Abstract
Bamboo propagation and seed collection are severely limited by a protracted and unpredictable flowering cycle. Dendrocalamus hamiltonii Nees et Arn. ex Munro was renowned for its delectable, bitter-free bamboo shoots, making it an exceptional choice for culinary purposes, which has significant economic value in China. To date, no fruit has been collected, and there are currently no comprehensive studies available on the floral morphology and embryology of D. hamiltonii. The morphological and anatomical characteristics of floral organs were described, and the developmental process of male and female gametophytes was elucidated, through anatomical observation. It was found that the floret of D. hamiltonii was composed of one lemma, palea, pistil, and six stamens, but lacked lodicules. The single ovule, possessing a parietal placenta, was anatropous and featured a double integument. The floral organs showed normal in external morphology, whereas male and female gametophyte development was abnormal, such as pollen grain shrinkage, hollow anther chambers, and underdeveloped ovules, which might be the important factors of its low seed setting rate. This study provided important information on the developmental stages of D. hamiltonii flowers and offered a theoretical basis for understanding the low seed setting rate of bamboo.
1. Introduction
Bamboos (Bambusoideae), as herbaceous plants characterized by rapid growth and high productivity, represent a significant non-wood forest resource. Approximately 14 million hectares of the earth’s surface are bamboo-covered, with 80% of this occurring in Asia [1]. China, home to one of the most substantial bamboo resources, boasts over 800 distinct species distributed across 43 genera. Bamboo forests in China, spanning approximately 5.4 million hectares, account for approximately one-third of global bamboo germplasm resources [2].
The consumption of bamboo shoots, which are rich in various vitamins, amino acids, and other essential nutrients, is beneficial for both humans and animals. Freshly collected bamboo shoots contain high concentrations of thiamine, niacin, vitamin A, vitamin B6, and vitamin E, as well as amino acids, including tyrosine, arginine, histidine, and leucine. Tyrosine serves as the primary precursor of adrenaline and is crucial for metabolic activities within the body. Moreover, bamboo shoots are abundant in essential minerals, such as potassium, phosphorus, sodium, calcium, magnesium, and iron, which are vital for maintaining proper metabolic function [3]. Furthermore, bamboo has applications in various fields, including biofuel production, construction, and soil conservation [4].
As perennial plants, most bamboo species have a long seeding cycle and sporadic, unsynchronized, and unpredictable flowering dates [5,6]. According to previous studies, the reproductive cycle of bamboo is protracted, ranging from 3 to 120 years [5]. Depending on their flowering cycle, bamboos can be categorized into three major groups: annually flowering bamboos (e.g., Indocalamus wightianus and Ochlandra sp.), sporadically or irregularly flowering bamboos (e.g., Chimonobambusa sp. and D. hamiltonii), and synchronously flowering bamboos (e.g., Bambusa bambos, Bambusa tulda, Dendrocalamus strictus, and Thamnocalamus spathiflora) [1]. During synchronous flowering, all members of a common cohort (plants derived from seeds of common origin) enter the reproductive phase simultaneously and subsequently perish [1]. Due to the idiosyncratic and unpredictable nature of bamboo flowering, it is uncommon [7], severely constricting seed collection and reproduction.
Bamboo reproduction is based mainly on traditional methods such as burying whips, stalks, or nodes [8]. Due to its long-term asexual reproduction, bamboo exhibits limited genetic diversity. Consequently, employing conventional breeding methods to alter traits for the development of new varieties is a considerable challenge [9]. Historically, bamboo taxonomy has been based on vegetative morphological characteristics, such as stalk or sheath. Variations in bamboo stems, leaves, or rhizomes may occur during different developmental stages or environmental conditions; thus, establishing a distinct taxonomic boundary and methodology solely based on morphology is problematic. Furthermore, ambiguous taxonomic classifications based on morphological characteristics have been observed in newly identified species, including homonyms and synonyms [10]. The floral characteristics of most plants serve as the basis for identification keys, rendering characterization and identification using these characteristics invaluable.
The investigation of the morphology, anatomy, and systematic classification of bamboo plants based on their reproductive organs has faced considerable challenges, particularly in the field of embryology. Consequently, an in-depth examination of the reproductive characteristics of bamboo is required. The discovery of new flowering bamboo species, coupled with morphological and anatomical studies of the flower organs of bamboo plants in China, has significantly advanced our understanding of bamboo flower organs. Moreover, successive embryological descriptions of bamboo species have been undertaken, including those of Bambusa eutuldoides, Bambusa intermedia, Bambusa rigida, Bambusa rutila, Dendrocalamus sinicus, Fargesia fungosa, Menstruocalamus sichuanensis, Phyllostachys edulis, and Phyllostachys praecox [11,12,13,14,15,16,17,18,19]. Nonetheless, research concerning sexual reproduction in bamboo remains limited in comparison to other economically significant crops or trees.
Dendrocalamus hamiltonii is a tall, woody bamboo that is extensively cultivated in southern Yunnan of China, India, Myanmar, Nepal, Sikkim, Bhutan, and Laos for its applications in construction, handicrafts, and fuel. The shoots of this species can reach heights of 15–25 m and are known for their sweet flavor when consumed fresh, earning it the local name of “sweet bamboo” [20]. Dendrocalamus hamiltonii displays both sporadic and gregarious flowering patterns, with a ~30 years flowering cycle [21]. However, the precise mechanism behind the mass flowering and subsequent decline of D. hamiltonii remains elusive due to unknown natural clone ages [22]. According to Fan Du et al.’s records [23], D. hamiltonii becomes almost leafless during blooming, the entire cluster dies after 2–3 years of successive blooming, and no fruit production was observed throughout the stage. While extensive research has been conducted on the nutritional components of bamboo shoots, preservation techniques, and other related research [24,25], limited attention has been given to the embryo development of D. hamiltonii and its floral anatomy. The species confronts major challenges in its natural and conventional propagation due to its long flowering cycle, short seed viability, seed sterility, and irregular or low seed production [26]. To date, the embryology of D. hamiltonii has not been investigated previously.
In this investigation, the spikelets and florets of D. hamiltonii at various developmental stages were scrutinized to (1) observe the fundamental floral architecture and the development of male and female gametophytes in D. hamiltonii and (2) reveal the underlying factors contributing to the low seed setting rate of D. hamiltonii. This study supplied foundational insights into the pattern of embryonic development, documenting the sequential progression of anther wall formation, microspore development, and male gametogenesis in D. hamiltonii, thus establishing a scientific basis for breeding strategies in this species.
2. Materials and Methods
2.1. Sample Collection
The spikelets of D. hamiltonii were gathered from the bamboo grove in Mengla County, Xishuangbanna City, Yunnan Province, China (100.80°, 22.00°) in 2015. All spikelets from various developmental stages were fixed in FAA fixative (50% ethanol/40% formaldehyde/glacial acetic acid = 18:1:1) and pumped with a vacuum pump for 8 h. No fruits were collected on-site.
