Abstract
The globose scale (GS) (Sphaerolecanium prunastri Boyer de Fonscolombe) is a sucking insect that feeds on saps of wild apricot tree (Prunus armeniaca L.) in the Xinjiang Uygur Autonomous Region of northwestern China. It feeds on branches of wild apricot leading to poor growth, leaf yellowing and leaf drop, and sometimes mortality. Since the initial infestation in 2018, all the main valleys of wild apricot trees have been affected, but there is little research on the host’s physiological response to GS infestation. We measured the differences in growth between infested and non-infested wild apricots. The results showed that the diameter of shoot branches, the fresh weight, dry weight, length, width, area, and chlorophyll content of leaves, and the longitudinal diameter of fruit, were all significantly less for infested wild apricot trees than for un-infested wild apricot. The branch length of shoots, cross diameter, and weight of fruit also decreased, although the differences were not significant. Overall, GS infestation significantly reduced the growth of wild apricot trees.
1. Introduction
The interaction of biological and abiotic factors determines the dynamic equilibrium in forest ecosystems, of which insects and plants are important components. Their interaction greatly influences species diversity, which is related to forest productivity [1]. Changes in the insect community have a great influence on the composition of forest ecosystems, and can directly or indirectly affect the abundance and distribution of other species [2]. Increasing of phytophagous insect populations can have a significant, negative impact on forest ecosystems by reducing the growth of trees [3,4]. By affecting their physiology and biochemistry, including chlorophyll content and photosynthesis rate, thus affecting their growth, even leading to their death [5,6,7]. The relationship between insect feeding and plant growth has long been the focus of research on forest ecosystem health, including mechanisms of tolerance to feeding [8,9].
The wild apricot (Prunus armeniaca L.), the ancestors of the cultivated apricot [10], is mainly distributed in the Tianshan and Himalayan regions of Central Asia, including the mountainous areas of northern and northeastern China, the northern and western Himalayas of India, and the Dzunggar and Zagara mountains of Central Asia [11,12]. It is also found in small numbers at high altitudes in Afghanistan and Pakistan [13].
It is an important wild fruit producing species in the forests of Yili, Tianshan, China, where it is a relict species in tertiary, deciduous, broad-leaved forest [14]. It is also an important genetic resource for the improvement of cultivated apricot varieties [13,15], an important tourism resource with significant economic and social value, and has been listed as a key national protected species in China at level II and a key protected wild plant at the sub-level of Xinjiang conservation priorities species list [16]. However, with the influence of environmental change, including human factors and pests, the area of wild fruit forest available for wild apricot collection in Yili, Xinjiang has shrunk, due in part to the difficulty in plant regeneration [17,18]. The survival of wild apricot is also under serious threats from the insect pest globose scale (GS) (Sphaerolecanium prunastri Boyer de Fonscolombe) (Hemiptera: Coccidae), a wax bug that sucks sap from the stem of the host plant.
Globose scale is native to the subtropical areas of the Palearctic Region and now is widely distributed in the Mediterranean Region, southern Europe, the Middle East, North America, China, some areas of Central Asia and the Far East [19,20]. In China, GS has caused damages to fruit producing trees such as apricots and peaches in Liaoning, Hebei, Shandong and Shaanxi Provinces [19,21]. Since 2018, GS has been widespread in the wild apricot forests in the Yili River Valley. Infestation has resulted in a large number of dead branches and a small number of dead wild apricot trees, which has reduced the health and ecological services functions of the forest ecosystem. Nevertheless, the relationship between the damage caused by GS and the growth rate of its host plants has not been reported.
The feeding of GS slows down the growth of wild apricot trees, with the level of infestationt actually posing a great threat to the survival of wild apricot forests. At present, the research on GS mainly focuses on its biological characteristics; occurrence patterns; damage; prevention of infestation, and control techniques including parasitism and predatory natural enemies [22,23,24]. Understanding the effects of GS on its host plants is critical to develop mitigation strategies for conservation. Therefore, the effects of the damage of GS on the growth of its host, the wild apricot tree, were studied, with the expectation of also better understanding the theoretical basis for the effects of sap sucking insects on the growth and health of plants more broadly.
2. Materials and Methods
2.1. Study Site and Treatment
The experiments were conducted at in Kuerdening town in Gongliu County, Yili, China to investigate the impact of GS infestation on the branch, and leaf growth, chlorophyll SPAD values and fruit characteristics of the wild apricot tree, P. armeniaca. The experimental site was located at 43°14′53.41″ N–43°15′25.40″ N and 82°49′39.79″ E–82°49′40.15″ E, where P. armeniaca was a dominant species in the experimental forest stands with other tree and shrub species including Malus sieversii (Ledeb.) Roem, Lonicera hispida Pallas ex Schultes, Crataegus sanguine Pallas, and Berberis nummularia Bunge. The region has a typical temperate, alpine, continental climate. The climate is mild and humid, with large temperature differences between day and night, and an average annual temperature of 5–7 °C. Rainfall is concentrated in spring and summer, and the average annual precipitation ranges from 200 mm to 800 mm. The frost-free period is about 150 days [17,25].
The heights of the experimental trees, randomly selected, ranged from 3.5 m to 4.5 m, and the basal diameter ranged from 6.5 cm to 8.5 cm. At the stage of female adult enlargement of S. prunastri, on the 25 May 2020, 120 leaves were collected from six GS-infested trees and 60 leaves were collected from three control, uninfested, wild apricot trees (there were very few uninfested trees in the study area). The diameters of the wild apricot fruits were measured at ripening stage. 30 fruits were collected from both the six the GS-infested trees and three control wild apricot trees, respectively.
2.2. Shoot Growth
The length and basal diameter of three current year shoots were measured. Measurements were taken from the base of the new shoot to the shoot tip with a steel tape measure and 1 cm from the base with a digital electronic vernier caliper (0–300 mm, Shanghai Jiuliang Hardware Tools Co., Ltd., Shanghai, China), respectively. Measurements were taken twice and the mean value was used.
2.3. Leaf Traits
The area of the leaves of wild apricot trees was measured with a portable YMJ-B living leaf area meter (Hangzhou Tuopu Instrument Co., Ltd., Hangzhou, China), and the leaf length and width were recorded. Each leaf trait was measured three times and the average value was taken.
2.4. Chlorophyll SPAD Values
The chlorophyll SPAD values were measured by using a portable daily chlorophyll tester spad-502plus (Konica Minolta Holdings, Inc., Tokyo, Japan). Each leaf was tested 3 times in 6 different areas, including the proximal, middle and distal parts, and the average values were taken.
2.5. Leaf Biomass
An electronic balance (0.0001 g, HUAZHI PTX-FA210, Fuzhou Huazhi Scientific Instrument Co., Ltd., Fuzhou, China) was used to measure the fresh weight of the leaves. After measurement, they were placed in an oven and dried to a constant weight at 80 °C, removed and cooled. The dry weight of the leaves was determined immediately, with the average value of three weightings calculated.
2.6. Fruit Traits
The length and width of fruit were measured with digital electronic vernier calipers (0–300 mm, Shanghai Jiuliang Hardware Tools Co., Ltd., Shanghai, China), and the fresh weight of single fruits was measured with an electronic balance.
