Influence of Phoebe bournei (Hemsl.) Replanting on Soil Carbon Content and Microbial Processes in a Degraded Fir Forest
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Site
2.2. Sampling
2.3. Measurement of Basic Physiochemical and Enzyme Activity
2.4. Shotgun Metagenomic Sequencing
2.5. Statistical Analysis
3. Results
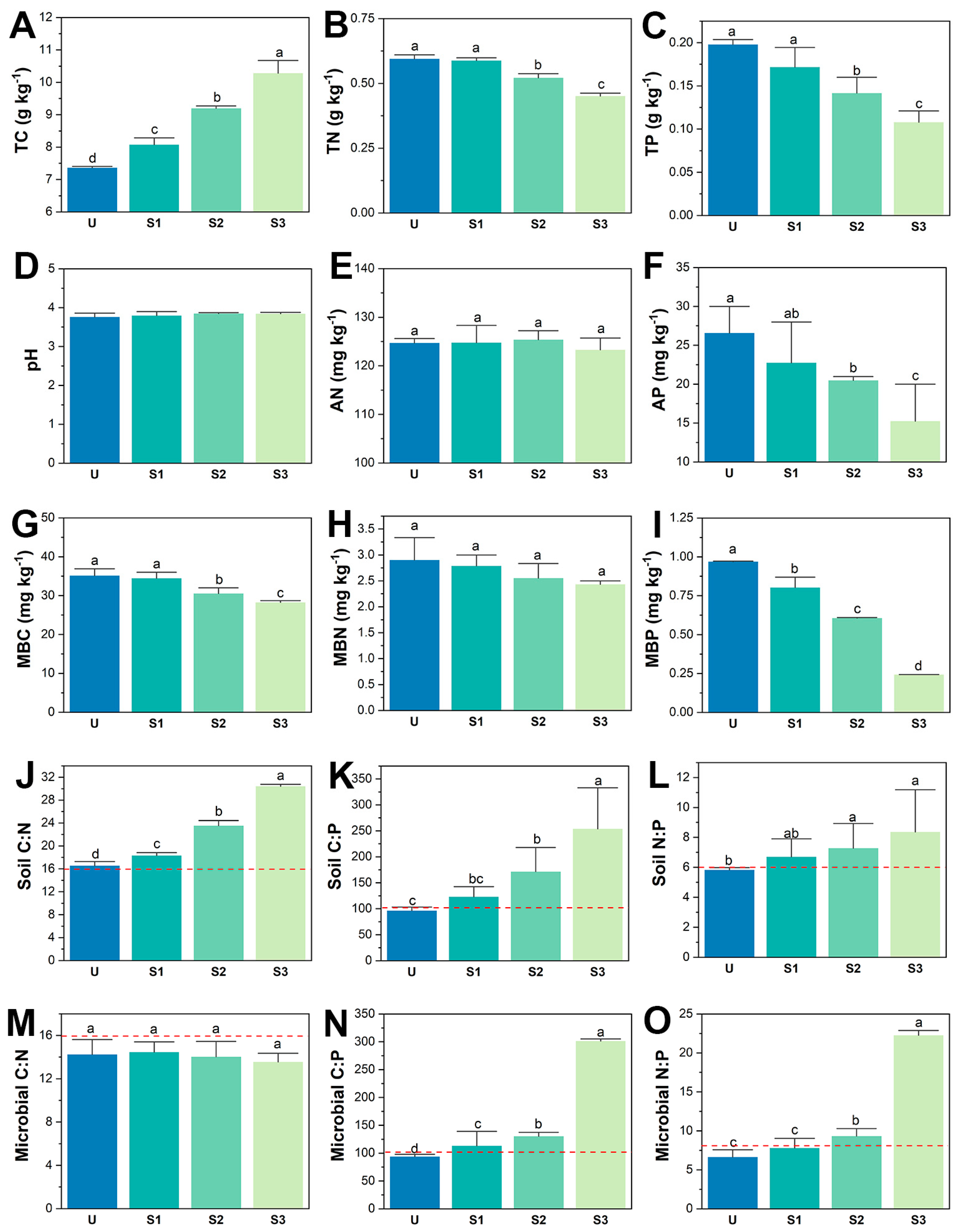
3.1. Soil and Microbial Carbon, Nitrogen, and Phosphorus Content and Stoichiometry of Different Stages
3.2. Soil Enzyme Activity of Different Stages
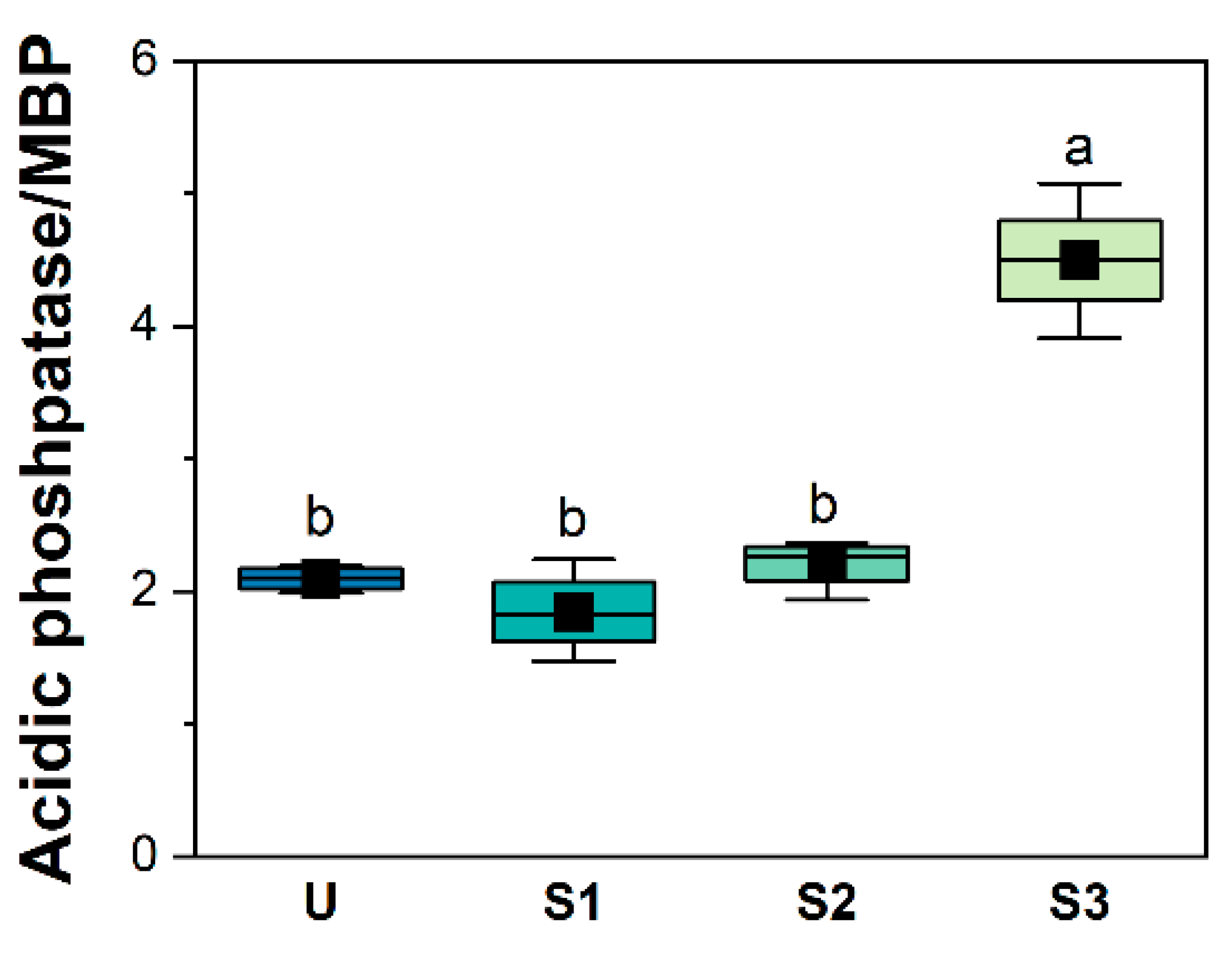
3.3. Variation in Soil Enzyme Activity
3.4. SEM for Soil Carbon Accumulation
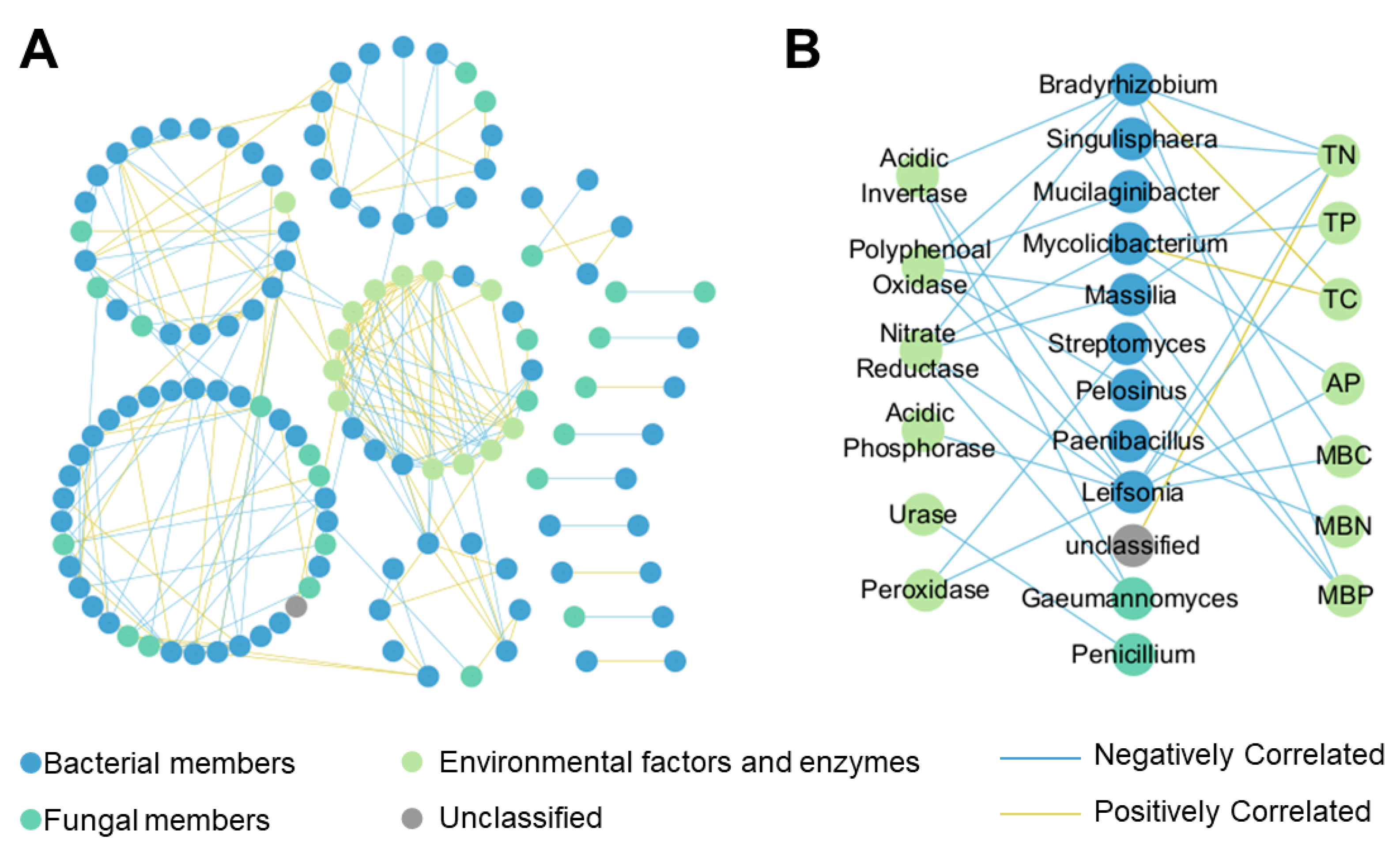
3.5. Network Analysis on Microbial Community
4. Discussion
4.1. P. bournei Replanting Enhances Soil Nitrogen and Phosphorus Limitation
4.2. Effects of P. bournei Replanting on Microbial Community Properties
4.3. P. bournei Replanting Enhances Soil Carbon Accumulation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Le Quéré, C.; Raupach, M.R.; Canadell, J.G.; Marland, G.; Bopp, L.; Ciais, P.; Conway, T.J.; Doney, S.C.; Feely, R.A.; Foster, P.; et al. Trends in the sources and sinks of carbon dioxide. Nat. Geosci. 2009, 2, 831–836. [Google Scholar] [CrossRef]
- Cavicchioli, R.; Ripple, W.J.; Timmis, K.N.; Azam, F.; Bakken, L.R.; Baylis, M.; Behrenfeld, M.J.; Boetius, A.; Boyd, P.W.; Classen, A.T.; et al. Scientists’ warning to humanity: Microorganisms and climate change. Nat. Rev. Microbiol. 2019, 17, 569–586. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.K.; Solomon, A.M.; Brown, S.; Houghton, R.A.; Trexier, M.C.; Wisniewski, J. Carbon pools and flux of global forest ecosystems. Science 1994, 263, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Angst, G.; Mueller, K.E.; Castellano, M.J.; Vogel, C.; Wiesmeier, M.; Mueller, C.W. Unlocking complex soil systems as carbon sinks: Multi-pool management as the key. Nat. Commun. 2023, 14, 2967. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Hansel, C.M.; Kaiser, C.; Kleber, M.; Maher, K.; Manzoni, S.; Nunan, N.; Reichstein, M.; Schimel, J.P.; Torn, M.S.; et al. Persistence of soil organic carbon caused by functional complexity. Nat. Geosci. 2020, 13, 529–534. [Google Scholar] [CrossRef]
- Thompson, I.D.; Okabe, K.; Parrotta, J.A.; Brockerhoff, E.; Jactel, H.; Forrester, D.I.; Taki, H. Biodiversity and ecosystem services: Lessons from nature to improve management of planted forests for REDD-plus. Biodivers. Conserv. 2014, 23, 2613–2635. [Google Scholar] [CrossRef]
- Bongers, F.J.; Schmid, B.; Bruelheide, H.; Bongers, F.; Li, S.; von Oheimb, G.; Li, Y.; Cheng, A.; Ma, K.; Liu, X. Functional diversity effects on productivity increase with age in a forest biodiversity experiment. Nat. Ecol. Evol. 2021, 5, 1594–1603. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Jeffries, T.C.; Gaitan, J.J.; Encinar, D.; Berdugo, M.; Campbell, C.D.; Singh, B.K. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun. 2016, 7, 10541. [Google Scholar] [CrossRef]
