Expression and Functional Analysis of the PaPIP1-2 Gene during Dormancy and Germination Periods of Kernel-Using Apricot (Prunus armeniaca L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Recognition of PIP Subfamily Components in P. armeniaca by Phylogenetic Analyses
2.2. Analysis of Structural Features of Putative PaPIPs
2.3. Subcellular Localization Analysis of PaPIP1-2 in A. thaliana Protoplasts
2.4. cDNA-Library Preparation and Illumina Sequencing for Transcriptome Analysis
2.5. Gene Expression Analysis and qRT–PCR
2.6. Co-Expression Network Construction and Analysis
2.7. Yeast Transformation and Cold-Condition Handling
2.8. Plant Transformants and Low-Temperature Resistance Assessment
2.9. Statistical Assessments
3. Results
3.1. PaPIP1-2 Showed High Expression in the Dormancy and Sprouting Periods of P. armeniaca
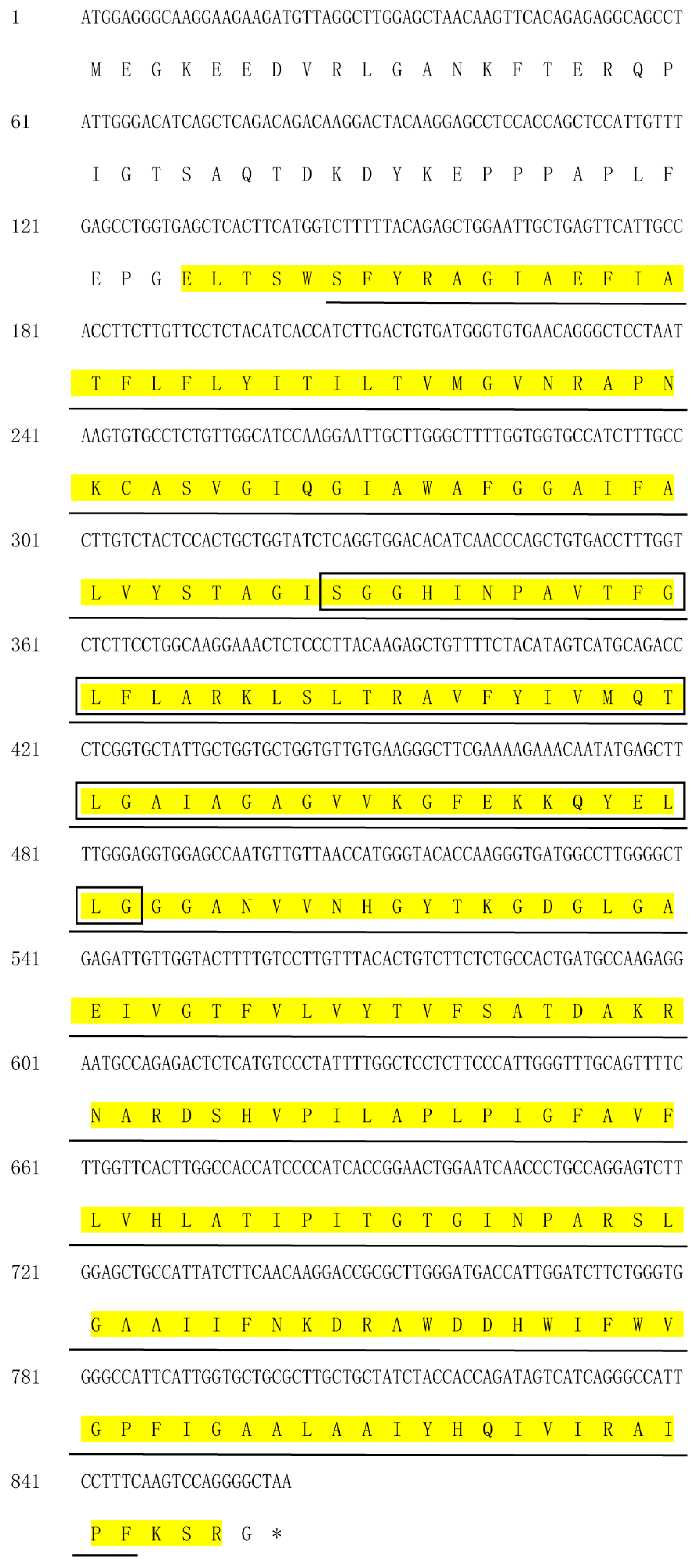
3.2. Isolation and Characterization of PaPIP1-2
3.3. Subcellular Localization of PaPIP1-2 Protein
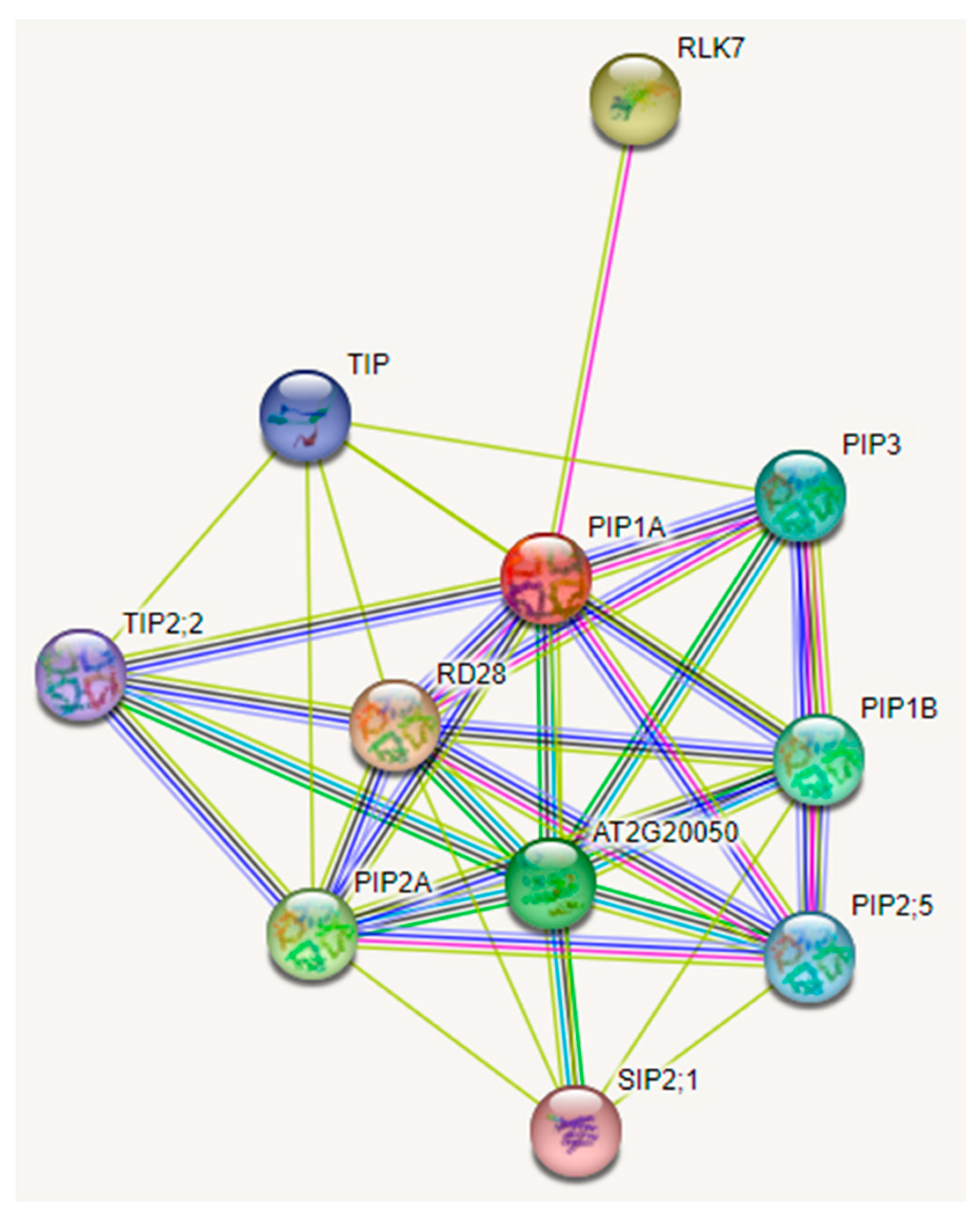
3.4. Protein–Protein Interaction Network of PaPIP1-2
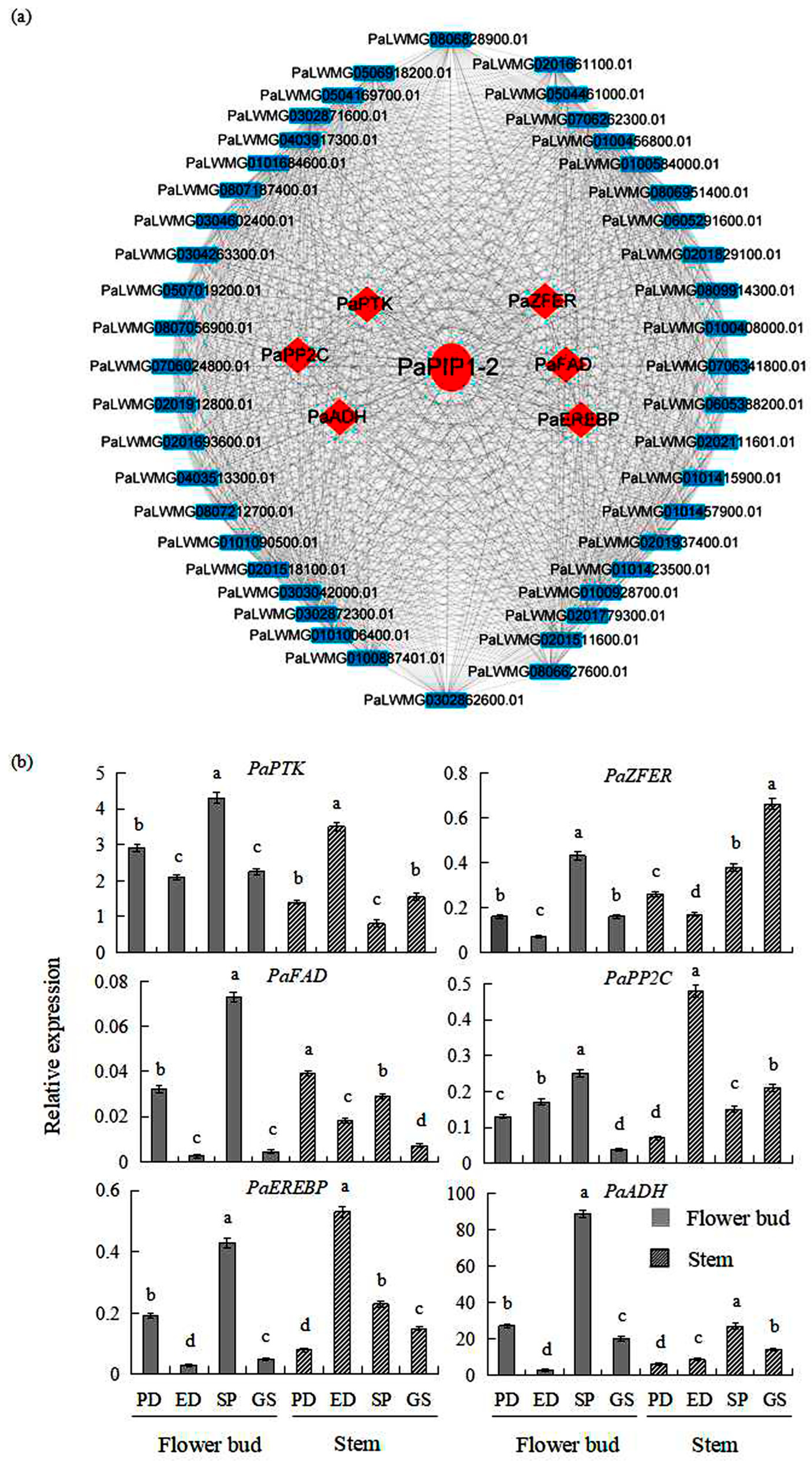
3.5. PaPIP1-2 Co-Expression Network in P. armeniaca
3.6. The PaPIP1-2 Gene Conferred Cold Resistance to Yeast
3.7. PaPIP1-2 Was Transformed in A. thaliana and Conferred Low-Temperature Tolerance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Maurel, C.; Santoni, V.; Luu, D.T.; Wudick, M.M.; Verdoucq, L. The cellular dynamics of plant aquaporin expression and functions. Curr. Opin. Plant Biol. 2009, 12, 690–698. [Google Scholar] [CrossRef]
- Maurel, C.; Verdoucq, L.; Luu, D.T.; Santoni, V. Plant aquaporins: Membrane channels with multiple integrated functions. Annu. Rev. Plant Biol. 2008, 59, 595–624. [Google Scholar] [CrossRef]