2.7. Data Analysis
SPSS 19.0 software was used to conduct the Independent sample t-test, and Origin 7.0 software was used to construct the graphs. Prior to all analyses, to satisfy the requirement for normal distribution of data, all of the original data were normally transformed through the Normal scores in Rank Cases using SPSS 19.0 software. The Independent sample t-test was used to analyze the differences between the values for the GS-infested trees and the control trees. All analyses were done at the 1% significance level.
3. Results
3.1. Shoot Growth
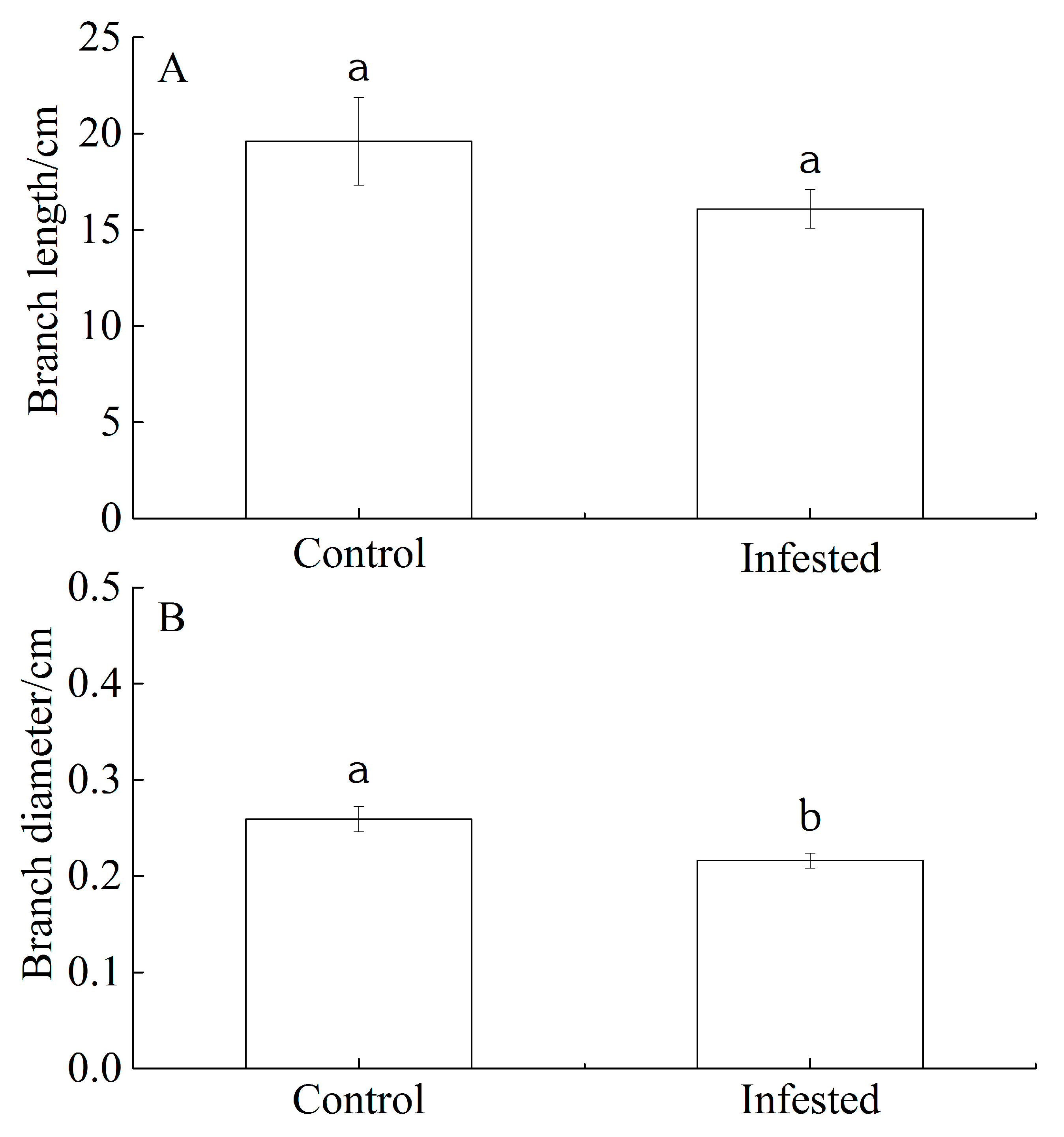
The branch length and branch diameter of GS-infested wild apricot trees decreased by 17.92% and 16.61%, respectively, compared with those of the control trees. We interpreted this as evidence for GS-induced wild apricot growth reduction. However, there was no significant difference for branch length (p = 0.202; Figure 1A). but there was a significant difference for branch diameter (p < 0.01; Figure 1B).
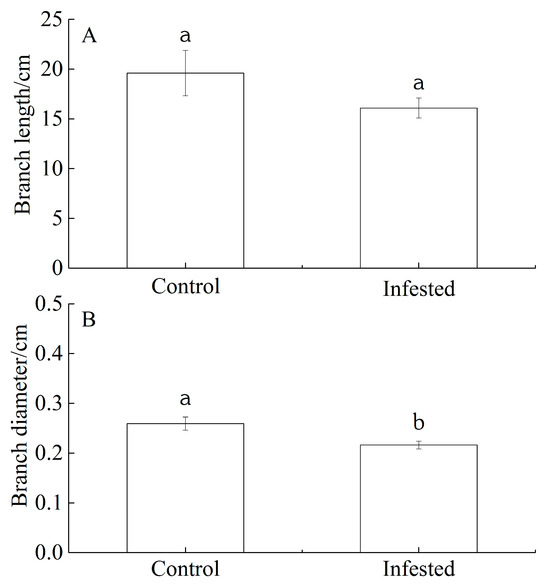
Figure 1.
Differences in (A) branch length and (B) branch diameter of Prunus armeniaca infested with Sphaerolecanium prunastri (infested) and in the uninfested controls. Data: mean ± SE. Different lowercase letters indicate a significant difference (Independent two-sample t-test; p < 0.01).
3.2. Leaf Traits
GS-infested trees had significantly lower relative leaf length, leaf width and leaf area than the control trees (Table 1). We interpret this as evidence of GS-induced wild apricot leaf deficit.

Table 1.
Differences in leaf length, leaf width and leaf area of Prunus armeniaca infested with Sphaerolecanium prunastri and in the non-infested controls.
