Ecophysiological Behavior of Fagus sylvatica L. Growing at Its Southern Distribution Limit: Insights for Understanding the Fate of the European Beech under Warmer and Dryer Growth Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Seasonal Changes of Plant Water Relations
2.3. Anatomical Measurements
- the mean xylem conduit diameter D;
- the hydraulically weighted mean xylem conduit diameter [25], Dh, estimated as: Σd5/Σd4;
- the potential cross-sectional conductive area, estimated as: Σπr2, where r is the inner conduit radius;
- the relative xylem conduit area, estimated as: Σπr2/Ax, where Ax is the xylem area;
- the efficiency of water transport estimated as: Σr4 (according to the Hagen–Poiseuille equation).
2.4. Statistical Analysis
3. Results
3.1. Study Sites
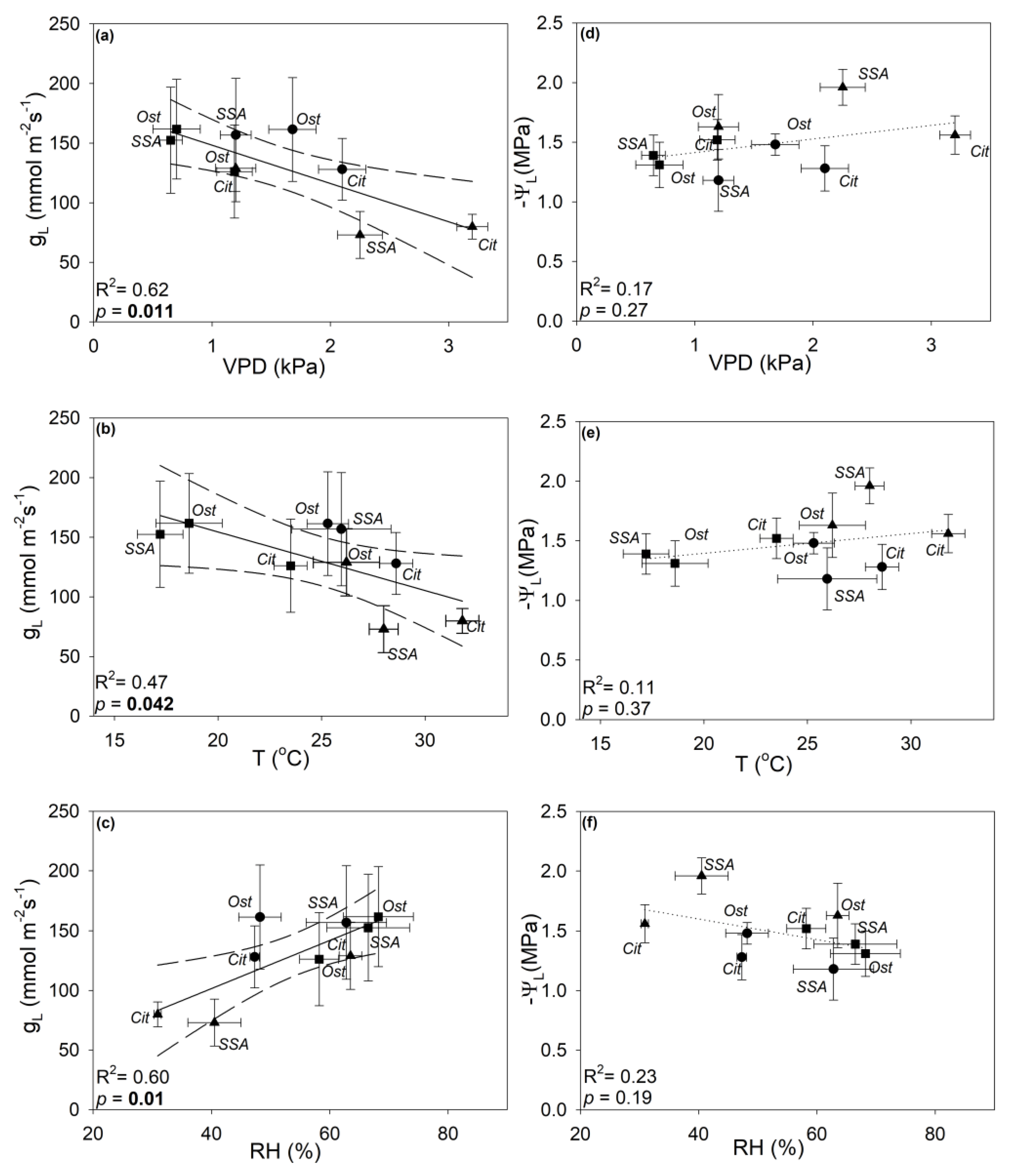
3.2. Seasonal Changes of Plant Water Relations
3.3. Anatomical Measurements
4. Discussion
4.1. Phenotypic Plasticity of Beech Populations at Its Southern Distribution Limit
4.2. Climate-Driven Plasticity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Durrant, T.H.; de Rigo, D.; Caudullo, G. Fagus sylvatica and other beeches in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publication Office of EU: Luxembourg, 2016; pp. 40–45. [Google Scholar]
- Spampinato, G.; Crisarà, R.; Cameriere, P.; Cano-Ortiz, A.; Musarella, C.M. Analysis of the forest landscape and its transformations through phytotoponyms: A case study in Calabria (Southern Italy). Land 2022, 11, 518. [Google Scholar] [CrossRef]
- Schuldt, B.; Buras, A.; Arend, M.; Vitasse, Y.; Beierkuhnlein, C.; Damm, A.; Kahmen, A. A first assessment of the impact of the extreme 2018 summer drought on Central European forests. Basic Appl. Ecol. 2020, 45, 86–103. [Google Scholar] [CrossRef]
- Leuschner, C. Drought response of European beech (Fagus sylvatica L.)—A review. Perspect. Plant Ecol. Evolut. Syst. 2020, 47, 12557. [Google Scholar] [CrossRef]
- Farahat, E.; Linderholm, H.W. Growth-climate relationship of European beech at its northern distribution limit. Europ. J. Forest Res. 2018, 137, 619–629. [Google Scholar] [CrossRef]
- Obladen, N.; Dechering, P.; Skiadaresis, G.; Tegel, W.; Keßler, J.; Höllerl, S.; Kaps, S.; Hertel, M.; Dulamsuren, C.; Seifert, T.; et al. Tree mortality of European beech and Norway spruce induced by 2018–2019 hot droughts in central Germany. Agric. Forest Meteorol. 2021, 307, 108482. [Google Scholar] [CrossRef]
- Martinez del Castillo, E.; Zang, C.S.; Buras, A.; Hacket-Pain, A.; Esper, J.; Serrano-Notivoli, R.; de Luis, M. Climate-change-driven growth decline of European beech forests. Communicat. Biol. 2022, 5, 163. [Google Scholar] [CrossRef]