2.2. Sample Measurement and Slice Preparation
Fifteen spikelets were randomly selected, and each floret within a spikelet was dissected for measurement. Floret images and dimensional measurements of its respective components were acquired utilizing an LEICA S8AP0 (Leica, Wetzlar, Germany) stereomicroscope. The paraffin section experiments were conducted in accordance with the methodologies detailed by Zhengli Li [27].
All spikelets were taken out of FAA fixative and then were dehydrated in a graded series of alcohol (begins at 50%) and technology of paraffin section was used. Transverse sections (7 μm) were cut using a rotary microtome and double stained with 1% alcoholic Safranin O (Solarbio, Beijing, China) (in 50% ethanol), distilled water, and 1% Fast green (Solarbio, Beijing, China) and dehydrated in a graded series of ethanol. The sections were permanently mounted in Canadian balsam. The sections were observed and captured via a video camera linked to a lightmicroscope (PH100-3B41L-IPL, Phenix, Shangrao, China) and a Lenovo computer. The structure of each floret was observed with an LEICA S8AP0 anatomical lens and images were taken by using the LY-WN-HPCCD 50 Imaging systems (Liyang, Sichuan, China), and measurements were taken employing Toup View 3.7 (ToupTek Photonic, Hangzhou, China) measurement software. A total of three to five replicates were performed for each experiment.
3. Results
3.1. Inflorescence and Morphology
The spikelets of D. hamiltonii emerged from the branch nodes (Figure 1A,B), accompanied by two bracts at the base of the spikelets (Figure 1C). Each spikelet possessed latent buds at its base, which subsequently developed into new spikelets (Figure 1D). Therefore, the spikelets of D. hamiltonii were classified as the ‘pseudo spikelet’ type [28]. The average lengths of spikelet and rachilla (Figure 1E) were recorded as 9.74 ± 0.51 mm and 0.85 ± 0.02 mm, respectively.

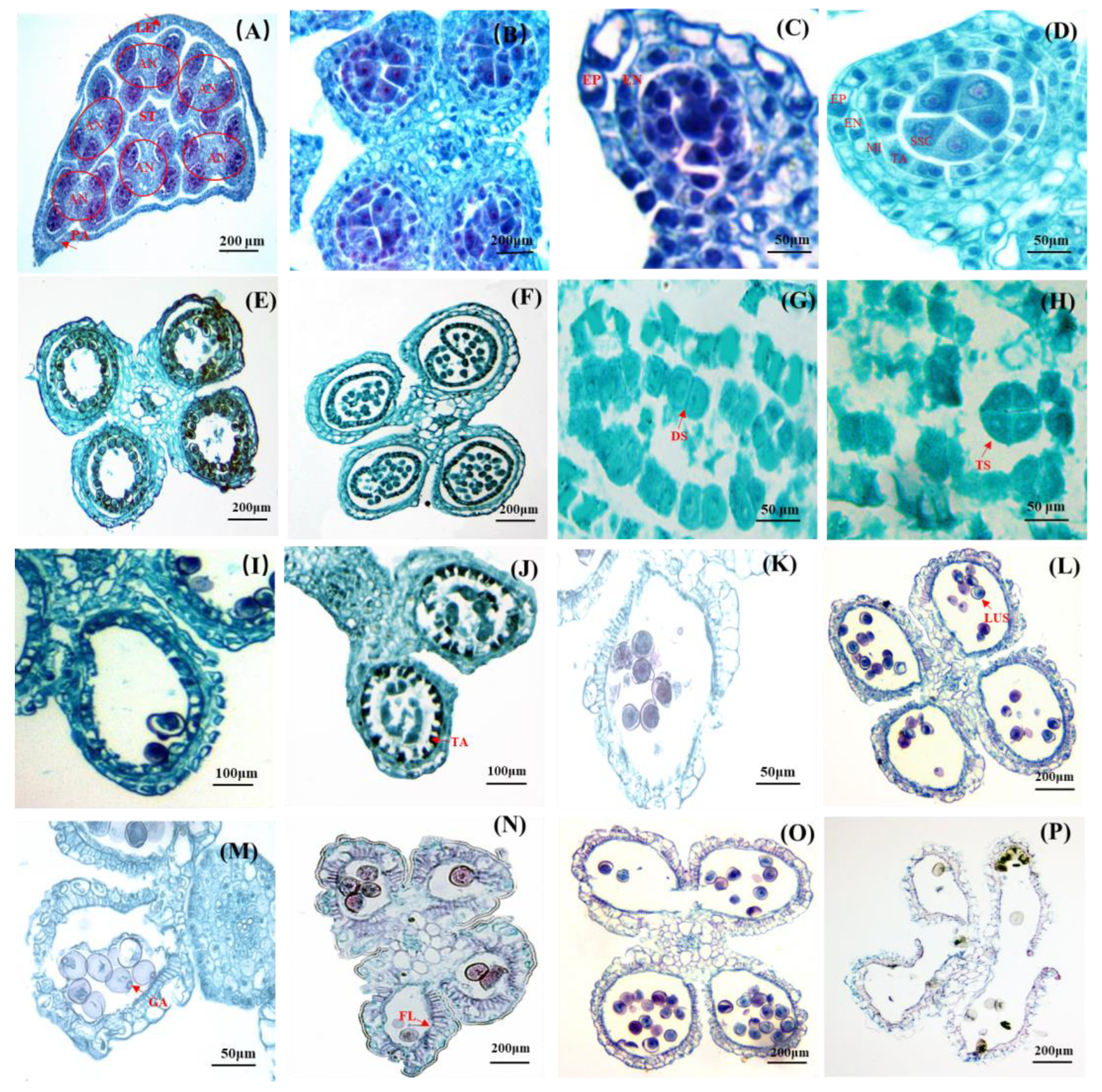
Figure 1.
Floret morphological anatomy of D. hamiltonii Nees et Arn. ex Munro. (A) Immature floret. (B) Blooming floret (The entire floral structure was positioned in the bottom left corner). (C) BR: Bracts. (D) LB: Latent bud. (E) RA: Rachilla. (F) Whole floret. (G) Lemma. (H) Palea. (I) Stamens. (J) Browning stamen. (K) Immature pistil. (L) Mature pistil, browning stigma.
