Effects of Climate Change and Fire on the Middle and Late Holocene Forest History in Yenisei Siberia
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Area
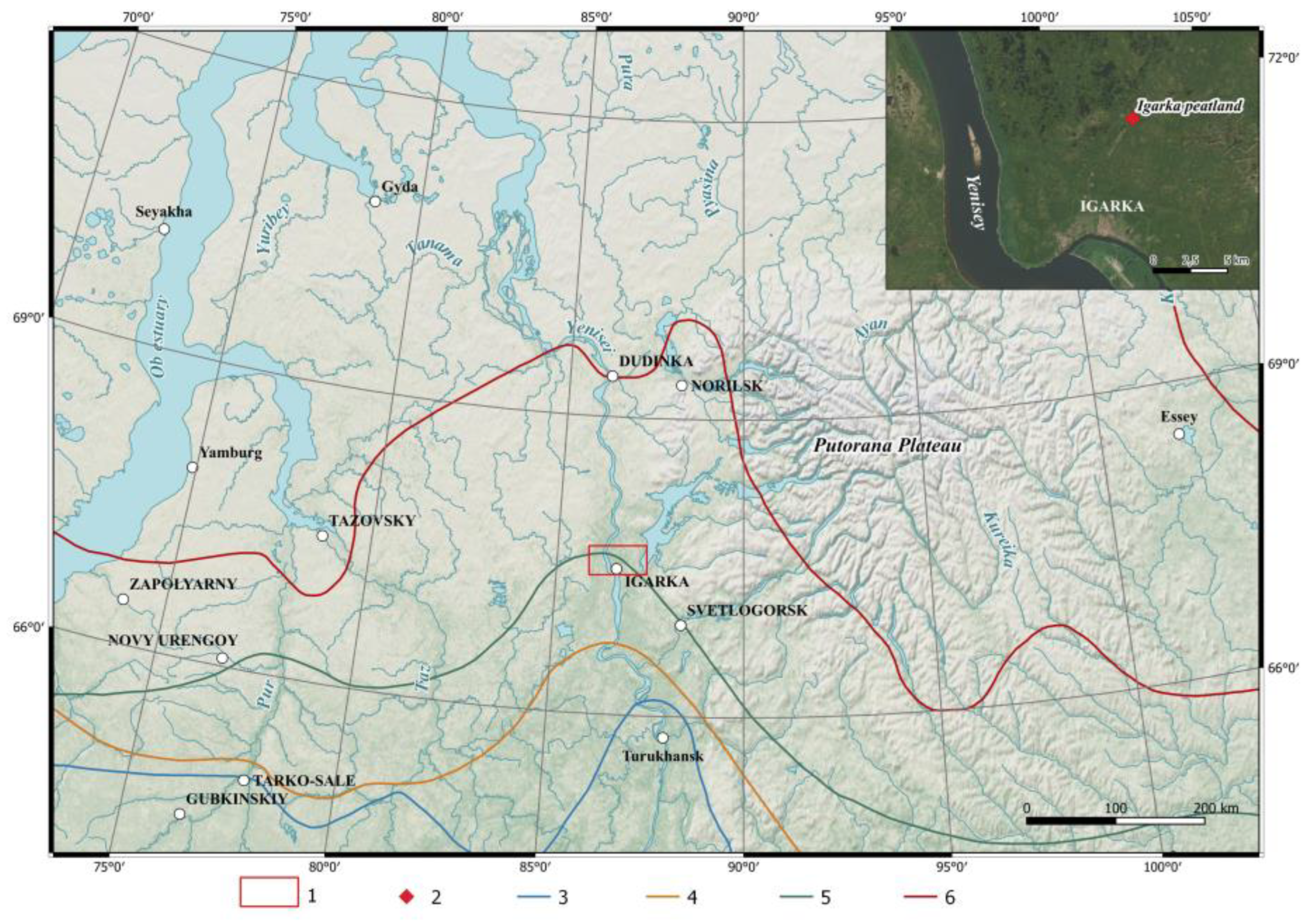
2.2. Sediment Sampling and Radiocrbon Dating
2.3. Pollen Analysis
2.4. Charcoal Analysis
2.5. Climate Reconstruction
3. Results
3.1. Chronology
3.2. Pollen Analysis and Vegetation History
3.3. Macroscopic Charcoal and Fire History
4. Discussion
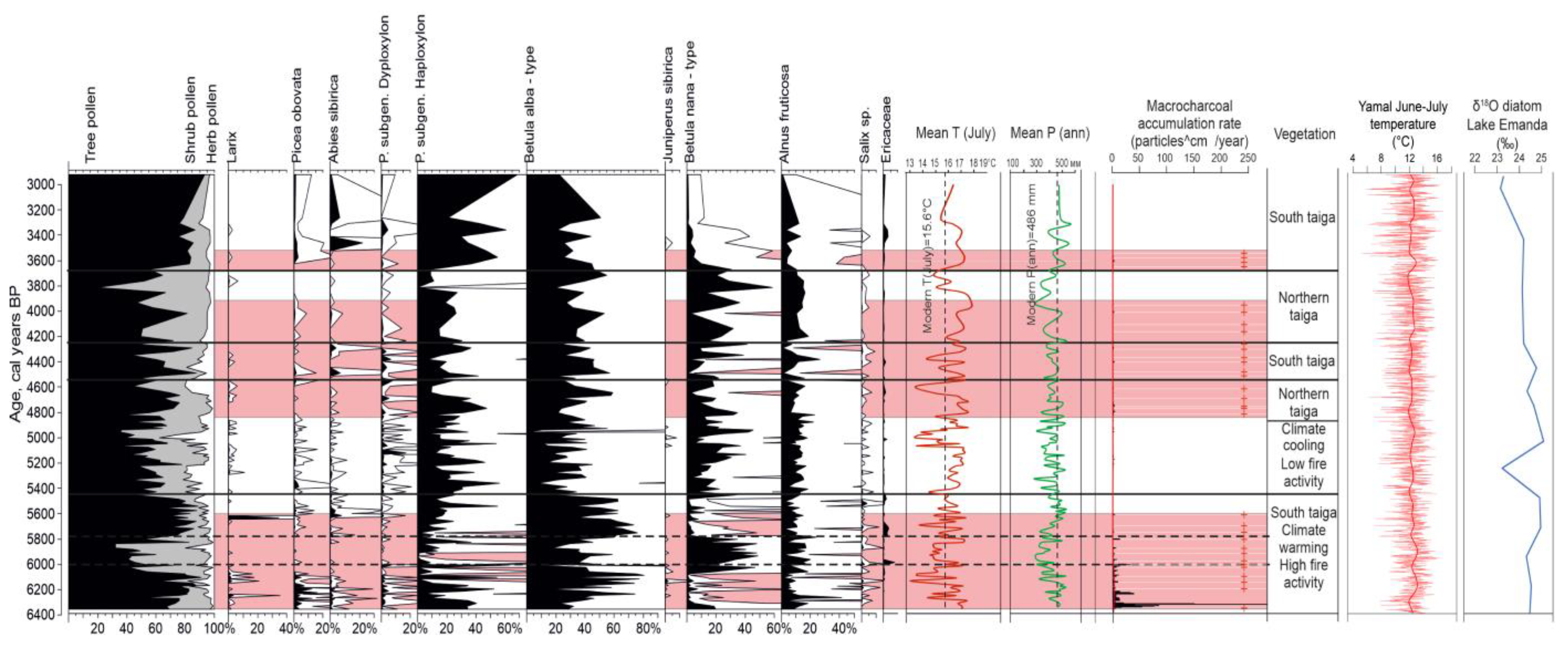
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2022: Impacts, Adaptation, and Vulnerability; Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Pörtner, H.-O., Roberts, D.C., Tignor, M., Poloczanska, E.S., Mintenbeck, K., Alegría, A., Craig, M., Langsdorf, S., Löschke, S., Möller, V., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022; 3056p. [Google Scholar] [CrossRef]
- Callaghan, T.V.; Björn, L.O.; Chernov, Y.; Chapin, T.; Christensen, T.R.; Huntley, B.; Ims, R.A.; Johansson, M.; Jolly, D.; Jonasson, S.; et al. Biodiversity, Distributions and Adaptations of Arctic Species in the Context of Environmental Change. AMBIO 2004, 33, 404–417. [Google Scholar] [CrossRef]
- Groisman, P.Y.; Sherstyukov, B.G.; Razuvaev, V.N.; Knight, R.W.; Enloe, J.G.; Stroumentova, N.S.; Whitfield, P.H.; Førland, E.; Hannsen-Bauer, I.; Tuomenvirta, H.; et al. Potential forest fire danger over Northern Eurasia: Changes during the 20th century. Glob. Planet. Chang. 2007, 56, 371–386. [Google Scholar] [CrossRef]
- Bowman, D.M.J.S.; Kolden, C.A.; Abatzoglou, J.T.; Johnston, F.H.; van der Werf, G.R.; Flannigan, M. Vegetation fires in the Anthropocene. Nat. Rev. Earth Environ. 2020, 1, 500–515. [Google Scholar] [CrossRef]
- Roberts, K.E.; Lamoureux, S.F.; Kyser, T.K.; Muir, D.C.G.; Lafrenière, M.J.; Iqaluk, D.; Pieńkowski, A.J.; Normandeau, A. Climate and permafrost effects on the chemistry and ecosystems of High Arctic Lakes. Sci. Rep. 2017, 7, 13292. [Google Scholar] [CrossRef]
- Gibson, C.M.; Chasmer, L.E.; Thompson, D.K.; Quinton, W.L.; Flannigan, M.D.; Olefeldt, D. Wildfire as a major driver of recent permafrost thaw in boreal peatlands. Nat. Commun. 2018, 9, 3041. [Google Scholar] [CrossRef]
- Biskaborn, B.K.; Smith, S.L.; Noetzli, J.; Matthes, H.; Vieira, G.; Streletskiy, D.A.; Schoeneich, P.; Romanovsky, V.E.; Lewkowicz, A.G.; Abramov, A.; et al. Permafrost is warming at a global scale. Nat. Commun. 2019, 10, 264. [Google Scholar] [CrossRef]