- Mitani-Ueno, N.; Yamaji, N.; Zhao, F.J.; Ma, J.F. The aromatic/arginine selectivity filter of NIP aquaporins plays a critical role in substrate selectivity for silicon, boron, and arsenic. J. Exp. Bot. 2011, 62, 4391–4398. [Google Scholar] [CrossRef]
- Anderberg, H.I.; Kjellbom, P.; Johanson, U. Annotation of Selaginella moellendorffii Major Intrinsic Proteins and the Evolution of the Protein Family in Terrestrial Plants. Front. Plant Sci. 2012, 3, 33. [Google Scholar] [CrossRef]
- Yang, X.; Li, J.; Ji, C.; Wei, Z.; Zhao, T.; Pang, Q. Overexpression of an aquaporin gene EsPIP1;4 enhances abiotic stress tolerance and promotes flowering in Arabidopsis thaliana. Plant Physiol. Biochem. 2022, 193, 25–35. [Google Scholar] [CrossRef]
- Kayum, M.A.; Park, J.I.; Nath, U.K.; Biswas, M.K.; Kim, H.T.; Nou, I.S. Genome-wide expression profiling of aquaporin genes confer responses to abiotic and biotic stresses in Brassica rapa. BMC Plant Biol. 2017, 17, 23. [Google Scholar] [CrossRef]
- Bansal, A.; Sankararamakrishnan, R. Homology modeling of major intrinsic proteins in rice, maize and Arabidopsis: Comparative analysis of transmembrane helix association and aromatic/arginine selectivity filters. BMC Struct. Biol. 2007, 7, 27. [Google Scholar] [CrossRef]
- Hove, R.M.; Bhave, M. Plant aquaporins with non-aqua functions: Deciphering the signature sequences. Plant Mol. Biol. 2011, 75, 413–430. [Google Scholar] [CrossRef]
- Froger, A.; Tallur, B.; Thomas, D.; Delamarche, C. Prediction of functional residues in water channels and related proteins. Protein Sci. 1998, 7, 1458–1468. [Google Scholar] [CrossRef]
- Rahman, A.; Kawamura, Y.; Maeshima, M.; Rahman, A.; Uemura, M. Plasma Membrane Aquaporin Members PIPs Act in Concert to Regulate Cold Acclimation and Freezing Tolerance Responses in Arabidopsis thaliana. Plant Cell Physiol. 2020, 61, 787–802. [Google Scholar] [CrossRef]