3.3. Chlorophyll SPAD Values
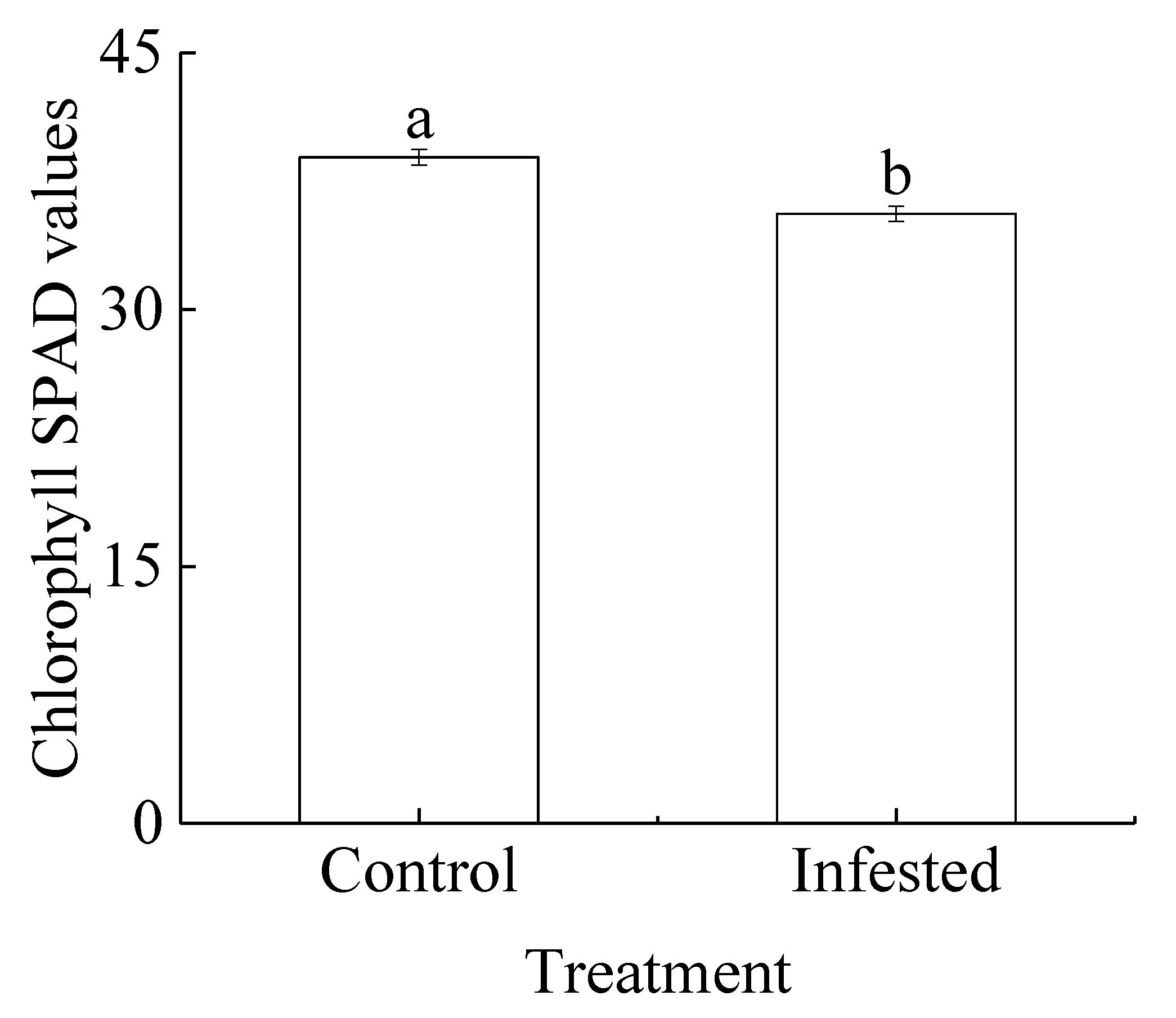
The mean chlorophyll SPAD value for the GS-infested wild apricot trees was 8.49% lower than that of the control trees, and the difference was significant (p < 0.01; Figure 2).
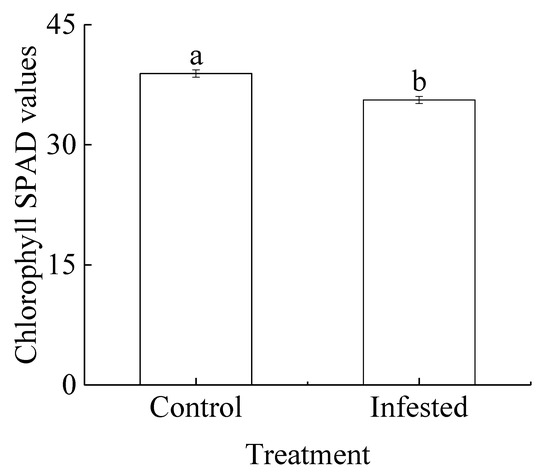
Figure 2.
Comparison of chlorophyll SPAD values (mean ± SE) for Prunus armeniaca trees infested with Sphaerolecanium prunastri and the controls. Different lowercase letters indicate a significant difference (Independent two-sample t-test; p < 0.01).
3.4. Leaf Biomass
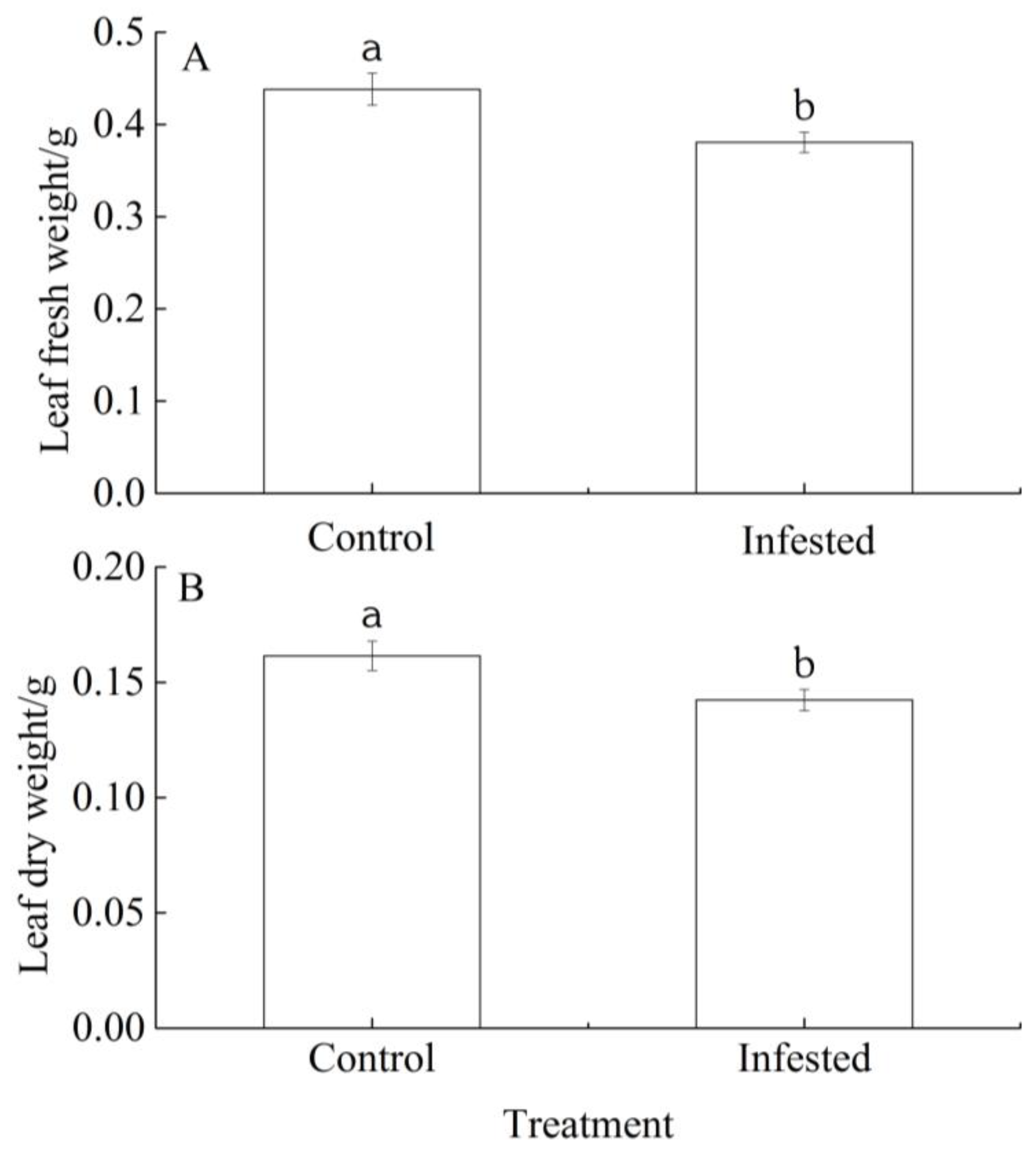
The fresh and dry leaf weights of the GS-infested trees were 13.14% and 11.89% lower, respectively, than those of the controls, and in both cases the differences were significant (p < 0.01; Figure 3A,B).