- Zhou, J.; Deng, Y.; Shen, L.; Wen, C.; Yan, Q.; Ning, D.; Qin, Y.; Xue, K.; Wu, L.; He, Z.; et al. Temperature mediates continental-scale diversity of microbes in forest soils. Nat. Commun. 2016, 7, 12083. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Balser, T.C. Microbial production of recalcitrant organic matter in global soils: Implications for productivity and climate policy. Nat. Rev. Microbiol. 2011, 9, 75. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Chang. Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Schimel, J.; Schaeffer, S. Microbial control over carbon cycling in soil. Front. Microbiol. 2012, 3, 348. [Google Scholar] [CrossRef]
- Rui, Y.; Jackson, R.D.; Cotrufo, M.F.; Sanford, G.R.; Spiesman, B.J.; Deiss, L.; Culman, S.W.; Liang, C.; Ruark, M.D. Persistent soil carbon enhanced in Mollisols by well-managed grasslands but not annual grain or dairy forage cropping systems. Proc. Natl. Acad. Sci. USA 2022, 119, e2118931119. [Google Scholar] [CrossRef] [PubMed]
- Meier, C.L.; Bowman, W.D. Links between plant litter chemistry, species diversity, and below-ground ecosystem function. Proc. Natl. Acad. Sci. USA 2008, 105, 19780–19785. [Google Scholar] [CrossRef]
- Liang, C.; Amelung, W.; Lehmann, J.; Kästner, M. Quantitative assessment of microbial necromass contribution to soil organic matter. Glob. Chang. Biol. 2019, 25, 3578–3590. [Google Scholar] [CrossRef]
- Keiluweit, M.; Bougoure, J.J.; Nico, P.S.; Pett-Ridge, J.; Weber, P.K.; Kleber, M. Mineral protection of soil carbon counteracted by root exudates. Nat. Clim. Chang. 2015, 5, 588–595. [Google Scholar] [CrossRef]
- Chari, N.R.; Taylor, B.N. Soil organic matter formation and loss are mediated by root exudates in a temperate forest. Nat. Geosci. 2022, 15, 1011–1016. [Google Scholar] [CrossRef]
- Chen, X.; Taylor, A.R.; Reich, P.B.; Hisano, M.; Chen, H.Y.H.; Chang, S.X. Tree diversity increases decadal forest soil carbon and nitrogen accrual. Nature 2023, 618, 94–101. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.Y.H. Plant mixture balances terrestrial ecosystem C:N:P stoichiometry. Nat. Commun. 2021, 12, 4562. [Google Scholar] [CrossRef]
- Ben Keane, J.; Hartley, I.P.; Taylor, C.R.; Leake, J.R.; Hoosbeek, M.R.; Miglietta, F.; Phoenix, G.K. Grassland responses to elevated CO2 determined by plant–microbe competition for phosphorus. Nat. Geosci. 2023, 16, 704–709. [Google Scholar] [CrossRef]
- Zhu, Q.; Riley, W.J.; Tang, J.; Koven, C.D. Multiple soil nutrient competition between plants, microbes, and mineral surfaces: Model development, parameterization, and example applications in several tropical forests. Biogeosciences 2016, 13, 341–363. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Liptzin, D. C:N:P stoichiometry in soil: Is there a “Redfield ratio” for the microbial biomass? Biogeochemistry 2007, 85, 235–252. [Google Scholar] [CrossRef]
- Averill, C.; Waring, B. Nitrogen limitation of decomposition and decay: How can it occur? Glob. Chang. Biol. 2018, 24, 1417–1427. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, S.; Zhang, J. Assessing the effects of vegetation types on carbon storage fifteen years after reforestation on a Chinese fir site. For. Ecol. Manag. 2009, 258, 1437–1441. [Google Scholar] [CrossRef]