- Czerniawska, J.; Chlachula, J. Climate-Change Induced Permafrost Degradation in Yakutia, East Siberia. Arctic 2020, 73, 509–528. [Google Scholar] [CrossRef]
- Heijmans, M.M.P.D.; Magnússon, R.Í.; Lara, M.J.; Frost, G.V.; Myers-Smith, I.H.; van Huissteden, J.; Jorgenson, M.T.; Fedorov, A.N.; Epstein, H.E.; Lawrence, D.M.; et al. Tundra vegetation change and impacts on permafrost. Nat. Rev. Earth Environ. 2022, 3, 68–84. [Google Scholar] [CrossRef]
- MacDonald, G.M.; Velichko, A.A.; Kremenetski, C.V.; Borisova, O.K.; Goleva, A.A.; Andreev, A.A.; Cwynar, L.C.; Riding, R.T.; Forman, S.L.; Edwards, T.W.D.; et al. Holocene treeline history and climate change across Northern Eurasia. Quat. Res. 2000, 53, 302–311. [Google Scholar] [CrossRef]
- Bigelow, N.H.; Brubaker, L.B.; Edwards, M.E.; Harrison, S.P.; Prentice, I.C.; Anderson, P.M.; Andreev, A.A.; Bartlein, P.J.; Christensen, T.R.; Cramer, W.; et al. Climate change and Arctic ecosystems: 1. Vegetation changes north of 55° N between the last glacial maximum, mid-Holocene, and present. J. Geophys. Res. 2003, 108, 8170. [Google Scholar] [CrossRef]
- Andreev, A.A.; Klimanov, V.A. Quantitative Holocene climatic reconstruction from Arctic Russia. J. Paleolimnol. 2000, 24, 81–91. [Google Scholar] [CrossRef]
- Andreev, A.A.; Tarasov, P.E.; Klimanov, V.A.; Melles, M.; Lisitsyna, O.M.; Hubberten, H.-W. Vegetation and climate changes around the Lama Lake, Taymyr Peninsula, Russia during the Late Pleistocene and Holocene. Quat. Int. 2004, 122, 69–84. [Google Scholar] [CrossRef]
- Andreev, A.A.; Tarasov, P.E.; Siegert, C.; Ebel, T.; Klimanov, V.A.; Melles, M.; Bobrov, A.A.; Dereviagin, A.Y.; Lubinski, D.J.; Hubberten, H.-W. Late Pleistocene and Holocene vegetation and climate on the northern Taymyr Peninsula, Arctic Russia. Boreas 2003, 32, 484–505. [Google Scholar] [CrossRef]
- Müller, S.; Tarasov, P.E.; Andreev, A.A.; Diekmann, B. Late Glacial to Holocene environments in the present-day coldest region of the Northern Hemisphere inferred from a pollen record of Lake Billyakh, Verkhoyansk Mts, NE Siberia. Clim. Past. 2009, 5, 73–84. [Google Scholar] [CrossRef]
- Liu, S.; Stoof-Leichsenring, K.R.; Kruse, S.; Pestryakova, L.A.; Herzschuh, U. Holocene vegetation and plant diversity changes in the North-Eastern Siberian treeline region from pollen and sedimentary ancient DNA. Front. Ecol. Evol. 2020, 8, 560243. [Google Scholar] [CrossRef]
- Novenko, E.Y.; Rudenko, O.V.; Mazei, N.G.; Kupriyanov, D.A.; Batalova, V.A.; Volkova, E.M.; Phelps, L.N.; Davis, B.A.S. Late-Holocene vegetation and fire history in Western Putorana Plateau (subarctic Siberia, Russia). Holocene 2022, 32, 433–441. [Google Scholar] [CrossRef]
