Abstract
The objective of this work was to examine the effects of Scots pine blister rust on the quality of Scots pine wood. The research material was taken from tree parts with visible symptoms of fungal infection as well as from unaffected tree parts. Our results show that the effects of the fungus Cronartium pini (Willd.) Jørst. are local and do not prevent the use of wood, and especially its unaffected fragments. Statistical analysis was used to describe the ways in which the pathogen altered wood. While the fungus statistically increased wood density, it significantly diminished its strength parameters. The structural properties of infected and healthy wood from affected trees were found to be similar and much higher than the average values for Scots pine. The higher transverse parameters of wood fibers had a beneficial effect on morphological properties. Scots pine blister rust significantly decreased wood homogeneity, which, together with stem deformation, lowered the quality of timber and reduced its yield.
1. Introduction
While Pucciniales (previously known as Uredinales) are among the most widespread fungi found around the world [1], little research has been devoted to their effects on wood properties. Cronartium pini (Willd.) Jørst. is a common pathogen. In Poland, Scots pine blister rust disease caused by Cronartium pini affects Scots pines [2,3]. Scots pine blister rust used to pose a major problem for silviculture, but according to current forecasts issued by the Forestry Research Institute, its impact has somewhat lessened [4]. First symptoms appear 2 to 4 years after inoculation [5] as local swellings near the whorl or in branches as a result of fungal growth into the wood tissue. Aeciospores appear annually on the bark, causing cracks that lead to a diamond-shaped resinous canker [6], where the opposite part of the stem swells causing eccentricity. One of the results of fungal activity is an increased amount of resin, which is seen on the wood. Resin canker usually occurs in the middle part of the stem [6] and the infection may persist for many years [3,5]. The fungus tends to grow down the stem to a greater extent than circumferentially, up to several centimeters per year [5,6,7]. Infection primarily results in a lower tree height and volume, stem deformation, and even mortality [8,9,10], reducing the commercial value of the timber [10].
It is often said that wood infected by fungi is only fit to be used for fuel due to its high resin content leading to increased wood density and durability. However, the defects resulting from this kind of fungal infection occur only locally in the tree, and so the entire stem does not need to be relegated to the lowest quality classes. According to a study by Mirski et al. [11], most wood defects are concentrated in the middle region of the stem, and this is also the case with Cronartium pini defects, which are not included in the polish technical conditions of pine wood.
The rational evaluation of wood quality affected by a pathogen requires knowledge of the way it impacts wood and should be based on tests of strength properties as well as on visual assessment [11]. The wood industry values high-quality timber with versatile properties and wide availability. These criteria are met by the Scots pine, which is one the most important tree species in the world. It has been primarily studied in terms of its structure [12,13] and physical properties [14,15,16,17,18,19,20,21] (and especially density [13,22,23,24,25,26,27,28]) as well as mechanical properties [29,30,31,32,33].
On the other hand, the anatomical characteristics and morphological properties of various species of production trees are among the least-studied areas in wood research in Poland, as evidenced by the limited availability of the literature data [34,35,36,37,38]. To expand the knowledge in this field, it is necessary to examine the results and insights of international authors [14,39,40]. The present work makes a substantial contribution in this area, additionally providing valuable information about the influence of pathogens on the structural properties of P. sylvestris wood.
Most of the cited publications focus on the effects of Cronartium pini on tree development, and they completely ignore impact on wood quality and its technical suitability. The lack of scientific studies shows that the current study is much needed. This is the first research on the properties of pine wood infected with Scots pine blister rust in Poland. On the other hand, ample research on the properties of pine wood has enabled comparative evaluation of the obtained results for healthy timber from infected trees with a view to determining its quality. It was assumed that Scots pine blister rust had an effect on the properties of the wood. The objective of the work was to investigate the structural, physical, and mechanical parameters of Scots pine wood affected by Cronartium pini and compare them with the corresponding properties of healthy wood from affected trees. Our work focuses on those properties of wood that are of importance to its applications in the wood industry.
2. Materials and Methods
The research material came from the central part of Poland, the Mińsk Forest District (Warsaw State Forests Regional Directorate, Warszawa, Poland). It is located about 60 km east of Warsaw. The trees mostly grew on a fresh coniferous forest site; with a few exceptions, they were obtained from a fresh mixed coniferous forest site. The research material was selected based on the degree of fungal damage as assessed by the proportion of the crown that was dead. Field work included the determination of tree age as well as the following parameters: tree height and diameter at breast height (DBH), height to the beginning and to the end of the resinous area, the length of the resinous area, and the evaluation of crown health. The fieldwork assessment resulted in thirteen trees being qualified for study with an average age of 83 years, determined by the number of annual rings after felling, an average height of 21.49 m and a DBH of 26 cm. The trees were subsequently harvested and 3 m logs were cut from the middle part of stem. Each log consisted of a part visibly affected by Scots pine blister rust (infected wood) as well as a part without visible rust symptoms (healthy wood). The logs were debarked, slit and seasoned to 12% moisture content. Then, 20 mm × 20 mm × 30 mm samples were taken from the logs pursuant to the standard ISO 3129:2019 [41]. Studies were conducted in accordance with the international standards ISO 13061-2:2014 [42], ISO13061-17:2014 [43], and ISO 13061-1:2014 [44].
Structural studies involved the measurement of 271 samples in terms of annual ring width (ARW [mm]) and latewood proportion (LW [%]). These parameters were determined on the basis of digital images of sample cross-sections acquired using CooRecorder 7.8 software [45]. In the next step, measurement results were exported to the CDendro 7.6 program [46], to obtain readings in millimeters.
Mechanical studies and wood density at a moisture content of 12% (air-dry density) (, kgm−3) were determined for 271 samples. Measurements were performed using the stereometric method (in the radial, tangential, and longitudinal directions) using a SYLVAC S_Cal Evo IP67 150/0.01 mm electronic caliper (SYLVAC, Yverdon-les-Bains, Switzerland) with a precision of 0.01 mm. The samples were weighed using a RADWAG WTC 200 laboratory balance (Radwag, Radom, Poland) with a precision of 0.001 g. The following physical parameters were determined as a result of measuring 220 wood samples at a moisture content of 30% and 0%: (a) wood density at a moisture content of 0% (oven-dry density) (, kgm−3); (b) basic wood density (, kgm−3); (c) total radial shrinkage (, %); (d) total tangential shrinkage (, %); (e) total longitudinal shrinkage (, %); (f) total volumetric shrinkage (, %); (g) total radial shrinkage coefficient (); (h) total tangential shrinkage coefficient (); (i) total longitudinal shrinkage coefficient (); (j) total volumetric shrinkage coefficient (); (k) shrinkage anisotropy index (); (l) the proportion of wood substance (D, %); and (m) porosity (c, %).
A ZD-10 universal tester (VEB WPM, Lipsk, Germany), measuring strength and displacement with a precision of 100 N and 0.1 mm, respectively, was used to determine the following parameters: (a) compression strength parallel to grain (, MPa); (b) coefficient of compression strength parallel to grain (compressive collapse strength) (, km).
Wood samples used in anatomical studies were subjected to tangential cutting upon reaching maximum moisture content by means of a Leica SM 2000 R sliding microtome (Leica Biosystems Nussloch GmbH, Nussloch, Germany), with a separation into earlywood and latewood. Due to high resin content, the obtained material varied in thickness, which affected maceration time. The obtained fragments were placed in Eppendorf tubes and immersed in a maceration solution consisting of 30% hydrogen peroxide and 99.5% glacial acetic acid in a 1:1 ratio. Maceration was conducted at 60 °C in a Memmert UN 30 laboratory incubator (Memmert GmbH, Schwabach, Germany) for 48 h. After maceration, the fibers were washed with distilled water several times to remove the maceration solution, and then immersed in a mixture of water and glycerol in a 1:1 ratio and left for 7 days. In the next step, the samples were stained with 1% safranin solution for 24 h and washed with distilled water. Microscopic slides were prepared under an Olympus SZH10 Research stereomicroscope (Olympus Optical Co.GmbH, Hamburg, Germany) using a 1:1 water–glycerol mixture. After depositing a drop of the mixture on a slide, fibers were placed on the drop and spread with a needle to separate individual fibers. Fifteen fibers were taken for analysis from each slide, which amounted to a total of 720 fibers (360 earlywood and 360 latewood fibers). The preparations were photographed using an Olympus Provis AX70 microscope coupled to an Olympus UC90 camera (Olympus Optical Co.GmbH, Hamburg, Germany). Images were acquired at a magnification of ×2 or ×4 for fiber length measurements and at ×40 for cell width and lumen measurements. Fibers were measured by means of image analysis using ImageJ 1.8.0 software (LOCI, University of Wisconsin), which enabled readouts in millimeters. The following wood fiber parameters and coefficients were determined: (a) fiber length (L, mm); (b) fiber width (D, mm); (c) lumen width (d, mm); (d) cell wall thickness (); (e) slenderness ratio (); (f) Runkel ratio (); (g) flexibility coefficient (); (h) rigidity index (); (i) Mühlsteph index (); and (j) solid factor ().
The results were statistically analyzed to provide characterization of the effects of Cronartium pini on the properties of P. sylvestris wood. Descriptive statistics, including the arithmetic mean (M), median (Me), standard deviation, as well as extreme values (Max and Min) are presented in Table 1 for structural, physical, and mechanical properties and in Table 2 for anatomical properties. Further statistical analysis was contingent on the results of the Shapiro–Wilk test, which determined the normality of distribution of the study material. Since the obtained values of the physical, mechanical, and structural parameters were not normally distributed, the nonparametric Mann–Whitney test was conducted to categorize the results according to wood health status. The same was true of the results obtained for anatomical parameters. Additionally, due to the subdivision of wood samples into earlywood and latewood in infected and healthy wood, the Kruskal–Wallis test was performed. Subsequently, a multiple-comparison Dunn’s test was conducted for values with p < 0.05 to determine significant differences between groups. In the diagram, significant differences are marked with * for p < 0.05, ** for p = 0.01, and *** for p = 0.001. Differences between healthy wood and the remaining groups are shown in red, while those for infected wood are shown in green. The results of both statistical tests are given in Table 2. All statistical analyses were conducted using Statistica 13.5 software [47].

Table 1.