- Chen, L.-C.; Wang, S.-L. Allelopathic behaviour of Chinese fir from plantations of different ages. For. Int. J. For. Res. 2013, 86, 225–230. [Google Scholar] [CrossRef][Green Version]
- Bennett, A.J.; Bending, G.D.; Chandler, D.; Hilton, S.; Mills, P. Meeting the demand for crop production: The challenge of yield decline in crops grown in short rotations. Biol. Rev. Camb. Philos. Soc. 2012, 87, 52–71. [Google Scholar] [CrossRef]
- Liu, L.; Duan, Z.; Xu, M.; Hu, J.; Wang, S.; Hu, Z.; Zhang, Q.; Wang, S. Effect of monospecific and mixed Cunninghamia lanceolata plantations on microbial community and two functional genes involved in nitrogen cycling. Plant Soil 2010, 327, 413–428. [Google Scholar] [CrossRef]
- Zhou, H.; Ma, A.; Zhou, X.; Chen, X.; Zhang, J.; Zhang, Q.; Qi, X.; Liu, G.; Zhuang, G. Phosphorus shapes soil microbial community composition and network properties during grassland expansion into shrubs in Tibetan dry valleys. Front. Plant Sci. 2022, 13, 704–709. [Google Scholar] [CrossRef]
- Kpemoua, T.P.I.; Leclerc, S.; Barré, P.; Houot, S.; Pouteau, V.; Plessis, C.; Chenu, C. Are carbon-storing soils more sensitive to climate change? A laboratory evaluation for agricultural temperate soils. Soil Biol. Biochem. 2023, 183, 109043. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Chhin, S.; Zhang, J.; Duan, A. Disentangling the effects of stand and climatic variables on forest productivity of Chinese fir plantations in subtropical China using a random forest algorithm. Agric. For. Meteorol. 2021, 304–305, 108412. [Google Scholar] [CrossRef]
- Wang, Q.-K.; Wang, S.-L.; Zhong, M.-C. Ecosystem carbon storage and soil organic carbon stability in pure and mixed stands of Cunninghamia lanceolata and Michelia macclurei. Plant Soil 2013, 370, 295–304. [Google Scholar] [CrossRef]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setälä, H.; van der Putten, W.H.; Wall, D.H. Ecological linkages between aboveground and belowground biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef] [PubMed]
- Sumner, M.E. Measurement of soil pH: Problems and solutions. Commun. Soil Sci. Plant Anal. 1994, 25, 859–879. [Google Scholar] [CrossRef]
- Sommers, L.E.; Nelson, D.W. Determination of total phosphorus in soils: A rapid perchloric acid digestion procedure. Soil Sci. Soc. Am. J. 1972, 36, 902–904. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Brookes, P.C.; Powlson, D.S.; Jenkinson, D.S. Measurement of microbial biomass phosphorus in soil. Soil Biol. Biochem. 1982, 14, 319–329. [Google Scholar] [CrossRef]
- Li, D.; Liu, C.-M.; Luo, R.; Sadakane, K.; Lam, T.-W. MEGAHIT: An ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef]
- Barrett, P. Structural equation modelling: Adjudging model fit. Personal. Individ. Differ. 2007, 42, 815–824. [Google Scholar] [CrossRef]
- Deng, Y.; Jiang, Y.-H.; Yang, Y.; He, Z.; Luo, F.; Zhou, J. Molecular ecological network analyses. BMC Bioinform. 2012, 13, 113. [Google Scholar] [CrossRef] [PubMed]
- Chapuis-Lardy, L.; Vanderhoeven, S.; Dassonville, N.; Koutika, L.S.; Meerts, P. Effect of the exotic invasive plant Solidago gigantea on soil phosphorus status. Biol. Fertil. Soils 2006, 42, 481–489. [Google Scholar] [CrossRef]