The spikelets were composed of six to eight florets, each comprising a lemma, a palea, a pistil, and six stamens devoid of lodicules (Figure 1F). The lemma had an approximate length of 5.52 ± 0.32 mm, and displayed 9–12 longitudinal veins with a sparse distribution of cilia on the surface (Figure 1G). The palea displayed two ciliated ridges on its surface, accompanied by three to four veins on each side of the ridge (Figure 1H), with an average length of 5.67 ± 0.26 mm. The length of the palea was found to be greater than that of the lemma. Each floret contained six yellow stamens measuring approximately 2.70 ± 0.41 mm in length. The normal stamens exhibited light yellow with pointed tips (Figure 1I), and some exhibited browning (Figure 1J). The pistil measured approximately 4.07 ± 0.37 mm in length, and its stigma was solitary and unbranched in nature. Mature pistils displayed curled stigmas with denser and yellow cilia compared to immature ones, while also exhibiting slightly greater elongation in style and stigma than the features of immature pistils. The ovary surface exhibited small yellowish cilia on its juvenile stage (Figure 1K), which gradually darkened and elongated as it matured (Figure 1L).
3.2. Development of Male Gametophyte
The florets and anthers of D. hamiltonii were transversely sectioned. Each spikelet contained six anthers (Figure 2A), with each anther comprising four pollen sacs (Figure 2B). Two anther chambers were present on each side, which were separated by an anther septum. The cross-sectional shape of each chamber was oval, with six stamens around the pistil (Figure 2A). The number of anther wall layers varied with the developmental stage. During sporulation, the epidermal cells possessed regularly shaped rectangular, which were noticeably larger than those of the other cell layers. After reaching the microspore stage, the shape of epidermal cells became irregular and nearly devoid of nuclei, while the cuticle began to emerge. The archesporial cells underwent peripheral division, giving rise to primary parietal cells in the outer layer, and differentiating into primary sporogenous cells in the inner layer (Figure 2C). The outer layer of the primary parietal cell developed to form the inner wall of the chamber, while the inner layer developed into the middle layer and the conspicuous tapetum. Primary sporogenous cells were divided continuously to form secondary sporogenous cells, and the wall of the pollen sac consisted of four layers: the epidermis, endothecium, middle layer, and conspicuous tapetum (Figure 2D). The cells of the middle layer were rectangular, while the tapetum displayed remarkably dense cytoplasm (Figure 2D).
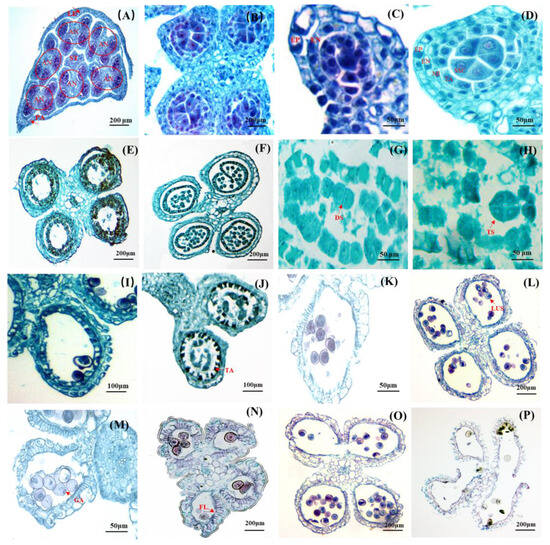
Figure 2.
Anatomical structure of D. hamiltonii anther. (A) The position of anthers and ovary in a floret, and a stigma surrounded by six anthers. (B) Four pollen sacs. (C) Stage of primary sporogenous cells. (D) Stage of secondary sporogenous cells. (E) Transformation from secondary cell to mature pollen grains. (F) Microsporocyte. (G) Dyad stage. (H) Tetrad stage. (I) The stage between the middle cells and the chamber wall (fibrous layer) was beginning to blur. (J) Tapetum degeneration. (K) systolic microspores. (L) Microspores with the nucleus located aside. (M) GA: Germinal aperture. (N) Anther maturation leaded to a significant thickening of the fibrous layer. (O) Mature anthers split into two adjacent chambers. (P) Pollen grains released. EN: endothecium; EP: epidermis; MI: middle layer; SSC: secondary sporogenous cell; TA: tapetum; LUS: late uninucleate stage; FL: fibrous layer; AN: anther; ST: stigma; LE: lemma; PA: palea.
With further development, the secondary sporogenous cells underwent differentiation into microsporocytes (Figure 2E,F). Subsequently, dyads were formed (Figure 2G), followed by tetrad formation (Figure 2H), and microspores were eventually formed. Meanwhile, the middle layer gradually diminished, and the demarcation between the inner walls of the chamber was indistinct (Figure 2I). After the completion of microsporocyte meiosis, the tapetum cells initiated their degeneration (Figure 2J). However, it was noteworthy that the tapetum cells maintained their original position throughout anther development; thereby indicating a glandular nature of the tapetum. The cytoplasm during the initial stage of microspore formation exhibited a relatively high density, characterized by prominent centrally located nuclei, which were referred to as “systolic” or “early” microspores (Figure 2K). No vacuoles were observed at this developmental stage. The nuclei of the early uninucleate microspore were positioned centrally. Subsequently, during the “late uninucleate stage” (Figure 2L), small vacuoles emerged within the cytoplasm, eventually coalescing into a single large vacuole that pushed the nuclei towards the periphery of the microspore. During this period, the tapetal cells underwent degeneration, resulting in the retention of only a thin layer in the final stage, accompanied by the observation of a circular germination aperture on the pollen wall (Figure 2M). The middle layer of the anther walls disappeared, and significant fibrosis occurred at the endothecium during anther maturation. The radial wall fibers thickened to form a fiber layer, which played a crucial role in the chamber cracking and successful pollen grains releasing. The anther septum between two adjacent pollen sacs was split to allow for the release of mature pollen (Figure 2O,P).
