Spatial Distribution and Determinants of Aboveground Biomass in a Subalpine Coniferous Forest in Southwestern China
Abstract
:1. Introduction
2. Materials and Methods
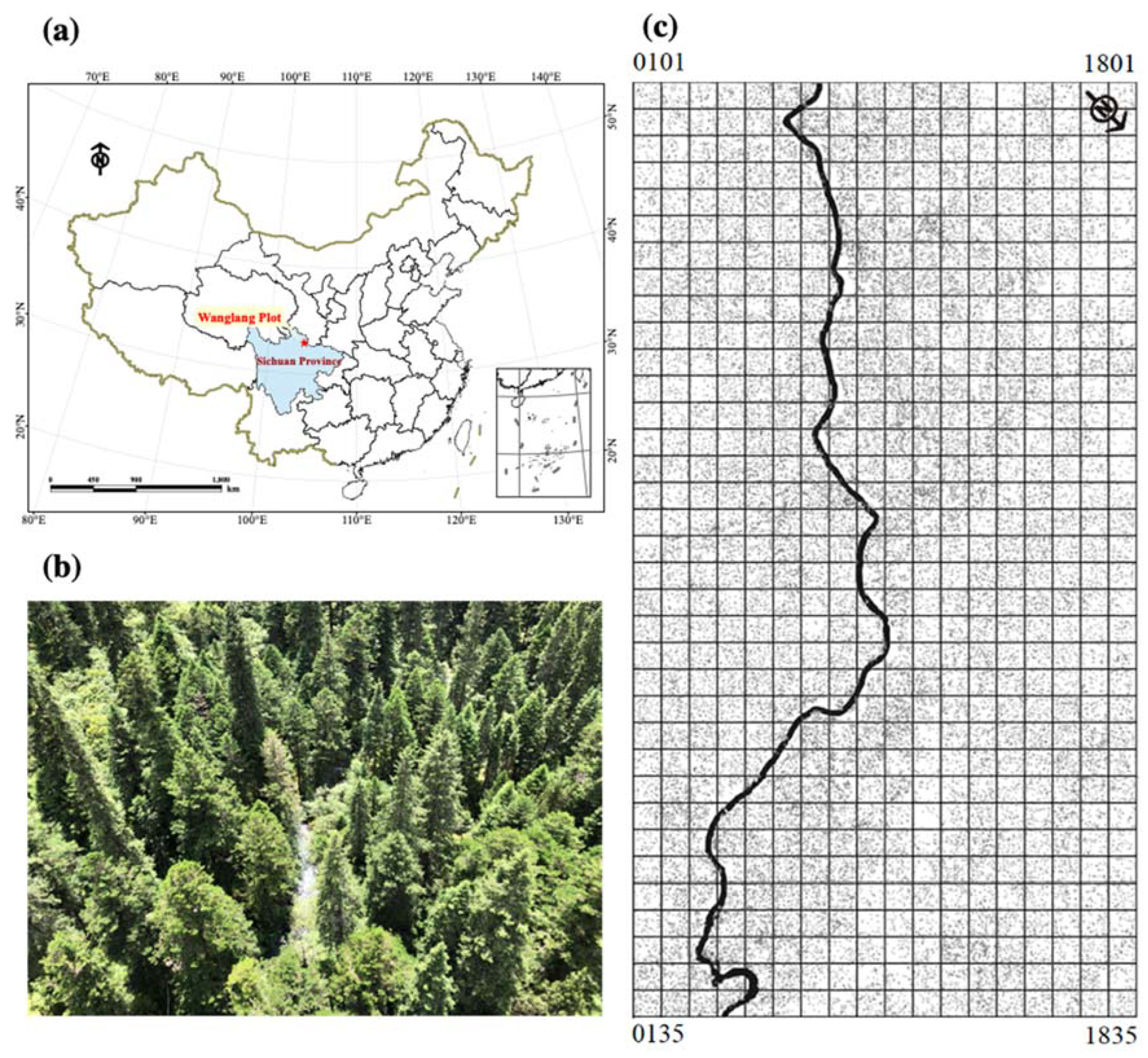
2.1. Study Sites
2.2. Vegetation Investigation and AGB Estimation
2.3. Soil Sampling and Determination
2.4. Topography Acquisition
2.5. Statistical Analysis
3. Results
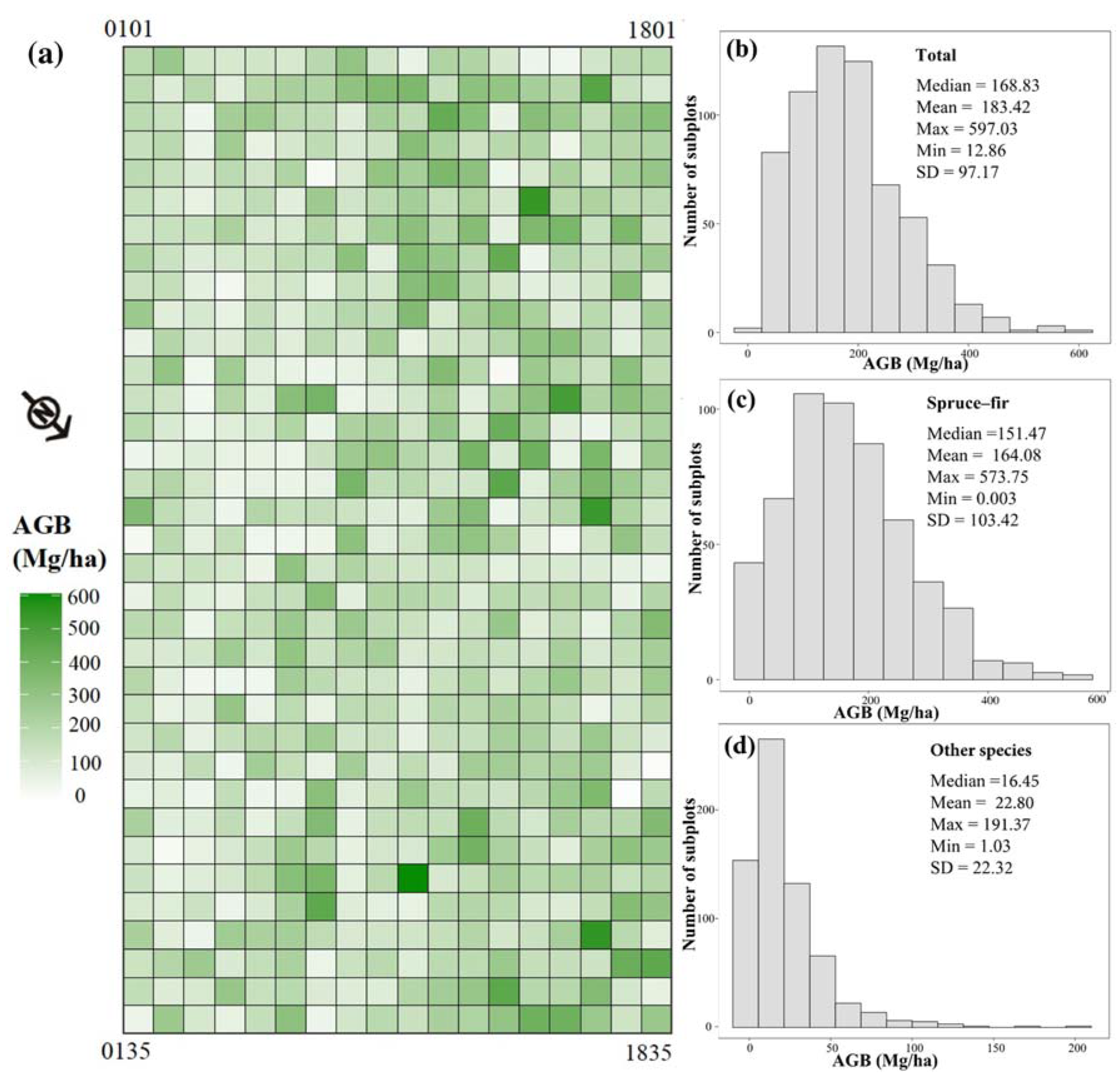
3.1. Spatial Distribution of Forest AGB
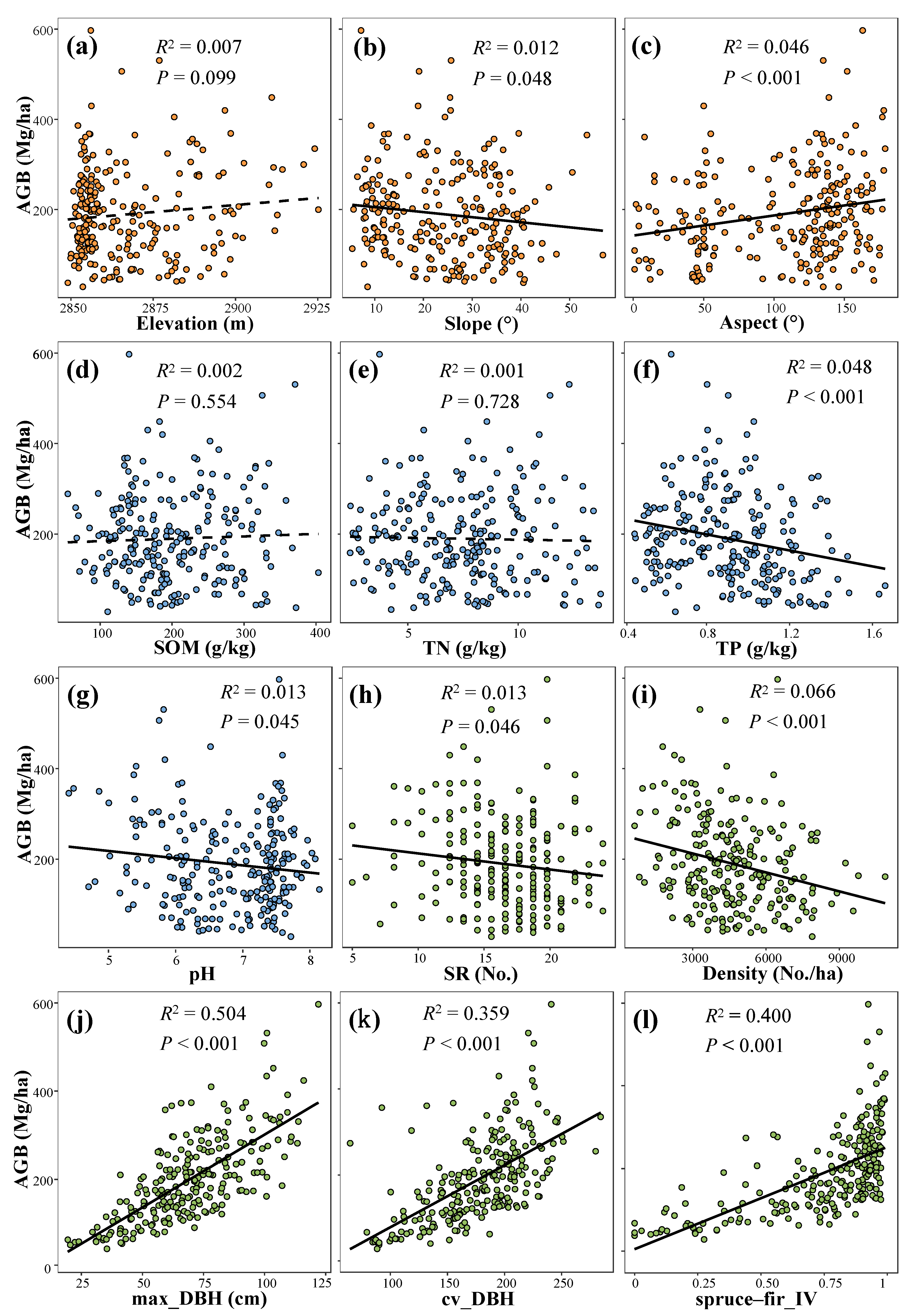
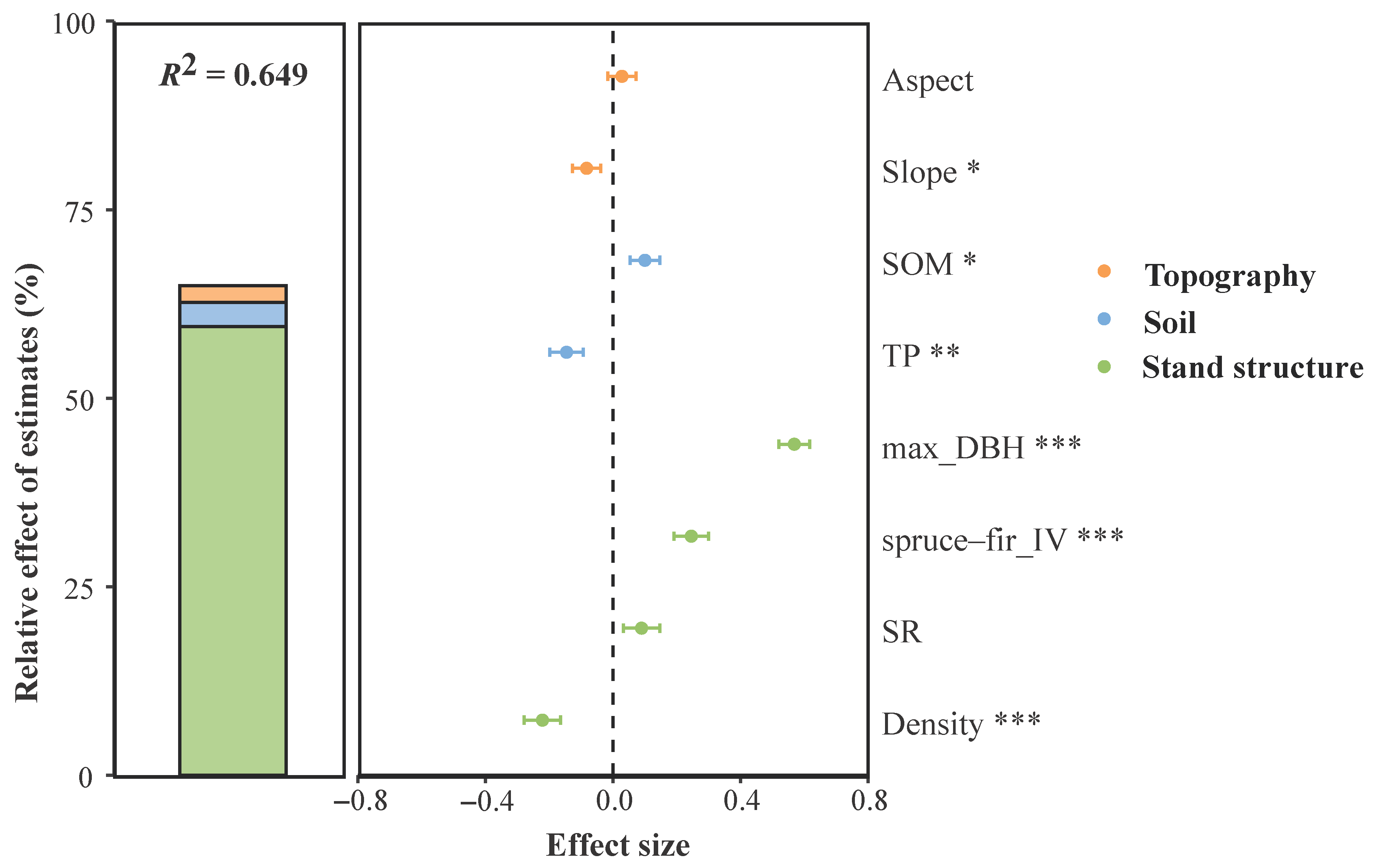
3.2. Topography, Soil, and Stand Structure Factors Affecting Forest AGB
4. Discussion
4.1. Stand Structure Determined Forest AGB
4.2. Influence of Topography and Soil on Forest AGB
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tamiminia, H.; Salehi, B.; Mahdianpari, M.; Beier, C.M.; Johnson, L. Mapping two decades of New York State Forest aboveground biomass change using remote sensing. Remote Sens. 2022, 14, 4097. [Google Scholar] [CrossRef]
- Beer, C.; Reichstein, M.; Tomelleri, E.; Ciais, P.; Jung, M.; Carvalhais, N.; Rödenbeck, C.; Arain, M.A.; Baldocchi, D.; Bonan, G.B.; et al. Terrestrial gross carbon dioxide uptake: Global distribution and covariation with climate. Science 2010, 329, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Birdsey, R.; Fang, J.; Houghton, R.; Kauppi, P.; Kurz, W.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- King, A.W.; Hayes, D.J.; Huntzinger, D.N.; WesSt, T.O.; Post, W.M. North American carbon dioxide sources and sinks: Magnitude, attribution, and uncertainty. Front. Ecol. Environ. 2012, 10, 512–519. [Google Scholar] [CrossRef]
- Houghton, R.A. Aboveground Forest biomass and the global carbon balance. Glob. Chang. Biol. 2005, 11, 945–958. [Google Scholar] [CrossRef]
- Fahey, T.J.; Woodbury, P.B.; Battles, J.J.; Goodale, C.L.; Hamburg, S.P.; Ollinger, S.V.; Woodall, C.W. Forest carbon storage: Ecology, management, and policy. Front. Ecol. Environ. 2010, 8, 245–252. [Google Scholar] [CrossRef]
- Slik, J.F.; Paoli, G.; McGuire, K.; Amaral, I.; Barroso, J.; Bastian, M.; Blanc, L.; Bongers, F.; Boundja, P.; Clark, C.; et al. Large trees drive forest aboveground biomass variation in moist lowland forests across the tropics. Glob. Ecol. Biogeogr. 2013, 22, 1261–1271. [Google Scholar] [CrossRef]
- Liu, X.; Trogisch, S.; He, J.S.; Niklaus, P.A.; Bruelheide, H.; Tang, Z.; Erfmeier, A.; Scherer-Lorenzen, M.; Pietsch, K.A.; Yang, B.; et al. Tree species richness increases ecosystem carbon storage in subtropical forests. Proc. R. Soc. B 2018, 285, 20181240. [Google Scholar] [CrossRef]
