Rapid Detection of Phytophthora cambivora Using Recombinase Polymerase Amplification Combined with CRISPR/Cas12a
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolate Preservation and DNA Extraction
2.2. Screening Phytophthora cambivora Target Genes
2.3. Designing Primers, crRNA, and ssDNA Reporter
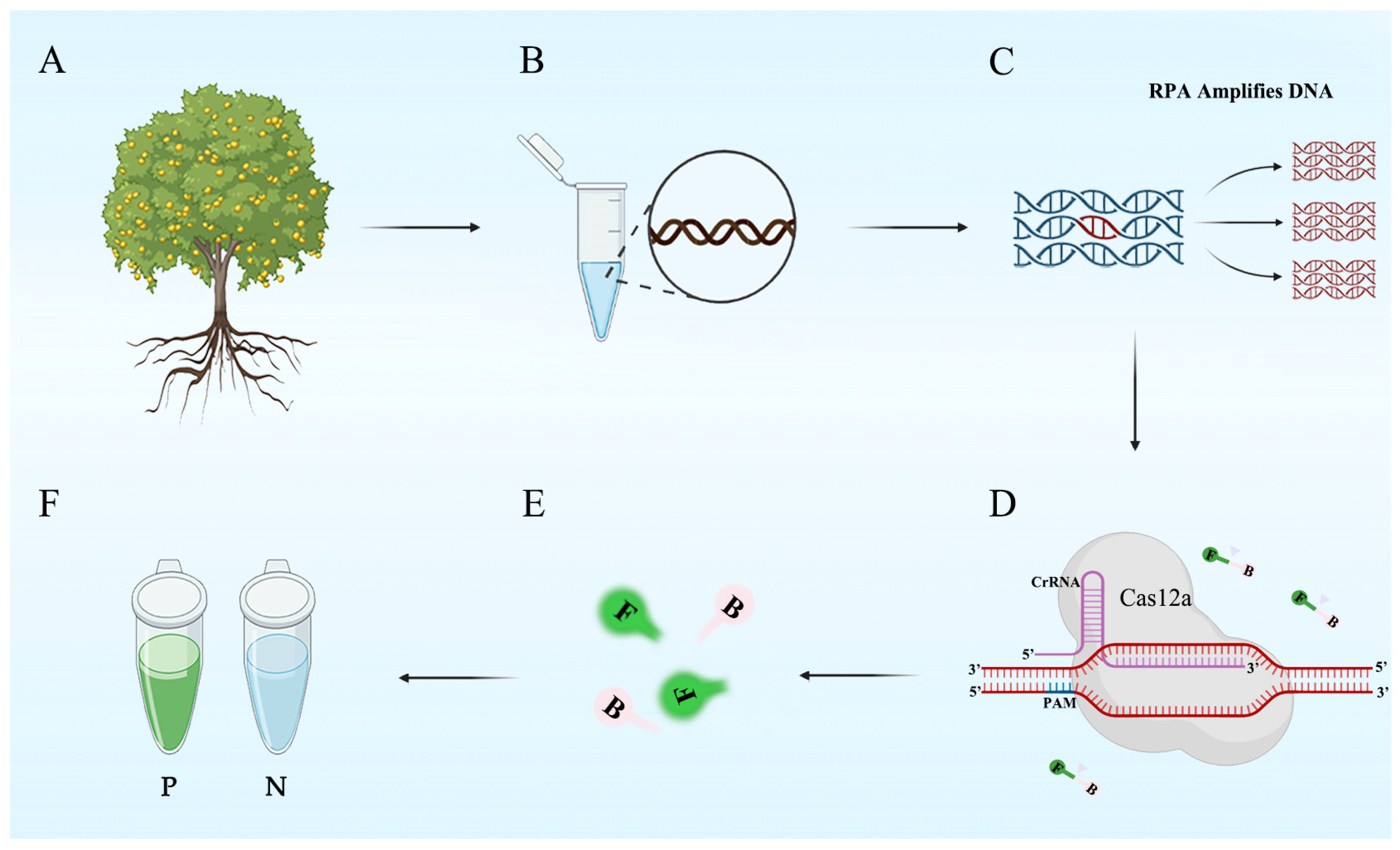
2.4. RPA-CRISPR/Cas12a Assay
2.5. Specificity and Sensitivity of the RPA-CRISPR/Cas12a Assay
2.6. Detection of P. cambivora in Artificially Inoculated Apple Fruits Using the RPA-CRISPR/Cas12a Detection System
3. Results
3.1. Optimization of the RPA-CRISPR/Cas12a Assay for the Detection of Phytophthora cambivora
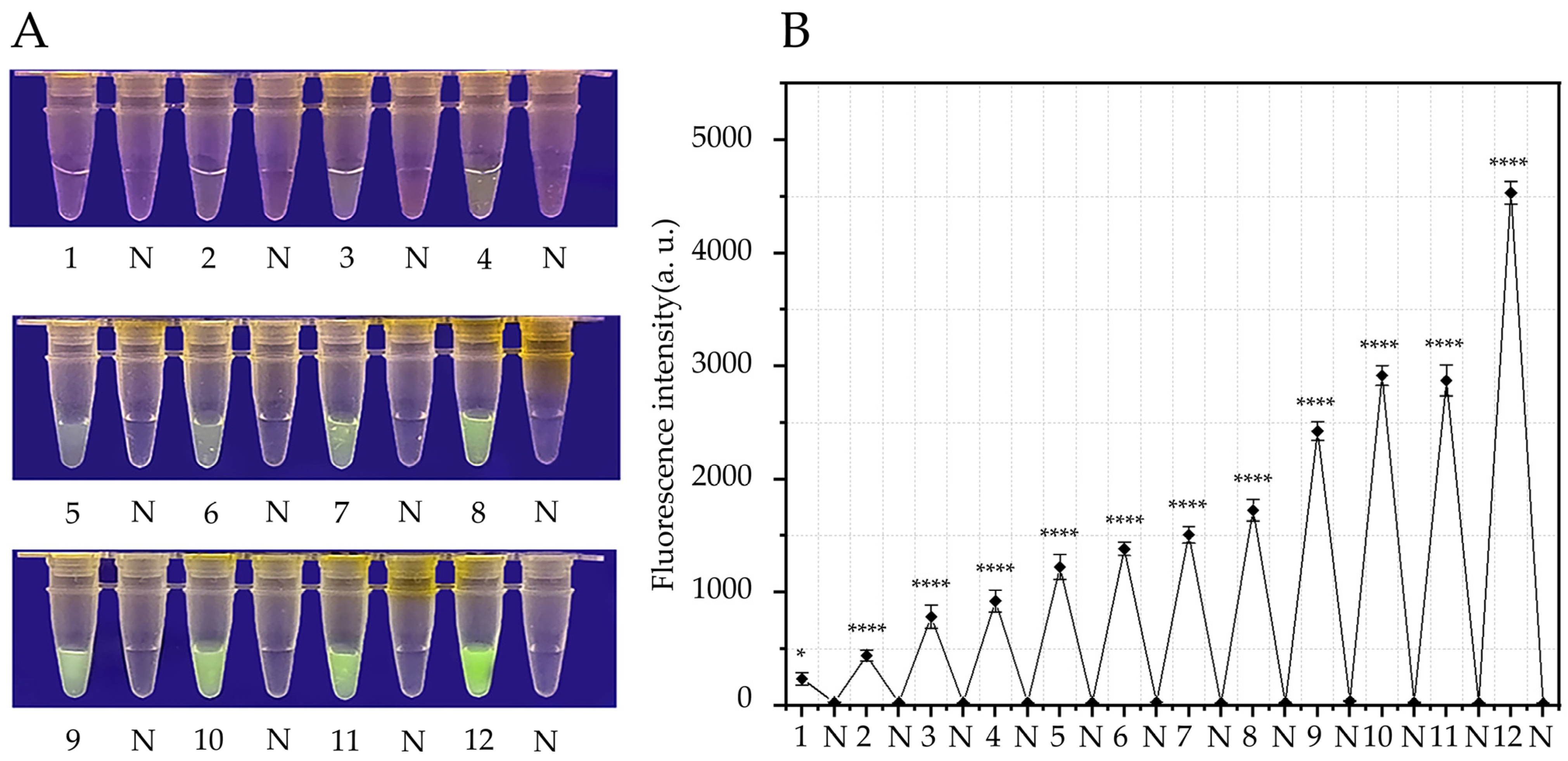