- Kobe, F.; Hoelzmann, P.; Gliwa, J.; Olschewski, P.; Peskov, S.A.; Shchetnikov, A.A.; Danukalova, G.A.; Osipova, E.M.; Goslar, T.; Leipe, C.; et al. Lateglacial–Holocene environments and human occupation in the Upper Lena region of Eastern Siberia derived from sedimentary and zooarchaeological data from Lake Ochaul. Quat. Int. 2022, 623, 139–158. [Google Scholar] [CrossRef]
- Pestryakova, L.A.; Herzschuh, U.; Wetterich, S.; Ulrich, M. Present-day variability and Holocene dynamics of permafrost-affected lakes in central Yakutia (Eastern Siberia) inferred from diatom records. Quat. Sci. Rev. 2012, 51, 56–70. [Google Scholar] [CrossRef]
- Nazarova, L.; Lüpfert, H.; Subetto, D.; Pestryakova, L.A.; Diekmann, B. Holocene climate conditions in central Yakutia (Eastern Siberia) inferred from sediment composition and fossil chironomids of Lake Temje. Quat. Int. 2013, 290–291, 264–274. [Google Scholar] [CrossRef]
- Murton, J.B.; Edwards, M.E.; Lozhkin, A.V.; Anderson, P.M.; Savvinov, G.N.; Bakulina, N.; Bondarenko, O.V.; Cherepanova, M.V.; Danilov, P.P.; Boeskorov, V.; et al. Preliminary paleoenvironmental analysis of permafrost deposits at Batagaika megaslump, Yana uplands, northeast Siberia. Quat. Res. 2017, 87, 314–330. [Google Scholar] [CrossRef]
- Feurdean, A.; Gałka, M.; Florescu, G.; Diaconu, A.-C.; Tant, I.; Kirpotin, S.; Hutchinson, S.M. 2000 years of variability in hydroclimate and carbon accumulation in western Siberia and the relationship with large-scale atmospheric circulation: A multi-proxy peat record. Quat. Sci. Rev. 2019, 226, 105948. [Google Scholar] [CrossRef]
- Novenko, E.Y.; Mazei, N.G.; Kupriyanov, D.A.; Babeshko, K.V.; Kusilman, M.V.; Zyuganova, I.S.; Tsyganov, A.N.; Mazei, Y.A.; Phelps, L.N.; Davis, B.A. A 1300-year multi-proxy palaeoecological record from the northwest Putorana Plateau (Russian Subarctic): Environmental changes, vegetation dynamics and fire history. Holocene 2023, 33, 181–193. [Google Scholar] [CrossRef]
- Clark, J.S.; Lynch, J.; Stocks, B.J.; Goldammer, J.G. Relationships between charcoal particles in air and sediments in west-central Siberia. Holocene 1998, 8, 19–29. [Google Scholar] [CrossRef]
- De Groot, W.J.; Cantin, A.S.; Flannigan, M.; Soja, A.J.; Gowman, L.M.; Newbery, A. A comparison of Canadian and Russian boreal forest fire regimes. For. Ecol. Manag. 2013, 294, 23–34. [Google Scholar] [CrossRef]