- Chen, C.R.; Condron, L.M.; Davis, M.R.; Sherlock, R.R. Effects of plant species on microbial biomass phosphorus and phosphatase activity in a range of grassland soils. Biol. Fertil. Soils 2004, 40, 313–322. [Google Scholar] [CrossRef]
- Cong, J.; Wang, X.; Liu, X.; Zhang, Y. The distribution variation and key influencing factors of soil organic carbon of natural deciduous broadleaf forests along the latitudinal gradient. Acta Ecol. Sin. 2016, 36, 333–339. [Google Scholar] [CrossRef]
- Chiti, T.; Díaz-Pinés, E.; Rubio, A. Soil organic carbon stocks of conifers, broadleaf and evergreen broadleaf forests of Spain. Biol. Fertil. Soils 2012, 48, 817–826. [Google Scholar] [CrossRef]
- Ehrenfeld, J.G. Effects of exotic plant invasions on soil nutrient cycling processes. Ecosystems 2003, 6, 503–523. [Google Scholar] [CrossRef]
- Geddes, P.; Grancharova, T.; Kelly, J.J.; Treering, D.; Tuchman, N.C. Effects of invasive Typha × glauca on wetland nutrient pools, denitrification, and bacterial communities are influenced by time since invasion. Aquat. Ecol. 2014, 48, 247–258. [Google Scholar] [CrossRef]
- Yuan, Y.; Guo, W.; Ding, W.; Du, N.; Luo, Y.; Liu, J.; Xu, F.; Wang, R. Competitive interaction between the exotic plant Rhus typhina L. and the native tree Quercus acutissima Carr. in Northern China under different soil N:P ratios. Plant Soil 2013, 372, 389–400. [Google Scholar] [CrossRef]
- Dunn, R.M.; Mikola, J.; Bol, R.; Bardgett, R.D. Influence of microbial activity on plant–microbial competition for organic and inorganic nitrogen. Plant Soil 2006, 289, 321–334. [Google Scholar] [CrossRef]
- Moreau, D.; Pivato, B.; Bru, D.; Busset, H.; Deau, F.; Faivre, C.; Matejicek, A.; Strbik, F.; Philippot, L.; Mougel, C. Plant traits related to nitrogen uptake influence plant-microbe competition. Ecology 2015, 96, 2300–2310. [Google Scholar] [CrossRef] [PubMed]
- Stein, L.Y.; Klotz, M.G. The nitrogen cycle. Curr. Biol. 2016, 26, R94–R98. [Google Scholar] [CrossRef] [PubMed]
- Hou, E.; Chen, C.; Luo, Y.; Zhou, G.; Kuang, Y.; Zhang, Y.; Heenan, M.; Lu, X.; Wen, D. Effects of climate on soil phosphorus cycle and availability in natural terrestrial ecosystems. Glob. Chang. Biol. 2018, 24, 3344–3356. [Google Scholar] [CrossRef] [PubMed]
- Ferenci, T. Regulation by nutrient limitation. Curr. Opin. Microbiol. 1999, 2, 208–213. [Google Scholar] [CrossRef]
- Waring, B.G.; Weintraub, S.R.; Sinsabaugh, R.L. Ecoenzymatic stoichiometry of microbial nutrient acquisition in tropical soils. Biogeochemistry 2014, 117, 101–113. [Google Scholar] [CrossRef]
- Manzoni, S.; Taylor, P.; Richter, A.; Porporato, A.; Ågren, G.I. Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytol. 2012, 196, 79–91. [Google Scholar] [CrossRef]
- Mooshammer, M.; Wanek, W.; Hämmerle, I.; Fuchslueger, L.; Hofhansl, F.; Knoltsch, A.; Schnecker, J.; Takriti, M.; Watzka, M.; Wild, B.; et al. Adjustment of microbial nitrogen use efficiency to carbon:nitrogen imbalances regulates soil nitrogen cycling. Nat. Commun. 2014, 5, 3694. [Google Scholar] [CrossRef]