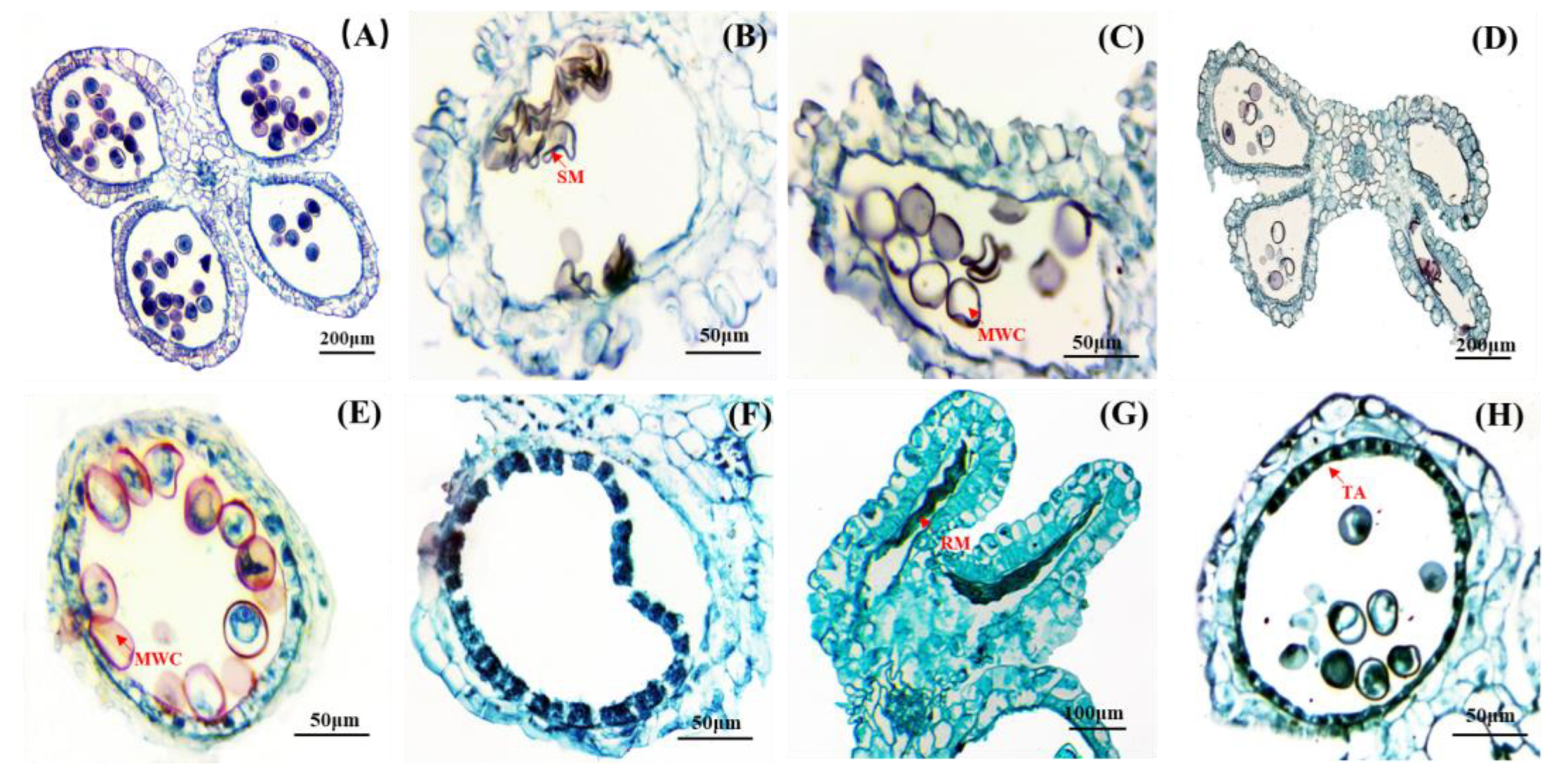
3.3. Abortions in Development of Stamens
The flowering and seed production of bamboo were rare occurrences, resulting in limited or negligible seed collection from the flowering clusters. During the developmental stage, various types of anther abortions were observed in D. hamiltonii: (1) In some anther chambers, pollen grains shrank and became deformed (Figure 3B). (2) In others, microspores with irregular shapes lost their nuclei and cytoplasm (Figure 3C). (3) Some anthers contracted without forming normal pollen grains and were emptied (Figure 3D). (4) A mixture of formed and empty pollen grains was observed within the same anther chamber (Figure 3E). (5) In some cases, no microsporocytes formed (Figure 3F). (6) Residual micropores were found in deformed chambers (Figure 3G). (7) After the maturation, the tapetum layer remained intact without dissolution occurring (Figure 3H).
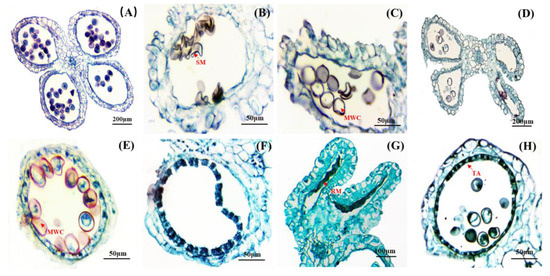
Figure 3.
Anatomical of the abortive anther. (A) The microspores were normal development. (B) Pollen grains were shrunken and deformed. (C) Pollen grains without nuclei and cytoplasm. (D) The anther chamber was without normal pollen grains or hollow. (E) Some pollen grains without contents. (F) Microsporocytes were not formed (G) Deformed anther with remnants of microspore. (H) The tapetum layer did not dissolve normally. SM: Shrunken microspore; RM: residual microspore; MWC: microspore without contents; TA: tapetum.
3.4. Development of Female Gametophyte
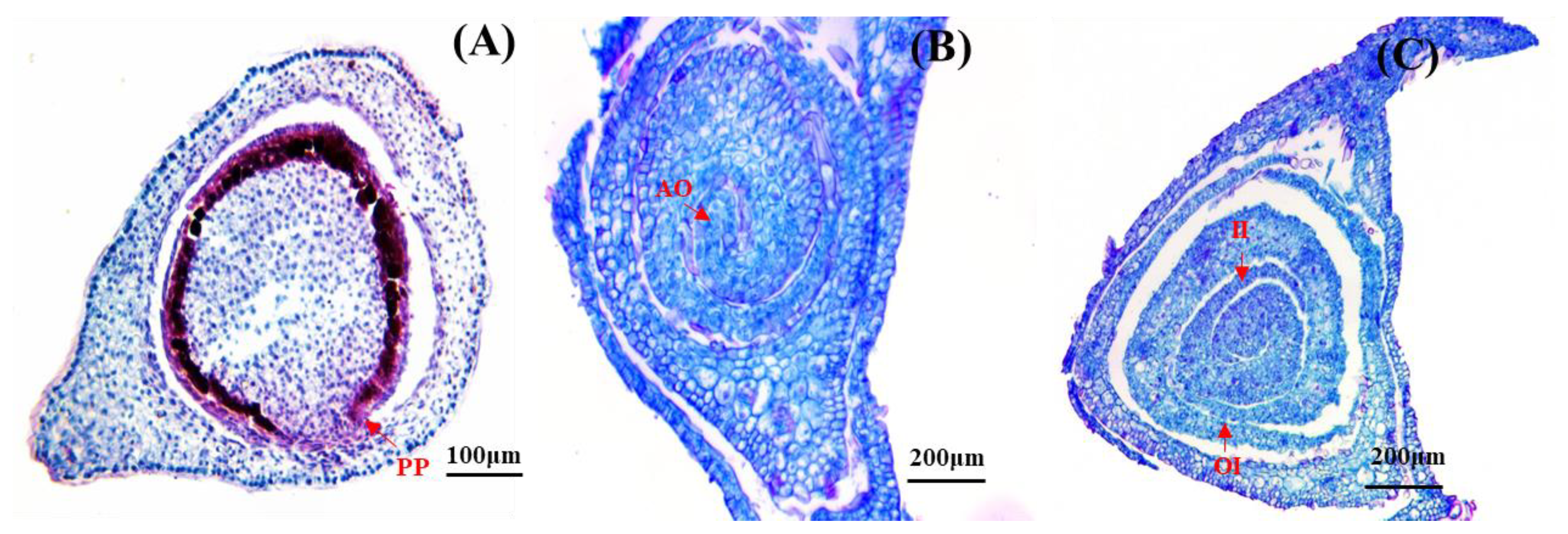
The placenta of D. hamiltonii was located on the inner wall of the ovary and exhibited parietal placentation (Figure 4A). It was unilocular with an anatropous, characterized by differential growth rates on opposite sides. The ovule underwent a reversal of 180° from the slower growing side, resulting in dual-integument ovules (Figure 4B,C). As they grew upwards, the integument enveloped and surrounded the nucleus. Archesporial cells were situated beneath a single layer of the nucellar epidermis and differentiated into megasporocytes in the following stages.