- Barhoumi, C.; Vogel, M.; Dugerdil, L.; Limani, H.; Joannin, S.; Peyron, O.; Ali, A.A. Holocene Fire Regime Changes in the Southern Lake Baikal Region Influenced by Climate-Vegetation-Anthropogenic Activity Interactions. Forests 2021, 12, 978. [Google Scholar] [CrossRef]
- Glückler, R.; Herzschuh, U.; Kruse, S.; Andreev, A.; Vyse, S.A.; Winkler, B.; Biskaborn, B.K.; Pestrykova, L.; Dietze, E. Wildfire history of the boreal forest of south-western Yakutia (Siberia) over the last two millennia documented by a lake-sediment charcoal record. Biogeosciences 2021, 18, 4185–4209. [Google Scholar] [CrossRef]
- Feurdean, A.; Diaconu, A.-C.; Pfeiffer, M.; Gałka, M.; Hutchinson, S.M.; Butiseaca, G.; Gorina, N.; Tonkov, S.; Niamir, A.; Tantau, I.; et al. Holocene wildfire regimes in forested peatlands in western Siberia: Interaction between peatland moisture conditions and the composition of plant functional types. Clim. Past. 2022, 18, 1255–1274. [Google Scholar] [CrossRef]
- Novenko, E.Y.; Kupryanov, D.A.; Mazei, N.G.; Prokushkin, A.S.; Phelps, L.N.; Buri, A.; Davis, B.A.S. Evidence that modern fires may be unprecedented during the last 3400 years in permafrost zone of Central Siberia, Russia. Environ. Res. Lett. 2022, 17, 025004. [Google Scholar] [CrossRef]
- Chen, A.; Yang, L.; Sun, L.; Gao, Y.; Xie, Z. Holocene changes in biomass burning in the boreal Northern Hemisphere, reconstructed from anhydrosugar fluxes in an Arctic sediment profile. Sci. Total Environ. 2023, 867, 161460. [Google Scholar] [CrossRef]
- Katz, N.Y. Types of Bogs of the USSR and Western Europe and Their Geographical Distribution; State Publishing House of Geographical Literature: Moscow, Russia, 1948. [Google Scholar]
- Vasil’chuk, Y.; Vasil’chuk, A.; Budantseva, N.; Chizhova, J. Palsa of Frozen Peat Mires; Moscow University Press: Moscow, Russia, 2008. [Google Scholar]
- Pyavchenko, N.I. Bugristye Bolota; Publishing House of the USSR Academy of Sciences: Moscow, Russia, 1955. [Google Scholar]
- Levkovskaya, G.M.; Kind, N.B.; Zavelski, F.S.; Forova, V.S. The absolute age of peatlands in Igarka region and subdivision of the Holocene in West Siberia. Bull. Comm. Study Quat. 1970, 37, 94–101. [Google Scholar]
- Beck, H.; Zimmermann, N.; McVicar, T.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef] [PubMed]
- Veselov, V.M.; Pribyl’skaya, I.P.; Mirzeabasov, O.A. Nauchno-Prikladnoy Spravochnik “Klimat Rossii”; Scientific and Applied Reference Book “Climate of Russia”; VNIIGMI-MCD: Obninsk, Russia, 2022; Available online: http://aisori-m.meteo.ru/climsprn (accessed on 1 May 2023).