Selected structural, physical, and mechanical properties of Scots pine wood expressed as mean (M), minimum (Min), maximum (Max), median (Me), and standard deviation (SD), as well as Mann–Whitney test results (M–W) at a significance level of p < 0.05.

Table 2.
Basic characteristics of parameters and indicators of the structure of Scots pine wood fibers expressed as mean (M), minimum (Min), maximum (Max), standard deviation (SD), and median (Me) as well as Mann–Whitney (M–W)test results for health status and Kruskal–Wallis (K–W) test for wood type at a significance level of p < 0.05.
3. Results
The results of our studies clearly indicate the impact of Cronartium pini on wood properties. This impact was the strongest for properties such as the mean annual ring width, density, porosity, the proportion of wood substance, total radial, tangential, and volumetric shrinkage, total radial, tangential, and volumetric shrinkage coefficients, compression strength parallel to the grain, and the coefficient of compression strength parallel to the grain (Table 1), as well as the fiber width and lumen and the solid factor (Table 2). On the other hand, Scots pine blister rust did not affect the proportion of latewood, total longitudinal shrinkage and the coefficient of that shrinkage, the shrinkage anisotropy index, fiber length, cell wall thickness, the rigidity index, the Runkel ratio, or the Mühlsteph index (Table 2).
The statistical characterization of the structural, physical, and mechanical properties is given in Table 1. Pathogenic activity significantly affected the mean annual ring width, leading to its higher values as compared to healthy wood as well as considerable variation between individual rings. On the other hand, our study showed that the proportion of latewood is not susceptible to biotic factors, as differences between healthy and infected wood were small in this respect. The presence of resin significantly affected