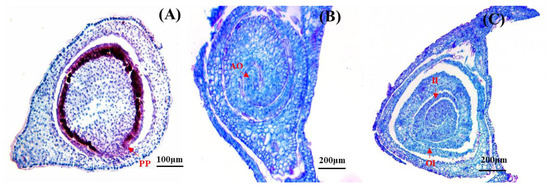
Figure 4.
Anatomical structure of D. hamiltonii ovary. (A) PP: Parietal placentation. (B) AO: Anatropous ovule. (C) Dual-integument, OI: outer integuments; II: inner integuments.
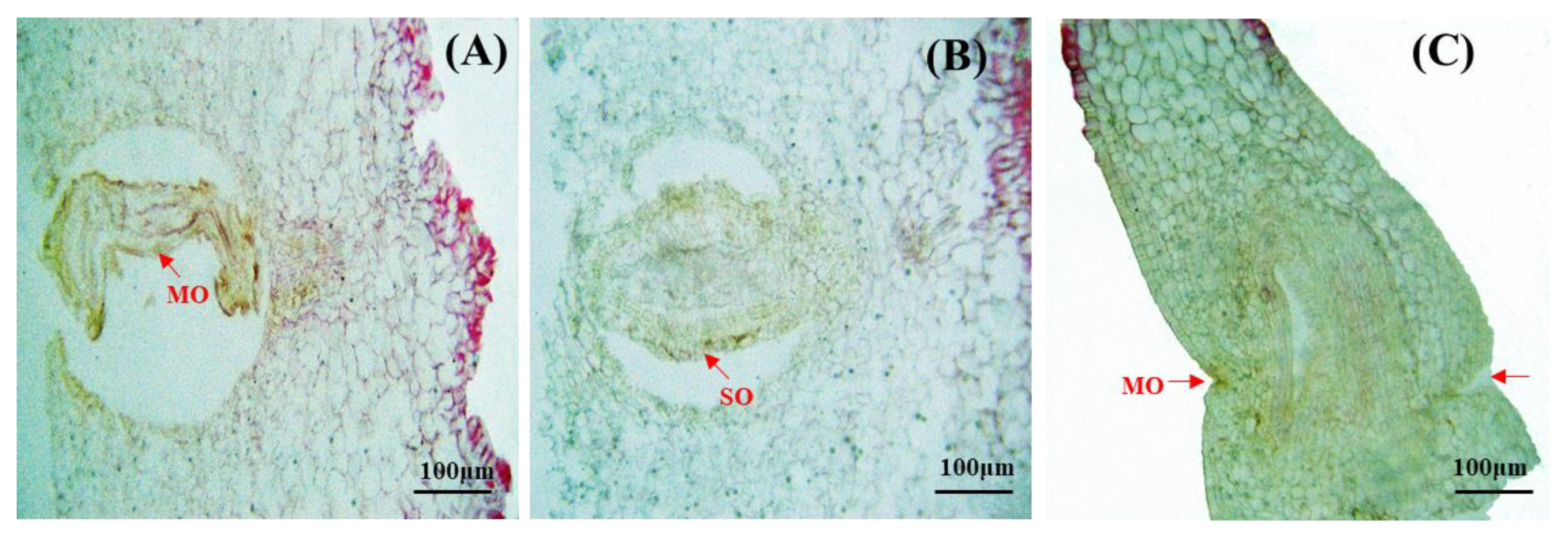
3.5. Abortions in Development of Pistils
Several aberrant ovules were observed in mature pistils during development. A normal ovary was shown in Figure 4B, and the aberrant ovules were completely maldeveloped, leading to the failure of the egg cells to fertilize, and subsequent abortion shown in Figure 5A. Notably, Ovule shrinking was observed (Figure 5B). Furthermore, impaired ovarian wall development resulted in ovule malformation and ultimately decreased fertility (Figure 5C).
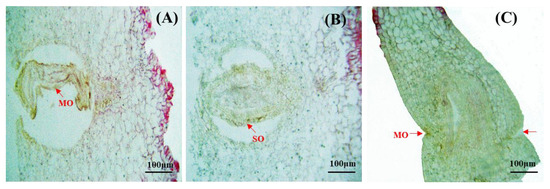
Figure 5.
Anatomy of abortive ovary. (A) MO: Maldeveloped ovule. (B) SO: Shrunken ovule. (C) MO: Malformed ovary.
4. Discussion
4.1. Morphological Characteristics
The morphological structures of inflorescence (spikelet, spikelet axis, spikelet stalk, floret, lemma, palea, slice, stamen, and pistil) exhibit significant variations among bamboo genera. Each genus demonstrates distinct characteristics closely associated with biological characteristics [29].
There were distinct variations in spikelet morphology and length among different bamboo species. The spikelet of D. hamiltonii was characterized by its compact and planar morphology, displaying densely arranged florets on both sides of the rachilla. This similar morphological pattern was also observed in Bambusa oldhami and D. sinicus [15,30]. Conversely, the bamboo species such as Chimonobambusa utilis and Sasaela kongosanensis [31,32] exhibited an alternative morphotype.
The morphological features of floral organs served as a crucial basis for the taxonomy of bamboo species [33]. Bamboo flowers typically exhibited bisexual traits, featuring three and six stamens. Notably, certain bamboo species such as P. praecox, C. utilis, and Shibataea chinensisa possessed precisely three stamens [19,31,33]. The number of stamens in D. hamiltonii was six, which was consistent with the stamen count observed in B. intermedia, Bambusa multiplex, and D. sinicus [12,15,34].
