Does the Distance from the Formal Path Affect the Richness, Abundance and Diversity of Geophytes in Urban Forests and Parks?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling
2.2. Measurement of Abiotic and Biotic Traits
2.3. Statistical Analyses
3. Results
3.1. Characteristics of Abiotic Conditions
3.2. Characteristics of Plant Cover and Number of Species
3.3. Characteristics of Geophytes
3.4. The Relationship between the Number of Geophytes and Habitat Conditions
3.5. The Relationship between the Share of Geophytes and Habitat Conditions
3.6. The Relationship between the Cover-Abundance of Geophytes and Habitat Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smaniotto Costa, C.; Šuklje Erjavec, I.; Mathey, J. Green spaces—A key resources for urban sustainability The Green Key sapproach for developing greenspaces. Urbani Izziv. 2008, 19, 199–211. [Google Scholar] [CrossRef]
- World Health Organization. Urban Green Spaces: A Brief for Action; World Health Organization, Regional Office for Europe: Copenhagen, Denmark, 2017; p. 24. Available online: https://www.euro.who.int/__data/assets/pdf_file/0010/342289/Urban-Green-Spaces_EN_WHO_web3.pdf (accessed on 11 November 2023).
- Cavender, N.; Donnelly, G. Intersecting urban forestry and botanical gardens to address big challenges for healthier trees, people, and cities. Plants People Planet 2019, 1, 315–322. [Google Scholar] [CrossRef]
- Pearlmutter, D.; Calfapietra, C.; Samson, R.; O’Brien, L.; Krajter Ostoić, S.; Giovanni Sanesi, G.; del Amo, R.A. (Eds.) The Urban Forest. Future City; Springer: Cham, Switzerland, 2017; Volume 7, p. 351. [Google Scholar] [CrossRef]
- Lüttge, U.; Buckeridge, M. Trees: Structure and function and the challenges of urbanization. Trees 2020, 37, 9–16. [Google Scholar] [CrossRef]
- Pataki, D.E.; Alberti, M.; Cadenasso, M.L.; Felson, A.J.; McDonnell, M.J.; Pincetl, S.; Pouyat, R.V.; Setälä, H.; Whitlow, T.H. The Benefits and Limits of Urban Tree Planting for Environmental and Human Health. Front. Ecol. Evol. 2021, 9, 603757. [Google Scholar] [CrossRef]
- Seymour, M.; Byrne, J.; Martino, D.; Wolch, J. Green Visions Plan for 21st Century Southern California: A Guide for Habitat Conservation, Watershed Health, and Recreational Open Space. 9. Recreationist-Wildlife Interactions in Urban Parks; University of Southern California GIS Research Laboratory and Center for Sustainable Cities: Los Angeles, CA, USA, 2006; p. 87. [Google Scholar]
- Zdanowicz, E.; Skłodowski, J. Ocena zmian w środowisku wokół szlaków rekreacyjnych na przykładzie rezerwatu Las Bielański w Warszawie. Stud. I Mater. CEPL W Rogowie 2013, 37, 348–355. [Google Scholar]
- Verlič, A.; Arnberger, A.; Japelj, A.; Simončič, P.; Pirnat, J. Perceptions of recreational trail impacts on an urban forest walk: A controlled field experiment. Urban For. Urban Green. 2015, 14, 89–98. [Google Scholar] [CrossRef]
- Sujetovienė, G.; Baranauskienė, T. Impact of visitors on soil and vegetation characteristics in urban parks of central Lithuania. Environ. Res. Eng. Manag. 2016, 72, 51–58. [Google Scholar]
- Talal, M.L.; Santelmann, M.V. Vegetation management for urban park visitors: A mixed methods approach in Portland, Oregon. Ecol. Appl. 2020, 30, e02079. [Google Scholar] [CrossRef] [PubMed]
- Hamzah, H.; Shaari, S.J.; Shamsuddin, M.S. Assessment of Tree Vandalism Level in Kuala Kangsar Urban Park, Perak, Malaysia. Int. J. Acad. Res. Bus. Soc. Sci. 2022, 12, 39–49. [Google Scholar] [CrossRef]
- Dafni, A.; Cohen, D.; Noy-Mier, I. Life-cycle variation in geophytes. Ann. Mo. Bot. Gard. 1981, 68, 652–660. [Google Scholar] [CrossRef]
- Rundel, P.W.; Anderson, M.K. What is a geophyte? Fremontia 2016, 44, 5–6. [Google Scholar]
- Howard, C.C.; Folk, R.A.; Beaulieu, J.M.; Cellinese, N. The monocotyledonous underground: Global climatic and phylogenetic patterns of geophyte diversity. Am. J. Bot. 2019, 106, 850–863. [Google Scholar] [CrossRef] [PubMed]
- Tribble, C.M.; Martínez-Gómez, J.; CoyoteeHoward, C.; Males, J.; Sosa, V.; Sessa, E.B.; Cellinese, N.; Specht, C.D. Get the shovel: Morphological and evolutionary complexities of belowground organ sin geophytes. Am. J. Bot. 2021, 108, 372–387. [Google Scholar] [CrossRef]
- Noy-Meir, I.; Oron, T. Effects of grazing on geophytes in Mediterranean vegetation. J. Veg. Sci. 2001, 12, 749–760. [Google Scholar] [CrossRef]
- Popović, Z.; Vidaković, V. Ecophysiological and growth-related traits of two geophytes three years after the fire event in grassland steppe. Plants 2022, 11, 734. [Google Scholar] [CrossRef]