- Lin, D.; Lai, J.; Muller-Landau, H.C.; Mi, X.; Ma, K. Topographic variation in aboveground biomass in a subtropical evergreen broad-leaved forest in China. PLoS ONE 2012, 7, e48244. [Google Scholar] [CrossRef]
- Liang, J.; Crowther, T.W.; Picard, N.; Wiser, S.; Zhou, M.; Alberti, G.; Schulze, E.D.; McGuire, A.D.; Bozzato, F.; Pretzsch, H.; et al. Positive biodiversity-productivity relationship predominant in global forests. Science 2016, 354, aaf8957. [Google Scholar] [CrossRef]
- Babst, F.; Bouriaud, O.; Poulter, B.; Trouet, V.; Girardin, M.P.; Frank, D.C. Twentieth century redistribution in climatic drivers of global tree growth. Sci. Adv. 2019, 5, eaat4313. [Google Scholar] [CrossRef]
- Zhao, F.; Guo, Q.; Kelly, M. Allometric equation choice impacts lidar-based forest biomass estimates: A case study from the Sierra National Forest, CA. Agric. For. Meteorol. 2012, 165, 64–72. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Phillips, O.L.; Jackson, R.B. The structure, distribution, and biomass of the world’s forests. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 593. [Google Scholar] [CrossRef]
- Yang, Q.; Su, Y.; Hu, T.; Jin, S.; Liu, X.; Niu, C.; Liu, Z.; Kelly, M.; Wei, J.; Guo, Q. Allometry-based estimation of forest aboveground biomass combining lidar canopy height attributes and optical spectral indexes. For. Ecosyst. 2022, 9, 617–629. [Google Scholar] [CrossRef]
- Swetnam, T.L.; Brooks, P.D.; Barnard, H.R.; Harpold, A.A.; Gallo, E.L. Topographically driven differences in energy and water constrain climatic control on forest carbon sequestration. Ecosphere 2017, 8, e01797. [Google Scholar] [CrossRef]
- Xiong, X.; Zhu, J.; Li, S.; Fan, F.; Cai, Q.; Ma, S.; Su, H.; Ji, C.J.; Tang, Z.; Fang, J. Aboveground biomass and its biotic and abiotic modulators of a main food bamboo of the giant panda in a subalpine spruce–fir forest in southwestern China. J. Plant Ecol. 2022, 15, 1–12. [Google Scholar] [CrossRef]
- Luizão, R.C.; Luizão, F.J.; Paiva, R.Q.; Monteiro, T.F.; Sousa, L.S.; Kruijt, B. Variation of carbon and nitrogen cycling processes along a topographic gradient in a central Amazonian Forest. Glob. Chang. Biol. 2004, 10, 592–600. [Google Scholar] [CrossRef]
- Culmsee, H.; Leuschner, C.; Moser, G.; Pitopang, R. Forest aboveground biomass along an elevational transect in Sulawesi, Indonesia, and the role of Fagaceae in tropical montane rain forests. J. Biogeogr. 2010, 37, 960–974. [Google Scholar] [CrossRef]