Each individual bamboo flower possessed a single ovary accompanied by a style, while the stigma was divided into two or three segments [33]. Dendrocalamus hamiltonii possessed a pistil characterized by densely packed cilia with an unlobed apex; however, within the same genus, Dendrocalamus latiflorus exhibited dense and distinctive white villi with a one-to-three-lobed apex [35]. Latent buds were observed at the base of D. hamiltonii spikelets, which had the potential to develop into new spikelets; hence, they were considered pseudo spikelets. According to Zhang et al. [36], bamboo flowers could be classified into long and short styles based on the length of extension stigmas. Considering the blooming of D. hamiltonii and the protrusion of its stigma beyond the palea, it could be categorized as a long-type pistil stigma for this species.
According to the floral structure and flowering characteristics, bamboo flowers could be divided into two types: One type was represented by Bambusa, the florals of which were composed of a lemma, palea, stamen, pistil, and lodicules. During flowering, lodicules absorbed water and expanded to open the lemma, stamens extended out, pistils were separated into three phases, and homogamy occurs. Thus, the timing of pollination could be determined easily. The other type was represented by Dendrocalamus, the florals of which had no lodicules, and the lemma was not open during flowering. The stamens and pistils did not extend simultaneously and were dichogamous; therefore, determining the timing of pollination in closed-flower species was difficult [6]. The present study found that D. hamiltonii florals had no lodicules, and therefore belonged to the second type.
4.2. Factors Influencing of Low Seed Setting Rate
Field observations revealed that D. hamiltonii exhibited sporadically flowering in its natural habitat, followed by a significantly low fruiting rate. No seeds or fruits were observed after the flowering period D. hamiltonii, thereby, resulting in the naturally occurring seedlings not being seen.
Several factors might have contributed to the subsequent flowering period low seed-setting rate of this species. In cases where the stigma protruded from the palea of D. hamiltonii, there was no simultaneous protrusion of the anther from the palea; thus indicating a typical diplogamy phenomenon. This suggested that achieving precise the timing of pollination might be challenging, which could potentially explain the low fruit-set rate observed in D. hamiltonii. The fusion of male and female gametes were necessary for seed production, while abnormal development led to reproductive failure [37].
During floral organ development in D. hamiltonii, pollen abortions were observed, characterized by shrunken and deformed pollen grains, irregularly shaped microspores in the anther chamber with loss of nuclei and cytoplasm, the contraction of the anther chamber preventing normal pollen grain formation, contentless pollen grains, and the abnormal dissolution of the tapetum layer.
The primary function of the tapetum was to provide nutrients and structural components for microspores [38]. During the later stages of anther development, the tapetum layer underwent degeneration and released the lipids or phenols needed for normal anther development [39]. Consequently, the delayed degradation of the tapetum would lead to empty shells or aberrant pollen grains formed from microspores within the anther chambers. Abnormal tapetum development had been reported in various bamboo species, including B. multiplex, Bambusa sinospinosa, Neosinocalamus affinis, S. chinensis [34,40,41], For D. hamiltonii, the tapetum layer did not dissolve normally and may be one of the causes of stamen abortion.
In this research, the contraction of the anther chamber preventing normal pollen grain formation and contentless pollen grains were observed in D. hamiltonii. Similarly, pollen grains deformation had been observed in B. eutuldoides, B. intermedia, B. rigida, Dendrocalamus giganteus, Drepanostachyum microphyllum, and S. chinensis [11,12,13,40,41,42]. Furthermore, hollow anthers had been observed in Neomicrocalamus prainii and Bambusa tuldoides [37,43]. Meanwhile, hollowed pollen grains without nuclei or cytoplasm had been found in B. rigida, F. fungosa, and Fargesia yuanjianggensis [13,16,44]. Shrunken anther chambers had also been observed in B. intermedia and N. prainii [12,37]. Therefore, the occurrence of pollen abortion was prevalent among bamboo plants, which may contribute to the low seed-setting rate of D. hamiltonii.
Several cases of pistil abortion were observed in D. hamiltonii during the present study, with several pistils containing maldeveloped ovules, shrunken ovules and ovaries. Abnormal pistil development had been observed in other bamboo species. For instance, B. multiplex [45] did not possess the structure of an ovule or experience ovule abortion within the ovary. S. chinensis [41] had shown abnormal embryo sac development, and B. tuldoides [43] showed identified ovule shrinkage. Thus, pistil abortion may significantly impact the seed-setting rate. Obviously, abnormalities in the pistil and stamen impeded successful seed formation.
Generally, bamboo possessed inherent fruit-bearing capabilities. Bamboo plants underwent anemophily pollination; however, the efficiency of wind-mediated pollination in these plants was typically lower compared to insect-pollinated plants [6]. The low seed setting rate of bamboo might be attributed to various other contributing factors. Under natural field conditions, pollen easily underwent desiccation and the subsequent loss of germination capacity due to exposure to sunlight or dry environments. Conversely, excessive water could lead to the swelling of bamboo pollen and protoplasm overflow, ultimately resulting in impaired germination ability. Consequently, bamboo pollen exhibiting these characteristics would inevitably show reduced or absent fruit production [33]. In cultivated bamboo species with a long history of artificial long-term nutritional reproductive intervention, sexual reproductive dysfunction or the gradual deterioration of sexual reproductive function had been observed. Consequently, the majority of cultivated bamboo species gradually lost their capacity for natural reproduction via sexual reproduction [6].
Additionally, nutrient elements might contribute to the low seed-setting rate of D. hamiltonii. Zhenguo Xu et al. [46] proposed that nutrient supply was one of the reasons for the flowering but sterility of D. latiflorus. After bamboo flowering, a significant nutrient consumption by the plant, resulted in flowering without fruiting. The low seed-setting rate of D. hamiltonii might be attributed to the dichogamy of pistil and stamen. Furthermore, the sporadic occurrence of flowering events and the limited effectiveness of wind pollination might account for the observed low fruiting rate in D. hamiltonii [17]. Jasmonic acid and its derivatives had been demonstrated to impact viable pollen production, with jasmonic acid exhibiting inhibitory effects on pollen formation. This observation might provide an explanation for the observed low rates of both pollen and seed setting [39,47]. The high expression of the jasmonic acid synthesis gene in the pistil could potentially account for the occurrence of D. latiflorus pollen abortion [47]. Given that JA level and the JA biosynthetic pathway played a crucial role in rice pollen fertility [39]. Therefore, it was plausible to suggest that jasmonic acid contributes to reduced seed setting rates.
In conclusion, the low seed-setting rate of D. hamiltonii might be primarily attributed to the abortion of pistil and stamen. However, future investigations should incorporate additional factors to validate this hypothesis. It was worth noting that incomplete collection of spikelets at various developmental stages in the paraffin section limited the observation of certain periods in female and male gametophyte development.
