W.H.; Shi, S.L.; Cao, W.X. Nitrogen fertilizer regulates soil respiration by altering the organic carbon storage in root and topsoil in alpine meadow of the north-eastern Qinghai-Tibet Plateau. Sci. Rep. 2019, 9, 13735. [Google Scholar] [CrossRef]
- Raich, J.W.; Schlesinger, W.H. The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus B. 1992, 44, 81–89. [Google Scholar] [CrossRef]
- Ryan, M.G.; Law, B.E. Interpreting, measuring, and modeling soil respiration. Biogeochemistry 2005, 73, 3–27. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, J.; Lin, Y.; He, X.; He, Z.; Kong, X. Drought Exerted a Stronger Controlling Effect on Soil Carbon Release than Moisturizing in a Global Meta-Analysis. Forests 2023, 14, 1957. https://doi.org/10.3390/f14101957
Xiao J, Lin Y, He X, He Z, Kong X. Drought Exerted a Stronger Controlling Effect on Soil Carbon Release than Moisturizing in a Global Meta-Analysis. Forests. 2023; 14(10):1957. https://doi.org/10.3390/f14101957
Chicago/Turabian StyleXiao, Jiamin, Yonghui Lin, Xingbing He, Zaihua He, and Xiangshi Kong. 2023. "Drought Exerted a Stronger Controlling Effect on Soil Carbon Release than Moisturizing in a Global Meta-Analysis" Forests 14, no. 10: 1957. https://doi.org/10.3390/f14101957
APA StyleXiao, J., Lin, Y., He, X., He, Z., & Kong, X. (2023). Drought Exerted a Stronger Controlling Effect on Soil Carbon Release than Moisturizing in a Global Meta-Analysis. Forests, 14(10), 1957. https://doi.org/10.3390/f14101957