- Larsen, D.R.; Speckman, P.L. Multivariate regression trees for analysis of abundance data. Biometrics 2004, 60, 543–549. [Google Scholar] [CrossRef]
- Méndez-Toribio, M.; Meave, J.A.; Zermeño-Hernández, I.; Ibarra-Manríquez, G. Effects of slope aspect and topographic position on environmental variables, disturbance regime and tree community attributes in a seasonal tropical dry forest. J. Veg. Sci. 2016, 27, 1094–1103. [Google Scholar] [CrossRef]
- Sundqvist, M.K.; Sanders, N.J.; Wardle, D.A. Community and ecosystem responses to elevational gradients: Processes, mechanisms, and insights for global change. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 261–280. [Google Scholar] [CrossRef]
- de Castilho, C.V.; Magnusson, W.E.; de Araújo, R.N.O.; Luizao, R.C.; Luizao, F.J.; Lima, A.P.; Higuchi, N. Variation in aboveground tree live biomass in a central Amazonian Forest: Effects of soil and topography. For. Ecol. Manag. 2006, 234, 85–96. [Google Scholar] [CrossRef]
- McEwan, R.W.; Lin, Y.C.; Sun, I.F.; Hsieh, C.F.; Su, S.H.; Chang, L.W.; Song, G.Z.M.; Wang, H.H.; Hwong, J.L.; Lin, K.C.; et al. Topographic and biotic regulation of aboveground carbon storage in subtropical broad-leaved forests of Taiwan. For. Ecol. Manag. 2011, 262, 1817–1825. [Google Scholar] [CrossRef]
- Fotis, A.T.; Murphy, S.J.; Ricart, R.D.; Krishnadas, M.; Whitacre, J.; Wenzel, J.W.; Queenborough, S.A.; Comita, L.S. Aboveground biomass is driven by mass-ratio effects and stand structural attributes in a temperate deciduous forest. J. Ecol. 2017, 106, 561–570. [Google Scholar] [CrossRef]
- Ding, Y.; Zang, R.G. Determinants of aboveground biomass in forests across three climatic zones in China. For. Ecol. Manag. 2021, 482, 118805. [Google Scholar] [CrossRef]
- Ali, A.; Lin, S.-L.; He, J.-K.; Kong, F.-M.; Yu, J.-H.; Jiang, H.-S. Climate and soils determine aboveground biomass indirectly via species diversity and stand structural complexity in tropical forests. For. Ecol. Manag. 2019, 432, 823–831. [Google Scholar] [CrossRef]
- Laurance, W.F.; Fearnside, P.M.; Laurance, S.G.; Delamonica, P.; Lovejoy, T.E.; Rankin-de Merona, J.M.; Chambers, J.Q.; Gascon, C. Relationship between soils and Amazon Forest biomass: A landscape-scale study. For. Ecol. Manag. 1999, 118, 127–138. [Google Scholar] [CrossRef]
- Paoli, G.D.; Curran, L.M.; Slik, J.W.F. Soil nutrients affect spatial patterns of aboveground biomass and emergent tree density in southwestern Borneo. Oecologia 2008, 155, 287–299. [Google Scholar] [CrossRef]