3.2. Verification of the Specificity of the RPA-CRISPR/Cas12a Assay
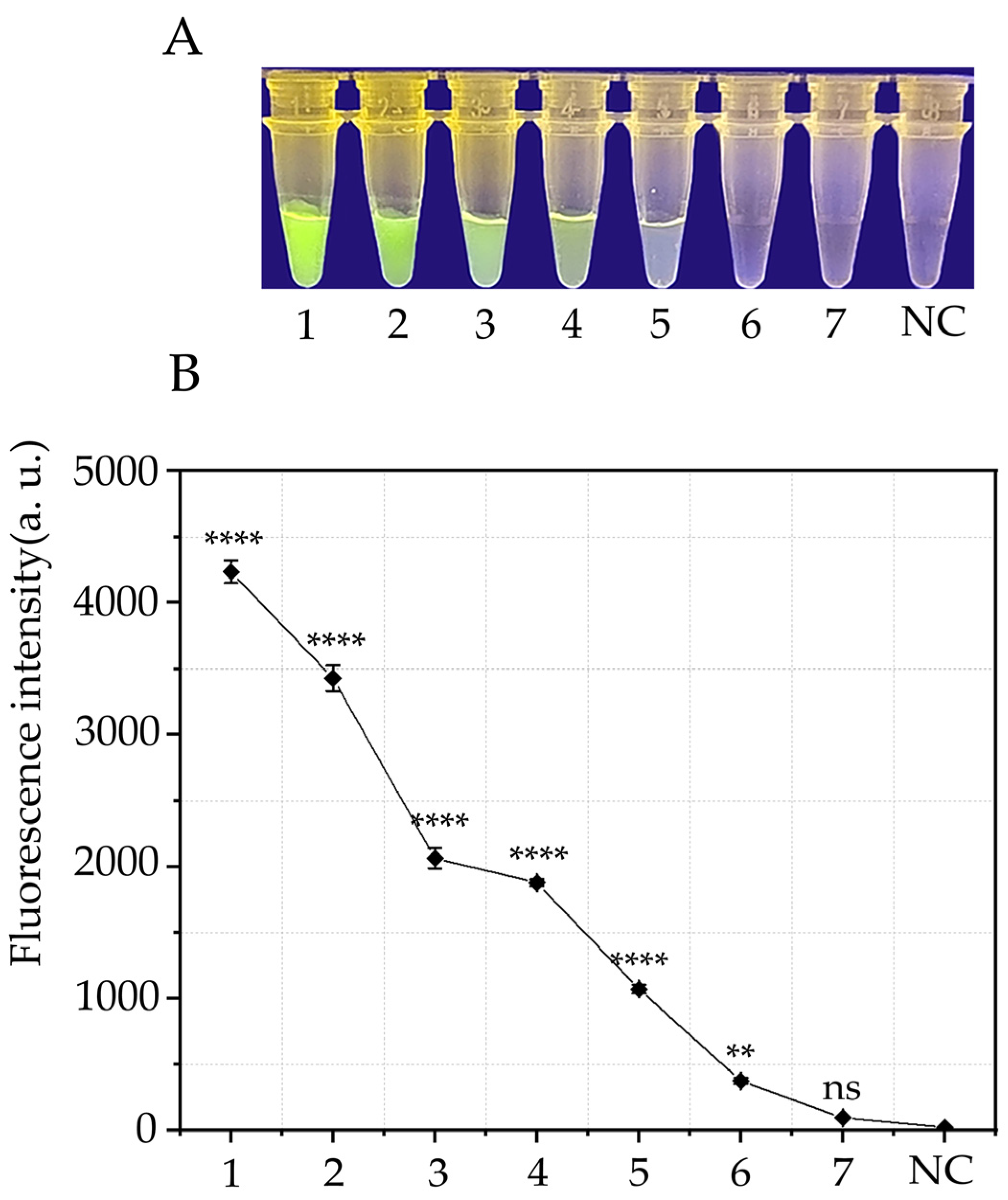
3.3. Sensitivity of the RPA-CRISPR/Cas12a Assay
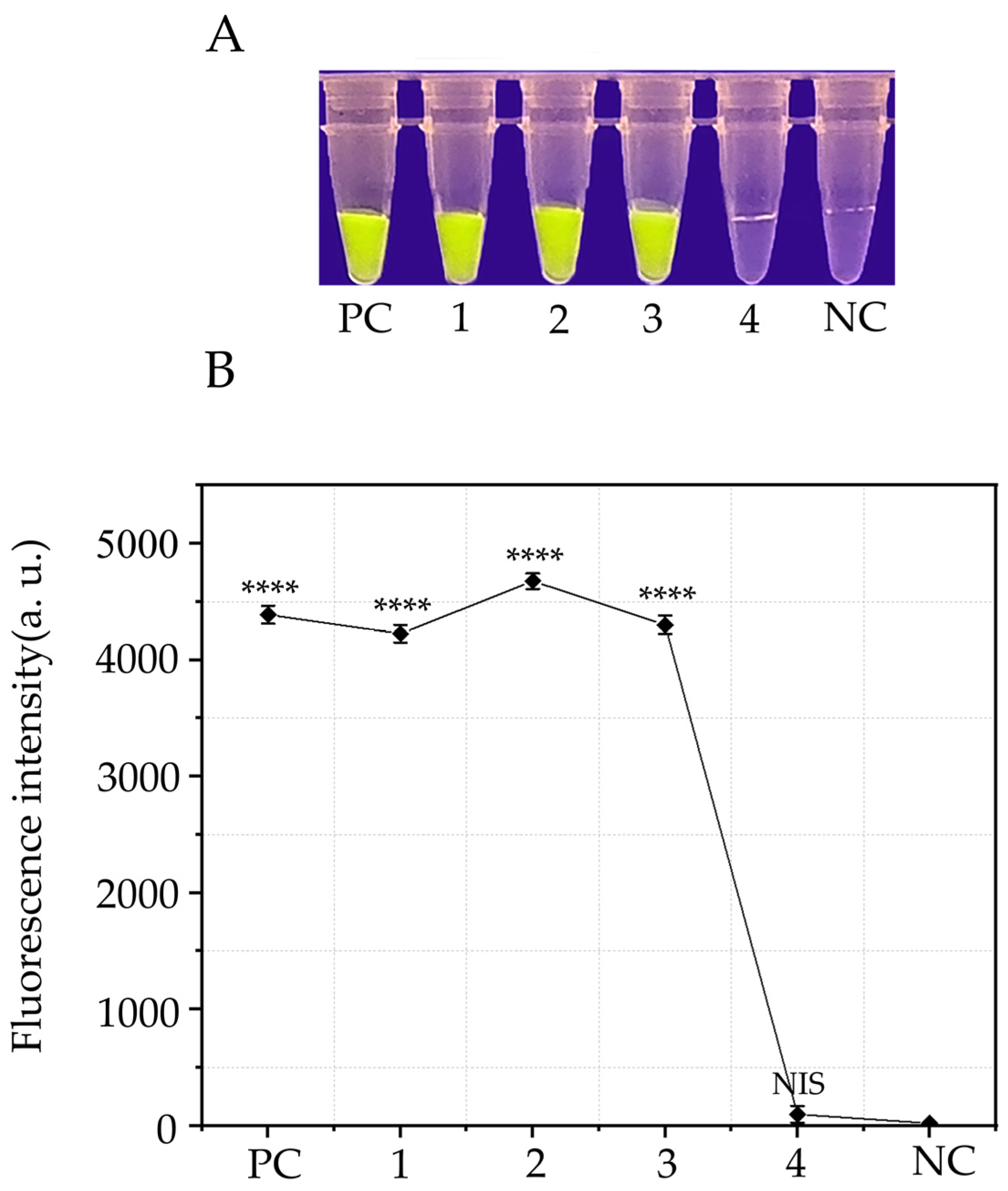
3.4. Detection of P. cambivora in Artificially Inoculated Apples Using the RPA-CRISPR/Cas12a Detection System
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, J.; Xu, T.; Xu, X.; Dai, T.; Liu, T. The New Report of Root Rot on Fatsia jaonica Caused by Phytophthora nicotianae in China. Forests 2023, 14, 1459. [Google Scholar] [CrossRef]
- Nagy, Z.A.; Bakonyi, J.; Ersek, T.T. Standard and Swedish variant types of the hybrid alder Phytophthora attacking alder in Hungary. Pest Manag. Sci. 2003, 59, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Félix-Gastélum, R.; Mircetich, S.M. Influence of flooding duration on the development of root and crown rot of Lovell peach [Prunus persica (L.) Bstsch] caused by three different Phytophthora species. Rev. Mex. Fitopatol. 2005, 23, 33–41. Available online: https://www.redalyc.org/pdf/612/61223105 (accessed on 8 April 2022).
- Blair, J.E.; Coffey, M.D.; Park, S.Y.; Geiser, D.M.; Kang, S. A multi-locus phylogeny for Phytophthora utilizing markers derived from complete genome sequences. Fungal Genet. Biol. 2008, 45, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Dunstan, W.A.; Rudman, T.; Shearer, B.L. Containment and spot eradication of a highly destructive, invasive plant pathogen (Phytophthora cinnamomi) in natural ecosystems. Biol. Invas. 2010, 12, 913–925. [Google Scholar] [CrossRef]
- Vannini, A.; Breccia, M.; Bruni, N.; Tomassini, A.; Vettraino, A.M. Behaviour and survival of Phytophthora cambivora inoculum in soil-like substrate under different water regimes. For. Pathol. 2012, 42, 362–370. [Google Scholar] [CrossRef]
- Zambounis, A.; Xanthopoulou, A.; Madesis, P.; Tsaftaris, A.; Vannini, A.; Bruni, N.; Tomassini, A.; Chilosi, G.; Vettraino, A.M. A tool to assess genetic diversity of Phytophthora cambivora isolates. J. Plant Pathol. 2016, 9, 611–616. Available online: http://www.jstor.org/stable/44280509 (accessed on 8 April 2022).