- Kominoski, J.S.; Hoellein, T.J.; Kelly, J.J.; Pringle, C.M. Does mixing litter of different qualities alter stream microbial diversity and functioning on individual litter species? Oikos 2009, 118, 457–463. [Google Scholar] [CrossRef]
- Thoms, C.; Gattinger, A.; Jacob, M.; Thomas, F.M.; Gleixner, G. Direct and indirect effects of tree diversity drive soil microbial diversity in temperate deciduous forest. Soil Biol. Biochem. 2010, 42, 1558–1565. [Google Scholar] [CrossRef]
- Wang, C.; Liu, D.; Bai, E. Decreasing soil microbial diversity is associated with decreasing microbial biomass under nitrogen addition. Soil Biol. Biochem. 2018, 120, 126–133. [Google Scholar] [CrossRef]
- Vellend, M. Effects of diversity on diversity: Consequences of competition and facilitation. Oikos 2008, 117, 1075–1085. [Google Scholar] [CrossRef]
- Liu, C.; Dong, Y.; Hou, L.; Deng, N.; Jiao, R. Acidobacteria Community Responses to Nitrogen Dose and Form in Chinese Fir Plantations in Southern China. Curr. Microbiol. 2017, 74, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ding, K.; Yrjälä, K.; Liu, H.; Tong, Z.; Zhang, J. Introduction of broadleaf species into monospecific Cunninghamia lanceolata plantations changed the soil Acidobacteria subgroups composition and nitrogen-cycling gene abundances. Plant Soil 2021, 467, 29–46. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, C.; Yu, W.; Turak, A.; Chen, D.; Huang, Y.; Ao, J.; Jiang, Y.; Huang, Z. Effects of nitrogen and phosphorus inputs on soil bacterial abundance, diversity, and community composition in Chinese fir plantations. Front. Microbiol. 2018, 9, 1543. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.-C.; Kong, C.-H.; Chen, L.-C.; Wang, S.-L. Allelochemical-mediated soil microbial community in long-term monospecific Chinese fir forest plantations. Appl. Soil Ecol. 2015, 96, 52–59. [Google Scholar] [CrossRef]
- Meng, M.; Lin, J.; Guo, X.; Liu, X.; Wu, J.; Zhao, Y.; Zhang, J. Impacts of forest conversion on soil bacterial community composition and diversity in subtropical forests. CATENA 2019, 175, 167–173. [Google Scholar] [CrossRef]
- Córdova, S.C.; Olk, D.C.; Dietzel, R.N.; Mueller, K.E.; Archontouilis, S.V.; Castellano, M.J. Plant litter quality affects the accumulation rate, composition, and stability of mineral-associated soil organic matter. Soil Biol. Biochem. 2018, 125, 115–124. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Cornelissen, J.H.C.; Amatangelo, K.; Dorrepaal, E.; Eviner, V.T.; Godoy, O.; Hobbie, S.E.; Hoorens, B.; Kurokawa, H.; Pérez-Harguindeguy, N.; et al. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol. Lett. 2008, 11, 1065–1071. [Google Scholar] [CrossRef]
- Trivedi, P.; Delgado-Baquerizo, M.; Trivedi, C.; Hu, H.; Anderson, I.C.; Jeffries, T.C.; Zhou, J.; Singh, B.K. Microbial regulation of the soil carbon cycle: Evidence from gene–enzyme relationships. ISME J. 2016, 10, 2593–2604. [Google Scholar] [CrossRef]