- Ershov, E.D. (Ed.) Geocryologya SSSR, Srednya Sibir; Geocryology of USSR, Middle Siberia; Nedra-Press: Moscow, Russia, 1989. [Google Scholar]
- Novenko, E.Y.; Mazei, N.G.; Prokushkin, A.S.; Kupryanov, D.A.; Shatunov, A.E.; Andreev, R.A.; Serikov, S.I. The Holocene palaeoecology of the palsa mire near Igarka (Yenisei Siberia). IOP Conf. Ser. Earth Environ. Sci. 2022, 1093, 012029. [Google Scholar] [CrossRef]
- Novenko, E.Y.; Mazei, N.G.; Kupryanov, D.A.; Shatunov, A.E.; Andreev, R.A.; Makarova, E.A.; Borodina, K.A.; Rudenko, O.V. Vegetation changes in Yenisei Siberia over the last 4700 years: New palaeoecological data from Igarka area, Krasnoyarsk Region. Geomorfol. I Paleogeogr. 2022, 53, 51–60. [Google Scholar]
- Sokolov, S.Y.; Svyazeva, O.A.; Kubli, V.A. Arealy Derev’ev i Kustarnikov SSSR [Areals of Trees and Shrubs of USSR]; Nauka-Press Leningrad Branch: Leningrad, Russia, 1977; Volume 1. [Google Scholar]
- Blaauw, M.; Christen, J.A. Flexible paleoclimate age-depth models using an autoregressive gamma process. Bayesian Anal. 2011, 6, 457–474. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: www.R-project.org/ (accessed on 10 July 2023).
- Reimer, P.; Austin, W.E.N.; Bard, E.; Bayliss, A.; Blackwell, P.G.; Bronk Ramsey, C.; Butzin, M.; Edwards, R.L.; Friedrich, M.; Grootes, P.M.; et al. The Intcal20 Northern Hemisphere radiocarbon age calibration curve (0–55 cal kBP). Radiocarbon 2020, 4, 725–757. [Google Scholar] [CrossRef]
- Moore, P.D.; Webb, J.A.; Collinson, M.E. Pollen Analysis; Blackwell: Oxford, UK, 1991. [Google Scholar]
- Bennett, K.D.; Willis, K.J. Pollen. Tracking Environmental Change Using Lake Sediments Volume 3: Terrestrial, Algal, and Siliceous Indicators; Smol, J.P., Birks, H.J., Last, W.M., Bradley, R.S., Alverson, K., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 5–32. [Google Scholar]
- Mazei, N.G.; Novenko, E.Y. The use of propionic anhydride in the sample preparation for pollen analysis. Nat. Conserv. Res. 2021, 6, 110–112. [Google Scholar] [CrossRef]
- Stockmarr, J. Tablets with spores used in absolute pollen analysis. Pollen Et Spores 1971, 1, 615–621. [Google Scholar]
- Reille, M. Pollen Et Spores d’Europe Et d’Afrique Du Nord; Laboratoire de Botanique Historique et Palynologie: Marseille, France, 1992. [Google Scholar]
- Beug, H.-J. Leitfaden der Pollenbestimmung für Mitteleuropa und Angrenzende Gebiete; Verlag Friedrich Pfeil: Munich, Germany, 2004. [Google Scholar]
- Van Geel, B. A palaeoecological study of Holocene peat bog sections in Germany and the Netherlands, based on the analysis of pollen, spores and macro- and microscopic remains of fungi, algae cormophytes and animals. Rev. Palaeobot. Palynol. 1978, 25, 1–120. [Google Scholar] [CrossRef]