- Shekhawat, U.K.S.; Ganapathi, T.R. MusaWRKY71 Overexpression in Banana Plants Leads to Altered Abiotic and Biotic Stress Responses. PLoS ONE 2013, 8, e75506. [Google Scholar] [CrossRef]
- Zhou, S.; Hu, W.; Deng, X.; Ma, Z.; Chen, L.; Huang, C.; Wang, C.; Wang, J.; He, Y.; Yang, G.; et al. Overexpression of the wheat aquaporin gene, TaAQP7, enhances drought tolerance in transgenic tobacco. PLoS ONE 2012, 7, e52439. [Google Scholar] [CrossRef]
- Qu, J.; Xu, S.; Zhang, Z.; Chen, G.; Zhong, Y.; Liu, L.; Zhang, R.; Xue, J.; Guo, D. Evolutionary, structural and expression analysis of core genes involved in starch synthesis. Sci. Rep. 2018, 8, 12736. [Google Scholar] [CrossRef]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef]
- Kong, W.; Yang, S.; Wang, Y.; Bendahmane, M.; Fu, X. Genome-wide identification and characterization of aquaporin gene family in Beta vulgaris. PeerJ 2017, 5, e3747. [Google Scholar] [CrossRef]
- Chou, K.-C.; Shen, H.-B. Plant-mPLoc: A Top-Down Strategy to Augment the Power for Predicting Plant Protein Subcellular Localization. PLoS ONE 2010, 5, e11335. [Google Scholar] [CrossRef]
- Horton, P.; Park, K.-J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007, 35, W585–W587. [Google Scholar] [CrossRef]
- Sheen, J. Signal Transduction in Maize and Arabidopsis Mesophyll Protoplasts. Plant Physiol. 2001, 127, 1466–1475. [Google Scholar] [CrossRef]
- Nelson, B.K.; Cai, X.; Nebenführ, A. A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J. 2007, 51, 1126–1136. [Google Scholar] [CrossRef]
- Song, C.; Yin, M.; Jiang, Z.; Li, H.; Wuyun, T.; Song, J. Relationship between flower bud differentiation and change of endogenous hormone of ‘Youyi’ during dormancy period. J. Northwest A F Univ. Nat. Sci. Ed. 2017, 45, 170–184. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, L.; Liang, X.; Zhang, S. CpLEA5, the Late Embryogenesis Abundant protein gene from Chimonanthus praecox, possesses low temperature and osmotic resistances in Prokaryote and Eukaryotes. Int. J. Mol. Sci. 2015, 16, 26978–26990. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Wang, L.; Xu, C.; Wang, C.; Wang, Y. Characterization of a eukaryotic translation initiation factor 5A homolog from Tamarix androssowii involved in plant abiotic stress tolerance. BMC Plant Biol. 2012, 12, 118. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Zhang, Y.; Zhu, G.; Zhu, X.; Xia, Y.; Li, J.; Gao, X.; Wang, S.; Zhang, J.; et al. Genome-Wide identification and function of aquaporin genes during dormancy and sprouting periods of Kernel-Using Apricot (Prunus armeniaca L.). Front. Plant Sci. 2021, 12, 690040. [Google Scholar] [CrossRef]
- Tähtiharju, S.; Palva, T. Antisense inhibition of protein phosphatase 2C accelerates cold acclimation in Arabidopsis thaliana. Plant J. 2001, 26, 461–470. [Google Scholar] [CrossRef]
- Chen, X.; Ding, Y.; Yang, Y.; Song, C.; Wang, B.; Yang, S.; Guo, Y.; Gong, Z. Protein kinases in plant responses to drought, salt, and cold stress. J. Integr. Plant Biol. 2021, 63, 53–78. [Google Scholar] [CrossRef]
- Jung, Y.J.; Lee, I.H.; Nou, I.S.; Lee, K.D.; Rashotte, A.M.; Kang, K.K. BrRZFP1 a Brassica rapa C3HC4-type RING zinc finger protein involved in cold, salt and dehydration stress. Plant Biol. 2013, 15, 274–283. [Google Scholar] [CrossRef]
- Jingu, D.; Iino, M.; Kawasaki, J.; Urano, E.; Kusakari, S.; Hayashi, Y.; Matozaki, T.; Ohnishi, H. Protein tyrosine phosphatase Shp2 positively regulates cold stress-induced tyrosine phosphorylation of SIRPα in neurons. Biochem. Biophys. Res. Commun. 2021, 569, 72–78. [Google Scholar] [CrossRef]