wood density, which was higher for infected wood and revealed high variation. It also affected wood shrinkage (lower in infected samples) and compression strength parallel to the grain (higher in infected samples), while decreasing the coefficient of compression strength parallel to the grain. Infected wood was also found to have a higher proportion of wood substance and lower porosity, in direct contrast to healthy wood.
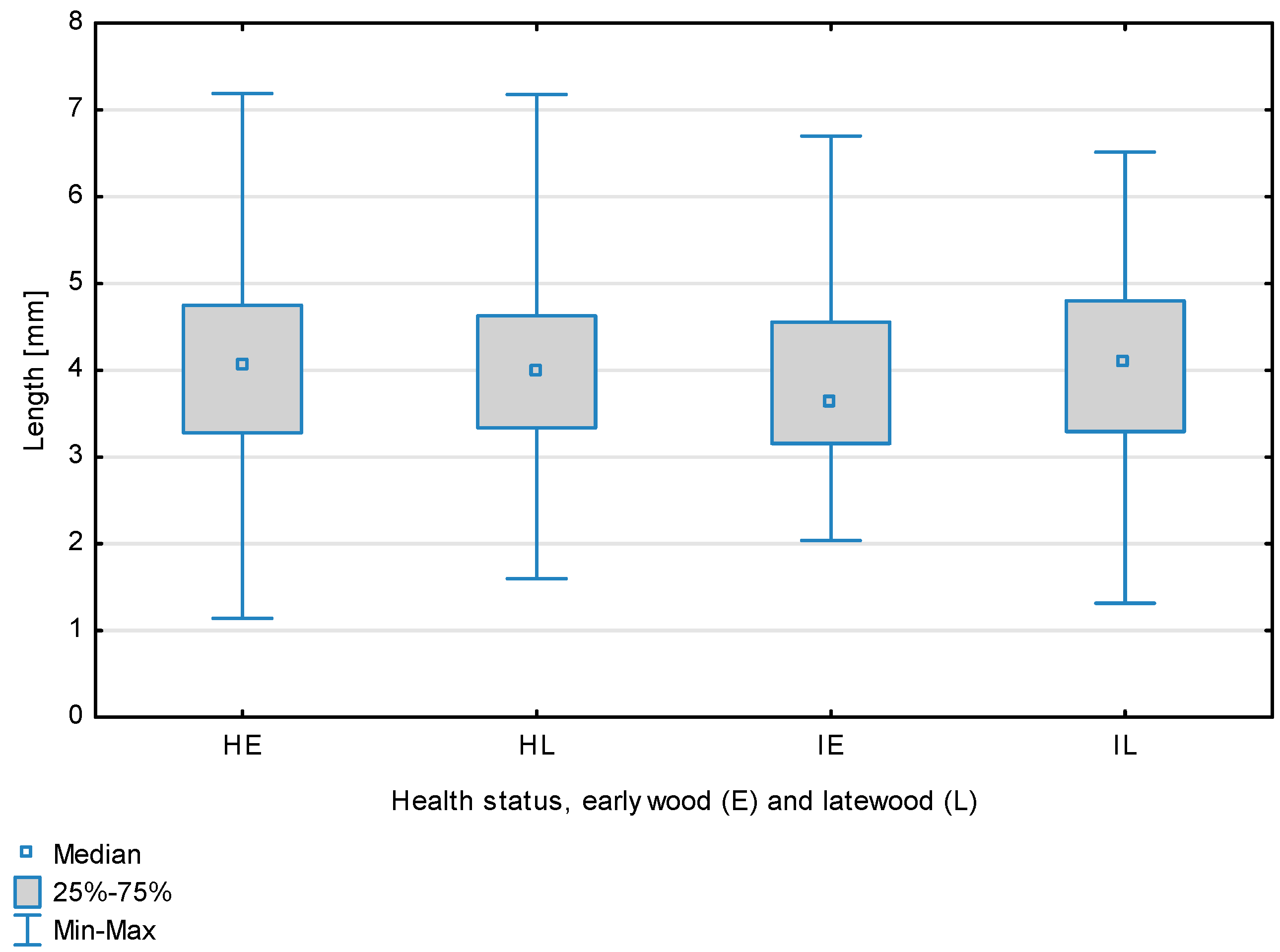
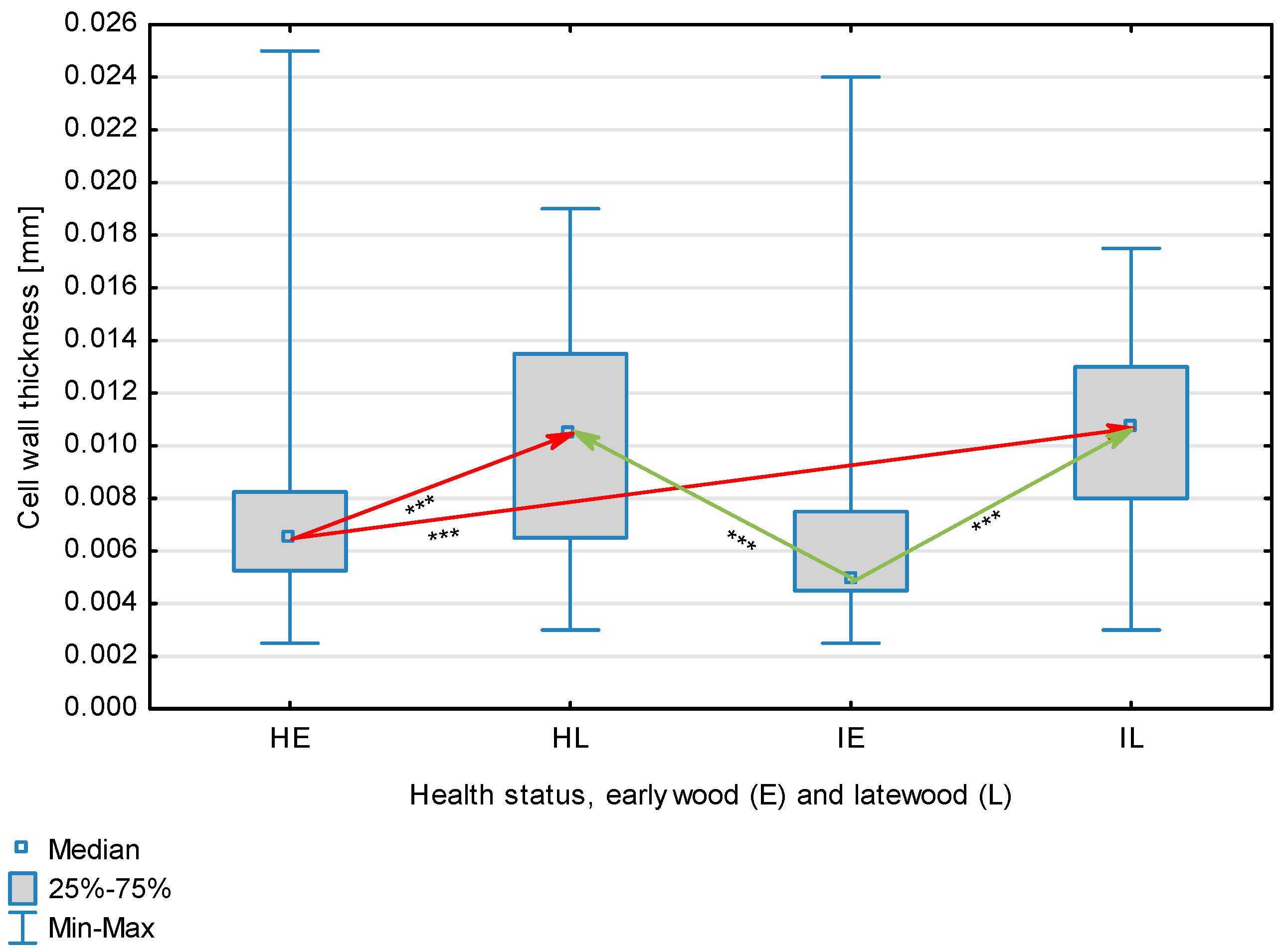
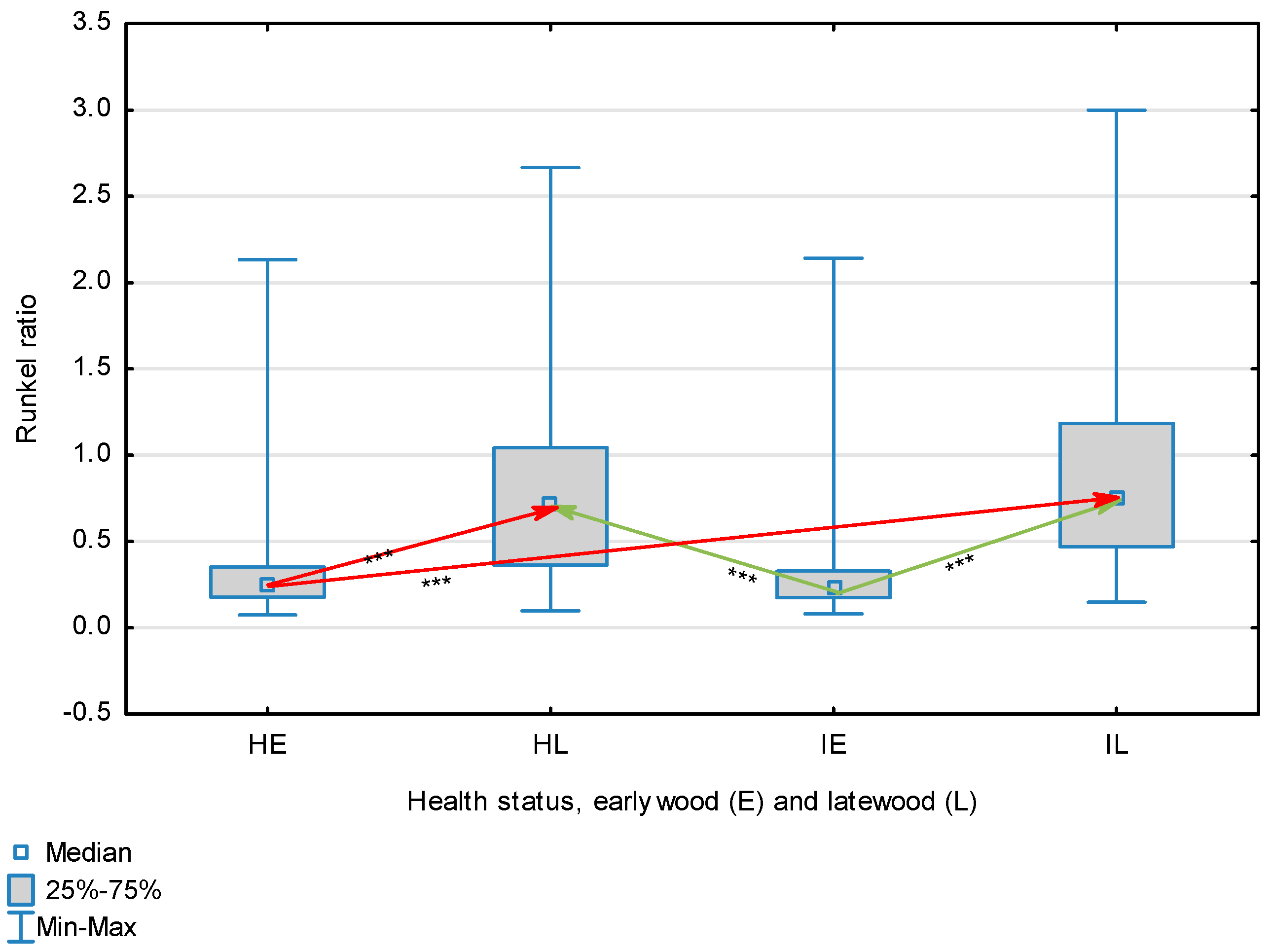
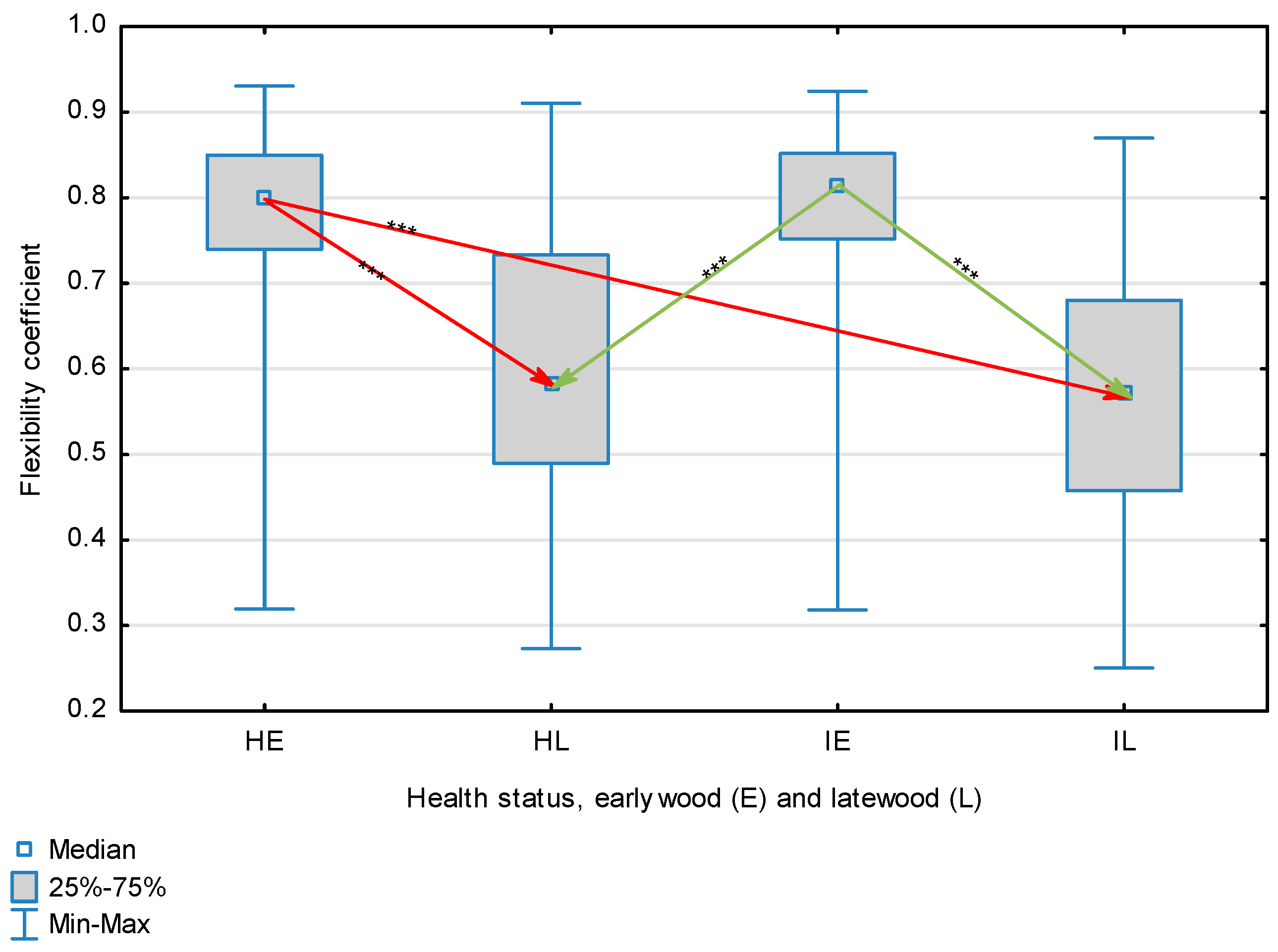
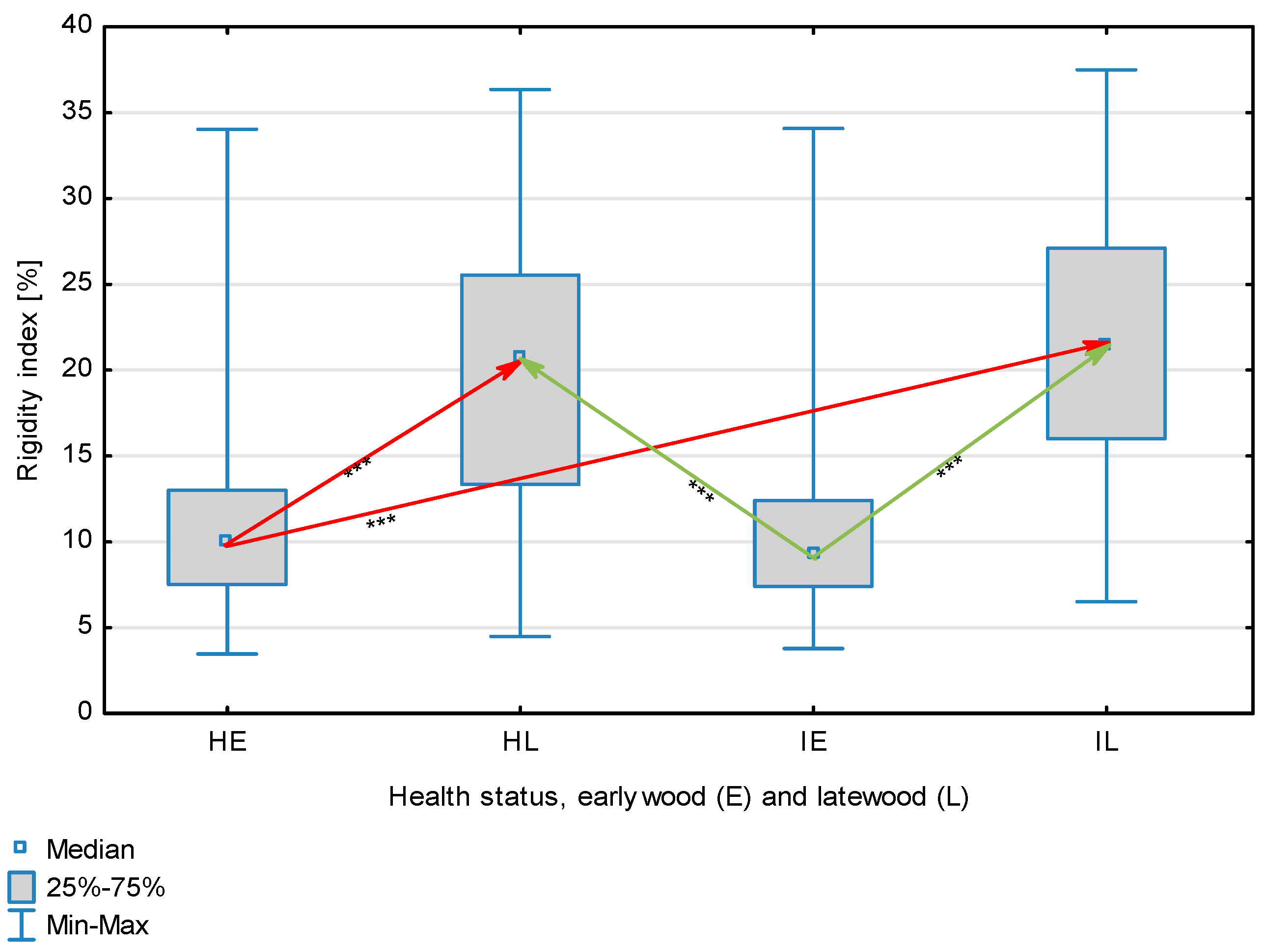
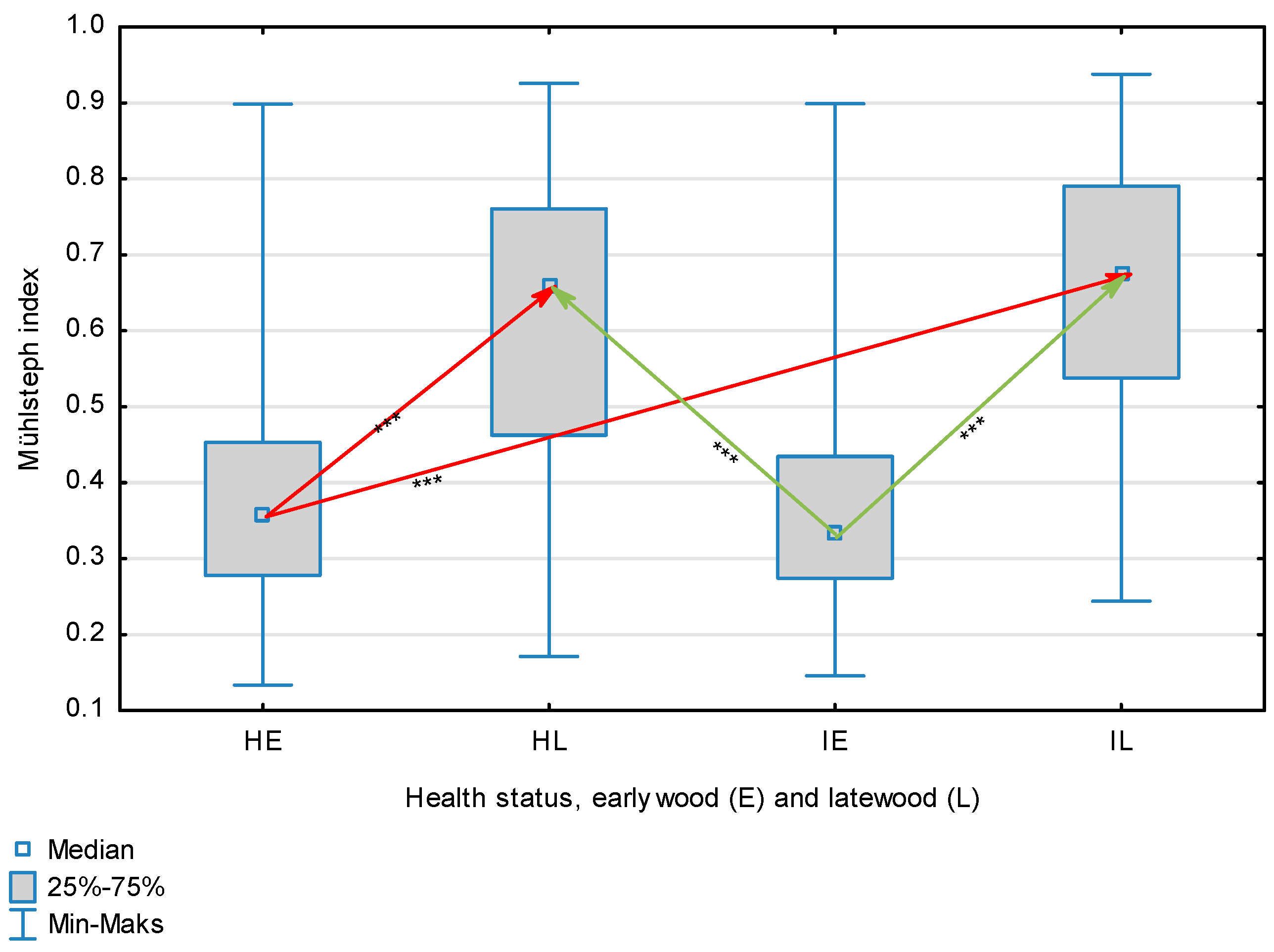
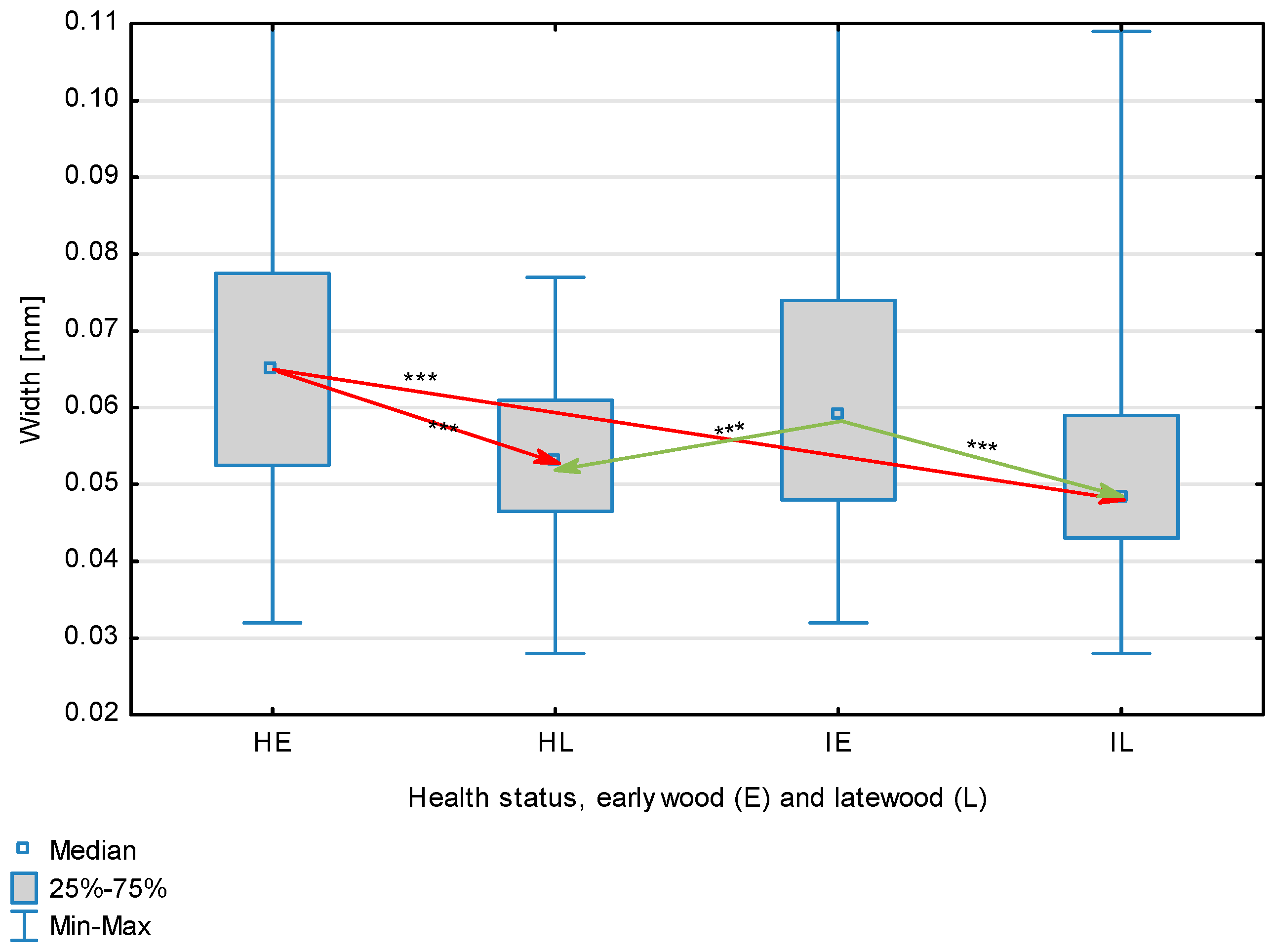
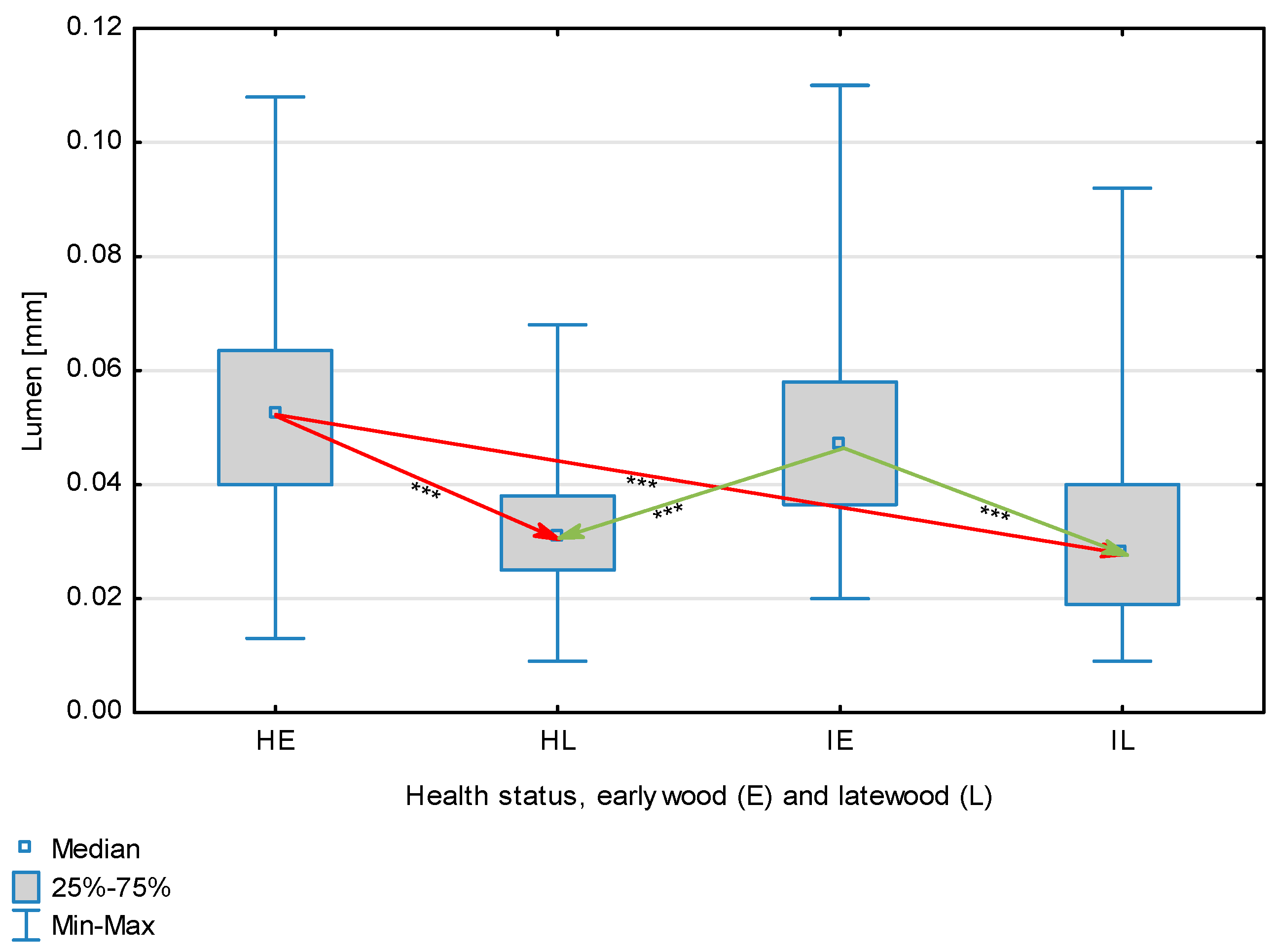
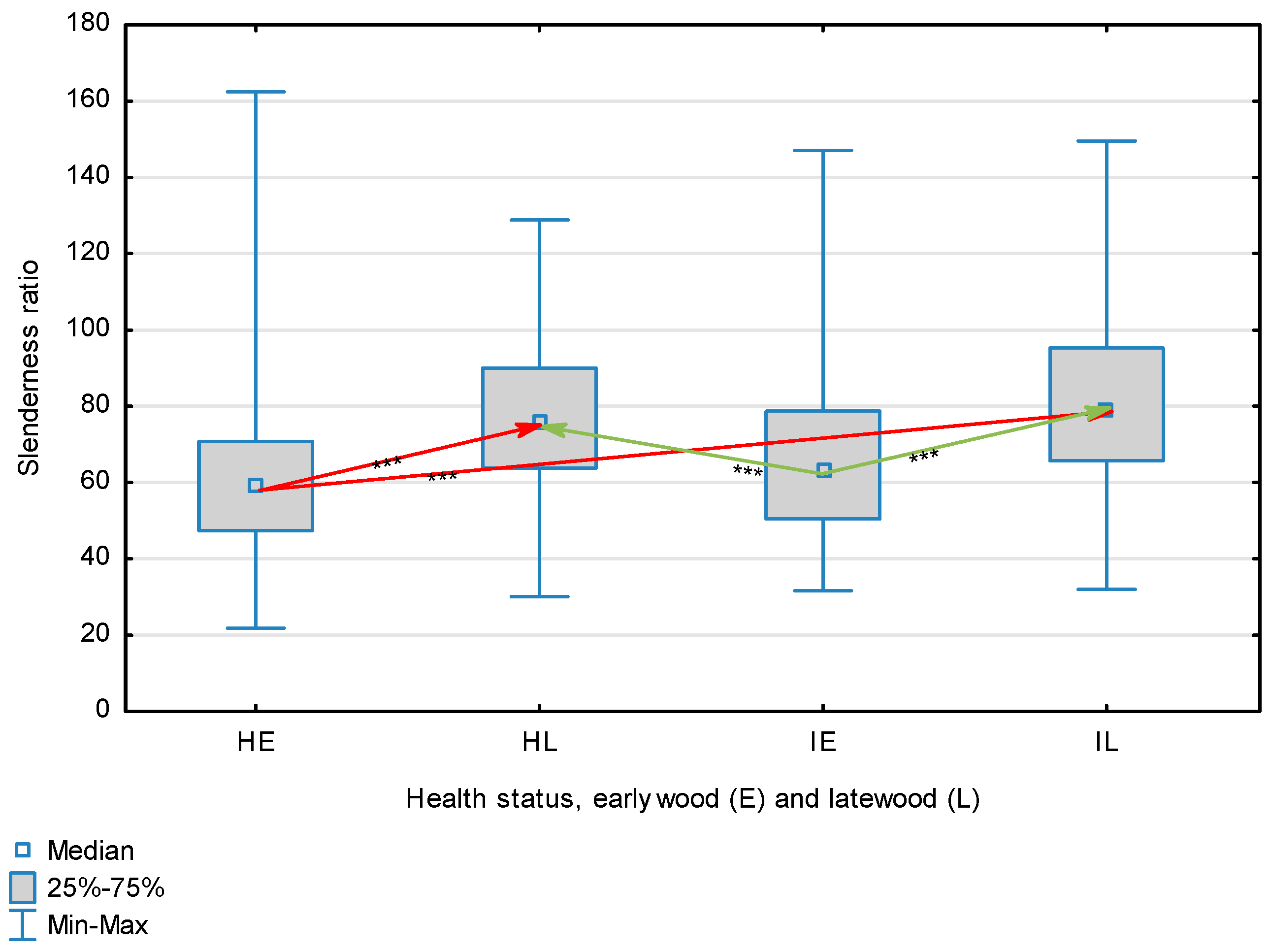
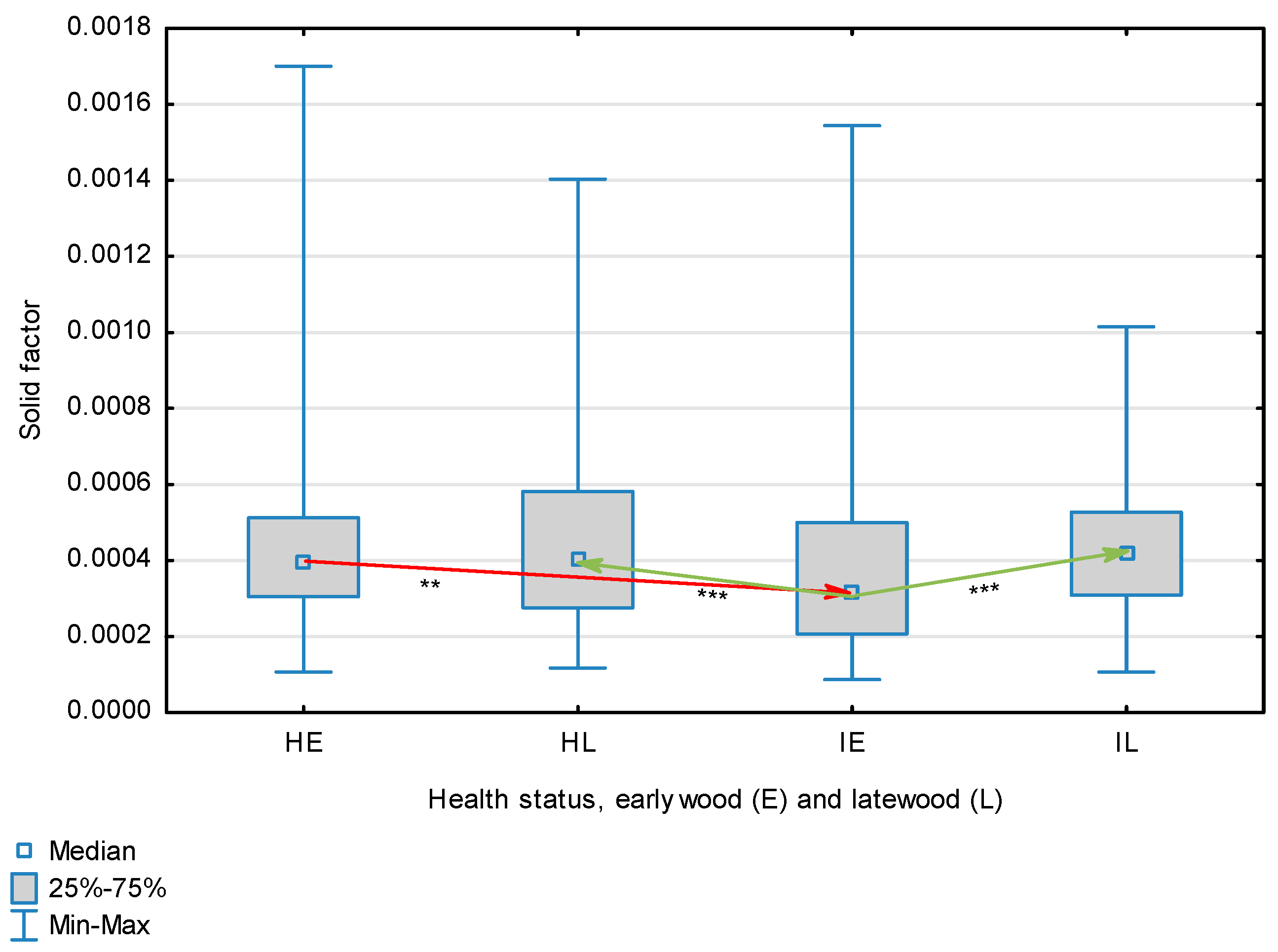
The statistical characterization of the parameters and indicators for wood fibers is presented in Table 2. Statistical analysis did not show an impact of Cronartium pini, and did not indicate differences between the studied groups in terms of fiber length (Table 2, Figure 1). In the case of fiber thickness, the Runkel ratio, the flexibility coefficient, the rigidity index, and the Mühlsteph index, pathogen impact was not found (Table 2), but some differences between groups were noted (Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6). Cronartium pini was found to affect fiber width and lumen, the slenderness ratio, and the solid factor (Table 2), and inter-group differences were identified (Figure 7, Figure 8, Figure 9 and Figure 10). Fiber parameters unaffected by Scots pine blister rust were fiber length and cell wall thickness, which were nearly identical for healthy and infected wood. In the case of parameters influenced by the fungi, their values were much higher than average for pine wood, with the infected wood exhibiting lower values as compared to healthy wood from affected trees.