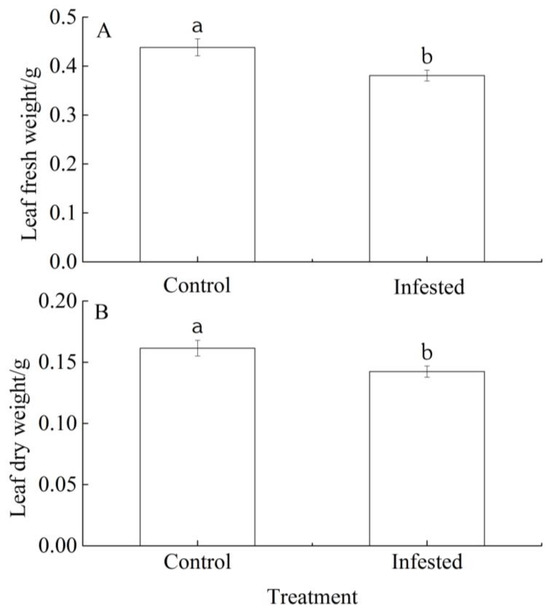
Figure 3.
Differences in (A) leaf fresh weight and (B) leaf dry weight of Prunus armeniaca infested with Sphaerolecanium prunastri and the uninfested controls. Data: mean ± SE. Different lowercase letters indicate a significant difference (Independent two-sample t-test; p < 0.01).
3.5. Fruit Traits
The GS-infested trees had significantly lower fruit length than the control trees (Table 2). Additionally, although there were trends for greater fruit width and fruit weight in the controls than the GS trees, the differences were not significant (Table 2).

Table 2.
Fruit length, fruit width, and fruit weight of Prunus armeniaca infested with Sphaerolecanium prunastri and uninfested controls.
4. Discussion
4.1. Effects of GS Infestation on the Growth of Wild Apricot Trees
Insects can affect the overall physiological health, appearance and photosynthetic performance of plants, and thus change the species diversity and social and economic value of forest ecosystems. For example, infestation by bud moth larvae (Lasiognatha cellifera Meyrick) reduced the leaf area and amount of flowering and increased the amount of deciduous leafs, in a mangrove ecosystem [3]. In the forests of southern California, the invasion of the gold-spotted oak borer (Agrilus coxalis Waterhouse) resulted in the death of oak trees and reduced the landscape value of the forest [26].
Our study showed that GS had a significant adverse effect on the length of new shoots and leaves, branch diameter and leaf area of wild apricot trees. Similar results have also been reported for date palm trees infected by Parlatoria blanchardii (Targ.) [27], Vitis vinifera infested by Parthenolecanium pruinosum Cocquillet [28] and Hibiscus rosa-sinensis L. infested by Phenacoccus solenopsis Tinsley [29]. GS infection leads to poor growth, decreased nutrient levels, reduced stress resistance and increased vulnerable to GS, other insect pests and diseases [8]. Thus a “vicious cycle” of deteriorating health afflicts the host tree until it dies, and GS is transferred to or moves to the next host. Globose scale is therefore a major, direct threat to the survival of wild apricot trees, which implies the ongoing loss of genetic and phenotypic diversity and reductions in its economic and landscape values.
4.2. Effects of GS Infestation on the Leaf Traits of Wild Apricot Trees
Leaves are the largest organs with which plants interact with their environment, and their morphology and physiology changes under different ecological conditions. Under pest stress, leaf morphology can change, leading to leaf yellowing or even shedding. For example, the transpiration rate of the leaves of Euonymus fortunei (Turcz.) Hand Mazz infested by euonymus scale (Unaspis euonymi Comstock) were vulnerable to withering and leaf fall [30,31]. Leaf yellowing of E. fortunei was caused by the destruction of chloroplasts due to euonymus scale infestation, with the chlorophyll content decreased by 49% on average due to the feeding of two generations of scale [32]. Also, feeding by Toumeyella sp. caused a reduction in the leaf area, leaf dry weight and chlorophyll content of Guaiacum sanctum L. in comparison to uninfested trees, leading to decreased tree growth [33]. Our study found that the fresh weight, dry weight, leaf length, leaf width, leaf area and relative chlorophyll content (chlorophyll SPAD value) of GS-infested wild apricot trees were significantly lower than those of uninfested trees, which essentially corroborates the research results above. Similar results were also found for palm trees fed by P. blanchardii (Targ.) [27]. Similar results have also been reported for other piercing-sucking pests, such as the feeding of adults of Bagrada hilaris Burmeister which significantly reduced the leaf area, relative chlorophyll content and dry weight of its host plant [34]. The above studies buttress our assertion that infestation by GS causes leaf diminution, yellowing and even shedding in wild apricot trees. Premature leaf fall inhibited branch growth, and the survival rate of branches with fewer leaves was lower [35], which may explain the large number of withered branches observed on in the wild apricot trees in the present study.
4.3. Effects of GS Infestation on Photosynthetic Products of Wild Apricot Trees
The source-sink relationships of plants are relative and they change at different stages of plant development. During the reproductive stage,, the leaf is the “source” and the fruit is the main “sink” [36]. Pests affect photosynthesis and can change the proportion of source-sink tissues in plants and affect photosynthesis [33]. Leaves are the main organs of photosynthesis and transpiration in green plants, and they also have certain functions related to absorption, reproduction and storage. In the study of the relationship between insect pest and plant growth, the change of chlorophyll content can reflect the damage level of the host plant [37]. Under pest stress, leaf size diminished, chlorophyll content declined, and then photosynthate production reduced, and dry matter accumulation decreased [27,38,39]. Similar results were reported for the feeding of Phenacoccus solenopsis (Tinsley) on Solanum lycopersicum L. [40], mango shield scale (Kilifia acuminata Signoret) on mango [41], and Coccus hesperidum L. on Citrus limon and Nephrolepis biserrata [42]. Scale insects cause the loss of the green coloring of plant leaves and ultimately reduce photosynthesis [43] by reducing the maximum rate of electron transport in plants [44].