- Kulla, L.; Roessiger, J.; Bošea, M.; Kucbel, S.; Murgaš, V.; Vencurik, J.; Pittner, J.; Jaloviar, P.; Šumichrast, L.; Saniga, M. Changing patterns of natural dynamics in old-growth European beech (Fagus sylvatica L.) forests can inspire forest management in Central Europe. Forest Ecol. Manag. 2023, 529, 120633. [Google Scholar] [CrossRef]
- Tomasella, M.; Beikircher, B.; Häberle, K.; Hesse, B.; Kallenbach, C.; Matyssek, R.; Mayr, S. Acclimation of branch and leaf hydraulics in adult Fagus sylvatica and Picea abies in a forest through-fall exclusion experiment. Tree Physiol. 2018, 38, 198–211. [Google Scholar] [CrossRef]
- Tomasella, M.; Nardini, A.; Hesse, B.D.; Machlet, A.; Matyssek, R.; Häberle, K. Close to the edge: Effects of repeated severe drought on stem hydraulics and non-structural carbohydrates in European beech saplings. Tree Physiol. 2019, 39, 717–728. [Google Scholar] [CrossRef]
- Meyer, B.F.; Buras, A.; Rammig, A.; Zang, C.S. Higher susceptibility of beech to drought in comparison to oak. Dendrocronology 2020, 64, 125780. [Google Scholar] [CrossRef]
- Peuke, A.D.; Schraml, C.; Hartung, W.; Rennenberg, H. Identification of drought-sensitive beech ecotypes by physiological parameters. New Phytol. 2002, 154, 373–387. [Google Scholar] [CrossRef]
- Rose, L.; Leuschner, C.; Kockemann, B.; Buschmann, H. Are marginal beech (Fagus sylvatica L.) provenances a source for drought tolerant ecotype? Europ. J. Forest Res. 2009, 128, 335–343. [Google Scholar] [CrossRef]
- Weber, P.; Bugmann, H.; Pluess, a.R.; Walthert, L.; Rigling, A. Drought response and changing mean sensitivity of European vìbeech close to dry distribution limit. Trees Struct. Funct. 2013, 27, 171–181. [Google Scholar] [CrossRef]
- Pfenninger, M.; Reuss, F.; Kiebler, A.; Schönnenbeck, P.; Caliendo, C.; Gerber, S.; Cocchiararo, B.; Reuter, S.; Blüthgen, N.; Mody, K.; et al. Genomic basis for drought resistance in European beech forests threatened by climate change. eLife 2021, 10, e65532. [Google Scholar] [CrossRef] [PubMed]
- Knutzen, F.; Meier, I.C.; Leuschner, C. Does reduced precipitation trigger physiological and morphological drought adaptations in European beech (Fagus sylvatica L.)? Comparing provenances across a precipitation gradient. Tree Physiol. 2015, 35, 949–963. [Google Scholar] [CrossRef] [PubMed]
- Stolz, J.; van der Maaten, E.; Kalanke, H.; Martin, J.; Wilmking, M.; van der Maaten-Theunissen, M. Increasing climate sensitivity of beech and pine is not mediated by adaptation and soil characteristics along a precipitation gradient in northeastern Germany. Dendrochrology 2021, 67, 125834. [Google Scholar] [CrossRef]