- Shumilovskikh, L.S.; Schlütz, F.; Achterberg, I.; Bauerochse, A.; Leuschner, H.H. Nonpollen palynomorphs from mid-Holocene peat of the raised bog Borsteler Moor (Lower Saxony, Germany). Stud. Quat. 2015, 32, 5–18. [Google Scholar]
- Shumilovskikh, L.S.; Shumilovskikh, E.S.; Schlütz, F.; van Geel, B. NPP-ID: Non-Pollen Palynomorph Image Database as a research and educational platform. Veget. Hist. Archaeobot. 2022, 31, 323–328. [Google Scholar] [CrossRef]
- Clark, R.L. Point count estimation of charcoal in pollen preparations and thin sections of sediments. Pollen Spores 1982, 24, 523–535. [Google Scholar]
- Grimm, E. TILIA and TILIA*GRAPH.PC spreadsheet and graphics software for pollen data. INQUA Work. Group Data-Handl. Methods Newsl. 1990, 4, 5–7. [Google Scholar]
- Benkova, V.E.; Schweingruber, F.H. Anatomy of Russian Woods. An Atlas for the Identification of Trees, Shrubs, Dwarf Shrubs and Woody Lianas from Russia; Haupt Verlag: Bern, Switzerland, 2004. [Google Scholar]
- Mooney, S.; Tinner, W. The analysis of charcoal in peat and organic sediments. Mires Peat 2011, 7, 1–18. [Google Scholar]
- Finsinger, W.; Bonnici, I. Tapas: An R Package to Perform Trend and Peaks Analysis. 2022. Available online: https://zenodo.org/records/6344463 (accessed on 11 August 2023).
- Overpeck, J.T.; Webb, T., III; Prentice, I.C. Quantitative interpretation of fossil pollen spectra: Dissimilarity coefficients and the method of modern analogs. Quat. Res. 1985, 23, 87–108. [Google Scholar] [CrossRef]
- Guiot, J. Methodology of the last climatic cycle reconstruction in France from pollen data. Palaeogeogr. Palaeoclim. Palaeoecol. 1990, 80, 49–69. [Google Scholar] [CrossRef]
- Nakagawa, T.; Tarasov, P.; Kotoba, N.; Gotanda, K.; Yasuda, Y. Quantitative pollen-based climate reconstruction in Japan: Application to surface and late Quaternary spectra. Quat. Sci. Rev. 2002, 21, 2099–2113. [Google Scholar] [CrossRef]
- Williams, J.W.; Shuman, B. Obtaining accurate and precise environmental reconstructions from the modern analogue technique and North American surface pollen dataset. Quat. Sci. Rev. 2008, 27, 669–687. [Google Scholar] [CrossRef]
- Chevalier, M.; Davis, B.A.S.; Heiri, O.; Seppä, H.; Chase, B.M.; Gajewski, K.; Lacourse, T.; Telford, R.J.; Finsinger, W.; Guiot, J.; et al. Pollen-based climate reconstruction techniques for late Quaternary studies. Earth-Sci. Rev. 2020, 210, 103384. [Google Scholar] [CrossRef]
- Simpson, G.L.; Oksanen, J. Analogue: Analogue and Weighted Averaging Methods for Palaeoecology. R Package Version 0.17-6. 2021. Available online: https://cran.r-project.org/package=analogue (accessed on 5 August 2023).