- Khosa, J.; Bellinazzo, F.; Kamenetsky Goldstein, R.; Macknight, R.; Immink, R.G.H. Phosphatidylethanolamine-Binding Proteins: The conductors of dual reproduction in plants with vegetative storage organs. J. Exp. Bot. 2021, 72, 2845–2856. [Google Scholar] [CrossRef]
- Dzwonko, Z.; Loster, S. Wskaźnikowe gatunki roślin starych lasów i ich znaczenie dla ochrony przyrody i kartografii roślinności. Prace Geogr. 2001, 178, 119–132. [Google Scholar]
- Sikorska, D.; Sikorski, P.; Wierzba, M. Ancient forest species in tree stands of different age as indicators of the continuity of forest habitat. Ann. Wars. Univ. Life Sci. SGGW Hortic. Landsc. Archit. 2008, 29, 155–162. [Google Scholar]
- Jabs-Sobocińska, Z.; Affek, A.N.; Matuszkiewicz, J.M. The list of ancient-forest plant species revisited—Field verification in the Carpathian ancient and recent forests. For. Ecol. Manag. 2022, 512, 120152. [Google Scholar] [CrossRef]
- Seyidoğlu, N.; Zencirkıran, M.; Ayaşlıgil, A. Position and application areas of geophytes within landscape design. Afr. J. Agric. Res. 2009, 4, 1351–1357. [Google Scholar]
- Nagase, A.; Dunnett, N. Performance of geophytes on extensive green roofs in the United Kingdom. Urban For. Urban Green. 2013, 12, 509–521. [Google Scholar] [CrossRef]
- Ros, M.; Garcia, C.; Hernandez, T.; Andres, M.; Barja, A. Short-Term Effects of Human Trampling on Vegetation and Soil Microbial Activity. Commun. Soil Sci. Plant Anal. 2004, 35, 1591–1603. [Google Scholar] [CrossRef]
- Dumitraşcu, M.; Marin, A.; Preda, E.; Ţîbîrnac, M.; Vădineanu, A. Trampling effects on plant species morphology. Rom. J. Biol. Plant Biol. 2010, 55, 89–96. [Google Scholar]
- Lehvävirta, S.; Vilisics, F.; Hamberg, L.; Malmivaara-Lämsä, M.; Kotze, J.D. Fragmentation and recreational use affect tree regeneration in urban forests. Urban For. Urban Green. 2014, 13, 869–877. [Google Scholar] [CrossRef]
- Pescott, O.L.; Stewart, G.B. Assessing the impact of human trampling on vegetation: A systematic review and meta-analysis of experimental evidence. PeerJ 2014, 2, e360. [Google Scholar] [CrossRef]
- Jägerbrand, A.K.; Alatalo, J.M. Effects of human trampling on abundance and diversity of vascular plants, bryophytes and lichens in alpine heath vegetation, Northern Sweden. SpringerPlus 2015, 4, 95. [Google Scholar] [CrossRef]
- Kuss, F.R. A review of major factors influencing plant responses to recreation impacts. Environ. Manag. 1986, 10, 637–650. [Google Scholar] [CrossRef]
- Sun, D.; Liddle, M.J. A survey of trampling effects on vegetation and soil in eight tropical and subtropical sites. Environ. Manag. 1993, 17, 497–510. [Google Scholar] [CrossRef]
- Kostrakiewicz-Gierałt, K.; Pliszko, A.; Gmyrek, K. The effect of informal tourist trails on the abiotic conditions and floristic composition of deciduous forest undergrowth in an urban area. Forests 2021, 12, 423. [Google Scholar] [CrossRef]
- Vakhlamova, T.; Rusterholz, H.-P.; Kamkin, V.; Baur, B. Recreational use of urban and suburban forests affects plant diversity in a Western Siberian city. Urban For. Urban Green. 2016, 17, 92–103. [Google Scholar] [CrossRef]
- Fornal-Pieniak, B.; Ollik, M.; Schwerk, A. Impact of different levels of anthropogenic pressure on the plant species composition in woodland sites. Urban For. Urban Green. 2019, 38, 295–304. [Google Scholar] [CrossRef]
- Erfanian, M.B.; Alatalo, J.M.; Ejtehadi, H. Severe vegetation degradation associated with different disturbance types in a poorly managed urban recreation destination in Iran. Sci. Rep. 2021, 11, 19695. [Google Scholar] [CrossRef] [PubMed]
- Kostrakiewicz-Gierałt, K.; Gmyrek, K.; Pliszko, A. The effect of the distance from a path on abiotic conditions and vascular plant species in the undergrowth of urban forests and parks. Int. J. Environ. Res. Public Health 2022, 19, 5621. [Google Scholar] [CrossRef] [PubMed]
- Hagiladi, A.; Umiel, N.; Ozeri, Y.; Elyasi, R.; Abramsky, S.; Levy, A.; Lobovsky, O.; Matan, E. The effect of planting depth on emergence and development of some geophytic plants. Acta Hortic. 1992, 325, 131–138. [Google Scholar] [CrossRef]
- Statistics Poland. Statistical Yearbook of the Republic of Poland; Statistics Poland: Warsaw, Poland, 2022; p. 791.
- Municipal Greenery Management in Kraków. 2023. Available online: https://zzm.krakow.pl/zzm/parki.html (accessed on 11 November 2023).