- Wang, F.; Israel, D.; Ramirez-Valiente, J.A.; Sanchez-Gomez, D.; Aranda, I.; Aphalo, P.J.; Robson, T.M. Seedlings from marginal and core populations of European beech (Fagus sylvatica L.) respond differently to imposed drought and shade. Trees Struct. Funct. 2021, 35, 53–67. [Google Scholar] [CrossRef]
- Lo Gullo, M.A.; Salleo, S.; Rosso, R.; Trifilò, P. Drought resistance of 2 years old saplings of Mediterranean forest trees in the field: Relations between water relations, hydraulics and productivity. Plant Soil 2003, 250, 259–272. [Google Scholar] [CrossRef]
- Niinemets, U. Responses of forest trees to single and multiple environmental stresses from seedlings to mature plants: Past stress history, stress interactions, tolerance and acclimation. Forest Ecol. Manag. 2010, 260, 1623–1639. [Google Scholar] [CrossRef]
- Andivia, E.; Ruiz-Benito, P.; Díaz-Martínez, P.; Carro-Martínez, N.; Zavala, M.A.; Madrigal-González, J. Inter-specific tolerance to recurrent droughts of pine species revealed in saplings rather than adult trees. Forest Ecol. Manag. 2020, 459, 117848. [Google Scholar] [CrossRef]
- Pignatti, S. Flora d’Italia—Volume II, 2nd ed.; Edagricole: Bologna, Italy, 1982; ISBN 978-88-506-5243-3. [Google Scholar]
- Fotelli, M.N.; Nahm, M.; Radoglou, K.; Rennenberg, H.; Halyvopoulos, G.; Matzarakis, A. Seasonal and interannual ecophysiological responses of beech (Fagus sylvatica) at its south-eastern distribution limit in Europe. Forest Ecol. Manag. 2009, 257, 1157–1164. [Google Scholar] [CrossRef]
- Tyree, M.T.; Hammel, H.T. The measurement of the turgor pressure and the water relations of plants by the pressure-bomb technique. J. Exp. Bot. 1972, 23, 267–282. [Google Scholar] [CrossRef]
- Kolb, K.J.; Sperry, J.S. Differences in drought adaptation between subspecies of sagebrush (Artemisia tridentata). Ecology 1999, 80, 2373–2384. [Google Scholar] [CrossRef]
- Jansen, S.; Gortan, E.; Lens, F.; Lo Gullo, M.A.; Salleo, S.; Scholz, A.; Stein, A.; Trifilò, P.; Nardini, A. Do quantitative vessel and pit characters account for ion-mediated changes in the hydraulic conductance of angiosperm xylem? New Phytol. 2011, 189, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, J.; Bates, D.; Debroy, S.; Sarkar, D.; R Core Team. Nlme Nonlinear MIXED Effects Models. R Package. Version 3.1-124. 2016. Available online: https://CRAN.R-project.org/package=nlme (accessed on 14 January 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 14 January 2022).