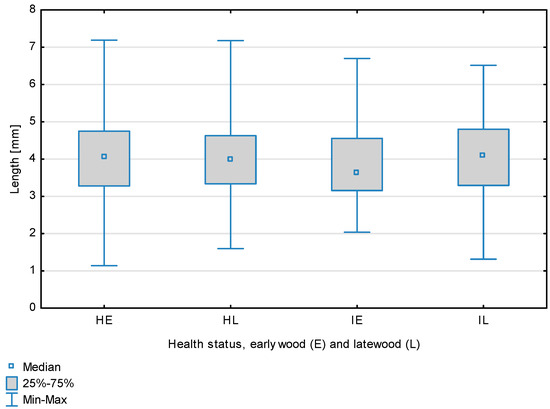
Figure 1.
Fiber length depending on health status (H—healthy wood, I—infected wood) and broken down into earlywood (E) and latewood (L). Differences between healthy wood and the remaining groups are shown in red, while those for infected wood are shown in green.
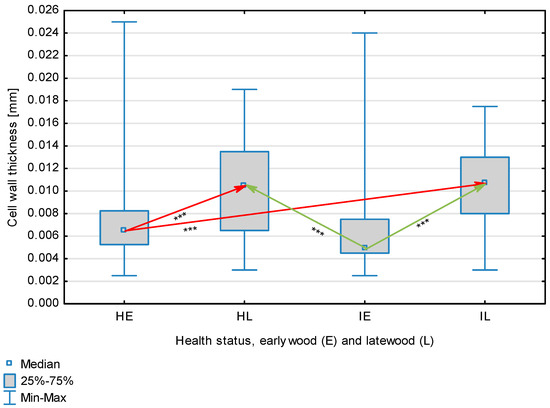
Figure 2.
Fiber wall thickness depending on health status (H—healthy wood, I—infected wood) and broken down into earlywood (E) and latewood (L). Statistically significant differences are marked *** for p < 0.001. Differences between healthy wood and the remaining groups are shown in red, while those for infected wood are shown in green.
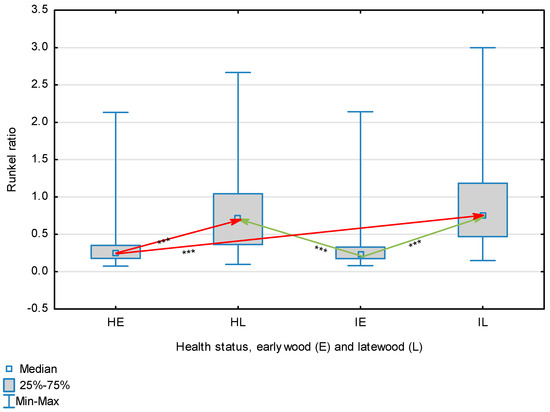
Figure 3.
Runkel index for wood fibers depending on health status (H—healthy wood, I—infected wood) and broken down into earlywood (E) and latewood (L). Statistically significant differences are marked *** for p < 0.001. Differences between healthy wood and the remaining groups are shown in red, while those for infected wood are shown in green.
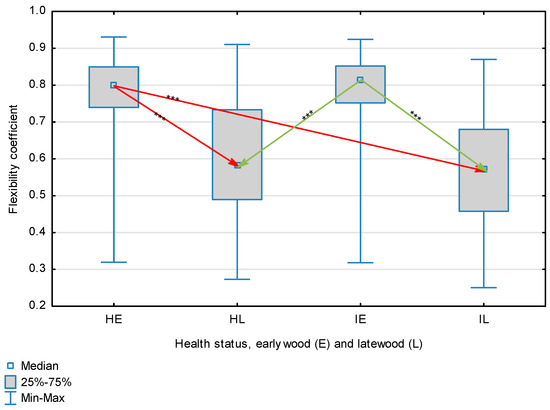
Figure 4.
Flexibility coefficient for wood fibers depending on health status (H—healthy wood, I—infected wood) and broken down into earlywood (E) and latewood (L). Statistically significant differences are marked *** for p < 0.001. Differences between healthy wood and the remaining groups are shown in red, while those for infected wood are shown in green.
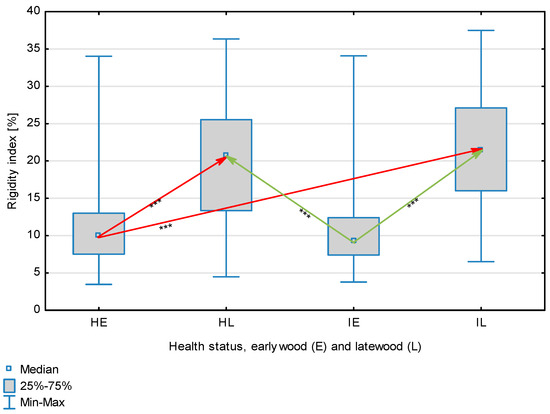
Figure 5.
Rigidity index for wood fibers depending on health status (H—healthy wood, I—infected wood) and broken down into earlywood (E) and latewood (L). Statistically significant differences are marked *** for p < 0.001. Differences between healthy wood and the remaining groups are shown in red, while those for infected wood are shown in green.
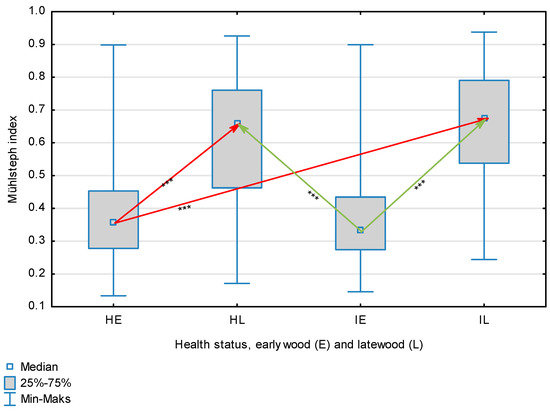
Figure 6.
Mühlsteph index for wood fibers depending on health status (H—healthy wood, I—infected wood) and broken down into earlywood (E) and latewood (L). Statistically significant differences are marked *** for p < 0.001. Differences between healthy wood and the remaining groups are shown in red, while those for infected wood are shown in green.
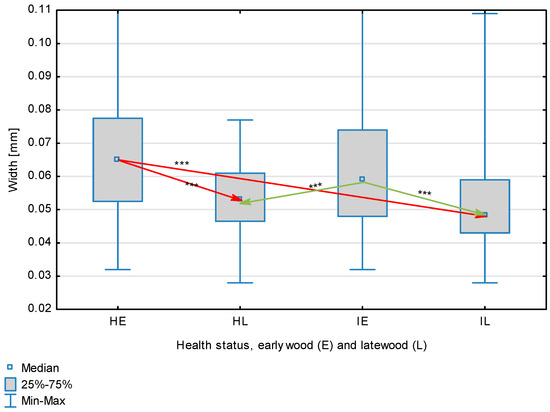
Figure 7.
Fiber width depending on health status (H—healthy wood, I—infected wood) and broken down into earlywood (E) and latewood (L). Statistically significant differences are marked *** for p < 0.001. Differences between healthy wood and the remaining groups are shown in red, while those for infected wood are shown in green.
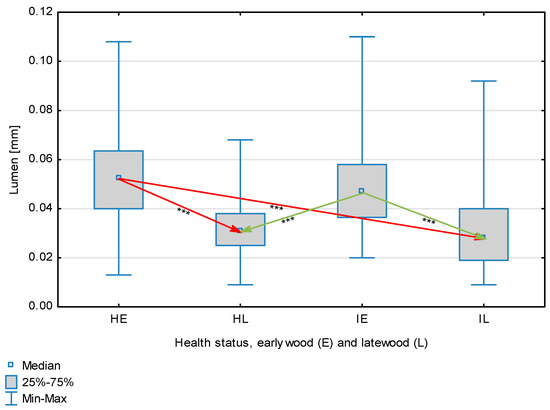
Figure 8.
Fiber lumen depending on health status (H—healthy wood, I—infected wood) and broken down into earlywood (E) and latewood (L). Statistically significant differences are marked *** for p < 0.001. Differences between healthy wood and the remaining groups are shown in red, while those for infected wood are shown in green.
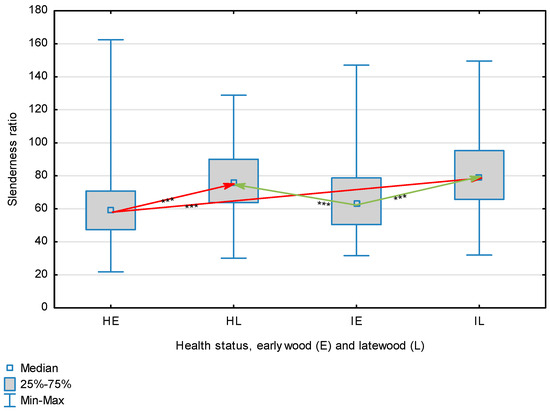
Figure 9.
Slenderness ratio of wood fibers depending on health status (H—healthy wood, I—infected wood) and broken down into earlywood (E) and latewood (L). Statistically significant differences are marked *** for p < 0.001. Differences between healthy wood and the remaining groups are shown in red, while those for infected wood are shown in green.
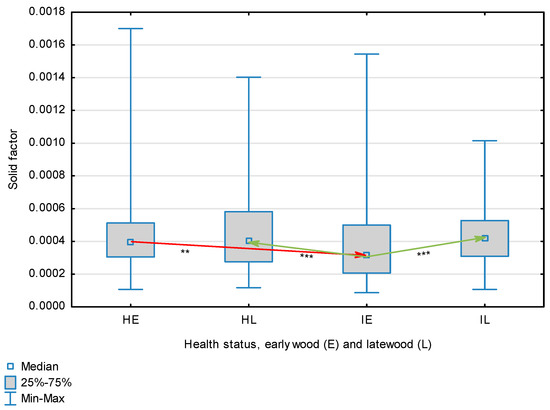
Figure 10.