- Kwartnik-Pruc, A.; Trembecka, A. Public Green Space Policy Implementation: A Case Study of Krakow, Poland. Sustainability 2021, 13, 538. [Google Scholar] [CrossRef]
- Braun-Blanquet, J. Pflanzensoziologie. Grundzüge der Vegetationskunde, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 1964; p. 631. [Google Scholar]
- Csapodý, V. Keimlingsbestimmungsbuch der Dikotyledonen; Akademiai Kiado: Budapeszt, Hungary, 1968; p. 286. [Google Scholar]
- Muller, F.M. Seedlings of the North-Western European Lowland. In A Flora of Seedlings, 1st ed.; Springer: Wageningen, The Netherlands, 1978; p. 653. [Google Scholar]
- Rutkowski, L. Klucz do Oznaczania Roślin Naczyniowych Polski Niżowej; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2004; p. 814. [Google Scholar]
- POWO. Plants of the World Online. 2023. Available online: http://www.plantsoftheworldonline.org (accessed on 21 March 2023).
- Klotz, S.; Kühn, I.; Durka, W. BIOLFLOR—Eine Datenbankmitbiologisch-ökologischen Merkmalenzur Flora von Deutschland; Bundesamt für Naturschutz: Bonn, Germany, 2002; p. 334.
- BiolFlor. BiolFlor, Search and Information System on Vascular Plants in Germany. 2023. Available online: https://www.ufz.de/biolflor/index.jsp (accessed on 20 February 2023).
- Zielińska, K. The influence of roads on the species diversity of forest vascular flora in Central Poland. Biodiv. Res. Conserv. 2007, 5–8, 71–80. [Google Scholar]
- Avon, C.; Dumas, Y.; Bergès, L. Management practices increase the impact of roads on plant communities in forests. Biol. Conserv. 2013, 159, 24–31. [Google Scholar] [CrossRef]
- Sikorski, P.; Szumacher, I.; Sikorska, D.; Kozak, M.; Wierzba, M. Effects of visitor pressure on understory vegetation in Warsaw forested parks (Poland). Environ. Monit. Assess. 2013, 185, 5823–5836. [Google Scholar] [CrossRef]
- Mucina, L. Conspectus of classes of European vegetation. Folia Geobot. Phytotax. 1997, 32, 117–172. [Google Scholar] [CrossRef]
- Matuszkiewicz, W. Przewodnik do Oznaczania Zbiorowisk Roślinnych Polski; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2008; p. 540. [Google Scholar]
- Dzwonko, Z. Rośliny runa wskaźnikami pochodzenia i przemian lasów. Stud. I Mater. CEPL W Rogowie 2015, 42, 27–37. [Google Scholar]
- Pirożnikow, E. Demography of Anemone nemorosa L. in dry-site deciduous forest (Tilio-Carpinetum) in the Bialowieza Forest. Ekol. Pol. 1994, 42, 155–172. [Google Scholar]
- Kosiński, I. The influence of shoot harvesting on the size and fecundity of Convallaria majalis L. Acta Soc. Bot. Pol. 2001, 70, 303–312. [Google Scholar] [CrossRef]
- Vandepitte, K.; DeMeyer, T.; Jacquemyn, H.; Honnay, O.; Roldán-Ruiz, I. The impact of extensive clonal growth on fine-scale mating patterns: A full paternity analysis of a lily-of-the-valley population (Convallaria majalis). Ann. Bot. 2013, 111, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Vandepitte, K.; Jacquemyn, H.; Roldán-Ruiz, I.; Honnay, O. Extremely low genotypic diversity and sexual reproduction in isolated populations of the self-incompatible lily-of-the-valley (Convallaria majalis) and the role of the local forest environment. Ann. Bot. 2019, 105, 769–776. [Google Scholar] [CrossRef]
- Eriksson, O. Seedling recruitment in deciduous forest herbs: The effects of litter, soil chemistry and seed bank. Flora 1995, 190, 65–70. [Google Scholar] [CrossRef]