- Lenth, R.V. Emmeans: Estimated Marginal Means, aka LeastSquares Means. R Package Version 1.7.2. 2022. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 14 June 2022).
- Bartoń, K. MuMIn: Multi-Model Inference. R Package Version 1.47.5. 2023. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 5 August 2023).
- Bartlett, M.K.; Scoffoni, C.; Sack, L. The determinants of leaf turgor loss point and prediction of drought tolerance of species and biomes: A global meta-analysis. Ecol. Lett. 2012, 15, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Chen, Y.; Ye, Q.; He, P.; Liu, H.; Li, R.; Fu, P.; Jiang, G.; Cao, K. Leaf turgor loss point is correlated with drought tolerance and leaf carbon economics traits. Tree Physiol. 2018, 38, 658–663. [Google Scholar] [CrossRef]
- Schuldt, B.; Knutzen, F.; Delzon, S.; Jansen, S.; Müller-Haubold, H.; Burlett, R.; Clough, Y.; Leuschner, C. How adaptable is the hydraulic system of European beech in the face of climate change-related precipitation reduction? New Phytol. 2016, 210, 443–458. [Google Scholar] [CrossRef] [PubMed]
- Vicente, E.; Didion-Gency, M.; Morcillo, L.; Morin, X.; Vilagrosa, A.; Grossiord, C. Aridity and cold temperatures drive divergent adjustments of European beech xylem anatomy, hydraulics and leaf physiological traits. Tree Physiol. 2022, 42, 1720–1735. [Google Scholar] [CrossRef] [PubMed]
- Peters, R. Beech Forests in Geobotany; No. 24; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1997; p. 169. ISBN 978-079-234-485-8. [Google Scholar]
- Wright, I.J.; Reich, P.B.; Cornelissen, J.H.C.; Falster, D.S.; Groom, P.K.; Hikosaka, K.; Lee, W.; Lusk, C.H.; Niinemets, Ü.; Oleksyn, J.; et al. Modulation of leaf economic traits and trait relationships by climate. Glob. Ecol. Biogeogr. 2005, 14, 411–421. [Google Scholar] [CrossRef]
- Schönbeck, L.C.; Schuler, P.; Lehmann, M.M.; Mas, E.; Mekarni, L.; Pivovaroff, A.L.; Turberg, P.; Grossiord, C. Increasing temperature and vapour pressure deficit lead to hydraulic damages in the absence of soil drought. Plant Cell Environ. 2022, 45, 3275–3289. [Google Scholar] [CrossRef]
- Zhu, J.; Thimonier, A.; Etzold, S.; Meusburger, K.; Waldner, P.; Schmitt, M.; Schleppi, P.; Schaub, M.; Thormann, J.; Lehmann, M.M. Variation in leaf morphological traits of European beech and norway spruce over two decades in Switzerland. Front. For. Glob. Chang. 2022, 4, 778351. [Google Scholar] [CrossRef]
- Gago, J.; de Menezes Daloso, D.; Figueroa, C.M.; Flexas, J.; Fernie, A.R.; Nikoloski, Z. Relationships of leaf net photosynthesis, stomatal conductance, and mesophyll conductance to primary metabolism: A multispecies meta-analysis approach. Plant Physiol. 2016, 171, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Schreel, J.D.M.; Leroux, O.; Goossens, W.; Brodersen, C.; Rubinstein, A.; Steppe, K. Identifying the pathways for foliar water uptake in beech (Fagus sylvatica L.): A major role for trichomes. Plant J. 2020, 103, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Schreel, J.D.M.; Brodersen, C.; De Schryver, T.; Dierick, M.; Rubinstein, A.; Dewettinck, K.; Boone, M.N.; Van Hoorebeke, L.; Steppe, K. Foliar water uptake does not contribute to embolism repair in beech (Fagus sylvatica L.). Ann. Bot. 2022, 129, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Choat, B.; Medek, D.E.; Stuart, S.A.; Pasquet-Kok, J.; Egerton, J.J.G.; Salari, H.; Sack, L.; Ball, M.C. Xylem traits mediate a trade-off between resistance to freeze–thaw-induced embolism and photosynthetic capacity in overwintering evergreens. New Phytol. 