Our research results showed that the leaf area and relative chlorophyll content of wild apricot trees decreased significantly after GS infestation, so it can be inferred that the photosynthesis of wild apricot trees also decreased. In contrast, Retuerto et al. found that the photosynthetic rate of European holly (Ilex aquifolium L.) was enhanced after being infested with Coccus sp., which was considered to be a compensatory response [45]. The plant compensation effect is common, but under high population pressure or long term harm, the compensation effect is insufficient to make up for the loss caused by insect feeding, and the plants show serious deterioration [46,47,48]. This phenomenon was clearly observed in wild apricot trees seriously infested with GS.
A considerable number of studies have demonstrated that herbivorous insects inhibit the photosynthesis of plants and reduce photosynthesis, while the compensatory enhancement of plant photosynthesis is rarely found [49]. The study of Velikova et al. showed that both the feeding and oviposition behaviors of herbivorous insects inhibit the photosynthesis of host plants [50]. In addition, wild apricot trees seriously infested by GS have large areas of leafless dead branches, which reduced the rate of photosynthesis of the plant [51].
When the “source” of the plant changes, the corresponding “sink” will also be affected. Scale insect infestation can be equated to an additional pools of plants that compete with an individual plant’s own absorption pools and ability to consume nutrients while changing the distribution of plant nutrients [46]. There is some evidence that the production of honeydew by scale insects, in addition to the inhibition of photosynthesis, also consumes plant nutrients [52,53,54]. Furthermore, the fruit yield and quality of host plants decreased after being affected by scale insects [55], e.g., kiwifruit (Actinidia chinensis Planch.) infested by scale insect pests, Hemiberlesia rapax Comstock and H. lataniae Signoret [56], mango plants infested by Icerya seychellarum Westwood and Aonidiella aurantii Maskell [57,58], and coconut palms infested by Aspidiotus rigidus Reyne [7]. The current study also found that the fruit appearance deteriorated, and yield of wild apricot trees decreased after GS infestation, which was essentially consistent with the above research results. Similar results have been reported for other sucking pests, such as a decline in the yield of Brassica carinata A. Braun infested by Brevicoryne brassicae L. and Lipahis eyrsimi Kalt [59].
The decrease in leaf dry weight can also be used as an argument for a decrease in photosynthate production due to reduced photosynthesis in wild apricot trees, which is also attributable to a decrease in leaf area [60,61]. Lu and Ge observed the stylet damage of Matsucoccus matsumurae (Kuwana) in slices of damaged shoots and further reported punctures in the phloem cambium, and destroyed xylem and tracheids [62]. They suggested that scale insects are not only depleting plants of nutrients but also inhibiting the transportation of water and nutrients in the tree itself, thereby weakening growth and, in severe cases, killing plants. Similarly, the removal of nutrients by GS to wild apricot trees can also explain, at least in part, the decline in the health of their shoots and even the large number of dead branches observed [63].
4.4. Effects of GS Infestation on Wild Apricot Trees
The growth and survival of wild apricot are threatened by GS, and the ecological balance of the forests in which they grow is also affected. When large aggregations of some herbivorous insect species occur, they cause foliar damage, crown loss and under high feeding pressure, the mortality of host trees, and the composition, structure and age distribution of the forest changes to varying degrees [64,65]. Similar studies have been reported for wild apples (Malus sieversii (Ledeb.) M. Roem) [66], mangroves in South China [3], and eastern hemlock (Tsuga canadensis (L.) Carrière) in Massachusetts, USA [67]. Compared with mechanical damage, herbivorous insects caused a greater reduction in the physiological indices of plants [68]. Compared to the leaf-eaters, pests with sap-sucking and stem feeding mouthparts cause more adverse effects to host plants [67,69,70]. However, regardless of the type of herbivorous insect, its damage will have an impact on growth and reproduction of the host plant, and also on the plant’s ecosystem [71,72]. Delucia et al. also asserted that effects of herbivorous insects on host plants can unbalance ecosystem [73]. Liu et al. pointed out that the mobility of herbivorous insects enables them to spread to a wider ranges [74]. Also, Ancheta and Heard, who reviewed 37 articles on the relationship between herbivorous insects and rare plants, asserted that herbivorous insects greatly reduce the survival ability and reproduction, and may even cause the extinction of some species [75]. Myers and Sarfeaz also reported similar review results [76].
From our research results, we can conclude that the GS- infestation of wild apricot trees has a negative impact on their growth and reproduction, which is not conducive to their survival and therefore has a negative impact on the forest ecosystem. Domec et al. reported that the death mechanism of eastern hemlock trees was attributable to a decrease in water use and carbon assimilation caused by hemlock woolly adelgid (Adelges tsugae Borchsenius) infestation [77]. Also, Haavik et al. suggested that carbon imbalance is a potential mechanism for the death of oaks infested by pests and diseases [78]. The mechanism of GS infestation leading to the death of wild apricot trees needs further study. However, it is predictable that the death of a large number of wild apricot tree branches, or of the trees themselves, caused by GS, will ultimately lead to the decline of apricot population [46], which will also exacerbate the imbalance of the wild fruit forest ecosystem.
4.5. Management and Conservation
Sustaining the health of the wild apricot forest ecosystem is more critical than merely reducing pest density. Natural enemies [79] and pruning [80] may contribute to pest management of the GS and resilience of wild apricot forests. Releasing of the natural enemies at the enlargement period of 2nd-instar overwintering nymphs and the dispersal period of 1st-instar nymphs, and pruning of infested branches in spring may effectively reduce the number of GS and improve the wild apricot forest regeneration and ecosystem health. Rebuilding and enhancing ecosystem resilience at various levels, from the individual tree to the forest ecosystem, should be considered in the management.
5. Conclusions
In general, our results showed that after GS infestation, the growth of branches and leaves, and the relative chlorophyll content and fruit quality of wild apricot trees, all decreased. These physiological deterioration are helpful in elucidating the mechanisms of GS-induced leaf shrinkage and withering, and ultimately the death of wild apricot trees. Long-term monitoring of GS populations in wild fruit forests, from initial GS infestation to the death of wild apricot trees, and their ecosystem, is recommended. This is necessary to better clarify the mechanisms the GS-induced death of wild apricot trees, and more broadly, the factors contributing to the collapse of their forest ecosystem.