- Mullett, M.S.; Van, P.K.; Haegeman, A.; Focquet, F.; Cauldron, N.C.; Knaus, B.J.; Horta, J.M.; Kageyama, K.; Hieno, A.; Masuja, H.; et al. Phylogeography and population structure of the global, wide host-range hybrid pathogen Phytophthora × cambivora. IMA Fungus 2023, 14, 4. [Google Scholar] [CrossRef]
- Feng, L.P.; Liu, H.M.; Wang, Y.C.; Li, Y.; Wu, X.H.; Ji, Y.; Wu, P.S. The Entry-Exit Inspection and Quarantine Industry Standards of the People’s Republic of China-Quarantine and Identification of Phytophthora cambivora (Petri) Buisman; China Standards Press: Beijing, China, 2011. [Google Scholar]
- Liao, F.; Huang, G.M.; Zhu, L.H.; Lv, D.J.; Zhang, D.D.; Luo, J.F.; Li, G.R. Quadruplex PCR detection of three quarantine Phytophthora pathogens of berries. Eur. J. Plant Pathol. 2019, 154, 1041–1049. [Google Scholar] [CrossRef]
- Zhu, L.H. Molecular Detection of the Ouarantine Phytophthora Pathogens of the Customs-Entry Fruits and Seedlings. Southwest University. 2016. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=1015337659.nh&DbName=CMFD2016 (accessed on 8 April 2022).
- Hüberli, D.; Tommerup, I.C.; Hardy, G.E.S.J. False-negative isolations or absence of lesions may cause mis-diagnosis of diseased plants infected with Phytophthora cinnamomi. Australas. Plant Pathol. 2000, 29, 164–169. [Google Scholar] [CrossRef]
- Lane, C.R.; Hobden, E.; Walker, L.; Barton, V.C.; Inman, A.J.; Hughes, K.J.D.; Swan, H.; Colyer, A.; Barker, I. Evaluation of a rapid diagnostic field test kit for identification of Phytophthora species, including P. ramorum and P. kernoviae at the point of inspection. Plant Pathol. 2007, 56, 828–835. [Google Scholar] [CrossRef]
- Jung, T. Beech decline in Central Europe driven by the interaction between Phytophthora infections and climatic extremes. For. Pathol. 2009, 39, 73–94. [Google Scholar] [CrossRef]
- Das, A.K.; Nerkar, S.G.; Kumar, A.; Bawage, S.S. Detection, identification and characterization of Phytophthora spp. infecting citrus in India. J. Plant Pathol. 2016, 98, 55–69. [Google Scholar] [CrossRef]
- Ahmed, Y.; Hubert, J.; Fourrier-Jeandel, C.; Dewdney, M.M.; Aguayo, J.; Ioos, R. A set of conventional and multiplex real-time PCR assays for direct detection of Elsinoe fawcettii, E. australis, and Pseudocercospora angolensis in citrus fruits. Plant Dis. 2019, 103, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Ristaino, J.B.; Madritch, M.; Trout, C.L.; Parra, G. PCR amplification of ribosomal DNA for species identification in the plant pathogen genus Phytophthora. Appl. Environ. Microbiol. 1998, 64, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Grote, D.; Olmos, A.; Kofoet, A.; Tuset, J.J.; Bertolini, E.; Cambra, M. Specific and sensitive detection of Phytophthora nicotianae by simple and nested-PCR. Eur. J. Plant Pathol. 2002, 108, 197–207. [Google Scholar] [CrossRef]
- Zhu, L.H.; Guo, J.Z.; Liao, F.; Luo, J.F.; Huang, G.M.; Ren, X.Y.; Li, G.R. Simultaneous triplex PCR detection of two quarantine fungal pathogens of citrus, Phytophthora hiberalis and Phytophthora syringae. J. Southwest Univ. 2015, 37, 1–8. [Google Scholar] [CrossRef]
- Ippolito, A.; Schena, L.; Nigro, F.; Ligorio, V.S.; Yaseen, T. Real-time detection of Phytophthora nicotianae and P. citrophthorain citrus roots and soil. Eur. J. Plant Pathol. 2004, 110, 833–843. [Google Scholar] [CrossRef]
- Schena, L.; Hughes, K.J.; Cooke, D.E. Detection and quantification of Phytophthora ramorum, P. kernoviae, P. citricola and P. quercina in symptomatic leaves by multiplex real-time PCR. Mol. Plant Pathol. 2006, 7, 365–379. [Google Scholar] [CrossRef]
- Stehlíková, D.; Beran, P.; Cohen, S.P.; Čurn, V. Development of real-time and colorimetric loop mediated isothermal amplification assay for detection of Xanthomonas gardneri. Microorganisms 2020, 8, 1301. [Google Scholar] [CrossRef]