- Holderegger, R.; Stehlik, I.; Schneller, J.J. Estimation of the relative importance of sexual and vegetative reproduction in the clonal woodland herb Anemone nemorosa. Oecologia 1998, 117, 105–107. [Google Scholar] [CrossRef]
- Axtell, A.E.; DiTommaso, A.; Post, A.R. Lesser Calendine (Ranunculus ficaria): A threat to woodland habitats in the northern United States and southern Canada. Invasive Plant Sci. Manag. 2010, 3, 190–196. [Google Scholar] [CrossRef]
- Sohrabi Kertabad, S.; Rashed Mohassel, M.H.; Nasiri Mahalati, M.; Gherekhloo, J. Some biological aspects of the weed Lesser celandine (Ranunculus ficaria). Planta Daninha 2013, 31, 577–585. [Google Scholar] [CrossRef]
- Rusterholz, H.-P.; Weisskopf-Kissling, M.; Baur, B. Single versus repeated human trampling events: Responses of ground vegetation in suburban beech forests. Appl. Veg. Sci. 2021, 24, e12604. [Google Scholar] [CrossRef]
- Littlemore, J.; Baker, S. The ecological response of forest ground flora and soils to experimental trampling in British urban woodlands. Urban Ecosyst. 2001, 5, 257–276. [Google Scholar] [CrossRef]
- Zarzycki, K.; Trzcińska-Tacik, H.; Różański, W.; Szeląg, Z.; Wołek, J.; Korzeniak, U. Ecological Indicator Values of Vascular Plants of Poland; W. Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2002; p. 183. [Google Scholar]
- Depauw, L.; Hu, R.; Dhungana, K.S.; Govaert, S.; Meeussen, C.; Vangansbeke, P.; Strimbeck, R.; Graae, B.J.; De Frenne, P. Functional trait variation of Anemone nemorosa along macro-and microclimatic gradients close to the northern range edge. Nord. J. Bot. 2022, 2022, e03471. [Google Scholar] [CrossRef]
- Baeten, L.; Bauwens, B.; De Schrijver, A.; De Keersmaeker, L.; Van Calster, H.; Vandekerkhove, K.; Roelandt, B.; Beeckman, H.; Verheyen, K. Compositional changes (1954–2000) in the forest herb layer following the cessation of coppice –with –standards management and soil acidification. Appl. Veg. Sci. 2009, 12, 187–197. [Google Scholar] [CrossRef]
- Baeten, L.; DeFrenne, P.; Verheyen, K.; Graae, B.J.; Hermy, M. Forest herbs in the face of global change: A single –species –multiple –threats approach for Anemone nemorosa. Plant Ecol. Evol. 2010, 143, 19–30. [Google Scholar] [CrossRef]
- Thomaes, A.; DeKeersmaeker, L.; DeSchrijver, A.; Baeten, L.; Vandekerkhove, K.; Verstraeten, G.; Verheyen, K. Can soil acidity and light help to explain tree species effects on forest herb layer performance in post-agricultural forests? Plant Soil 2013, 373, 183–199. [Google Scholar] [CrossRef]
- Tyler, G. Interacting effects of soil acidity and canopy cover on the species composition of field- layer vegetation in oak/hornbeam forests. For. Ecol. Manag. 1989, 28, 101–114. [Google Scholar] [CrossRef]
- Falkengren-Grerup, U.; Tyler, G. Soil chemical properties excluding field-layers pecies from beech forest mor. Plant Soil 1993, 148, 185–191. [Google Scholar] [CrossRef]
- Marrs, R.M.; Watt, A.S. Biological flora of the British Isles: Pteridium aquilinum (L.) Kuhn. J. Ecol. 2006, 94, 1272–1321. [Google Scholar] [CrossRef]
- Amouzgar, L.; Ghorbani, J.; Shokri, M.; Marrs, R.H.; Alday, J.G. Pteridium aquilinum performance is driven by climate, soil and land-use in Southwest Asia. Folia Geobot. 2020, 55, 301–314. [Google Scholar] [CrossRef]