2011, 191, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Lintunen, A.; Hölttä, T.; Kulmala, M. Anatomical regulation of ice nucleation and cavitation helps trees to survive freezing and drought stress. Sci. Rep. 2013, 3, 2031. [Google Scholar] [CrossRef]
- Hacke, U.G.; Spicer, R.; Schreiber, S.G.; Plavcová, L. An ecophysiological and developmental perspective on variation in vessel diameter. Plant Cell Environ. 2017, 40, 831–845. [Google Scholar] [CrossRef]
- Lens, F.; Gleason, S.M.; Bortolami, G.; Brodersen, C.; Delzon, S.; Jansen, S. Functional xylem characteristics associated with drought-induced embolism in angiosperms. New Phytol. 2022, 236, 2019–2036. [Google Scholar] [CrossRef]
- Isasa, E.; Link, R.M.; Jansen, S.; Tezeh, F.R.; Kaack, L.; Sarmento Cabral, J.; Schuldt, B. Addressing controversies in the xylem embolism resistance–vessel diameter relationship. New Phytol. 2023, 238, 283–296. [Google Scholar] [CrossRef]
- Pritzkow, C.; Brown, M.J.M.; Carins-Murphy, M.R.; Bourbia, I.; Mitchell, P.J.; Brodersen, C.; Choat, B.; Brodribb, T.J. Conduit position and connectivity affect the likelihood of xylem embolism during natural drought in evergreen woodland species. Ann. Bot. 2022, 130, 431–444. [Google Scholar] [CrossRef]
- Carlquist, S. Xylem heterochrony: An unappreciated key to angiosperm origin and diversifications. Bot. J. Linn. Soc. 2009, 161, 26–65. [Google Scholar] [CrossRef]
- Lens, F.; Sperry, J.S.; Christman, M.A.; Choat, B.; Rabaey, D.; Jansen, S. Testing hypotheses that link wood anatomy to cavitation resistance and hydraulic conductivity in the genus Acer. New Phytol. 2011, 190, 709–723. [Google Scholar] [CrossRef]
- Trifilò, P.; Barbera, P.M.; Raimondo, F.; Nardini, A.; Lo Gullo, M.A. Coping with drought-induced xylem cavitation: Coordination of embolism repair and ionic effects in three Mediterranean evergreens. Tree Physiol. 2014, 34, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Levionnois, S.; Jansen, S.; Wandji, R.T.; Beauchêne, J.; Ziegler, C.; Coste, S.; Stahl, C.; Delzon, S.; Authier, L.; Heuret, P. Linking drought-induced xylem embolism resistance to wood anatomical traits in Neotropical trees. New Phytol. 2021, 229, 1453–1466. [Google Scholar] [CrossRef] [PubMed]
- Knutzen, F.; Dulamsuren, C.; Meier, I.C.; Leuschner, C. Recent climate warming-related growth decline impairs Eueopean beech in the center of its distribution range. Ecosystems 2017, 20, 1494–1511. [Google Scholar] [CrossRef]
- Dulamsuren, C.; Hauck, M.; Kopp, G.; Ruff, M.; Leuschner, C. European beech responds to climate change with growth decline at lower, and growth increase at higher elevations in the center of its distribution range (>SW Germany). Trees Struct. Funct. 2017, 31, 676. [Google Scholar] [CrossRef]