- Fischer, M.S.; Stark, F.G.; Berry, T.D.; Zeba, N.; Whitman, T.; Traxler, M.F. Pyrolyzed substrates induce aromatic compound metabolism in the post-fire fungus, pyronema domesticum. Front. Microbiol. 2021, 12, 729289. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, S.; Hu, Y.; Chen, Y.; Jiang, H.; Duan, B.; Lu, X. Links between chemical composition of soil organic matter and soil enzyme activity in alpine grassland ecosystems of the Tibetan Plateau. CATENA 2022, 218, 106565. [Google Scholar] [CrossRef]
- Zhou, J.; Xue, K.; Xie, J.; Deng, Y.; Wu, L.; Cheng, X.; Fei, S.; Deng, S.; He, Z.; Van Nostrand, J.D.; et al. Microbial mediation of carbon-cycle feedbacks to climate warming. Nat. Clim. Chang. 2012, 2, 106–110. [Google Scholar] [CrossRef]
- Zhou, H.; Di, L.; Hua, X.; Deng, T.; Wang, X. Bacterial community drives the carbon source degradation during the composting of Cinnamomum camphora leaf industrial extracted residues. Microbiol. Res. 2023, 14, 229–242. [Google Scholar] [CrossRef]
- Zhou, H.; Di, L.; Hua, X.; Deng, T.; Wang, X. The addition of a small dose of Cinnamomum camphora biomass unexpectedly enhanced lignocellulose degradation during the compost of Stropharia rugosoannulata cultivation materials. Sustainability 2023, 15, 10483. [Google Scholar] [CrossRef]
- Frostegård, Å.; Vick, S.H.W.; Lim, N.Y.N.; Bakken, L.R.; Shapleigh, J.P. Linking meta-omics to the kinetics of denitrification intermediates reveals pH-dependent causes of N2O emissions and nitrite accumulation in soil. ISME J. 2022, 16, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Filippelli, G.M. The global phosphorus cycle: Past, present, and future. Elements 2008, 4, 89–95. [Google Scholar] [CrossRef]
- Dick, W.A.; Cheng, L.; Wang, P. Soil acid and alkaline phosphatase activity as pH adjustment indicators. Soil Biol. Biochem. 2000, 32, 1915–1919. [Google Scholar] [CrossRef]
- Peng, Z.; Wu, Y.; Guo, L.; Yang, L.; Wang, B.; Wang, X.; Liu, W.; Su, Y.; Wu, J.; Liu, L. Foliar nutrient resorption stoichiometry and microbial phosphatase catalytic efficiency together alleviate the relative phosphorus limitation in forest ecosystems. New Phytol. 2023, 238, 1033–1044. [Google Scholar] [CrossRef]
- Keane, J.B.; Hoosbeek, M.R.; Taylor, C.R.; Miglietta, F.; Phoenix, G.K.; Hartley, I.P. Soil C, N and P cycling enzyme responses to nutrient limitation under elevated CO2. Biogeochemistry 2020, 151, 221–235. [Google Scholar] [CrossRef]
- Luo, M.; Moorhead, D.L.; Ochoa-Hueso, R.; Mueller, C.W.; Ying, S.C.; Chen, J. Nitrogen loading enhances phosphorus limitation in terrestrial ecosystems with implications for soil carbon cycling. Funct. Ecol. 2022, 36, 2845–2858. [Google Scholar] [CrossRef]
- Zhen, Z.; Li, G.; Chen, Y.; Wei, T.; Li, H.; Huang, F.; Huang, Y.; Ren, L.; Liang, Y.; Zhang, D.; et al. Accelerated nitrification and altered community structure of ammonia-oxidizing microorganisms in the saline-alkali tolerant rice rhizosphere of coastal solonchaks. Appl. Soil Ecol. 2023, 189, 104978. [Google Scholar] [CrossRef]
- Ruyters, S.; Nicol, G.W.; Prosser, J.I.; Lievens, B.; Smolders, E. Activity of the ammonia oxidising bacteria is responsible for zinc tolerance development of the ammonia oxidising community in soil: A stable isotope probing study. Soil Biol. Biochem. 2013, 58, 244–247. [Google Scholar] [CrossRef]