- Davis, B.A.S.; Chevalier, M.; Sommer, P.; Carter, V.A.; Finsinger, W.; Mauri, A.; Phelps, L.N.; Zanon, M.; Abegglen, R.; Åkesson, C.M.; et al. The Eurasian Modern Pollen Database (EMPD), version 2. Earth Syst. Sci. Data 2020, 12, 2423–2445. [Google Scholar] [CrossRef]
- Ter Braak, C. Ordination. In Data Analysis in Community and Landscape Ecology; Jongman, R., Ter Braak, C., Van Tongeren, O., Eds.; Pudoc: Wageningen, The Netherlands, 1995; pp. 91–173. [Google Scholar]
- Borren, W.; Bleuten, W.; Lapshina, E.D. Holocene peat and carbon accumulation rates in the southern taiga of Western Siberia. Quat. Res. 2004, 61, 42–51. [Google Scholar] [CrossRef]
- Budantseva, N.A.; Chizhova, J.N.; Bludushkina, L.B.; Vasil’chuk, Y.K. Stable isotopes of oxygen, hydrogen and carbon and the age of palsas near the village of Yeletsky, north-east of the Bolshezemelskaya tundra. Arctic. Antarctic. 2017, 4, 38–56. [Google Scholar] [CrossRef]
- Carcaillet, C.; Ali, A.A.; Blarquez, O.; Genries, A.; Mourier, B.; Bremond, L. Spatial variability of fire history in subalpine forests: From natural to cultural regimes. Écoscience 2009, 16, 1–12. [Google Scholar] [CrossRef]
- Niemeyer, B.; Klemm, J.; Pestryakova, L.A.; Herzschuh, U. Relative pollen productivity estimates for common taxa of the northern Siberian Arctic. Rev. Palaeobot. Palynol. 2015, 221, 71–82. [Google Scholar] [CrossRef]
- Klemm, J.; Herzschuh, U.; Pisaric, M.F.J.; Telford, R.J.; Heim, B.; Pestryakova, L.A. A pollen-climate transfer function from the tundra and taiga vegetation in Arctic Siberia and its applicability to a Holocene record. Palaeogeog. Palaeoclimat. Palaeoecol. 2013, 386, 702–713. [Google Scholar] [CrossRef]
- Lopatina, D.A.; Zanina, O.G. Subrecent spore and pollen spectra from the Lower Kolyma River basin and their significance for reconstruction of Quaternary paleogeography of the region. Stratigr. Geol. Correl. 2016, 24, 103–105. [Google Scholar] [CrossRef]
- Raschke, E.A.; Savelieva, L.A. Subrecent Spore-Pollen Spectra and Modern Vegetation from the Lena River Delta, Russian Arctic. Contemp. Probl. Ecol. 2017, 4, 456–472. [Google Scholar] [CrossRef]
- Novenko, E.Y.; Mazei, N.G.; Kupriyanov, D.A.; Filimonova, L.V.; Lavrova, N.B. Subfossil Spore–Pollen Spectra from Larch Forests of Central Evenkia: Special Aspects of Interpretation for Paleoecological Research Purposes. Russ. J. Ecol. 2021, 52, 429–437. [Google Scholar] [CrossRef]
- Malyshev, L.I.; Peshkova, G.A. (Eds.) Flora of Central Siberia; Nauka-Press, Siberian Branch: Novosibirsk, Russia, 1979. [Google Scholar]
- Hantemirov, R.M.; Corona, C.; Guillet, S.; Shiyatov, S.G.; Stoffel, M.; Osborn, T.J.; Melvin, T.M.; Gorlanova, L.A.; Kukarskih, V.V.; Surkov, A.Y.; et al. Current Siberian heating is unprecedented during the past seven millennia. Nat. Commun. 2022, 13, 4968. [Google Scholar] [CrossRef]
- Klemm, J.; Herzschuh, U.; Pestryakova, L.A. Vegetation, climate and lake changes over the last 7000 years at the boreal treeline in north-central Siberia. Quat. Sci. Rev. 2016, 147, 422–434. [Google Scholar] [CrossRef]
- Kostrova, S.S.; Biskaborn, B.K.; Pestryakova, L.A.; Fernandoy, F.; Lenz, M.M.; Meyer, H. Climate and environmental changes of the Lateglacial transition and Holocene in northeastern Siberia: Evidence from diatom oxygen isotopes and assemblage composition at Lake Emanda. Quat. Sci. Rev. 2021, 259, 106905. [Google Scholar] [CrossRef]
- Kharuk, V.I.; Ponomarev, E.I.; Ivanova, G.A.; Dvinskaya, M.L.; Coogan, S.C.P.; Flannigan, M.D. Wildfires in the Siberian taiga. Ambio 2021, 50, 1953–1974. [Google Scholar] [CrossRef] [PubMed]
- Solomina, O.N.; Bradley, R.S.; Hodgson, D.A.; Ivy-Ochs, S.; Jomelli, V.; Mackintosh, A.N.; Nesje, A.; Owen, L.A.; Wanner, H.; Wiles, G.C.; et al. Holocene glacier fluctuations. Quat. Sci. Rev. 2015, 111, 9–34. [Google Scholar] [CrossRef]
- Berger, A.; Loutre, M.F. Insolation values for the climate of the last 10 million years. Quat. Sci. Rev. 1991, 10, 297–317. [Google Scholar] [CrossRef]