- Rosser, A.; Rollinson, D.; Forrest, M.; Webster, B.L. Isothermal recombinase polymerase amplification (RPA) of Schistosoma haematobium DNA and oligochromatographic lateral flow detection. Parasit. Vectors 2015, 8, 446. [Google Scholar] [CrossRef] [PubMed]
- Li, G.R.; Huang, G.M.; Zhu, L.H.; Lv, D.J.; Cao, B.H.; Liao, F.; Luo, J.F. Loop-mediated isothermal amplification (LAMP) detection of Phytophthora hibernalis, P. syringae and P. cambivora. J. Plant Pathol. 2019, 101, 51–57. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, S.W.; Wang, X.R. Application of CRISPR-Cas12a in rapid detection of pathogens. Chin. Vet. Sci. 2022, 52, 1031–1037. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Kellner, M.J.; Koob, J.G.; Gootenberg, J.S.; Abudayyeh, O.O.; Zhang, F. Sherlock: Nucleic acid detection with CRISPR nucleases. Nat. Protoc. 2019, 14, 2986–3012. [Google Scholar] [CrossRef]
- Kadam, U.S.; Cho, Y.; Park, T.Y.; Hong, J.C. Aptamer-based crispr-cas powered diagnostics of diverse biomarkers and small molecule targets. Appl. Biol. Chem. 2023, 66, 13. [Google Scholar] [CrossRef]
- Alon, D.M.; Partosh, T.; Burstein, D.; Pines, G. Rapid and sensitive on-site genetic diagnostics of pest fruit flies using CRISPR-Cas12a. Pest Manag. Sci. 2023, 79, 68–75. [Google Scholar] [CrossRef]
- Makarova, K.S.; Wolf, Y.I.; Iranzo, J.; Shmakov, S.A.; Alkhnbashi, O.S.; Brouns, S.J.J.; Charpentier, E.; Cheng, D.; Haft, D.H.; Horvath, P.; et al. Evolutionary classification of CRISPR-Cas systems: A burst of class 2 and derived variants. Nat. Rev. Microbiol. 2020, 18, 67–83. [Google Scholar] [CrossRef]
- Kuang, R.R.; Lei, R.; Jiang, L.; Duan, W.J.; Li, X.L.; Fu, N.; Fan, Z.F.; Li, Y.; Wu, P.S. Establishment of RPA/CRISPR-Cas12a rapid detection for Leptosphaeria lindquistii. Plant Prot. 2022, 48, 69–76. [Google Scholar] [CrossRef]
- Li, F.A.; Xiao, J.; Yang, H.M.; Yao, Y.; Li, J.Q.; Zheng, H.W.; Guo, Q.; Wang, X.T.; Chen, Y.Y.; Guo, Y.J.; et al. Development of a rapid and efficient RPA-CRISPR/Cas12a assay for Mycoplasma pneumoniae detection. Front. Microbiol. 2022, 13, 858806. [Google Scholar] [CrossRef]
- Chen, J.S.; Ma, E.; Harrington, L.B.; Da Costa, M.; Tian, X.; Palefsky, J.M.; Doudna, J.A. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science 2018, 360, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.X.; Lin, Z.Q.; Huang, X.H.; Lu, J.F.; Zhou, Y.; Zheng, L.B.; Lou, Y.L. Rapid and sensitive detection of Vibrio vulnificus using CRISPR/Cas12a combined with a recombinase-aided amplification assay. Front. Microbiol. 2021, 12, 767315. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, J.; Li, P.; Bai, L.; Jia, J.; Pan, A.; Long, X.; Cui, W.; Tang, X. Rapid detection of P-35S and T-nos in genetically modified organisms by recombinase polymerase amplification combined with a lateral flow strip. Food Control 2020, 107, 106775. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, J.; Yao, C.Y.; Xie, P.C.; Li, X.M.; Xu, Z.L.; Xian, Y.P.; Lei, H.T.; Shen, X. Alkaline lysis-recombinase polymerase amplification combined with CRISPR/Cas12a assay for the ultrafast visual identification of pork in meat products. Food Chem. 2022, 383, 132318. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Cheng, Q.X.; Wang, J.M.; Li, X.Y.; Zhang, Z.L.; Gao, S.; Cao, R.B.; Zhao, G.P.; Wang, J. CRISPR-Cas12a-assisted nucleic acid detection. Cell Discov. 2018, 4, 20. [Google Scholar] [CrossRef]
- Dai, T.T.; Chen, Z.P.; Guo, Y.F.; Ye, J.R. Rapid detection of the pine wood nematode Bursaphelenchus xylophilus using recombinase polymerase amplification combined with CRISPR/Cas12a. Crop Prot. 2023, 170, 106259. [Google Scholar] [CrossRef]
- Huang, J.H.; Ann, P.J.; Chiu, Y.H.; Tsai, J.N. First report of Phytophthora cambivora causing leaf and stem blight and root rot on Taiwan cherry (Prunus campanulata) in Taiwan. Plant Dis. 2012, 96, 1065. [Google Scholar] [CrossRef]