- Krüger, M.; Potthast, K.; Michalzik, B.; Tischer, A.; Küsel, K.; Deckner, F.F.K.; Herrmann, M. Drought and rewetting events enhance nitrate leaching and seepage-mediated translocation of microbes from beech forest soils. Soil Biol. Biochem. 2021, 154, 108153. [Google Scholar] [CrossRef]
- Fuchslueger, L.; Kastl, E.M.; Bauer, F.; Kienzl, S.; Hasibeder, R.; Ladreiter-Knauss, T.; Schmitt, M.; Bahn, M.; Schloter, M.; Richter, A.; et al. Effects of drought on nitrogen turnover and abundances of ammonia-oxidizers in mountain grassland. Biogeosciences 2014, 11, 6003–6015. [Google Scholar] [CrossRef]
- Mulder, C.; Elser, J. Soil pH, ecological stoichiometry, and allometric scaling in soil biota. Nat. Preced. 2010. [Google Scholar] [CrossRef]
- Fuchsman, C.A.; Devol, A.H.; Saunders, J.K.; McKay, C.; Rocap, G. Niche partitioning of the N cycling microbial community of an offshore oxygen deficient zone. Front. Microbiol. 2017, 8, 2384. [Google Scholar] [CrossRef]
- Von Lützow, M.; Kögel-Knabner, I.; Ekschmitt, K.; Flessa, H.; Guggenberger, G.; Matzner, E.; Marschner, B. SOM fractionation methods: Relevance to functional pools and to stabilization mechanisms. Soil Biol. Biochem. 2007, 39, 2183–2207. [Google Scholar] [CrossRef]
- Kleber, M.; Bourg, I.C.; Coward, E.K.; Hansel, C.M.; Myneni, S.C.B.; Nunan, N. Dynamic interactions at the mineral–organic matter interface. Nat. Rev. Earth Environ. 2021, 2, 402–421. [Google Scholar] [CrossRef]
- Jilling, A.; Keiluweit, M.; Contosta, A.R.; Frey, S.; Schimel, J.; Schnecker, J.; Smith, R.G.; Tiemann, L.; Grandy, A.S. Minerals in the rhizosphere: Overlooked mediators of soil nitrogen availability to plants and microbes. Biogeochemistry 2018, 139, 103–122. [Google Scholar] [CrossRef]
- Mueller, C.W.; Rethemeyer, J.; Kao-Kniffin, J.; Löppmann, S.; Hinkel, K.M.; Bockheim, J.G. Large amounts of labile organic carbon in permafrost soils of northern Alaska. Glob. Chang. Biol. 2015, 21, 2804–2817. [Google Scholar] [CrossRef]
- Tao, J.; Wang, S.; Liao, T.; Luo, H. Evolutionary origin and ecological implication of a unique nif island in free-living Bradyrhizobium lineages. ISME J. 2021, 15, 3195–3206. [Google Scholar] [CrossRef] [PubMed]
- Gourion, B.; Delmotte, N.; Bonaldi, K.; Nouwen, N.; Vorholt, J.A.; Giraud, E. Bacterial RuBisCO is required for efficient Bradyrhizobium/Aeschynomene symbiosis. PLoS ONE 2011, 6, e21900. [Google Scholar] [CrossRef] [PubMed]
- Horecker, B.L.; Hurwitz, J.; Smyrniotis, P.Z. The role of xylulose 5-phosphate in the transketolase reaction. J. Biol. Chem. 1956, 223, 1009–1019. [Google Scholar] [CrossRef]
- Fullam, E.; Pojer, F.; Bergfors, T.; Jones, T.A.; Cole, S.T. Structure and function of the transketolase from Mycobacterium tuberculosis and comparison with the human enzyme. Open Biol. 2012, 2, 110026. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Gu, Y.; Chen, Z.; Song, Y.; Sun, F.; Liu, J.; Waigi, M.G. Colonization and performance of a pyrene-degrading bacterium Mycolicibacterium sp. Pyr9 on root surfaces of white clover. Chemosphere 2021, 263, 127918. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.-R.; Yang, S.-R.; Zhen, C.; Ge, X.-F.; Chen, X.-K.; Wen, Z.-Q.; Li, Y.-N.; Liu, W.-Z. Genomic molecular signatures determined characterization of Mycolicibacterium gossypii sp. nov., a fast-growing mycobacterial species isolated from cotton field soil. Antonie Van Leeuwenhoek 2021, 114, 1735–1744. [Google Scholar] [CrossRef]