Author Contributions
Conceptualization, G.G. and Z.L. designed this study; W.L. and Y.W. collected the data; W.L. and G.G. wrote the first draft of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Central goverment finance in the project of forest and grass science and technology popularization demonstration project, grant number Xin 2020TG09 and Tianshan Talents Program of Xinjiang Uygur Autonomous Region.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liang, J.J.; Crowther, T.W.; Picard, N.; Wiser, S.K.; Zhou, M.; Alberti, G.; Schulze, E.D.; McGuire, A.D.; Bozzato, F.; Pretzsch, H.; et al. Positive biodiversity-productivity relationship predominant in global forests. Science 2016, 354, aaf8957. [Google Scholar] [PubMed]
- Zhang, H.Y.; Ou, X.H. Using insect for indicator to monitor and assess forest ecosystm health. World For. Res. 2006, 19, 22–25. [Google Scholar]
- Lu, W.Z.; Xiao, J.F.; Cui, X.W.; Xu, F.H.; Lin, G.X.; Lin, G.H. Insect occurs have transient effects on carbon fluxes and vegetative growth but longer-term impacts on reproductive growth in a mangrove forest. Agric. For. Meteorol. 2019, 279, 107747. [Google Scholar]
- Michael, A. Are changes in plants due to enhanced CO2 contributing to insect population declines? Environ. Entomol. 2019, 48, 274–275. [Google Scholar]
- Smith, J.P.; Schowalter, T.D. Aphid-induced reduction of shoot and root growth in Douglas-fir seedlings. Ecol. Entomol. 2010, 26, 411–416. [Google Scholar]
- Rubino, L.; Charles, S.; Sirulnik, A.G.; Tuininga, A.R.; Lewis, J.D. Invasive insect effects on nitrogen cycling and host physiology are not tightly linked. Tree Physiol. 2015, 35, 124–133. [Google Scholar]
- Watson, G.W.; Adalla, C.B.; Shepard, B.M.; Carner, G.R. Aspidiotus rigidus Reyne (Hemiptera: Diaspididae): A devastating pest of coconut in the Philippines. Agric. For. Entomol. 2015, 17, 1–8. [Google Scholar]
- Han, P.; Becker, C.; Bot, J.L.; Larbat, R.; Lavoir, A.V.; Desneux, N. Plant nutrient supply alters the magnitude of indirect interactions between insect herbivores: From foliar chemistry to community dynamics. J. Ecol. 2019, 108, 1497–1510. [Google Scholar]
- You, Y.R.; An, C.P.; Li, C.Y. Insect feeding assays with Spodoptera exigua on Arabidopsis thaliana. Bio-protocol 2020, 10, e3538. [Google Scholar] [CrossRef]
- Li, W.W.; Liu, L.Q.; Wang, Y.N.; Zhang, Q.P.; Fan, G.Q.; Zhang, S.K.; Wang, Y.T.; Liao, K. Genetic diversity, population structure, and relationships of apricot (Prunus) based on restriction site-associated DNA sequencing. Hortic. Res. 2020, 7, 69–82. [Google Scholar]
- Zeven, A.C.; Zhukovsky, P.M. Dictionary of Cultivated Plants and Their Centres of Diversity: Excluding Ornamentals, Forest Trees and Lower Plants; Centre for Agricultural Publishing and Documentation: Wageningen, The Netherlands, 1975; p. 36. [Google Scholar]
- Zhebentyayeva, T.; Reighard, G.; Gorina, V.; Abbott, A. Simple sequence repeat (SSR) analysis for assessment of genetic variability in apricot germplasm. Theor. Appl. Genet. 2003, 106, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.K.; Chaudhury, R.; Dhariwal, O.P.; Mir, S. Genetic diversity and traditional uses of wild apricot (Prunus armeniaca L.) in high-altitude north-western Himalayas of India. Plant Genet. Resour. 2010, 8, 249–257. [Google Scholar]
- Zhang, X.S. On the eco-geographical characters and the problems of classification of the wild fruit-tree forest in the Ili Valley of Sinkiang. Acta Bot. Sin. 1973, 15, 239–253. [Google Scholar]
- Khadivi-Khub, A.; Khalili, Z. A breeding project: The selection of promising apricot (Prunus armeniaca L.) genotypes with late blooming time and high fruit quality. Sci. Hortic. 2017, 216, 93–102. [Google Scholar]
- Liu, W.; Chen, X.S.; Liu, G.J.; Liang, Q.; He, T.M.; Feng, J.R. Interspecific hybridization of Prunus persica with P. armeniaca and P. salicina using embryo rescue. Plant Cell Tissue Organ Cult. 2007, 88, 289–299. [Google Scholar]
- Hou, B.; Xu, Z. Relationship of the occurences and evolutions of wild-fruit forests with climatic factors in the Tinshan Mountain. Acta Bot. Boreali-Occident. Sin. 2005, 25, 2266–2271. [Google Scholar]
- Fang, Z.Y.; Li, L.Y.; Maola, A.; Zhou, L.; Lu, B. Effects of human disturbance on plant diversity of wild fruit forests in western Tianshan Mountain. Bull. Soil Water Conserv. 2019, 39, 267–274. [Google Scholar]
- Wang, Z.Q. Zootaxa of China. Insecta XXII; Science Press: Beijing, China, 2001; pp. 237–243. [Google Scholar]
- Malumphy, C.; Anderson, H. Plant Pest Factsheet: Globose Scale, Sphaerolecanium prunastri [EB/OL]. Available online: https://planthealthportal.defra.gov.uk/assets/factsheets/Plant-Pest-Factsheet-S.-prunastri-June-2016v3.pdf (accessed on 28 September 2016).
- Wang, C. Investigation on the Species of Coccaidea from Shaanxi Garden (Hemiptera: Coccoidea); Northwest A & F University: Xianyang, China, 2010; p. 21. [Google Scholar]
- Karaca, I.; Japoshvili, G.; Demirozer, O. The chalcid parasitoid complex (Hymenoptera: Chalcidoidea) associated with the globose scale (Sphaerolecanium prunastri Fonscolombe) (Hemiptera: Coccoidea) in Isparta Province, Turkey and some east European countries. J. Plant Dis. Prot. 2003, 110, 505–511. [Google Scholar]
- Aksit, T.; Apak, F.K. Effects of some insecticides with infestation rate and some biological characteristics of Sphaerolecanium prunastri (Fonscolombe, 1834) (Hemiptera: Coccidae) on plum. Turkiye Entomol. Derg. 2013, 37, 133–144. [Google Scholar]
- Yiğit, T.; Tunaz, H. Determination of Sphaerolecanium prunastri Fonscolombe, 1834 (Hemiptera: Coccidae), and its parasitoids and predators in apricot areas of Malatya province, Turkey. Acta Hortic. 2018, 1214, 67–71. [Google Scholar]
- Yang, Y.H.; Chen, Y.N.; Li, W.H.; Chen, Y.P. Distribution of soil organic carbon under different vegetation zones in the Ili River Valley, Xinjiang. J. Geogr. Sci. 2010, 20, 729–740. [Google Scholar]
- Coleman, T.W.; Seybold, S.J. Previously unrecorded damage to oak, Quercus spp., in southern California by the goldspotted oak borer, Agrilus coxalis Waterhouse (Coleoptera: Buprestidae). Pan-Pac. Entomol. 2008, 84, 288–300. [Google Scholar] [CrossRef]