- Langrell, S.R.; Morel, O.; Robin, C. Touchdown nested multiplex PCR detection of Phytophthora cinnamomi and P. cambivora from French and English chestnut grove soils. Fungal Biol. 2011, 115, 672–682. [Google Scholar] [CrossRef]
- Lei, R.; Sun, X.W.; Jiang, L.; Wang, Z.H.; Li, G.Q.; Li, Y.; Liao, X.L.; Wu, P.S. Development of rapid detection for Phytophthora syringae based on RPA/CRISPR-Cas12a. Plant Quar. 2022, 36, 31–38. [Google Scholar] [CrossRef]
- Nguyen, P.Q.; Soenksen, L.R.; Donghia, N.M.; Angenent-Mari, N.M.; de Puig, H.D.; Huang, A.; Lee, R.; Slomovic, S.; Galbersanini, T.; Lansberry, G.; et al. Wearable materials with embedded synthetic biology sensors for biomolecule detection. Nat. Biotechnol. 2021, 39, 1366–1374. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.X.; Wang, C.; Liu, Q.; Fu, Y.P.; Wang, K.J. Targeted mutagenesis in rice using CRISPR-Cpf1 system. J. Genet. Genom. 2017, 44, 71–73. [Google Scholar] [CrossRef] [PubMed]


| Number | Species | Isolate 1 | Origin | RPA-CRISPR/Cas12 2 | |
|---|---|---|---|---|---|
| Source | Host/Substrate | ||||
| 1 | Phytophthora cambivora | Pc1 | Shanghai, China | Malus domestica Borkh | + |
| 2 | Phytophthora cambivora | Pc2 | Shanghai, China | Malus domestica Borkh | + |
| 3 | Phytophthora cambivora | Pc3 | Shanghai, China | Malus domestica Borkh | + |
| 4 | Phytophthora cambivora | Pc4 | Shanghai, China | Malus domestica Borkh | + |
| 5 | Phytophthora cambivora | Pc5 | Shanghai, China | Malus domestica Borkh | + |
| 6 | Phytophthora cambivora | Pc6 | Shanghai, China | Malus domestica Borkh | + |
| 7 | Phytophthora cambivora | CBS 248.60 | Boston, MA, USA | Castanea sativa | + |
| 8 | Phytophthora boehmeriae | Pb3 | Nanjing, China | Boehmeria nivea | − |
| 9 | Phytophthora cactorum | C1 | Nanjing, China | Malus pumila | − |
| 10 | Phytophthora capsici | Pc1 | Nanjing, China | Capsicum annuum | − |
| 11 | Phytophthora castaneae | CBS587.85 | Taiwan | Soil | − |
| 12 | Phytophthora citrophthora | Pcit | Nanjing, China | Citrus reticulata Blanco | − |
| 13 | Phytophthora cinnamomi | Pci1 | Anqing, China | Pinus sp. | − |
| 14 | Phytophthora cryptogea | Pcr1 | Nanjing, China | Solanum lycopersicum | − |
| 15 | Phytophthora citricola | Pcit | Nanjing, China | Rhododendron pulchrum | − |
| 16 | Phytophthora drechsleri | CBS 292.35 T | CA, USA | Beta vulgaris var. altissima | − |
| 17 | Phytophthora fragariae | CBS 209.46 | England, UK | Fragaria × ananassa | − |
| 18 | Phytophthora hibernalis | CBS 270.31 | Richmond, VA, USA | Citrus sinensis | − |
| 19 | Phytophthora infestans | Pi1 | Fuzhou, China | Solanum tuberosum | − |
| 21 | Phytophthora lateralis | CBS 168.42 | Vancouver, CA, USA | Cedrus deodara | − |
| 22 | Phytophthora megasperma | CBS305.36 | CA, USA | Matthiola incana | − |
| 23 | Phytophthora melonis | PMNJHG1 | Nanjing, China | Cucumis sativus | − |
| 24 | Phytophthora mississippiae | 57J3 | Commonwealth of Virginia, USA | Irrigation water | − |
| 25 | Phytophthora nicotianae | Pn1 | Fuzhou, China | Nicotiana tabacum | − |
| 26 | Phytophthora palmivora | Pp1 | Lijiang, China | Iridaceae | − |
| 27 | Phytophthora parvispora | CBS132771 | Basilicata, Italy | Arbutus unedo | − |
| 28 | Phytophthora pini | Ppini1 | Nanjing, China | Rhododendron pulchrum | − |
| 29 | Phytophthora plurivora | Pplu1 | Haikou, China | Manihot esculenta | − |
| 30 | Phytophthora quercina | CBS 789.95 | Brisbane, Australia | Quercus petraea | − |
| 31 | Phytophthora sojae | Psy1 | Lijiang, China | Glycine max | − |
| 32 | Phytophthora syringae | 9099 | Shanghai, China | Malus domestica Borkh | − |
| 33 | Phytophthora ramorum | EU1 2275 | Exeter, UK | Quercus palustris | − |