5. Conclusions
According to our observations, no seeds could be produced from D. hamiltonii. It was found that the flower of D. hamiltonii included two bracts at the base of each spikelet and latent buds capable of developing into new spikelets. Each floret was composed of one lemma, palea, and pistil, and six stamens, but lacked lodicules. The anther wall consisted of four layers, i.e., epidermis, endothecium, middle layer, and tapetum. The tapetum was glandular. The single ovule, possessing a parietal placenta, was anatropous and featured a double integument. Abortions of various types were observed in both anthers and ovaries, such as pollen grain shrinkage, hollow anther chambers, and underdeveloped ovules. Consequently, anther and ovary abortion could be the primary factor contributing to the low seed-setting rate of D. hamiltonii. This study offered a theoretical basis for understanding the low seed-setting rate of bamboo and enriched the research of bamboo embryo development. Further in-depth research will be conducted based on a continuous collection of D. hamiltonii flowers from various developmental stages.
Author Contributions
D.Y. co-designed the experiments and wrote the paper; S.W. supervised the research; H.Z., L.Y. and Y.M. performed the experiments; and J.L. designed the experiments and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was jointly supported by the Natural Science Foundation of the Yunnan Province (202001AT070133; NO. 202001AT070108), the National Natural Science Foundation of China (No. 31800506), the National Key R&D Program of China (2021YFD2200503-4), and the Yunnan Province Xingdian Talents Support Plan of 2022 (XDYC-QNRC-2022-0229).
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yeasmin, L.; Ali, M.N.; Gantait, S.; Chakraborty, S. Bamboo: An overview on its genetic diversity and characterization. 3 Biotech 2015, 5, 1–11. [Google Scholar] [CrossRef]
- Dlamini, L.C.; Fakudze, S.; Makombe, G.G.; Muse, S.; Zhu, J. Bamboo as a valuable resource and its utilization in historical and modern-day china. BioResources 2022, 17, 1926–1938. [Google Scholar] [CrossRef]
- Nongdam, P.; Tikendra, L. The nutritional facts of bamboo shoots and their usage as important traditional foods of northeast India. Int. Sch. Res. Not. 2014, 2014, 679073. [Google Scholar] [CrossRef] [PubMed]
- Emamverdian, A.; Ding, Y.L.; Ranaei, F.; Ahmad, Z.; Ren, H. Application of bamboo plants in nine aspects. Sci. World J. 2020, 2020, 7284203. [Google Scholar] [CrossRef]
- Janzen, D.H. Why bamboos wait so long to flower. Ann. Rev. Ecol. Syst. 1976, 7, 347–391. [Google Scholar] [CrossRef]
- Lin, S.Y.; Shi, W.W.; Miu, B.B.; Ding, Y.L. Research advances in reproduction biology of bamboos. J. Bamboo Ratt. 2018, 8, 1–6. [Google Scholar]
- Yi, T.P.; Shi, J.Y.; Ma, L.S.; Wang, H.T.; Yang, L. Iconographia Bambusoidearum Sinicarum; Science Press: Beijing, China, 2008. [Google Scholar]
- Li, R.; Zeng, B.S.; He, G.F.; Guo, Q.R. Research Advance and trend of bamboo tissue culture. J. Anhui Agric. Sci. 2008, 36, 4405–4407. [Google Scholar]
- Chen, G.C.; Ma, N.X. Advances in studies on genetics and breeding of bamboos. Rore. Res. 2005, 18, 749–754. [Google Scholar]
- Zhao, H.S.; Yang, L.; Peng, Z.H.; Sun, H.Y.; Yue, X.H.; Lou, Y.F.; Dong, L.L.; Wang, L.L.; Gao, Z.M. Developing genome-wide microsatellite markers of bamboo and their applications on molecular marker assisted taxonomy for accessions in the genus Phyllostachys. Sci. Rep. 2015, 5, 8018–8028. [Google Scholar] [CrossRef]
- Tang, G.J.; Yang, J.M.; Ding, Y.L.; Zhan, H.; Zhao, J.W.; Wang, Y.J.; Wang, S.G. Studies on the flower morphology and structure in Bambusa eutuldoides McClure var. viridi-vittata (W.T.Lin) Chia. J. Nanjing For. Univ. 2016, 40, 71–75. [Google Scholar]
- Wang, Y.J.; Luo, J.; Chen, N.N.; Lin, S.Y.; Ding, Y.L.; Wang, S.G. Floral morphology and development of female and male gametophyte of Bambusa intermedia Hsueh et Yi. Bull. Bot. Res. 2017, 37, 492–498. [Google Scholar]
- Li, J.; Wang, Y.F.; Chu, C.H.; Zhan, H.; Zhang, Y.G.; Wang, S.G. Studies on flower morphology and structure of Bambusa rigida. Fore. Res. 2020, 33, 28–34. [Google Scholar]
- Yang, N.; Cui, Y.J.; Wang, Q.; Wang, S.G. A study on the morphology and anatomical structure of Bambusa rutila spiklets. J. Nanjing For. Univ. 2021, 45, 90–96. [Google Scholar]
- Wang, S.G.; Pu, X.L.; Ding, Y.L. The structures of reproductive organs and development of the female and male gametophyte of Dendrocalamus sinicus. Bull. Bot. Res. 2006, 26, 270–274. [Google Scholar]
- Deng, L.; Huang, L.; Chu, C.H.; Wang, Q.; Zhan, H.; Wang, S.G. Study on the flower morphology and structure of Fargesia fungosa. Bull. Bot. Res. 2019, 39, 801–807. [Google Scholar]
- Lin, S.Y.; Hao, J.J.; Xin, H.; Ding, Y.L. The megasporogenesis, microsporogenesis and the development of their female and male gametophyte in Menstruocalamus sichuanensis. J. Nanjing For. Univ. 2009, 33, 9–12. [Google Scholar]
- Sun, L.F.; Guo, Q.R.; Wang, Q.; Feng, Y.; Mu, S.H. Flower organs morphology and structure of Phyllostachys edulis. Sci. Silvae Sin. 2012, 48, 124–129. [Google Scholar]
- Huang, J.Q.; Huang, H.H.; He, F.J.; Li, Z.J.; Pan, C.H. The formation of microspore and the development of male gametophyte of Phyllostachys praecox. J. Bam. Res. 1999, 18, 55–58. [Google Scholar]