- Molinari, C.; Lehsten, V.; Blarquez, O.; Carcaillet, C.; Davis, B.A.S.; Kaplan, J.O.; Clear, J.; Bradshaw, R.H.W. The climate, the fuel and the land use: Long-term regional variability of biomass burning in boreal forests. Glob. Chang. Biol. 2018, 24, 4929–4945. [Google Scholar] [CrossRef] [PubMed]
- Dvornikov, Y.; Novenko, E.; Korets, M.; Olchev, A. Wildfire Dynamics along a North-Central Siberian Latitudinal Transect Assessed Using Landsat Imagery. Remote Sens. 2022, 14, 790. [Google Scholar] [CrossRef]
- Knorre, A.; Kirdyanov, A.; Prokushkin, A.; Krusic, P.; Büntgen, U. Tree ring-based reconstruction of the long-term influence of wildfires on permafrost active layer dynamics in Central Siberia. Sci. Total Environ. 2019, 652, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Self, A.E.; Jones, V.J.; Brooks, S.J. Late Holocene environmental change in arctic western Siberia. Holocene 2015, 25, 150–165. [Google Scholar] [CrossRef]
- Kumke, T.; Kienel, U.; Weckström, J.; Korhola, A.; Hubberten, H.-W. Inferred Holocene Paleotemperatures from Diatoms at Lake Lama, Central Siberia. Arct. Antarct. Alp. Res. 2004, 36, 624–634. [Google Scholar] [CrossRef]
- Christiansen, B.; Ljungqvist, F.C. The extra-tropical Northern Hemisphere temperature in the last two millennia: Reconstructions of low-frequency variability. Clim. Past 2012, 8, 765–786. [Google Scholar] [CrossRef]
- PAGES 2k Consortium. Consistent multidecadal variability in global temperature reconstructions and simulations over the Common Era. Nat. Geosci. 2019, 12, 643–649. [Google Scholar] [CrossRef]
- Rudenko, O.; Taldenkova, E.; Ovsepyan, Y.; Stepanova, A.; Bauch, H.A.A. Multiproxy-based reconstruction of the mid- to late Holocene paleoenvironment in the Laptev Sea off the Lena River Delta (Siberian Arctic). Palaeogeog. Palaeoclimat. Palaeoecol. 2020, 540, 109502. [Google Scholar] [CrossRef]




| Laboratory Code IGANAMS | Depth, cm | Material | 14C yr. BP, 1σ | Age, cal. yr. BP, 2σ (Probability) |
|---|---|---|---|---|
| 8354 | 15 | TOC 1 | 1930 ± 20 | 1817–1896 (0.806) |
| 8332 | 50 | TOC | 3230 ± 20 | 3391–3468 (0.965) |
| 8333 | 60 | TOC | 3320 ± 20 | 3480–3574 (0.987) |
| 8334 | 101 | TOC | 3430 ± 20 | 3617–3723 (0.820) |
| 8335 | 150 | TOC | 3805 ± 20 | 4144–4248 (0.874) |
| 8336 | 202 | TOC | 4120 ± 20 | 4528–4654 (0.545) |
| 8337 | 252 | TOC | 4080 ± 20 | 4518–4624 (0.793) |
| 8338 | 301 | TOC | 4320 ± 20 | 4840–4888 (0.797) |
| 8339 | 351 | TOC | 4360 ± 20 | 4857–4973 (1.000) |
| 8340 | 402 | TOC | 4445 ± 20 | 4962–5070 (0.549) |
| 8341 | 449 | TOC | 4340 ± 20 | 4851–4960 (1.000) |
| 8342 | 500 | TOC | 4490 ± 20 | 5155–5288 (0.569) |
| 8343 | 550 | TOC | 4830 ± 20 | 5574–5596 (0.582) |
| 8344 | 599 | TOC | 4955 ± 25 | 5601–5729 (1.000) |
| 8345 | 650 | TOC | 4315 ± 25 | 4837–4888 (0.772) |
| 8346 | 700 | TOC | 5165 ± 20 | 5900–5942 (0.827) |
| 8347 | 749 | TOC | 5200 ± 20 | 5916–5994 (1.000) |
| 8348 | 798 | TOC | 5400 ± 20 | 6187–6282 (0.954) |
| 8349 | 845 | TOC | 5425 ± 20 | 6198–6287 (1.000) |
| 8350 | 860 | TOC | 5500 ± 20 | 6276–6315 (0.898) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novenko, E.; Rudenko, O.; Mazei, N.; Kupriyanov, D.; Andreev, R.; Shatunov, A.; Kusilman, M.; Prokushkin, A.; Olchev, A. Effects of Climate Change and Fire on the Middle and Late Holocene Forest History in Yenisei Siberia. Forests 2023, 14, 2321. https://doi.org/10.3390/f14122321
Novenko E, Rudenko O, Mazei N, Kupriyanov D, Andreev R, Shatunov A, Kusilman M, Prokushkin A, Olchev A. Effects of Climate Change and Fire on the Middle and Late Holocene Forest History in Yenisei Siberia. Forests. 2023; 14(12):2321. https://doi.org/10.3390/f14122321
Chicago/Turabian StyleNovenko, Elena, Olga Rudenko, Natalia Mazei, Dmitriy Kupriyanov, Rodion Andreev, Anton Shatunov, Maria Kusilman, Anatoly Prokushkin, and Alexander Olchev. 2023. "Effects of Climate Change and Fire on the Middle and Late Holocene Forest History in Yenisei Siberia" Forests 14, no. 12: 2321. https://doi.org/10.3390/f14122321