- Moussa, S.; Salman, A.; Bakry, M. The negative effects of Parlatoria blanchardii (Targ.) infestation on the morphological and chemical characters of certain varieties leaflets of date palm trees at Luxor governorate. Egypt. Acad. J. Biol. Sci. 2012, 5, 169–181. [Google Scholar]
- Simbiken, N.A.; Cooper, P.D.; Powell, K.S. Development and feeding effect of frosted scale Parthenolecanium pruinosum Cocquillet (Hemiptera: Coccidae) on selected Vitis vinifera L. cultivars. Aust. J. Grape Wine Res. 2015, 21, 451–457. [Google Scholar]
- Sun, F.; Ye, W.F.; Pan, Z.P.; Lu, Y.Y. Effect on population density of Phenacoccus solenopsis Tinsley on the growth of Hibiscus rosa-sinensis. J. Environ. Entomol. 2021, 43, 60–65. [Google Scholar]
- Cockfield, S.D.; Potter, D.A. Interaction of Euonymus scale (Homoptera: Diaspididae) feeding damage and severe water stress on leaf abscission and growth of Euonymus fortunei. Oecologia 1986, 71, 41–46. [Google Scholar]
- Cockfield, S.D.; Potter, D.A. Euonymus scale (Homoptera: Diaspididae) effects on plant growth and leaf abscission and implications for differential site selection by male and female scales. J. Econ. Entomol. 1990, 83, 995–1001. [Google Scholar]
- Cockfield, S.D.; Potter, D.A.; Houtz, R.L. Chlorosis and reduced photosynthetic CO2 assimilation of Euonymus fortunei infested with Euonynlus scale (Homoptera: Diaspididae). Environ. Entomol. 1987, 16, 1314–1318. [Google Scholar] [CrossRef]
- Schaffer, B.; Mason, L.J. Effects of scale insect herbivory and shading on net gas exchange and growth of a subtropical tree species (Guaiacum sanctum L.). Oecologia 1990, 84, 468–473. [Google Scholar]
- Huang, T.I.; Reed, D.A.; Perring, T.M.; Palumbo, J.C. Feeding damage by Bagrada hilaris (Hemiptera: Pentatomidae) and impact on growth and chlorophyll content of Brassicaceous plant species. Arthropod-Plant Interact. 2014, 8, 89–100. [Google Scholar]
- Canelo, T.; Gaytán, Á.; González-Bornay, G.; Bonal, R. Seed loss before seed predation: Experimental evidence of the negative effects of leaf feeding insects on acorn production. Integr. Zool. 2018, 13, 238–250. [Google Scholar] [PubMed]
- Proietti, P. Effect of fruiting on leaf gas Exchange in Olive (Olea europaea L.). Photosynthetica 2000, 38, 397–402. [Google Scholar] [CrossRef]
- Hura, T.; Hura, K.; Grzesiak, M.; Rzepka, A. Effect of long-term drought stress on leaf gas exchange and fluorescence parameters in C3 and C4 plants. Acta Physiol. Plant. 2007, 29, 103–113. [Google Scholar]
- Sharma, S.; Kumar, H.; Prasad, F.M.; Singh, K. Chlorophyll and carotenoid loss in Psidium guajva caused by Abgrallaspis cyanophylli. Ann. Plant Protect. Sci. 2009, 17, 245–246. [Google Scholar]
- Goławska, S.; Krzyżanowski, R.; Łukasik, I. Relationship between aphid infestation and chlorophyll content in fabaceae species. Acta Biol. Crac. Bot. 2010, 52, 76–80. [Google Scholar]
- Huang, J.; Zhang, P.J.; Zhang, J.; Lu, Y.B.; Huang, F.; Li, M.J. Chlorophyll content and chlorophyll fluorescence in tomato leafs tnfested with an invasive mealybug, Phenacoccus solenopsis (Hemiptera: Pseudococcidae). Environ. Entomol. 2013, 42, 973–979. [Google Scholar]
- Nabil, H.A. Relationship Between Kilifia acuminata (Signoret) and Chlorophyll Percentage Loss on Mango leafs. J. Entomol. 2013, 10, 110–114. [Google Scholar] [CrossRef][Green Version]
- Golan, K.; Rubinowska, K.; Kmiec, K.; Kot, I.; Gorska-Drabik, E.; Lagowska, B.; Michalek, W. Impact of scale insect infestation on the content of photosynthetic pigments and chlorophyll fluorescence in two host plant species. Arthropod-Plant Interact. 2015, 9, 55–65. [Google Scholar]
- Walstad, J.D.; Nielsen, D.G.; Johnson, N.E. Effect of the pine needle scale on photosynthesis of Scots pine. For. Sci. 1973, 19, 109–111. [Google Scholar]
- Moore, G.W.; Watts, D.A.; Goolsby, J.A. Ecophysiological responses of giant reed (Arundo donax) to herbivory. Invasive Plant Sci. Manag. 2010, 3, 521–529. [Google Scholar]
- Retuerto, R.; Fernandez-Lema, B.; Rodriguez-Roiloa; Obeso, J.R. Increased photosynthetic performance in holly trees infested by scale insects. Funct. Ecol. 2004, 18, 664–669. [Google Scholar]
- Vranjic, J.O.; Ash, J.E. Scale insects consistently affect roots more than shoots: The impact of infestation size on growth of eucalypt seedlings. J. Ecol. 1997, 85, 143–149. [Google Scholar] [CrossRef]
- Garcia, L.C.; Eubanks, M.D. Overcompensation for insect herbivory: A review and meta-analysis of the evidence. Ecology 2019, 100, 2585–2625. [Google Scholar]
- Nordkvist, M.; Klapwijk, M.J.; Edenius, L.R.; Björkman, C. Interacting effects of insect and ungulate herbivory on Scots pine growth. Sci. Rep. 2020, 10, 22341. [Google Scholar] [PubMed]
- Kerchev, P.I.; Fenton, B.; Foyer, C.H.; Hancock, R.D. Plant responses to insect herbivory: Interactions between photosynthesis, reactive oxygen species and hormonal signalling pathways. Plant Cell Environ. 2012, 35, 441–453. [Google Scholar]
- Velikova, V.; Salerno, G.; Frati, F.; Peri, E.; Conti, E.; Colazza, S.; Loreto, F. Influence of feeding and oviposition by phytophagous pentatomids on photosynthesis of herbaceous plants. J. Chem. Ecol. 2010, 36, 629–641. [Google Scholar] [PubMed]
- Delaney, K.J.; Haile, F.J.; Peterson, R.K.D.; Higley, L.G. Seasonal patterns of leaf photosynthesis after insect herbivory on Common Milkweed, Asclepias syriaca: Reflection of a physiological cost of reproduction, not defense? Am. Midl. Nat. 2009, 162, 224–238. [Google Scholar]
- Dungan, R.J.; Kelly, D.; Turnbull, M. Separating host-tree and environmental determinants of honeydew production by Ultracoelostoma scale insects in a Nothofagus forest. Ecol. Entomol. 2007, 32, 338–348. [Google Scholar]
- Dungan, R.J.; Turnbull, M.H.; Kelly, D. The carbon costs for host trees of a phloem-feeding herbivore. J. Ecol. 2007, 95, 603–613. [Google Scholar]