- Khodakovskaya, M.; McAvoy, R.; Peters, J.; Wu, H.; Li, Y. Enhanced cold tolerance in transgenic tobacco expressing a chloroplast omega-3 fatty acid desaturase gene under the control of a cold-inducible promoter. Planta 2006, 223, 1090–1100. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, X.; Gao, Y.; Li, K.; Guo, W. Transcriptome-based identification of AP2/ERF family genes and their cold-regulated expression during the dormancy phase transition of Chinese cherry flower buds. Sci. Hortic. 2021, 275, 109666. [Google Scholar] [CrossRef]
- Murata, K.; Mitsuoka, K.; Hirai, T.; Walz, T.; Agre, P.; Heymann, J.B.; Engel, A.; Fujiyoshi, Y. Structural determinants of water permeation through aquaporin-1. Nature 2000, 407, 599–605. [Google Scholar] [CrossRef]
- Deokar, A.A.; Tar’an, B. Genome-Wide Analysis of the Aquaporin Gene Family in Chickpea (Cicer arietinum L.). Front. Plant Sci. 2016, 7, 1802. [Google Scholar] [CrossRef]
- Gupta, A.B.; Sankararamakrishnan, R. Genome-wide analysis of major intrinsic proteins in the tree plant Populus trichocarpa: Characterization of XIP subfamily of aquaporins from evolutionary perspective. BMC Plant Biol. 2009, 9, 134. [Google Scholar] [CrossRef]
- Shivaraj, S.M.; Deshmukh, R.K.; Rai, R.; Bélanger, R.; Agrawal, P.K.; Dash, P.K. Genome-wide identification, characterization, and expression profile of aquaporin gene family in flax (Linum usitatissimum). Sci. Rep. 2017, 7, 46137. [Google Scholar] [CrossRef]
- Chaumont, F.; Barrieu, F.; Jung, R.; Chrispeels, M.J. Plasma membrane intrinsic proteins from maize cluster in two sequence subgroups with differential aquaporin activity. Plant Physiol. 2000, 122, 1025–1034. [Google Scholar] [CrossRef]
- Zou, Z.; Gong, J.; Huang, Q.; Mo, Y.; Yang, L.; Xie, G. Gene structures, evolution, classification and expression profiles of the Aquaporin gene family in Castor Bean (Ricinus communis L.). PLoS ONE 2015, 10, e0141022. [Google Scholar] [CrossRef]