- Godefroid, S.; Koedam, N. The impact of forest paths upon adjacent vegetation: Effects of the path surfacing material on the species composition and soil compaction. Biol. Conserv. 2004, 119, 405–419. [Google Scholar] [CrossRef]
- Yang, J.-L.; Zhang, G.-L. Formation, characteristics and eco-environmental implications of urban soils—A review. Soil Sci. Plant Nutr. 2015, 61, 30–46. [Google Scholar] [CrossRef]
- Paradeis, B.; Lovas, S.; Aipperspach, A.; Kazmierczak, A.; Boche, M.; He, Y.; Corrigan, P.; Chambers, K.; Gao, Y.; Norland, J.; et al. Dog-park soils: Concentration and distribution of urine-borne constituents. Urban Ecosyst. 2013, 16, 351–365. [Google Scholar] [CrossRef]
- Allen, J.A.; Setälä, H.; Kotze, D.J. Dog urine has acute impacts on soil chemistry in urban green spaces. Front. Ecol. Evol. 2020, 8, 615979. [Google Scholar] [CrossRef]
- Falkengren-Grerup, U. Effects on beech forest species of experimentally enhanced nitrogen deposition. Flora 1993, 188, 85–91. [Google Scholar] [CrossRef]
- Gordon, C.; Woodin, S.J.; Alexander, I.J.; Mullins, C.E. Effects of increased temperature, drought and nitrogen supply on two upland perennials of contrasting functional type: Calluna vulgaris and Pteridium aquilinum. New Phytol. 1999, 142, 243–258. [Google Scholar] [CrossRef]
- Falkengren-Grerup, U.; Quist, M.E.; Tyler, G. Relative importance of exchangeable and soil solution cations concentrations to the distribution of vascular plants. Environ. Exp. Bot. 1995, 35, 9–15. [Google Scholar] [CrossRef]
- Kermack, J.P.; Rauschert, E. Soil characteristics drive Ficaria verna abundance and reproductive output. Invasive Plant Sci. Manag. 2019, 12, 214–222. [Google Scholar] [CrossRef]
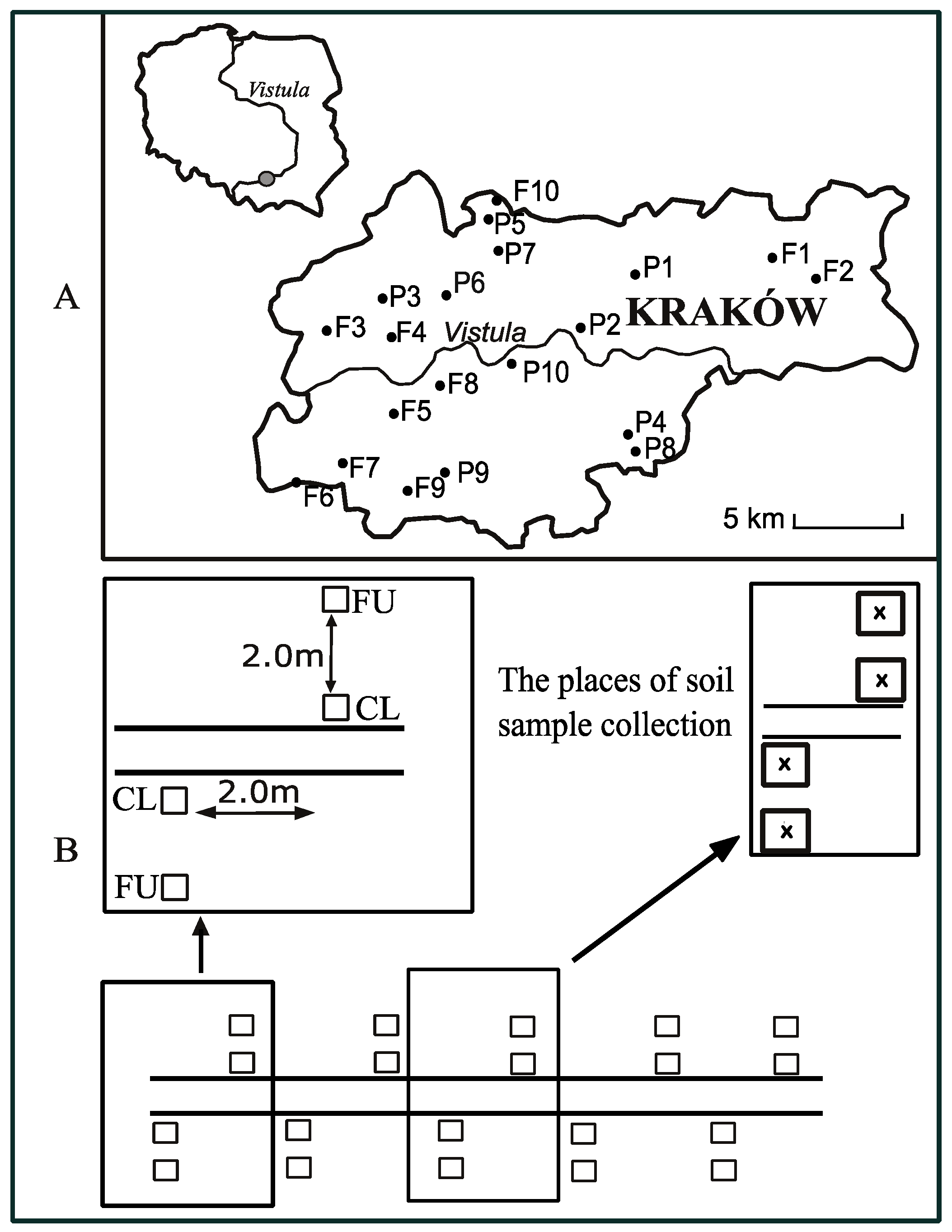

| Study Site | Symbol | GPS Coordinates | Elevation (m a.s.l.) | Width of the Path (cm) | Type of Path Surface |
|---|---|---|---|---|---|
| Łęgowski Forest | F1 | N 50°03.116′ | 199 | 300 | artificial (gravel) |
| E 20°02.092′ | |||||
| Mogilski Forest | F2 | N 50°03.228′ | 200 | 285 | artificial (asphalt) |
| E 20°03.195′ | |||||
| Wolski Forest | F3 | N 50°03.336′ | 334 | 160 | natural |
| E 19°51.445′ | |||||
| Forest in Sikornik Hill | F4 | N 50°03.466′ | 286 | 101 | natural |
| E 19°53.175′ | |||||
| Forest in Górka Pychowicka | F5 | N 50°01.908′ | 252 | 215 | natural |
| E 19°52.967′ | |||||
| Tyniec Forest | F6 | N 50°00.641′ | 245 | 260 | natural |
| E 19°49.709′ | |||||
| Forest in Skotniki | F7 | N 50°01.250′ | 218 | 200 | artificial (gravel) |
| E 19°51.118′ | |||||
| Forest of Twardowski Rocks | F8 | N 50°02.521′ | 234 | 305 | natural |
| E 19°54.546′ | |||||
| Borkowski Forest | F9 | N 50°00.666′ | 215 | 130 | natural |
| E 19°54.748′ | |||||
| Witkowice Forest | F10 | N 50°06.475′ | 238 | 100 | natural |
| E 19°57.000′ | |||||
| Polish Aviator’s Park | P1 | N 50°04.371′ | 229 | 175 | artificial (asphalt) |
| E 19°59.424′ | |||||
| Dąbie Park | P2 | N 50°03.579′ | 202 | 120 | natural |
| E 19°58.978′ | |||||
| Decius Park | P3 | N 50°03.957′ | 256 | 225 | artificial (asphalt) |
| E 19°52.379′ | |||||
| Anna and Erazm Jerzmanowski Park | P4 | N 50°01.030′ | 219 | 182 | artificial (sand and gravel) |
| E 19°59.657′ | |||||
| Stanisław Wyspiański’s Park | P5 | N 50°05.135′ | 235 | 300 | artificial (asphalt) |
| E 19°55.284′ | |||||
| Henryk Jordan’s Park | P6 | N 50°03.742′ | 204 | 310 | artificial (asphalt) |
| E 19°54.996′ | |||||