- Miezite, O.; Okmanis, M.; Indriksons, A.; Indriksons, A.; Ruba, J.; Polmanis, L.; Freimane, L. Assessment of sanitary conditions in stands of Norway spruce (Picea abies Karst.) damaged by spruce bud scale (Physokermes piceae Schrnk.). iForest-Biogeosci. For. 2013, 6, 73–78. [Google Scholar]
- Martins, D.S.; Fornazier, M.J.; Culik, M.P.; Ventura, J.A.; Ferreira, P.S.F.; Zanuncio, J.C. Scale insect (Hemiptera: Coccoidea) pests of Papaya (Carica papaya) in Brazil. Ann. Entomol. Soc. Am. 2015, 108, 35–42. [Google Scholar]
- Hill, M.G.; Mauchline, N.A.; Jones, M.K.; Sutherland, P.W. The response of resistant kiwifruit (Actinidia chinensis) to armoured scale insect (Diaspididae) feeding. Arthropod-Plant Interact. 2011, 5, 149–161. [Google Scholar]
- Salman, A.M.A.; Bakry, M.M.S. Relationship between the rate of infestation with the Mealybug, Icerya seychellarum (Westwood) (Margarodidae: Homoptera) and the yield loss of seedy Balady Mango trees at Luxor governorate. World Rural Observ. 2012, 4, 50–56. [Google Scholar]
- Bakry, M.M.S.; Mohamed, G.H. Relationship between the rates of infestation with the California red scale insect, Aonidiella aurantii (Mask.) (Hemiptera: Diaspidae) and the yield loss of mango trees at Luxor governorate, Egypt. Egypt. J. Agric. Res. 2015, 93, 41–60. [Google Scholar]
- Hussain, A.; Razaq, M.; Zaka, S.M.; Shahzad, W.; Mahmood, K. Effect of aphid infestation on photosynthesis, growth and yield of Brassica carinata A. Braun. Pak. J. Zool. 2015, 47, 1335–1340. [Google Scholar]
- May, B.M.; Carlyle, J.C. Effect of defoliation associated with Essigella californica on growth of mid-rotation Pinus radiata. For. Ecol. Manag. 2003, 183, 297–312. [Google Scholar]
- Mustafa, F. Switchgrass (Panicum virgatum L.) Yield and Photosynthetic Response to Simulated Insect Defoliation and Grasshopper (Orthoptera: Acrididae) Feeding; The University of Nebraska-Lincoln: Lincoln, NE, USA, 2013; pp. 85–98. [Google Scholar]
- Lu, Q.H.; Ge, Z.H. Observation on the stylet and the parasitical site segment of Japanese pine bast scale. For. Res. 1988, 1, 191–194. [Google Scholar]
- Miller-Pierce, M.R.; Orwig, D.A.; Preisser, E. Effects of hemlock woolly adelgid and elongate hemlock scale on eastern hemlock growth and foliar chemistry. Environ. Entomol. 2010, 39, 513–519. [Google Scholar] [CrossRef]
- Orwig, D.A.; Thompson, J.R.; Povak, N.A.; Manner, M.; Niebyl, D.; Forter, D.R. A foundation tree at the precipice: Tsuga canadensis health after the arrival of Adelges tsugae in central New England. Ecosphere 2012, 3, 1–16. [Google Scholar]
- Maclean, D.A. Impacts of insect occurs on tree mortality, productivity, and stand development. Can. Entomol. 2015, 148, S138–S159. [Google Scholar] [CrossRef]
- Zhang, P.; Cui, Z.J.; Xu, H.; Ali, A.; Zhang, X.; Liu, X.X.; Zhang, Y.M.; Zhou, X.B.; Lu, Z.Z. Thirst or malnutrition: The impacts of invasive insect Agrilus mali on the physiological status of wild apple trees. Forests 2020, 11, 440. [Google Scholar] [CrossRef]
- Radville, L.; Chaves, A.; Preisser, E.L. Variation in plant defense against invasive herbivores: Evidence for a hypersensitive response in eastern hemlocks (Tsuga canadensis). J. Chem. Ecol. 2011, 37, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Visakorpi, K.; Gripenberg, S.; Malhi, Y.; Bolas, C.; Oliveras, I.; Harris, N.; Rifai, S.; Riutta, T. Small-scale indirect plant responses to insect herbivory could have major impacts on canopy photosynthesis and isoprene emission. New Phytol. 2018, 220, 799–810. [Google Scholar] [CrossRef]
- Zvereva, E.L.; Lanta, V.; Kozlov, M.V. Effects of sap-feeding insect herbivores on growth and reproduction of woody plants: A meta-analysis of experimental studies. Oecologia 2010, 163, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Stephens, A.E.A.; Westoby, M. Effects of insect attack to stems on plant survival, growth, reproduction and photosynthesis. OIKOS 2014, 124, 266–273. [Google Scholar] [CrossRef]
- Takahashi, M.; Huntly, N. Herbivorous insects reduce growth and reproduction of big sagebrush (Artemisia tridentata). Arthropod-Plant Interact. 2010, 4, 257–266. [Google Scholar] [CrossRef]
- Halitschke, R.; Hamilton, J.G.; Kessler, A. Herbivore-specific elicitation of photosynthesis by mirid bug salivary secretions in the wild tobacco Nicotiana attenuata. New Phytol. 2011, 191, 528–535. [Google Scholar] [CrossRef]
- DeLucia, E.H.; Nabity, P.D.; Zavala, J.A.; Berenbaum, M.R. Climate change: Resetting plant-insect interactions. Plant Physiol. 2012, 160, 1677–1685. [Google Scholar] [CrossRef]
- Liu, Z.G.; Cai, Y.L.; Fang, Y.; Jing, J.; Li, K. Induced response in Schima superba: Effects of early-season herbivory on leaf traits and subsequent insect attack. Afr. J. Biotechnol. 2010, 9, 8731–8738. [Google Scholar]
- Ancheta, J.; Heard, S.B. Impacts of insect herbivores on rare plant populations. Biol. Conserv. 2011, 144, 2395–2402. [Google Scholar] [CrossRef]
- Myers, J.H.; Sarfraz, R.M. Impacts of insect herbivores on plant populations. Annu. Rev. Entomol. 2016, 62, 207–230. [Google Scholar] [CrossRef] [PubMed]
- Domec, J.C.; Rivera, L.N.; King, J.S.; Peszlen, I.; Hain, F.; Smith, B.; Frampton, J. Hemlock woolly adelgid (Adelges tsugae) infestation affects water and carbon relations of eastern hemlock (Tsuga canadensis) and Carolina hemlock (Tsuga caroliniana). New Phytol. 2013, 199, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Haavik, L.J.; Billings, S.A.; Guldin, J.M.; Stephen, F.M. Emergent insects, pathogens and drought shape changing patterns in oak decline in North America and Europe. For. Ecol. Manag. 2015, 354, 190–205. [Google Scholar] [CrossRef]
- Li, Z.R.; Huang, L.; Zhao, W.X.; Yao, Y.X. A new species of the genus Coccophagus (Hymenoptera: Aphelinidae) associated with Sphaerolecanium prunastri (Hemiptera: Coccoidea) from the Tianshan Mountains, Xinjiang. Entomotaxonomia 2022, 44, 228–239. [Google Scholar]
- Linghu, W.; Lu, Z.Z.; Wang, Y.L.; Wang, Q.; Gao, G.Z. Effects of pruning time and intensity on the population density of Sphaerolecanium prunastri (Boyer de Fonscolombe) and growth of Armeniaca vulgaris Lamarck. North. Hortic. 2021, 20, 34–41. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).