- Dewalt, S.J.; Chave, J. Structure and Biomass of Four Lowland Neotropical Forests. Biotropica 2004, 36, 7–19. [Google Scholar] [CrossRef]
- Baraloto, C.; Rabaud, S.; Molto, Q.; Blanc, L.; Fortunel, C.; Herault, B.; Dávila, N.; Mesones, I.; Rios, M.; Valderrama, E.; et al. Disentangling stand and environmental correlates of aboveground biomass in Amazonian forests. Glob. Chang. Biol. 2011, 17, 2677–2688. [Google Scholar] [CrossRef]
- Quesada, C.A.; Phillips, O.L.; Schwarz, M.; Czimczik, C.I.; Baker, T.R.; Patiño, S.; Fyllas, N.M.; Hodnett, M.G.; Herrera, R.; Almeida, S.; et al. Basin-wide variations in Amazon Forest structure and function are mediated by both soils and climate. Biogeosciences 2012, 9, 2203–2246. [Google Scholar] [CrossRef]
- Poorter, L.; van der Sande, M.T.; Arets, E.J.M.M.; Ascarrunz, N.; Enquist, B.; Finegan, B.; Licona, J.C.; Martínez-Ramos, M.; Mazzei, L.; Meave, J.A.; et al. Biodiversity and climate determine the functioning of Neotropical forests. Glob. Ecol. Biogeogr. 2017, 26, 1423–1434. [Google Scholar] [CrossRef]
- Prado-Junior, J.A.; Schiavini, I.; Vale, V.S.; Arantes, C.S.; van der Sande, M.T.; Lohbeck, M.; Poorter, L. Conservative species drive biomass productivity in tropical dry forests. J. Ecol. 2016, 104, 817–827. [Google Scholar] [CrossRef]
- Wang, S.; Jiménez-Alfaro, B.; Pan, S.; Yu, J.; Sanaei, A.; Sayer, E.J.; Ye, J.; Hao, Z.Q.; Fang, S.; Lin, F.; et al. Differential Responses of Forest Strata Species Richness to Paleoclimate and Forest Structure. For. Ecol. Manag. 2021, 499, 119605. [Google Scholar] [CrossRef]
- Vilà, M.; Vayreda, J.; Gracia, C.; Ibáñez, J.J. Does tree diversity increase wood production in pine forests? Oecologia 2003, 135, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Poorter, L.; van der Sande, M.T.; Thompson, J.; Arets, E.J.M.M.; Alarc’oõn, A.; Alvarez-Sanchez, J.; Ascarrunz, N.; Balvanera, P.; Barajas-Guzmán, G.; Boit, A.; et al. Diversity enhances carbon storage in tropical forests. Glob. Ecol. Biogeogr. 2015, 24, 1314–1328. [Google Scholar] [CrossRef]
- Ali, A.; Yan, E.R.; Chang, S.X.; Cheng, J.Y.; Liu, X.Y. Community-weighted mean of leaf traits and divergence of wood traits predict aboveground biomass in secondary subtropical forests. Sci. Total Environ. 2017, 574, 654–662. [Google Scholar] [CrossRef]