| Kleparski Park | P7 | N 50°04.583′ | 222 | 300 | artificial (asphalt) |
| E 19°56.241′ | |||||
| Aleksandra’s Park | P8 | N 50°00.727′ | 223 | 90 | natural |
| E 20°00.881′ | |||||
| Solvay Park | P9 | N 50°00.916′ | 226 | 175 | natural |
| E 19°55.599′ | |||||
| Florian Nowacki’s Park | P10 | N 50°02.625′ | 178 | 168 | artificial (sett) |
| E 19°56.577′ |
| Abiotic Traits | Forests | Parks | ||
|---|---|---|---|---|
| CL | FU | CL | FU | |
| Light intensity at ground level (lx) | 10,508.41 (±19,251.41) | 6449.63 (±13,293.03) | 11,940.01 (±15,231.39) | 9856.61 (±11,143.89) |
| Soil electrical conductivity (mS/cm) | 0.15 (±0.14) | 0.17 (±0.18) | 0.15 (±0.15) | 0.13 (±0.10) |
| Penetration depth of the penetrometer probe (cm) | 24.10 (±19.95) | 43.81 (±21.42) | 16.34 (±15.33) | 24.41 (±21.17) |
| Soil pH | 6.70 (±0.38) | 6.57 (±0.49) | 6.67 (±0.44) | 6.37 (±0.72) |
| Content of N-NH4 (mg/kg) | 23.99 (±70.73) | 16.38 (±38.54) | 3.90 (±6.33) | 3.71 (±6.26) |
| Content of N-NO3 (mg/kg) | 66.70 (±25.47) | 57.50 (±11.70) | 43.12 (±29.81) | 33.93 (±25.64) |
| Content of K (mg/kg) | 58.00 (±66.35) | 65.50 (±71.34) | 38.50 (±46.86) | 25.80 (±29.34) |
| Content of P (mg/kg) | 18.00 (±17.12) | 13.00 (±14.09) | 22.25 (±19.23) | 16.25 (±17.98) |
| Biotic Traits | Forests | Parks | ||
|---|---|---|---|---|
| CL | FU | CL | FU | |
| Total plant cover (%) | 32.85 (±23.56) | 36.94 (±29.19) | 33.10 (±21.06) | 41.55 (±25.85) |
| Height of the tallest shoot in herb layer (cm) | 36.32 (±23.39) | 51.60 (±26.39) | 25.51 (±16.90) | 34.21 (±22.35) |
| Number of species | 5.64 (±2.41) | 4.64 (±2.12) | 7.91 (±3.34) | 8.14 (±3.77) |
| Species | Abbreviation of Group | Storage Organ | Mean Frequency of Occurrence | |||
|---|---|---|---|---|---|---|
| Forests | Parks | |||||
| CL | FU | CL | FU | |||
| Allium oleraceum L. | BU | bulb | - | 0.10 (±0.32) | 0.10 (±0.32) | 0.30 (±0.95) |
| Anemonoides nemorosa (L.) Holub | RH | rhizome | 3.10 (±4.20) | 3.80 (±4.44) | 0.30 (±0.95) | 0.60 (±1.58) |
| Anemonoides ranunculoides (L.) Holub | RH | rhizome | - | - | 0.30 (±0.95) | 0.40 (±1.26) |
| Carex brizoides L. | RU-RH | runner, rhizome | 1.10 (±3.14) | 0.90 (±2.85) | - | - |
| Carex hirta L. | RU-RH | runner, rhizome | 0.20 (±0.63) | - | 0.40 (±0.97) | 0.30 (±0.67) |
| Circaea lutetiana L. | RU-TU | runner, runner with tuberous tip | 1.50 (±2.17) | 1.20 (±2.39) | - | - |
| Convallaria majalis L. | RH | rhizome | 0.40 (±1.26) | 0.70 (±2.21) | - | - |
| Corydalis solida (L.) Clairv. | HB | hypocotyl bulb | 1.00 (±2.83) | 0.50 (±1.58) | 0.10 (±0.32) | 0.10 (±0.32) |
| Elymus repens (L.) Gould | RL-RH | runner-like rhizome | - | - | 0.30 (±0.67) | - |
| Equisetum arvense L. | RU-ST | runner, shoot tuber | - | - | 0.10 (±0.32) | - |
| Gagea lutea (L.) Ker Gawl. | BU | bulb | 0.10 (±0.32) | 0.10 (±0.32) | 1.90 (±3.11) | 2.30 (±3.74) |
| Galium odoratum (L.) Scop. | RU | runner | 0.40 (±1.26) | 0.40 (±0.97) | - | - |
| Glechoma hederacea L. | RU | runner | 0.70 (±1.34) | 0.60 (±1.35) | 2.70 (±2.87) | 3.00 (±2.45) |
| Lathyrus vernus (L.) Bernh. | RH | rhizome | - | 0.10 (±0.32) | - | - |
| Lilium martagon L. | BU | bulb | 0.50 (±1.58) | 0.30 (±0.95) | - | - |
| Lysimachia vulgaris L. | RU | runner | 0.10 (±0.32) | - | - | - |
| Maianthemum bifolium (L.) F. W. Schmidt | RL-RH | runner-like rhizome | - | 0.10 (±0.32) | - | - |
| Mercurialis perennis L. | RU | runner | 0.70 (±2.21) | 0.90 (±2.51) | - | - |