- Kang, S.M.; Khan, A.L.; You, Y.H.; Kim, J.G.; Kamran, M.; Lee, I.J. Gibberellin production by newly isolated strain Leifsonia soli SE134 and its potential to promote plant growth. J. Microbiol. Biotechnol. 2014, 24, 106–112. [Google Scholar] [CrossRef]
- Garcia, F.H.S.; Barros, T.H.d.S.; Coelho, R.D.; Laborde, M.C.F.; Azevedo, R.A.; Lyra, D.H.; Kluge, R.A. Impact of the colonization of Leifsonia xyli subsp. xyli in a susceptible sugarcane genotype on water status and physiological traits. Eur. J. Plant Pathol. 2021, 159, 839–849. [Google Scholar] [CrossRef]
- Zavaglia, A.C.; Cia, M.C.; Popin, R.V.; Camargo, L.E.A. No alternative hosts of the sugarcane pathogen Leifsonia xyli subsp. xyli were identified among grass and non-grass species using novel PCR primers. Trop. Plant Pathol. 2016, 41, 336–339. [Google Scholar] [CrossRef]
- Wang, N.-Q.; Sun, J.; Huang, J.; Wang, P. Cloning, expression, and directed evolution of carbonyl reductase from Leifsonia xyli HS0904 with enhanced catalytic efficiency. Appl. Microbiol. Biotechnol. 2014, 98, 8591–8601. [Google Scholar] [CrossRef]
- Rastogi, A.; Banerjee, R. Production and characterization of cellulose from Leifsonia sp. Process Biochem. 2019, 85, 35–42. [Google Scholar] [CrossRef]
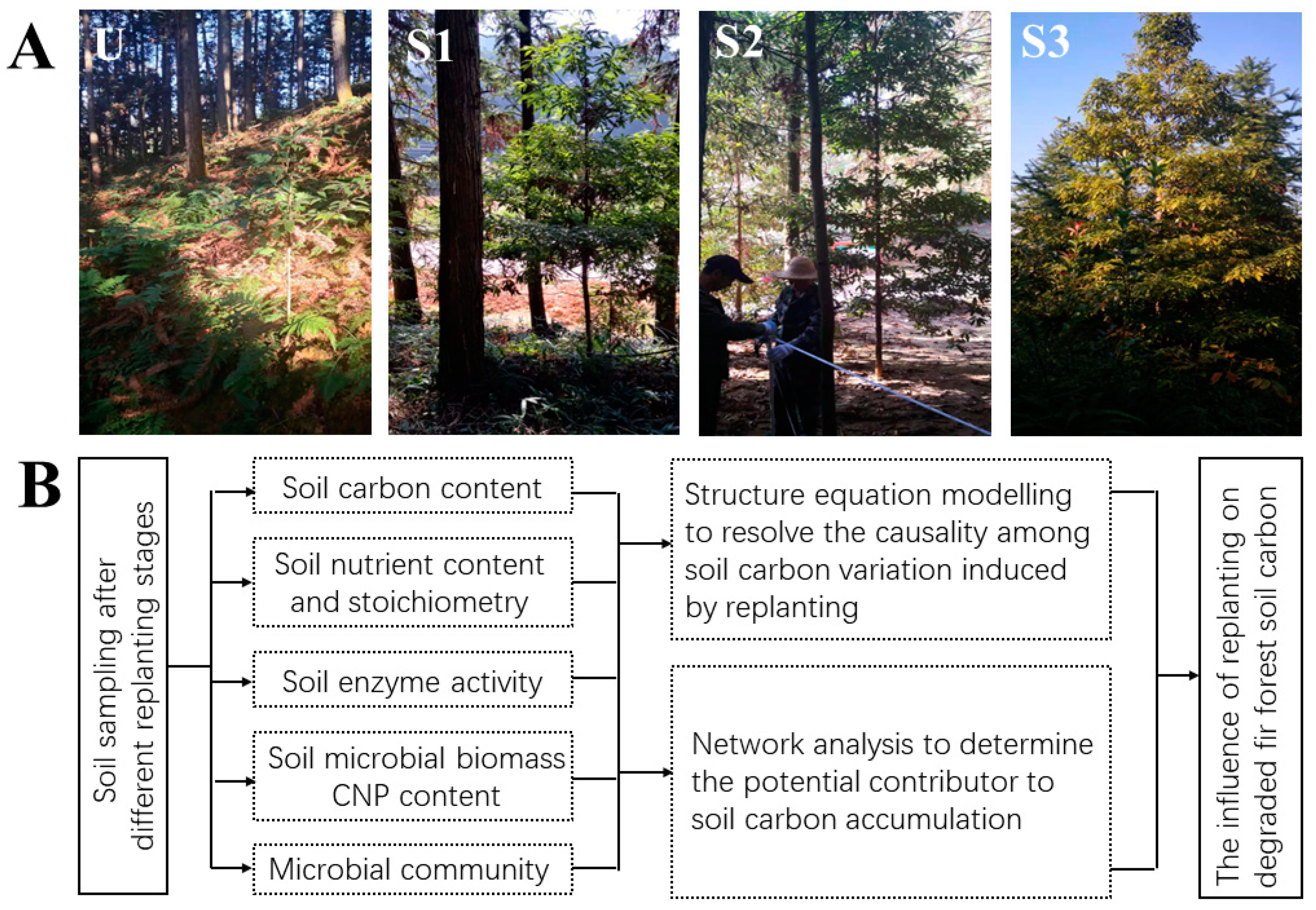



| Age | Altitude (m) | Slope (°) | Tree Species | Mean Tree Height (m) | Mean Diameter at Breast Height (cm) | Stand Density (n/ha) | |
|---|---|---|---|---|---|---|---|
| U | 0a | 174 | 24 | C. Lanceolata | 19.53 | 24.83 | 1190 |
| S1 | 5a | 165 | 21 | P. bournei | 1.74 | 3.52 | 1150 |
| C. Lanceolata | 20.17 | 25.15 | 765 | ||||
| S2 | 8a | 170 | 23 | P. bournei | 3.22 | 5.24 | 1190 |
| C. Lanceolata | 21.83 | 24.72 | 792 | ||||
| S3 | 12a | 170 | 24 | P. bournei | 5.65 | 8.15 | 1180 |
| C. Lanceolata | 22.34 | 25.31 | 788 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Zhou, H.; Xu, J.; Zhao, H.; Shen, J.; Liu, C.; Wang, L. Influence of Phoebe bournei (Hemsl.) Replanting on Soil Carbon Content and Microbial Processes in a Degraded Fir Forest. Forests 2023, 14, 2144. https://doi.org/10.3390/f14112144
Li T, Zhou H, Xu J, Zhao H, Shen J, Liu C, Wang L. Influence of Phoebe bournei (Hemsl.) Replanting on Soil Carbon Content and Microbial Processes in a Degraded Fir Forest. Forests. 2023; 14(11):2144. https://doi.org/10.3390/f14112144
Chicago/Turabian StyleLi, Ting, Hanchang Zhou, Jiawen Xu, Hong Zhao, Jiacheng Shen, Chunjiang Liu, and Liyan Wang. 2023. "Influence of Phoebe bournei (Hemsl.) Replanting on Soil Carbon Content and Microbial Processes in a Degraded Fir Forest" Forests 14, no. 11: 2144. https://doi.org/10.3390/f14112144
APA StyleLi, T., Zhou, H., Xu, J., Zhao, H., Shen, J., Liu, C., & Wang, L. (2023). Influence of Phoebe bournei (Hemsl.) Replanting on Soil Carbon Content and Microbial Processes in a Degraded Fir Forest. Forests, 14(11), 2144. https://doi.org/10.3390/f14112144