| 34 | Phytophthora rubi | CBS 967.95 | Scotland, UK | Rubus idaeus | − |
| 35 | Phytophthora vignae | CPHSTBL 30 | M. D. Coffey | Vigna sp. | − |
| 36 | Phytopythium litorale | PC-dj1 | Nanjing, China | Rhododendron simsii | − |
| 37 | Phytopythium helicoides | PH-C | Nanjing, China | Rhododendron simsii | − |
| 38 | Phytopythium vexans | DT10 | Nanjing, China | Rhododendron simsii | − |
| 39 | Pythium ultimum | Pul1 | Nanjing, China | Citrus sinensis | − |
| 40 | Pythium spinosum | Psp1 | Nanjing, China | Oryza sativa L. | − |
| 41 | Pythium aphanidermatum | Pap1 | Nanjing, China | Nicotiana tabacum | − |
| 42 | Fusarium oxysporium | Fox1 | Nanjing, China | Gossypium sp. | − |
| 43 | Fusarium solani | Fso1 | Nanjing, China | Gossypium sp. | − |
| 44 | Fusarium circinatum | A045-1 | Shanghai, China | Pinus sp. | − |
| 45 | Fusarium fujikuroi | Ffu1 | Nanjing, China | Oryza sativa | − |
| 46 | Fusarium graminearum | Fgr1 | Nanjing, China | Triticum aestivum | − |
| 47 | Fusarium acuminatum | Fac1 | Chengdu, China | Rhizophora apiculata | − |
| 48 | Fusarium asiaticum | Fas1 | Nanjing, China | Triticum aestivum | − |
| 49 | Fusarium avenaceum | Fav1 | Nanjing, China | Glycine max | − |
| 50 | Fusarium culmorum | Fcu1 | Suining, China | Glycine max | − |
| 51 | Fusarium commune | Fco1 | Harbin, China | Soil | − |
| 52 | Fusarium equiseti | Feq1 | Nanjing, China | Glycine max | − |
| 53 | Fusarium lateritium | Flat1 | Nanjing, China | Soil | − |
| 54 | Fusarium moniforme | Fmo1 | Nanjing, China | Oryza sativa | − |
| 55 | Fusarium nivale | Fniv | Nanjing, China | Triticum aestivum | − |
| 56 | Fusarium proliferatum | Fpr1 | Nanjing, China | Pinus sp. | − |
| 57 | Fusarium incarnatum | IL3HQ | Nanjing, China | Medicago sativa | − |
| 58 | Colletotrichum truncatum | Ctr1 | Nanjing, China | Glycine max | − |
| 59 | Colletotrichum glycines | Cgl1 | Nanjing, China | Glycine max | − |
| 60 | Colletotrichum orbiculare | Cor1 | Nanjing, China | Citrullus lanatus | − |
| 61 | Neofusicoccum parvum | BJ3-1 | Nanjing, China | Fatsia japonica | − |
| 62 | Verticilium dahliae | Vda1 | Nanjing, China | Gossypium sp. | − |
| 63 | Rhizoctonia solani | Rso1 | Nanjing, China | Gossypium sp. | − |
| 64 | Magnaporthe grisea | Guy11 | Tsukuba, Japan | Oryza sativa | − |
| 65 | Endothia parasitica | Epa1 | Nanjing, China | Castanea mollissima | − |
| 66 | Bremia lactucae | Bla1 | Nanjing, China | Lactuca sativa | − |
| 67 | Aspergillus flavus | NJC03 | Nantong, China | Actinidia chinensis | − |
| 68 | Botrytis cinerea | Bci1 | Nanjing, China | Cucumis sativus | − |
| 69 | Alternaria alternata | Aal1 | Nanjing, China | Soil | − |
| 70 | Tilletia indica | Tin1 | Nanjing, China | Triticum aestivum | − |
| 71 | Diaporthe mahothocarpus | DT1 | Nanjing, China | Kerria japonica | − |
| 72 | Diaporthe sapindicola | WHZ3 | Nanjing, China | Sapindus mukorossi | − |
| 73 | Botryosphaeria dothidea | Bci1 | Nanjing, China | Koelreuteria paniculata | − |
| 74 | Bursaphelenchus xylophilus | Js-1 | Nanjing, China | Pinus thunbergii | − |
| 75 | Bursaphelenchus mucronatus | Bmucro | Nanjing, China | Pinus sp. | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Dai, H.; Dai, T.; Liu, T. Rapid Detection of Phytophthora cambivora Using Recombinase Polymerase Amplification Combined with CRISPR/Cas12a. Forests 2023, 14, 2141. https://doi.org/10.3390/f14112141
Zhou J, Dai H, Dai T, Liu T. Rapid Detection of Phytophthora cambivora Using Recombinase Polymerase Amplification Combined with CRISPR/Cas12a. Forests. 2023; 14(11):2141. https://doi.org/10.3390/f14112141
Chicago/Turabian StyleZhou, Jing, Hanqian Dai, Tingting Dai, and Tingli Liu. 2023. "Rapid Detection of Phytophthora cambivora Using Recombinase Polymerase Amplification Combined with CRISPR/Cas12a" Forests 14, no. 11: 2141. https://doi.org/10.3390/f14112141