| Ornithogalum umbellatum L. | BU | bulb | - | - | - | 0.19 (±0.51) |
| Oxalis stricta L. | RU | runner | 0.20 (±0.42) | - | 0.30 (±0.67) | 0.10 (±0.32) |
| Polygonatum odoratum (Mill.) Druce | RH | rhizome | 0.50 (±1.58) | 0.30 (±0.95) | - | - |
| Pteridium aquilinum (L.) Kuhn | RH | rhizome | 0.10 (±0.32) | 0.20 (±0.63) | - | - |
| Ranunculus ficaria L. | RT | root tuber | 5.70 (±4.27) | 4.20 (±3.94) | 5.90 (±3.11) | 5.60 (±3.37) |
| Underground Storage Organ | Forests | Parks | ||
|---|---|---|---|---|
| CL | FU | CL | FU | |
| Bulb | 0.03 (±0.17) | 0.10 (±0.36) | 0.22 (±0.42) | 0.23 (±0.42) |
| Hypocotyl bulb | 0.06 (±0.24) | 0.01 (±0.10) | 0.02 (±0.14) | 0.04 (±0.20) |
| Rhizome | 0.41 (±0.65) a | 0.47 (±0.64) a | 0.06 (±0.24) b | 0.10 (±0.33) b |
| Runner | 0.22 (±0.50) a | 0.18 (±0.44) b | 0.31 (±0.46) b | 0.31 (±0.46) b |
| Runner and rhizome | 0.13 (±0.34) a | 0.09 (±0.29) a | 0.04 (±0.20) b | 0.03 (±0.17) b |
| Runner-like rhizome | 0.00 (±0.00) | 0.00 (±0.10) | 0.05 (±0.22) | 0.00 (±0.00) |
| Runner and runner with tuberous tip | 0.16 (±0.37) a | 0.12 (±0.33) a | 0.00 (±0.00) b | 0.00 (±0.00) b |
| Runner and shoot tuber | 0.00 (±0.00) | 0.00 (±0.00) | 0.01 (±0.10) | 0.00 (±0.00) |
| Root tuber | 0.57 (±0.50) | 0.42 (±0.50) | 0.59 (±0.49) | 0.56 (±0.50) |
| Underground Storage Organ | Forests | Parks | ||
|---|---|---|---|---|
| CL | FU | CL | FU | |
| Bulb | 0.39 (±2.31) a | 1.99 (±7.35) a | 4.07 (±8.39) b | 3.61 (±7.27) b |
| Hypocotyl bulb | 0.77 (±3.12) | 0.17 (±1.67) | 0.23 (±1.69) | 0.42 (±2.24) |
| Rhizome | 6.07 (±9.63) a | 7.98 (±10.71) a | 1.19 (±5.22) b | 1.71 (±6.04) b |
| Runner | 3.26 (±7.36) | 2.89 (±6.92) | 3.12 (±4.96) | 3.39 (±5.47) |
| Runner and rhizome | 2.48 (±6.80) a | 1.87 (±6.12) a | 0.45 (±2.21) b | 0.32 (±1.84) b |
| Runner-like rhizome | 0.00 (±0.00) | 0.11 (±1.11) | 0.57 (±2.57) | 0.00 (±0.00) |
| Runner and runner with tuberous tip | 2.55 (±6.26) a | 2.16 (±6.06) a | 0.00 (±0.00) b | 0.00 (±0.00) b |
| Runner and shoot tuber | 0.00 (±0.00) | 0.00 (±0.00) | 0.14 (±1.43) | 0.00 (±0.00) |
| Root tuber | 14.82 (±20.22) a | 11.94 (±19.26) a | 9.57 (±10.06) b | 7.60 (±7.98) b |
| Underground Storage Organ | Forests | Parks | ||
|---|---|---|---|---|
| CL | FU | CL | FU | |
| Bulb | 0.01 (±0.03) a | 0.02 (±0.11) a | 0.10 (±0.35) b | 0.15 (±0.47) b |
| Hypocotyl bulb | 0.01 (±0.03) | 0.01 (±0.10) | 0.00 (±0.01) | 0.02 (±0.20) |
| Rhizome | 0.62 (±1.10) a | 0.84 (±1.30) a | 0.02 (±0.14) b | 0.10 (±0.39) b |
| Runner | 0.10 (±0.38) | 0.18 (±0.57) | 0.05 (±0.14) | 0.14 (±0.43) |
| Runner and rhizome | 0.14 (±0.40) a | 0.14 (±0.47) a | 0.02 (±0.14) b | 0.01 (±0.10) b |
| Runner-like rhizome | 0.00 (±0.00) | 0.00 (±0.01) | 0.02 (±0.20) | 0.00 (±0.00) |
| Runner and runner with tuberous tip | 0.04 (±0.22) | 0.01 (±0.03) | 0.00 (±0.00) | 0.00 (±0.00) |
| Runner and shoot tuber | 0.00 (±0.00) | 0.00 (±0.00) | 0.00 (±0.01) | 0.00 (±0.00) |
| Root tuber | 1.28 (±1.47) a | 1.13 (±1.66) a | 0.75 (±1.01) b | 1.00 (±1.27) b |
| LI | SEC | PD | HTS | TPC | TNS | SR | N-NO3 | N-NH4 | P | K | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Forests | CL | RH | −0.23 | −0.08 | 0.27 | 0.14 | 0.21 | 0.39 | −0.42 | −0.50 | 0.11 | −0.59 | −0.43 |
| RT | 0.09 | 0.30 | 0.17 | −0.26 | 0.20 | −0.29 | 0.30 | 0.21 | −0.30 | 0.39 | 0.01 | ||