- Di Giorgio, J.A.; Bienert, G.P.; Ayub, N.D.; Yaneff, A.; Barberini, M.L.; Mecchia, M.A.; Amodeo, G.; Soto, G.C.; Muschietti, J.P. Pollen-specific Aquaporins NIP4;1 and NIP4;2 are required for pollen development and pollination in Arabidopsis thaliana. Plant Cell 2016, 28, 1053–1077. [Google Scholar] [CrossRef]
- He, Y.; Gan, S. A novel zinc-finger protein with a proline-rich domain mediates ABA-regulated seed dormancy in Arabidopsis. Plant Mol. Biol. 2004, 54, 1–9. [Google Scholar] [CrossRef]
- Kim, W.; Lee, Y.; Park, J.; Lee, N.; Choi, G. HONSU, a protein phosphatase 2C, regulates seed dormancy by inhibiting ABA signaling in Arabidopsis. Plant Cell Physiol. 2013, 54, 555–572. [Google Scholar] [CrossRef]
- Yooyongwech, S.; Horigane, A.K.; Yoshida, M.; Yamaguchi, M.; Sekozawa, Y.; Sugaya, S.; Gemma, H. Changes in aquaporin gene expression and magnetic resonance imaging of water status in peach tree flower buds during dormancy. Physiol. Plant. 2008, 134, 522–533. [Google Scholar] [CrossRef]
- Alexandersson, E.; Fraysse, L.; Sjövall-Larsen, S.; Gustavsson, S.; Fellert, M.; Karlsson, M.; Johanson, U.; Kjellbom, P. Whole gene family expression and drought stress regulation of aquaporins. Plant Mol. Biol. 2005, 59, 469–484. [Google Scholar] [CrossRef]
- Afzal, Z.; Howton, T.C.; Sun, Y.; Mukhtar, M.S. The Roles of Aquaporins in Plant Stress Responses. J. Dev. Biol. 2016, 4, 9. [Google Scholar] [CrossRef]
- Ding, Y.; Jia, Y.; Shi, Y.; Zhang, X.; Song, C.; Gong, Z.; Yang, S. OST1-mediated BTF3L phosphorylation positively regulates CBFs during plant cold responses. EMBO J. 2018, 37, e98228. [Google Scholar] [CrossRef]
- Jiang, B.; Shi, Y.; Zhang, X.; Xin, X.; Qi, L.; Guo, H.; Li, J.; Yang, S. PIF3 is a negative regulator of the CBF pathway and freezing tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA 2017, 114, E6695–E6702. [Google Scholar] [CrossRef]