- Feng, Y.; Schmid, B.; Loreau, M.; Forrester, D.I.; Fei, S.; Zhu, J.; Tang, Z.Y.; Zhu, J.L.; Hong, P.B.; Ji, C.J.; et al. Multispecies Forest Plantations Outyield Monocultures across a Broad Range of Conditions. Science 2022, 376, 865–868. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.Y. Individual size inequality links forest diversity and above-ground biomass. J. Ecol. 2015, 103, 1245–1252. [Google Scholar] [CrossRef]
- Dănescu, A.; Albrecht, A.T.; Bauhus, J. Structural diversity promotes productivity of mixed, uneven-aged forests in southwestern Germany. Oecologia 2016, 182, 319–333. [Google Scholar] [CrossRef]
- Ali, A.; Yan, E.R.; Chen, H.Y.; Chang, S.X.; Zhao, Y.T.; Yang, X.D.; Xu, M.S. Stand Structural Diversity rather than Species Diversity Enhances Aboveground Carbon Storage in Secondary Subtropical Forests in Eastern China. Biogeosciences 2016, 13, 4627–4635. [Google Scholar] [CrossRef]
- Ali, A.; Mattsson, E. Individual tree size inequality enhances aboveground biomass in homegarden agroforestry systems in the dry zone of Sri Lanka. Sci. Total Environ. 2017, 575, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Wang, S.; Ali, A.; Gazol, A.; Ruiz-Benito, P.; Wang, X.; Lin, F.; Ye, J.; Hao, Z.Q.; Loreau, M. Aboveground carbon storage is driven by functional trait composition and stand structural attributes rather than biodiversity in temperate mixed forests recovering from disturbances. Ann. For. Sci. 2018, 75, 67. [Google Scholar] [CrossRef]
- Fan, F.; Zhao, L.J.; Ma, T.Y.; Xiong, X.Y.; Zhang, Y.B.; Shen, X.L.; Li, S. Community composition and structure in a 25.2 hm2 subalpine dark coniferous forest dynamics plot in Wanglang, Sichuan, China. Chin. J. Plant Ecol. 2022, 46, 1005–1017. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity Hotspots for Conservation Priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Liu, Q. Ecological Research on Subalpine Coniferous Forests in China; Sichuan University Press: Chengdu, China, 2002. [Google Scholar]
- Barredo, J.I.; San-Miguel-Ayanz, J.; Caudullo, G.; Busetto, L. A European map of living forest biomass and carbon stock. Reference Report by the Joint Research Centre of the European Commission. EUR-Sci. Technol. Res. 2012, 25730, 5. [Google Scholar]
- Anderson-Teixeira, K.J.; Davies, S.J.; Bennett, A.C.; Gonzalez-Akre, E.B.; Muller-Landau, H.C.; Joseph Wright, S.; Abu Salim, K.; Almeyda Zambrano, A.M.; Alonso, A.; Baltzer, J.L.; et al. CTFS-Forest GEO: A worldwide network monitoring forests in an era of global change. Glob. Chang. Biol. 2015, 21, 528–549. [Google Scholar] [CrossRef]