| FU | RH | −0.12 | −0.20 | 0.13 | −0.01 | 0.17 | 0.48 | −0.42 | 0.50 | 0.28 | −0.43 | 0.15 | |
| RT | 0.28 | 0.18 | 0.36 | −0.32 | 0.48 | −0.08 | 0.32 | −0.25 | −0.29 | 0.18 | −0.02 | ||
| Parks | CL | RH | −0.13 | 0.13 | 0.40 | −0.19 | −0.06 | −0.09 | 0.17 | 0.21 | −0.04 | −0.29 | −0.17 |
| RT | 0.23 | 0.03 | 0.19 | −0.33 | −0.07 | −0.21 | −0.21 | 0.43 | −0.39 | −0.01 | 0.29 | ||
| FU | RH | −0.15 | 0.13 | 0.24 | −0.20 | 0.06 | 0.01 | −0.27 | 0.24 | −0.02 | −0.16 | −0.48 | |
| RT | 0.17 | 0.29 | 0.20 | −0.33 | 0.15 | 0.04 | −0.32 | 0.36 | −0.52 | 0.01 | −0.31 | ||
| LI | SEC | PD | HTS | TPC | TNS | SR | N-NO3 | N-NH4 | P | K | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Forests | CL | RH | −0.23 | 0.00 | 0.25 | 0.16 | 0.19 | 0.21 | −0.49 | −0.54 | 0.16 | −0.60 | −0.43 |
| RT | 0.16 | 0.12 | 0.04 | −0.38 | 0.22 | −0.61 | 0.30 | 0.14 | −0.31 | 0.53 | 0.04 | ||
| FU | RH | −0.13 | −0.28 | 0.05 | −0.09 | 0.17 | 0.34 | −0.40 | 0.35 | 0.14 | −0.50 | 0.02 | |
| RT | 0.24 | 0.09 | 0.40 | −0.40 | 0.14 | −0.30 | 0.38 | −0.24 | −0.33 | 0.22 | −0.05 | ||
| Parks | CL | RH | −0.10 | 0.15 | 0.53 | −0.17 | −0.01 | −0.20 | 0.17 | 0.22 | −0.04 | −0.31 | −0.19 |
| RT | 0.30 | 0.03 | 0.24 | −0.42 | −0.02 | −0.53 | −0.17 | 0.45 | −0.35 | −0.14 | 0.22 | ||
| FU | RH | −0.16 | 0.09 | 0.14 | −0.26 | −0.04 | −0.20 | −0.29 | 0.22 | −0.05 | −0.14 | −0.48 | |
| RT | 0.30 | 0.20 | 0.21 | −0.39 | 0.14 | −0.30 | −0.46 | 0.27 | −0.43 | 0.08 | −0.33 | ||
| LI | SEC | PD | HTS | TPC | TNS | SR | N-NO3 | N-NH4 | P | K | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Forests | CL | RH | −0.23 | −0.04 | 0.23 | 0.20 | 0.30 | 0.30 | −0.45 | −0.52 | 0.22 | −0.63 | −0.27 |
| RT | −0.02 | 0.28 | 0.23 | −0.31 | 0.58 | −0.36 | 0.37 | 0.12 | −0.40 | 0.34 | −0.24 | ||
| FU | RH | −0.14 | −0.25 | 0.05 | −0.05 | 0.24 | 0.29 | −0.47 | 0.39 | 0.39 | −0.20 | 0.27 | |
| RT | 0.21 | 0.13 | 0.40 | −0.27 | 0.71 | −0.26 | 0.36 | −0.24 | −0.32 | 0.15 | −0.02 | ||
| Parks | CL | RH | −0.09 | 0.07 | 0.44 | −0.12 | −0.03 | −0.17 | 0.14 | 0.18 | −0.01 | −0.27 | −0.17 |
| RT | 0.31 | 0.02 | 0.30 | −0.46 | 0.15 | −0.38 | −0.22 | 0.49 | −0.40 | 0.20 | −0.13 | ||
| FU | RH | −0.14 | 0.16 | 0.17 | −0.14 | 0.14 | 0.00 | −0.24 | 0.25 | −0.01 | −0.17 | −0.48 | |
| RT | 0.28 | 0.20 | 0.25 | −0.39 | 0.32 | −0.20 | −0.41 | 0.30 | −0.44 | 0.05 | −0.29 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostrakiewicz-Gierałt, K.; Gmyrek, K.; Pliszko, A. Does the Distance from the Formal Path Affect the Richness, Abundance and Diversity of Geophytes in Urban Forests and Parks? Forests 2023, 14, 2272. https://doi.org/10.3390/f14112272
Kostrakiewicz-Gierałt K, Gmyrek K, Pliszko A. Does the Distance from the Formal Path Affect the Richness, Abundance and Diversity of Geophytes in Urban Forests and Parks? Forests. 2023; 14(11):2272. https://doi.org/10.3390/f14112272
Chicago/Turabian StyleKostrakiewicz-Gierałt, Kinga, Katarzyna Gmyrek, and Artur Pliszko. 2023. "Does the Distance from the Formal Path Affect the Richness, Abundance and Diversity of Geophytes in Urban Forests and Parks?" Forests 14, no. 11: 2272. https://doi.org/10.3390/f14112272
APA StyleKostrakiewicz-Gierałt, K., Gmyrek, K., & Pliszko, A. (2023). Does the Distance from the Formal Path Affect the Richness, Abundance and Diversity of Geophytes in Urban Forests and Parks? Forests, 14(11), 2272. https://doi.org/10.3390/f14112272