- Hacket-Pain, A.J.; Cavin, L.; Friend, A.D.; Jump, A.S. Consistent limitation of growth by high temperature and low precipitation from range core to southern edge of European beech indicates widespread vulnerability to changing climate. Europ. J. Forest Res. 2016, 135, 897–909. [Google Scholar] [CrossRef]


| Study Site | Month | −Ψtlp (MPa) | −πo (MPa) | ε (MPa) |
|---|---|---|---|---|
| 750 m a.s.l. (Cit) | June | 2.30 ± 0.05 a | 1.69 ± 0.04 a | 24.5 ± 1.7 |
| September | 2.64 ± 0.10 b | 1.82 ± 0.02 bc | 30.5 ± 9.2 | |
| 976 m a.s.l. (Ost) | June | 2.19 ± 0.1 a | 1.66 ± 0.09 a | 23.5 ± 11.3 |
| September | 2.16 ± 0.09 a | 1.72 ± 0.11 ac | 20.1 ± 7.5 | |
| 1450 m a.s.l. (SSA) | June | 2.20 ± 0.04 a | 1.78 ± 0.08 a | 17.9 ± 5.9 |
| September | 2.59 ± 0.09 b | 1.88 ± 0.11 b | 32.1 ± 6.9 | |
| p-value | Site | <0.0001 | 0.054 | 1.779 |
| Month | <0.0001 | 0.002 | 1.297 | |
| Site × Month | <0.0001 | 0.341 | 1.187 |
| 750 m a.s.l. (Cit) | 976 m a.s.l. (Ost) | 1450 m a.s.l. (SSA) | p-Value | |
|---|---|---|---|---|
| AL (cm2) | 19.2 ± 6.6 | 15.7 ± 5.5 | 18.5 ± 6.4 | 0.352 |
| LMA (g m−2) | 42.9 ± 9.3 a | 30.0 ± 2.6 b | 32.4 ± 8.1 b | <0.001 |
| Ax (mm2) | 8.2 ± 0.6 | 10.4 ± 2.1 | 7.7 ± 1.3 | 1.0 |
| Vd | 375.8 ± 19.1 a | 564.5 ± 30.7 b | 869.6 ± 17.6 c | <0.001 |
| D (μm) | 24.3 ± 1.3 a | 22.8 ± 0.2 a | 19.7 ± 0.3 b | <0.001 |
| Dh (μm) | 30.8 ± 2.3 a | 30.8 ± 0.2 a | 27.2 ± 0.7 b | 0.017 |
| Σπr2 (mm2) | 1.45 ± 0.09 a | 2.78 ± 0.64 b | 2.24 ± 0.40 b | 0.002 |
| Σπr2/Ax (%) | 17.7 ± 2.0 a | 26.7 ± 2.9 b | 29.0 ± 0.4 b | <0.001 |
| Σr4 E-6 (mm2) | 98.9 ± 18.7 | 173.6 ± 42.2 b | 113.7 ± 25.5 | 0.052 |
| Vs | 0.69 ± 0.05 a | 0.69 ± 0.006 a | 0.5 ± 0.03 b | <0.001 |
| VG | 1.44 ± 0.08 a | 1.88 ± 0.08 b | 2.43 ± 0.06 c | <0.001 |
| FVM | 0.49 ± 0.07 a | 0.61 ± 0.01 b | 0.80 ± 0.01 c | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prigoliti, M.; Chiofalo, M.T.; Petruzzellis, F.; Lo Gullo, M.A.; Trifilò, P. Ecophysiological Behavior of Fagus sylvatica L. Growing at Its Southern Distribution Limit: Insights for Understanding the Fate of the European Beech under Warmer and Dryer Growth Conditions. Forests 2023, 14, 2058. https://doi.org/10.3390/f14102058
Prigoliti M, Chiofalo MT, Petruzzellis F, Lo Gullo MA, Trifilò P. Ecophysiological Behavior of Fagus sylvatica L. Growing at Its Southern Distribution Limit: Insights for Understanding the Fate of the European Beech under Warmer and Dryer Growth Conditions. Forests. 2023; 14(10):2058. https://doi.org/10.3390/f14102058
Chicago/Turabian StylePrigoliti, Maria, Maria Teresa Chiofalo, Francesco Petruzzellis, Maria Assunta Lo Gullo, and Patrizia Trifilò. 2023. "Ecophysiological Behavior of Fagus sylvatica L. Growing at Its Southern Distribution Limit: Insights for Understanding the Fate of the European Beech under Warmer and Dryer Growth Conditions" Forests 14, no. 10: 2058. https://doi.org/10.3390/f14102058
APA StylePrigoliti, M., Chiofalo, M. T., Petruzzellis, F., Lo Gullo, M. A., & Trifilò, P. (2023). Ecophysiological Behavior of Fagus sylvatica L. Growing at Its Southern Distribution Limit: Insights for Understanding the Fate of the European Beech under Warmer and Dryer Growth Conditions. Forests, 14(10), 2058. https://doi.org/10.3390/f14102058