- Taylor, A.H.; Qin, Z.; Liu, J. Tree regeneration in an Abies faxoniana forest after bamboo dieback, Wang Lang Natural Reserve, China. Can. J. For. Res. 1995, 25, 2034–2039. [Google Scholar] [CrossRef]
- Fan, F.; Bu, H.L.; McShea, W.J.; Shen, X.L.; Li, B.V.; Li, S. Seasonal habitat use and activity patterns of blood pheasant Ithaginis cruentusbe in the presence of free-ranging livestock. Glob. Ecol. Conserv. 2020, 23, e01155. [Google Scholar] [CrossRef]
- Zhou, G.; Yin, G.; Tang, X. Carbon Stocks of Forest Ecosystems in China: Biomass Equation; Science Press: Beijing, China, 2018. [Google Scholar]
- Graham, M.H. Confronting multicollinearity in ecological multiple regression. Ecology 2003, 84, 2809–2815. [Google Scholar] [CrossRef]
- García-Palacios, P.; Gross, N.; Gaitán, J.; Maestre, F.T. Climate mediates the biodiversity-ecosystem stability relationship globally. Proc. Natl. Acad. Sci. USA 2018, 115, 8400–8405. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Chave, J.; Andalo, C.; Brown, S.; Cairns, M.A.; Chambers, J.Q.; Eames, D.; Fölster, H.; Fromard, F.; Higuchi, N.; Kira, T.; et al. Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 2005, 145, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Stegen, J.C.; Swenson, N.G.; Enquist, B.J.; White, E.P.; Phillips, O.L.; Jørgensen, P.M.; Weiser, M.D.; Mendoza, A.M.; Vargas, P.N. Variation in Above-ground Forest Biomass across Broad Climatic Gradients. Glob. Ecol. Biogeogr. 2011, 20, 744–754. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; Euskirchen, E.S. Carbon Cycling and Storage in World Forests: Biome Patterns Related to Forest Age. Glob. Chang. Biol. 2004, 10, 2052–2077. [Google Scholar] [CrossRef]
- Feldpausch, T.R.; Banin, L.; Phillips, O.L.; Baker, T.R.; Lewis, S.L.; Quesada, C.A.; Affum-Baffoe, K.; Arets, E.J.M.M.; Berry, N.J.; Bird, M.; et al. Height–diameter allometry of tropical forest trees. Biogeosciences 2011, 8, 1081–1106. [Google Scholar] [CrossRef]
- Morin, X. Species richness promotes canopy packing: A promising step towards a better understanding of the mechanisms driving the diversity effects on forest functioning. Funct. Ecol. 2015, 29, 993–994. [Google Scholar] [CrossRef]
- Ali, A. Forest Stand Structure and Functioning: Current Knowledge and Future Challenges. Ecol. Indic. 2019, 98, 665–677. [Google Scholar] [CrossRef]
- Mao, Z.; van der Plas, F.; Corrales, A.; Anderson-Teixeira, K.J.; Bourg, N.A.; Chu, C.; Hao, Z.Q.; Jin, G.Z.; Lian, J.Y.; Lin, F.; et al. Scale-Dependent Diversity-Biomass Relationships Can Be Driven by Tree Mycorrhizal Association and Soil Fertility. Ecol. Monogr. 2023, 93, e1568. [Google Scholar] [CrossRef]