Solid factor for wood fibers depending on health status (H—healthy wood, I—infected wood) and broken down into earlywood (E) and latewood (L). Statistically significant differences are marked *** for p < 0.001. Differences between healthy wood and the remaining groups are shown in red, while those for infected wood are shown in green.
4. Discussion
The influence of Cronartium pini on the physical and mechanical properties of wood is consistent with the expected effects of fungal infection, leading to higher density and compression strength parallel to grain due to high resin content. However, our anatomical studies provided new knowledge in this respect in the absence of the literature data on the subject.
The mean fiber parameters obtained in this study were higher than the mean values reported by other authors or were close to those given as extreme values [34,35,38]; however, they fell within the ranges typically determined for this species [36]. Fiber length was found to be similar for healthy and infected wood because Cronartium. pini does not significantly affect wood. This was also the case for cell wall thickness. However, the analysis of earlywood and latewood measurements revealed strong deviations for earlywood, with values more than twice as high as those generally reported as average for the species [36]. Also, the fiber width and lumen exceeded average findings for pine wood (in our study, both parameters were higher for healthy wood). The greater transverse dimensions of fibers increased the values of several morphological parameters calculated in this study, so that they surpassed the average values indicated in the literature. The examined samples fell below the average slenderness ratio for pine of 90 [37], with the infected wood exhibiting a higher ratio than healthy wood due to a lower fiber width. Also, the Runkel index obtained for infected wood was higher than that for healthy wood. Its typical value for the species is 0.3, less than the value calculated in our study; however, this does not have a detrimental effect on wood properties as the maximum acceptable value of the Runkel ratio for good quality fibers is 1 [14,39]. The rigidity index for both materials was not high, translating into greater fiber resistance to tensile stress and tearing [14]. The flexibility coefficient determined for the fibers indicates that they are elastic [14,39], which facilitates bonding, increasing tensile and tearing strength [40]. The Mühlsteph index shows that the examined samples, both infected and healthy, can be readily felted. Thus, the obtained results do not disqualify the examined material from paper production, but due to the problems linked to the presence of resin in the infected wood, in practice only healthy wood is suitable for processing. In one of their conclusions, Kaitera et al. [9] observed that losses in sawtimber caused by blister rust translate into greater timber use for pulping.
Our study shows that Cronartium pini affected mean annual ring width, but not the proportion of latewood. Infected wood exhibited a greater mean annual ring width as compared to healthy wood, which is attributable to the nature of pathogen activity. The results obtained for healthy wood were higher than the average values reported by other researchers [12,15,16,31] because the material examined in this study was taken from higher tree segments. As compared to healthy wood, infected wood had a slightly lower proportion of latewood. Previous research has shown that the mean latewood proportion in P. sylvestris ranges widely from 20% to almost 50% [12,15,18,24,31]. This substantial variability may be associated with many causes, with the major ones being the location in the stem [13,31], annual ring width [16], tree age [33], and type of forest site [18].
In our study, Cronartium pini was shown to influence wood density, which was also the most obvious aspect of fungal infection due to the high resin content. As a result of abundant resin, infected wood exhibited much greater density than healthy wood. To be sure, this property is highly variable and may be affected, in addition to Scots pine blister rust, by sample location in the stem [22,23], tree age [23,28], and site conditions [23]. The density of healthy wood was found to be similar to the values reported for samples taken at mid-stem height [12,13]. The values reported by other researchers [15,18,24,25,26,31,32] are similar to ours or larger due to changes in density with tree height [13,15,18,20,21,22,26,48]. The basic density of infected wood was much greater than that of healthy wood. The latter was lower than the values reported in the literature for mid-stem height, amounting to 415 ± 55 kgm−3 [21], 417.15±3.54 kgm−3 [27], and 419 kmm−3 [23]. Infected wood exhibited a higher proportion of wood substance and lower porosity, with the opposite being true of healthy wood. The corresponding properties of healthy wood were found to be slightly lower as compared to those reported for this range of density and for mid-height location [27].
Cronartium pini was shown to affect wood shrinkage, which was lower for infected wood than for healthy wood, with the latter exhibiting values similar to average pine wood shrinkage [17,19,21,49]. According to Tomczak and Jelonek [21], wood shrinkage does not depend on the height on the stem, and so the values determined for healthy wood in our study are consistent with the reports of other authors who examined the basal part of the trunk. In our case, infected wood exhibited lower shrinkage and higher dimensional stability because of excessive content of resin, which decreases wood shrinkage due to cell wall buckling [50] and occupies space that would normally be taken by water [19]. Cronartium pini did not affect longitudinal shrinkage or anisotropy, which was found to be close to 2 both for infected and healthy wood, considerably exceeding the values generally accepted for the species.
Infected wood was characterized by higher compression strength parallel to the grain than healthy wood. The highest value of this parameter at mid-height for a Scots pine growing on a coniferous forest site has been reported to be 45.5 ± 1.5 MPa [33], which is more than the values found in this study and in other reports [26,29]. Compression strength parallel to the grain obtained by other researchers for the basal part of the trunk revealed higher values than those found by the present authors [30,31,32], as the compression strength of unaffected wood increases the closer to the base of the tree it is. The coefficient of compression strength parallel to the grain-infected wood was lower than that of healthy wood—our results for the latter were consistent with the values reported in the literature [32].
The above findings indicate that while Cronartium pini lowers the commercial value of wood, the pathogen does not prevent its use for various applications as healthy wood from infected trees exhibits average values for the species.
5. Conclusions
The anatomical structure of wood from trees infected with Cronartium pini was highly variable in terms of the studied features, while the results for infected and healthy samples were similar. Larger transverse fiber dimensions were found for both types of wood, which had a positive effect on the parameters associated with those dimensions. The obtained parameters, albeit much higher than average values for Scots pine, did not render the wood technically unsuitable.
Infected wood had wider annual rings than healthy wood. The fungus did not affect the proportion of latewood, which was only slightly lower in infected wood as compared to healthy wood.
Scots pine blister rust was shown to impact the physical properties of infected wood by increasing its density due to high resin content and decreased average radial and tangential shrinkage as compared to healthy wood. On the other hand, the fungus did not have an effect on anisotropy or longitudinal shrinkage.
As compared to healthy wood, infected wood was found to exhibit higher compression strength parallel to grain, but a lower coefficient of compression strength parallel grain. Healthy wood exhibited typical values of the studied mechanical properties, without any detrimental effects on its construction applications.
The activity of Cronartium pini led to annual ring in homogeneity and high resin content, limiting the suitability of the infected tree fragments to fuel applications, despite their good overall parameters. In contrast, the mean results obtained for healthy wood from affected trees are consistent with the average values reported for pine wood.
Author Contributions
Conceptualization, P.K., H.L., T.M. and A.G.; methodology, P.K., H.L., T.M., J.P., M.A. and A.G.; validation, H.L., T.M., M.A. and A.G.; formal analysis, P.K., H.L., M.A. and A.G.; investigation, P.K., H.L., T.M., J.P., M.A. and A.G.; resources, P.K., H.L., T.M., J.P., M.A. and A.G.; data curation, P.K., H.L., M.A. and A.G.; writing—original draft preparation, P.K. and H.L.; writing—review and editing, H.L., T.M., J.P., M.A. and A.G.; visualization, P.K. and H.L.; supervision, H.L., T.M. and A.G.; project administration, H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data not directly presented in the article will be made available on request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Brzezicka-Szymczyk, K. Grzyby rdzawnikowe (Uredinales)—Pasożyty roślin. Kosmos 1992, 41, 223–233. [Google Scholar]
- Samils, B.; Stenlid, J. A Review of Biology, Epidemiology and Management of Cronartium pini with Emphasis on Northern Europe. Scand. J. For. Res. 2022, 37, 153–171. [Google Scholar] [CrossRef]
- Kirk, P. Species Fungorum (Version Oct 2017). Catalogue of Life—2019 Annual Checklist. In Species 2000 & ITIS Catalogue of Life, 2019 Annual Checklist; Roskov, Y., Ower, G., Orrel, T., Nicolson, D., Kirk, P., Bourgoin, T., DeWalt, R., Decock, W., van Nieukerken, E., Zarucchi, J., et al., Eds.; Species 2000; Naturalis: Leiden, The Netherlands, 2019; ISSN 2405-884X. [Google Scholar]