- Wang, X.; Ding, Y.; Li, Z.; Shi, Y.; Wang, J.; Hua, J.; Gong, Z.; Zhou, J.M.; Yang, S. PUB25 and PUB26 promote plant freezing tolerance by degrading the cold signaling negative regulator MYB15. Dev. Cell 2019, 51, 222–235. [Google Scholar] [CrossRef]
- Ren, C.; Huayang, L.; Wang, Z.; Dai, Z.; Lecourieux, F.; Kuang, F.; Xin, H.; Li, S.; Liang, Z. Characterization of chromatin accessibility and gene expression upon cold stress reveals the transcription factor RAV1 functions in cold response in Vitis amurensis. Plant Cell Physiol. 2021, 62, 1615–1629. [Google Scholar] [CrossRef]
- Dolferus, R.; Jacobs, M.; Dennis, P. Differential interactions of promoter elements in stress responses of the Arabidopsis Adh gene. Plant Physiol. 1994, 105, 1075–1087. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Zheng, G.; Wang, F.; Yu, H.; Wang, S.; Guan, H.; Lv, F.; Xia, Y. Expression and Functional Analysis of the PaPIP1-2 Gene during Dormancy and Germination Periods of Kernel-Using Apricot (Prunus armeniaca L.). Forests 2023, 14, 2306. https://doi.org/10.3390/f14122306
Li S, Zheng G, Wang F, Yu H, Wang S, Guan H, Lv F, Xia Y. Expression and Functional Analysis of the PaPIP1-2 Gene during Dormancy and Germination Periods of Kernel-Using Apricot (Prunus armeniaca L.). Forests. 2023; 14(12):2306. https://doi.org/10.3390/f14122306
Chicago/Turabian StyleLi, Shaofeng, Guangshun Zheng, Fei Wang, Hai Yu, Shaoli Wang, Haohui Guan, Fenni Lv, and Yongxiu Xia. 2023. "Expression and Functional Analysis of the PaPIP1-2 Gene during Dormancy and Germination Periods of Kernel-Using Apricot (Prunus armeniaca L.)" Forests 14, no. 12: 2306. https://doi.org/10.3390/f14122306
APA StyleLi, S., Zheng, G., Wang, F., Yu, H., Wang, S., Guan, H., Lv, F., & Xia, Y. (2023). Expression and Functional Analysis of the PaPIP1-2 Gene during Dormancy and Germination Periods of Kernel-Using Apricot (Prunus armeniaca L.). Forests, 14(12), 2306. https://doi.org/10.3390/f14122306