- Soriano-Luna, M.D.l.Á.; Ángeles-Pérez, G.; Guevara, M.; Birdsey, R.; Pan, Y.; Vaquera-Huerta, H.; Valdez-Lazalde, J.R.; Johnson, K.D.; Vargas, R. Determinants of Above-Ground Biomass and Its Spatial Variability in a Temperate Forest Managed for Timber Production. Forests 2018, 9, 490. [Google Scholar] [CrossRef]
- Yuan, Z.; Ali, A.; Jucker, T.; Ruiz-Benito, P.; Wang, S.; Jiang, L.; Wang, X.G.; Lin, F.; Ye, J.; Hao, Z.Q.; et al. Multiple abiotic and biotic pathways shape biomass demographic processes in temperate forests. Ecology 2019, 100, e02650. [Google Scholar] [CrossRef]
- Maren, I.E.; Karki, S.; Prajapati, C.; Yadav, R.K.; Shrestha, B.B. Facing North or South: Does Slope Aspect Impact Forest Stand Characteristics and Soil Properties in a Semiarid Trans-Himalayan Valley? J. Arid Environ. 2015, 121, 112–123. [Google Scholar] [CrossRef]
- Warren, R.J. Mechanisms driving understory evergreen herb distributions across slope aspects, as derived from landscape position. Plant Ecol. 2008, 198, 297–308. [Google Scholar] [CrossRef]
- Yu, M.; Sun, O.J. Effects of forest patch type and site on herb layer vegetation in a temperate forest ecosystem. For. Ecol. Manag. 2013, 300, 14–20. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, M.; Nan, H. Abiotic and biotic drivers of species diversity in understory layers of cold temperate coniferous forests in North China. J. For. Res. 2018, 30, 2213–2225. [Google Scholar] [CrossRef]
- Xue, R.; Yang, Q.; Miao, F.; Wang, X.; Shen, Y. Slope Aspect Influences Plant Biomass, Soil Properties and Microbial Composition in Alpine Meadow on the Qinghai-Tibetan Plateau. J. Soil Sci. Plant Nutr. 2018, 18, 1–12. [Google Scholar] [CrossRef]
- Gaston, K.J. Global patterns in biodiversity. Nature 2000, 405, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Lutz, J.A.; Král, K.; Vrška, T.; Yin, X.; Myers, J.; Abiem, I.; Alonso, A.; Bourg, N.; Burslem, D.F.R.P.; et al. Direct and indirect effects of climate on richness drive the latitudinal diversity gradient in forest trees. Ecol. Lett. 2018, 22, 245–255. [Google Scholar] [CrossRef]
- Pachepsky, Y.A.; Timlin, D.J.; Rawls, W.J. Soil water retention as related to topographic variables. Soil Sci. Soc. Am. J. 2001, 65, 1787–1795. [Google Scholar] [CrossRef]
- Cai, W.H.; Yang, J.; Liu, Z.H.; Hu, Y.M.; Liu, S.J.; Jing, G.Z.; Zhao, Z.F. Controls of post-fire tree recruitment in Great Xing’an Mountains in Heilongjiang Province. Acta Ecol. Sin. 2012, 32, 3303–3312. [Google Scholar]