- DGLP. Raport o Stanie Lasów w Polsce 2010–2021; Centrum Informacyjne Lasów Państwowych: Warszawa, Poland, 2021; ISSN 1641-3229. [Google Scholar]
- Kaitera, J. Susceptibility and Lesion Development in Scots Pine Saplings Infected with Peridermium pini in Northern Finland. For. Pathol. 2003, 33, 353–362. [Google Scholar] [CrossRef]
- Kim, M.-S.; Hantula, J.; Kaitera, J.; Zambino, P.J.; Woodward, S.; Richardson, B.A.; Stewart, J.E.; Spaine, P.; Shaw, D.C.; Takeuchi, Y.; et al. Recovery Plan for Scots Pine Blister Rust Caused by Cronartium pini. Plant Health Prog. 2022, 23, 105–130. [Google Scholar] [CrossRef]
- Kaitera, J. Analysis of Cronartium Flaccidum Lesion Development on Pole-Stage Scots Pines. Silva Fenn. 2000, 34, 21–27. [Google Scholar] [CrossRef]
- Geils, B.W.; Jacobi, W.R. Effects of Comandra Blister Rust on Growth and Survival of Lodgepole Pine. Phytopathology 1993, 83, 638–644. [Google Scholar] [CrossRef]
- Kaitera, J.; Aalto, T.; Jalkanen, R. Effect of Resin-top Disease Caused by Peridermium pini on the Volume and Value of Pinus sylvestris Saw Timber and Pulpwood. Scand. J. For. Res. 1994, 9, 376–381. [Google Scholar] [CrossRef]
- Sullivan, M. CPHST Pest Datasheet for Cronartium Flaccidum (Alb. & Schwein) Winter; Revised July 2015 by D. Z. Mackesy; USDA-APHISPPQ-CPHST: Raleigh, NC, USA, 2010; pp. 1–18. [Google Scholar]
- Mirski, R.; Wieruszewski, M.; Malinowski, Z. Zmienność rozkładu wad drewna okrągłego w dojrzałych drzewostanach sosnowych. Sylwan 2019, 163, 913–923. [Google Scholar] [CrossRef]
- Karlman, L.; Mörling, T.; Martinsson, O. Wood Density, Annual Ring Width and Latewood Content in Larch and Scots Pine. EurasianJ. For. Res. 2005, 8, 91–96. [Google Scholar]
- Mańkowski, P.; Krzosek, S.; Burawska-Kupniewska, I.; Grześkiewicz, M.; Mirski, R. Correlation between the Share of Latewood and the Density of Sawn Timber from the Silesian Forestry Region. Ann. Wars. Univ. Life Sci. SGGW For. Wood Technol. 2020, 109, 70–75. [Google Scholar] [CrossRef]
- Bektas, İ.; Tutuş, A.; Eroğlu, H. A Study of the Suitability of Calabrian Pine (Pinus brutia Ten.) for Pulp and Paper Manufacture. Turk. J. Agric. For. 1999, 23, 589–598. [Google Scholar]
- Rikala, J. Spruce and Pine on Drained Peatlands—Wood Quality and Suitability for the Sawmill Industry; Publications 35; University of Helsinki, Department of Forest Resource Management: Helsinki, Finland, 2003; ISBN 951-45-9092-9. [Google Scholar]
- Gryc, V.; Vavrčík, H.; Horn, K. Density of Juvenile and Mature Wood of Selected Coniferous Species. J. For. Sci. 2011, 57, 123–130. [Google Scholar] [CrossRef]
- Farsi, M.; Kiaei, M.; Miar, S.; Mohammadnezhad Kiasari, S. Effect of Seed Source on Physical Properties of Scots Pine (a Case Study in Neka, Iran). Drv. Ind. 2013, 64, 183–191. [Google Scholar] [CrossRef]
- Kask, R. The Influence of Growth Conditions on Physico-Mechanical Properties of Scots Pine (Pinus sylvestris L.) Wood in Estonia. Ph.D. Thesis, Estonian University of Life Sciences, Tartu, Estonia, 2015. [Google Scholar]
- Schönfelder, O.; Zeidler, A.; Borůvka, V.; Bílek, L.; Lexa, M. Shrinkage of Scots Pine Wood as an Effect of Different Tree Growth Rates, a Comparison of Regeneration Methods. J. For. Sci. 2018, 64, 271–278. [Google Scholar] [CrossRef]
- Tomczak, A.; Jelonek, T.; Pazdrowski, W. Basic Density of Scots Pine Wood-Relationships between Values Calculated at Different Heights of the Trun. Ann. Wars. Univ. Life Sci. SGGW For. Wood Technol. 2013, 84, 241–246. [Google Scholar]
- Tomczak, A.; Jelonek, T. Comparison of selected physical properties of the juvenile and mature wood of Scots pine (Pinus sylvestris L.) from mature stands. Sylwan 2010, 154, 809–817. [Google Scholar] [CrossRef]
- Witkowska, J.; Lachowicz, H. Analysis of variation in pure density of Scots pine wood (Pinus sylvestris L.) along a trunk height depending on selected factors. Przegląd Pap. 2012, 68, 573–578. [Google Scholar]
- Witkowska, J.; Lachowicz, H. Variability of conventional wood density of Scots pine (Pinus sylvestris L.) depending on the selected factors. Sylwan 2013, 157, 336–347. [Google Scholar] [CrossRef]
- Mäkinen, H.; Hynynen, J. Wood Density and Tracheid Properties of Scots Pine: Responses to Repeated Fertilization and Timing of the First Commercial Thinning. For. Int. J. For. Res. 2014, 87, 437–447. [Google Scholar] [CrossRef]
- Janusz, S.; Danilov, D. Density of Wood of Pine and Spruce in the Postagrogenic Soil of the Boreal Zone. In Proceedings of the 24th International Scientific Conference Research for Rural Development, Jeglava, Latvia, 16–18 May 2018; pp. 92–96. [Google Scholar]
- Schönfelder, O.; Zeidler, A.; Borůvka, V.; Bílek, L. Impact of Silvicultural Measures on the Quality of Scots Pine Wood: Part II. Effect of Site. Wood Res. 2019, 64, 789–798. [Google Scholar]
- Kantieva, E.; Snegireva, S.; Platonov, A. Formation of Density and Porosity of Pine Wood in a Tree Trunk. IOP Conf. Ser.Earth Environ. Sci. 2021, 875, 012016. [Google Scholar] [CrossRef]
- Pikiński, P.M.; Szaban, J.; Šilingienė, G.; Korzeniewicz, R.; Pazdrowski, W. Selected Physical and Mechanical Properties of Scots Pine (Pinus sylvestris L.) Wood from Stands of Younger Age Classes as Criteria for Rational Utilization of Timber. Balt. For. 2021, 27, 363. [Google Scholar] [CrossRef]
- Pikk, J.; Kask, R. Mechanical Properties of Juvenile Wood of Scots Pine (Pinus sylvestris L.) on Myrtillus Forest Site Type. Balt. For. 2004, 10, 72–78. [Google Scholar]
- Roszyk, E.; Mania, P.; Iwańska, E.; Kusiak, W.; Broda, M. Mechanical Performance of Scots Pine Wood from Northwestern Poland—A Case Study. BioRes 2020, 15, 6781–6794. [Google Scholar] [CrossRef]
- Wąsik, R.; Michalec, K.; Barszcz, A.; Mudryk, K. Variability of Selected Macrostructure Features, Density and Compression Strength along the Grain of “Tabórz” Scots Pine Wood (Pinus sylvestris L.). Wood 2020, 63, 171–182. [Google Scholar] [CrossRef]
- Konofalska, E.; Kozakiewicz, P.; Buraczyk, W.; Szeligowski, H.; Lachowicz, H. The Technical Quality of the Wood of Scots Pine (Pinus sylvestris L.) of Diverse Genetic Origin. Forests 2021, 12, 619. [Google Scholar] [CrossRef]
- Kask, R.; Pikk, J.; Kangur, A. Effect of Growth Conditions on Wood Properties of Scots Pine (L.). For. Stud. 2021, 75, 176–187. [Google Scholar] [CrossRef]
- Pazdrowski, W.; Splawa-Neyman, S. Badania wybranych wlasciwosci drewna sosny zwyczajnej [Pinus silvestris L.] na tle klas biologicznych w drzewostanie. Folia For. Pol. Ser. B Drzew. 1994, 24, 133–145. [Google Scholar]
- Raczkowska-Helinska, L.; Raczkowski, J.; Krauss, A.; Ciazynska, I. Tracheids Length Variation in Scots Pine [Pinus silvestris L.] Trees Belonging to Different Tree Growth Classes. Folia For. Pol. Ser. B Drzew. 1994, 24, 123–132. [Google Scholar]
- Kokociński, W. Anatomia Drewna, 2nd ed.; Prodruk: Poznań, Poland, 2005; ISBN 83-88518-42-9. [Google Scholar]
- Przybysz, K. System Kontroli Jakości Masy Włóknistej. Cz. 2. Wymiary i Kształt Włókien. Przegląd Pap. 2005, 61, 212–216. [Google Scholar]
- Jelonek, T.; Gzyl, J.; Arasimowicz-Jelonek, M.; Tomczak, A.; Remlein, A. Wpływ wybranych wskaźników stabilności drzew na grubość ścian cewek u sosny zwyczajnej (Pinus sylvestris L.). Acta Sci. Pol. Silvarum Colendarum Ratio Ind. Lignaria 2016, 15, 13–21. [Google Scholar] [CrossRef]
- Area, M.C.; Popa, V. Characteristics of Cells and Properties of Pulps. In Wood Fibers for Papermaking, Smithers Pira; Smithers Information Ltd.: Shawbury, UK, 2014; Chapter 4; pp. 65–88. ISBN 978-1-909030-86-2. [Google Scholar]
- Sadiku, A.; Oluyege, A.; Ajayi, B. Fibre Dimension and Chemical Characterisation of Naturally Grown Bambusa Vulgaris for Pulp and Paper Production. J. Bamboo Ratt. 2016, 15, 33–43. [Google Scholar]
- ISO 3129:2019; Wood—Sampling Methods and General Requirements for Physical and Mechanical Testing of Small Clear Wood Specimens. International Organization for Standardization: Geneva, Switzerland, 2019.
- ISO 13061-2:2014; Physical and Mechanical Properties of Wood—Test Methods for Small Clear Wood Specimens—Part 2: Determination of Density for Physical and Mechanical Tests. International Organization for Standardization: Geneva, Switzerland, 2014.
- ISO 13061-17:2017; Physical and Mechanical Properties of Wood—Test Methods for Small Clear Wood Specimens—Part 17: Determination of Ultimate Stress in Compression Parallel to Grain. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 13061-1:2014; Physical and Mechanical Properties of Wood—Test Methods for Small Clear Wood Specimens—Part 1: Determination of Moisture Content for Physical and Mechanical Tests. International Organization for Standardization: Geneva, Switzerland, 2014.
- Cybis Elektronik & Data. CooRecorder—Cybis Coordinate Recorder, 64bit; Version 7.6; Cybis Elektronik & Data: Saltsjöbaden, Sweden, 2012. [Google Scholar]
- Cybis Elektronik & Data. CDendro—Cybis Dendro Dating Program, 64bit; Version 7.6; Cybis Elektronik & Data: Saltsjöbaden, Sweden, 2012. [Google Scholar]
- TIBCO. Statistica, an Advanced Analytics Software Package; Version 13.3; TIBCO: Palo Alto, CA, USA, 2017. [Google Scholar]
- Grekin, M.; Surini, T. Shear Strength and Perpendicular-to-Grain Tensile Strength of Defect-Free Scots Pine Wood from Mature Stands in Finland and Sweden. Wood Sci. Technol. 2008, 42, 75–91. [Google Scholar] [CrossRef]
- Bektas, I.; Alma, H.; As, N. The Effect of 120 Years of Service on Various Physical and Mechanical Properties of Scots Pine Wood Used as Roof Beam. Wood Res. 2005, 50, 27–32. [Google Scholar]
- Awoyemi, L. Reversibility of Dimensional Changes in Birch (Betula Pubescens) and Scots Pine (Pinus sylvestris L.) Wood. Taiwan J. For. Sci. 2004, 19, 97–101. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).