- Keng, P.; Wang, Z.P. FLORA; Science Press: Beijing, China, 1996; Volume 9, p. 183. [Google Scholar]
- Das, M.C.; Singnar, P.; Nath, A.J.; Das, A.K. Flowering of Dendrocalamus hamiltonii in Northeast India during recent years. NeBio J. Environ. Biodivers. 2018, 9, 304–306. [Google Scholar]
- Kaur, D.; Dogra, V.; Thapa, P.; Bhattacharya, A.; Sood, A.; Sreenivasulu, Y. In vitro flowering associated protein changes in Dendrocalamus hamiltonii. Proteomics 2015, 15, 1291–1306. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Xue, J.R.; Yang, Y.M.; Hui, C.M.; Wang, J. Study on the bamboo flowering phenomenon and its types in Yunnan in the past fifteen years. Sci. Silvae Sin. 2000, 36, 57–68. [Google Scholar]
- Wang, Q.; Wang, S.G.; Deng, L.; He, W.Z.; Wang, C.M.; Zhan, H. Nutrient Analysis of Dendrocalamus hamiltonii shoots from different provenances. J. Southwest For. Univ. 2017, 37, 188–193. [Google Scholar]
- Tan, H.C.; Yang, J.M. A study of quality and preservation technology of fresh Dendrocalamus hamitonii shoots. World Bamboo Ratt. 2018, 16, 25–27. [Google Scholar]
- Singh, S.R.; Dalal, S.; Singh, R.; Dhawan, A.K.; Kalia, R.K. Ascertaining clonal fidelity of micropropagated plants of Dendrocalamus hamiltonii Nees et Arn. ex Munro using molecular markers. Vitr. Cell. Dev. Biol.-Plant 2013, 49, 572–583. [Google Scholar] [CrossRef]
- Li, Z.L. Plant Tissue Production; Peking University Press: Beijing, China, 1996; pp. 15–50. [Google Scholar]
- Keng, P. A preliminary study of the inflorescence type arising from bamboos and its variation. J. Wuhan Bot. Res. 1986, 4, 323–336. [Google Scholar]
- Lin, S.Y.; Wan, Y.W.; Fu, H.J.; Zhang, L.; Jing, M.Y.; Yin, Z.F.; Ding, Y.L. Research on inflorescence establishment and revision of inflorescence type in bamboo plants. J. Nanjing For. Univ. 2018, 42, 1–6. [Google Scholar]
- Lin, S.Y.; Fu, H.J.; Wan, Y.W.; Zhang, S.X.; Zhu, R.J.; Wang, F.S.; Ding, Y.L. Anther development and floral morphology characteristics of Bambusa oldhami‘Xia Zao’ZSX. J. Nanjing For. Univ. 2019, 43, 7–13. [Google Scholar]
- Yang, M.; Zhang, Y.; Ding, Y.L.; Yao, W.J.; Lin, S.Y. Flowering characteristics and floral organ development characteristics of Chimonobambusa utilis. J. Northeast For. Univ. 2022, 50, 7–13. [Google Scholar]
- Yao, W.J.; Jiang, M.Y.; Wang, X.; Shi, W.S.; Ding, Y.L.; Lin, S.Y. Biological analysis of flowering and pollen germination in Sasaella kongosanensis ‘Aureostriatus’. J. Northeast. For. Univ. 2020, 48, 13–18. [Google Scholar]
- Lin, S.Y. Studies on the Reproductive Biology of Shibataea chinensis and Arundinaria simonii f. albostriatus. Ph.D. Thesis, Nanjing Forestry University, Nanjing, China, 2009. [Google Scholar]
- Lin, S.Y.; Li, J.; Zhao, R.; Dong, X.B.; Ding, Y.L. The development of flowering bud differentiation and male gametophyte of Bambusa multiplex. J. Nanjing For. Univ. 2015, 39, 51–56. [Google Scholar]
- Zhong, Y.B.; Yue, J.J.; Lou, C.; Yuan, J.L.; Gu, X.P. Floral organ and breeding system of Dendrocalamus latiflorus. Sci. Silvae Sin. 2017, 53, 1–10. [Google Scholar]
- Zhang, W.Y.; Ma, N.X. Vitality of bamboo pollens and natural pollination in bamboo plants. For. Res. 1990, 3, 250–255. [Google Scholar]
- Chu, C.H.; Huang, L.; Wang, S.G. Floral morphology and development of female and male gametophytes of Neomicrocalamus prainii. Acta Bot. Boreal.-Occident. Sin. 2019, 39, 763–769. [Google Scholar]
- Zhang, Y.T.; Yang, H.D.; Chenzhu, X.Z. Advances on the study of tapetum. Chin. Bull. Bot. 1996, 13, 6–13. [Google Scholar]
- He, Y.; Liu, C.; Zhu, L.; Fu, M.; Sun, Y.; Zeng, H. Jasmonic acid plays a pivotal role in pollen development and fertility regulation in different types of P(T)GMS rice lines. Int. J. Mol. Sci. 2021, 22, 7926. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.G.; Pu, X.L.; Lin, S.Y.; Ding, Y.L. Reproductive characteristics of three bamboo species. Pak. J. Bot. 2015, 47, 2301–2308. [Google Scholar]
- Lin, S.Y.; Ding, Y.L. Development of the male and female gametophytes in Shibataea chinensis (Bambusoideae). Acta Bot. Boreal.-Occident. Sin. 2012, 32, 907–914. [Google Scholar]
- Pang, Y.J.; Yu, F.G.; Hu, C.H.; Chen, S.L. Preliminary observation on abnormal development of the stamens of Drepanostachyum microphyllum. J. Bamboo Res. 1994, 13, 42–46. [Google Scholar]
- Long, H.; Chu, C.H.; Jin, D.K.; Lv, Z.; Wang, S.G. Anatomical observation and analysis on floral of Bamnusa tuldoides. Cuihaia 2022, 42, 174–182. [Google Scholar]
- Hua, L.; Deng, L.; Chu, C.H.; Zhan, H.; Wang, S.G. Morphological and anatomical oberservations of floral organs and sterility analysis of Fargesia yuanjianggensis. Sci. Silvae Sin. 2020, 56, 64–73. [Google Scholar]
- Lin, S.Y.; Shao, L.J.; Li, J.; Zhang, L.; Ding, Y.L. Studies on development of megasporogenesis and female gametophyte of Bambusa multiplex. J. Trop. Subtrop. Bot. 2018, 26, 278–284. [Google Scholar]
- Xu, Z.G.; Liang, X.J.; Liang, M.H.; Huang, D.Y.; Luo, S.X. Study on the flowering sterility development process and fertility regulation of Dendrocalamus latiflorus. Non-Wood For. Res. 2018, 36, 89–93. [Google Scholar]
- Yang, D.; Yang, J.; Wan, J.; Xu, Y.; Li, L.; Rong, J.; Chen, L.; He, T.; Zheng, Y. Genome-Wide identification of MIKCc-Type MADS-Box family gene and floral organ transcriptome characterization in Ma Bamboo (Dendrocalamus latiflorus Munro). Genes 2023, 14, 78. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).