| Variables | Abbreviations | Units | Mean | SD | Max | Min | Median |
|---|---|---|---|---|---|---|---|
| Topography | |||||||
| Elevation | Elevation | m | 2866 | 16 | 2925 | 2849 | 2858 |
| Slope | Slope | ° | 24 | 11 | 57 | 5 | 25 |
| Aspect | Aspect | ° | 105 | 50 | 179 | 1 | 124 |
| Soil | |||||||
| Soil organic matter | SOM | g/kg | 200.2 | 71.7 | 403.2 | 55.1 | 189.5 |
| Total nitrogen content | TN | g/kg | 0.91 | 0.26 | 1.66 | 0.45 | 0.91 |
| Total phosphorus content | TP | g/kg | 7.53 | 2.72 | 13.75 | 2.38 | 7.71 |
| Soil pH | pH | / | 6.79 | 0.81 | 8.14 | 4.41 | 7.06 |
| Stand structure | |||||||
| Species richness | SR | No. | 16 | 3 | 24 | 5 | 17 |
| Stem density | Density | No./ha | 4627 | 1744 | 10,875 | 625 | 4575 |
| Maximum DBH | max_DBH | cm | 68.5 | 20.1 | 122.0 | 19.4 | 68.7 |
| DBH variance | cv_DBH | / | 180.9 | 40.0 | 284.3 | 64.7 | 185.0 |
| Important value of spruce–fir | spruce–fir_IV | / | 74.11 | 24.99 | 99.14 | 0 | 82.98 |
| Direct Effects | Indirect Effects | Total Effects | |
|---|---|---|---|
| Aspect | 0 | 0.098 | 0.098 |
| Slope | 0 | −0.022 | −0.022 |
| TP | −0.173 | −0.084 | −0.256 |
| SOM | 0.088 | 0.012 | 0.100 |
| max_DBH | 0.561 | 0.142 | 0.703 |
| Density | −0.146 | −0.017 | −0.163 |
| spruce–fir_IV | 0.272 | 0 | 0.272 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, X.; Xiong, X.; Cai, Q.; Fan, F.; He, C.; Ji, C.; Li, S.; Shen, X.; Zhu, J. Spatial Distribution and Determinants of Aboveground Biomass in a Subalpine Coniferous Forest in Southwestern China. Forests 2023, 14, 2197. https://doi.org/10.3390/f14112197
Ni X, Xiong X, Cai Q, Fan F, He C, Ji C, Li S, Shen X, Zhu J. Spatial Distribution and Determinants of Aboveground Biomass in a Subalpine Coniferous Forest in Southwestern China. Forests. 2023; 14(11):2197. https://doi.org/10.3390/f14112197
Chicago/Turabian StyleNi, Xiaofeng, Xinyu Xiong, Qiong Cai, Fan Fan, Chenqi He, Chengjun Ji, Sheng Li, Xiaoli Shen, and Jiangling Zhu. 2023. "Spatial Distribution and Determinants of Aboveground Biomass in a Subalpine Coniferous Forest in Southwestern China" Forests 14, no. 11: 2197. https://doi.org/10.3390/f14112197
APA StyleNi, X., Xiong, X., Cai, Q., Fan, F., He, C., Ji, C., Li, S., Shen, X., & Zhu, J. (2023). Spatial Distribution and Determinants of Aboveground Biomass in a Subalpine Coniferous Forest in Southwestern China. Forests, 14(11), 2197. https://doi.org/10.3390/f14112197






