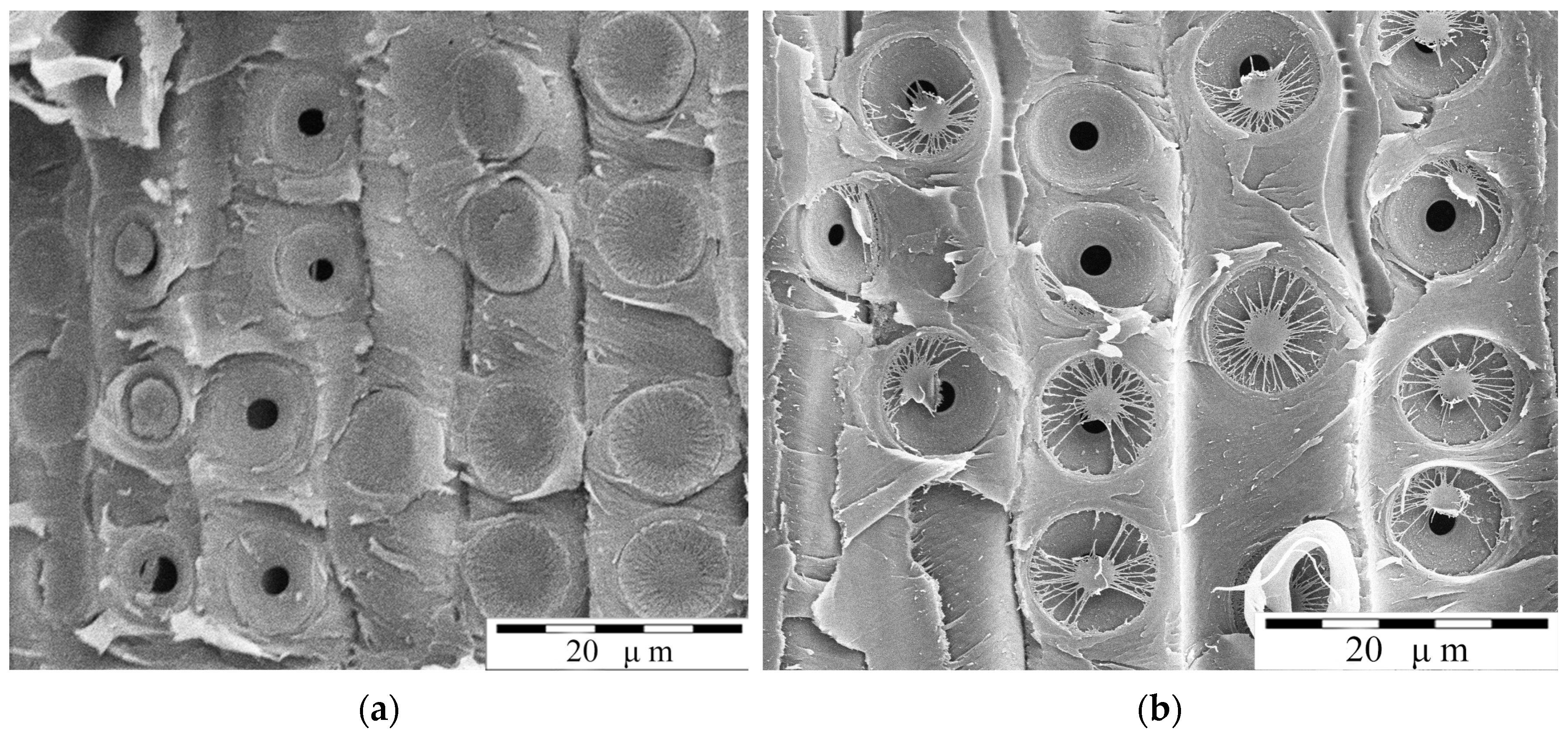
1. Introduction
The resistance of xylem to cavitation, which led to the embolization of water movement, was an essential characteristic for conifers in their ability to withstand drought-induced stress [
1,
2]. The presence of bordered pits in conifers has been recognized as a crucial evolutionary characteristic that facilitated water transfer in living trees by avoiding the spread of embolism from one tracheid to another under negative pressure [
3]. There have been global reports of hydraulic failure caused by drought in recent years [
4,
5]. There has been a growing interest in the influence of the bordering pit on conifer hydraulic safety [
6,
7,
8]. The literature extensively documents the observation of contact between the torus and the pit border in aspirated pits as a means to avoid air-seeding leakage between tracheids [
9,
10,
11]. This phenomenon (
Figure 1) was also found in a greenhouse experiment conducted at Northeast Forest University in Harbin, China, specifically investigating water stress conditions. However, it appeared to be less understood that the mechanical behavior of the torus and the pit border in the formation of a contact seal during pit aspiration.
The bordered pit in conifers was composed of a centrally thickened region known as the torus, and a porous region with radiating threads referred to as the margo [
12,
13]. A dense and non-porous layer of pectin [
14] was applied to the surface of the torus, which was commonly regarded as impervious to both water and air [
15,
16]. Evidence suggested that the torus–margo structure in bordered pits could serve as a safety mechanism to impede the movement of air bubbles between tracheids [
17]. Among a large number of conifer species, the cross-species correlations between structure parameters and cavitation resistance of bordered pits showed that a torus–aperture overlap and the valve effect were the best proxy to explain the variability of cavitation resistance [
18,
19,
20]. The results supported the seal capillary-seeding hypothesis that the contact and adhesion between the torus and the pit wall in aspirated pits contributed to sealing the air-seeding and blocking embolism spread [
21], although plasmodesmatal pores in the torus may also contribute to air-seeding in some species [
22]. In a study conducted by Zelinka et al. [
23], it was shown that torus displacement with margo stretching to an aspirated position required a force below 3 μN, and the torus was found to be extremely compliant. The results supported the suggestion that the pressures required for air seeding past the sealed torus were greater than the pressures needed to deflect the torus enough to seal the pit [
24]. Although substantial evidence suggested that the contact between the torus and the pit border was the key mechanism for preventing air-seeding spread in conifers, the mechanical characteristic between the contact seal and torus–margo structure have not been comprehensively understood.
To investigate the mechanical properties involved in the process of pit aspiration, the utilization of mechanical analysis has emerged as a significant approach alongside experimental measurement [
25]. A linear stress–strain model was devised by John and Uwe in order to determine the displacement of the pit membrane in angiosperm vessels when subjected to a pressure differential [
26]. The forces related to the displacement of pit membranes were investigated by Konrad and Roth-Nebelsick [
27]. A mechanical model was developed, incorporating the geometric characteristics of the pits, utilizing the Kirchhoff–Love thin plate theory [
28]. The methodology employed for constructing the mechanical model enabled the evaluation of how the pit’s geometry influences its mechanical characteristics. The torus–margo structure observed in conifer pits exhibited a higher level of complexity when contrasted with the pit structure found in angiosperm vessels. A linear elastic model was developed to forecast the closure pressure, utilizing a simplified margo structure consisting of a regular distribution of spokes [
29]. The variability of the margo structure across different species has been noted [
30]. In response, Schulte et al. [
31] devised a mechanics model utilizing the finite element method (FEM) to accurately represent the torus–margo structure. This model enabled the investigation of intricate structures involving higher-order freedoms, such as tracheid wall deformation [
32] and pit membrane natural frequency [
33]. In order to assess the forces related to displacements of the torus–margo, the investigation constructed a geometric model incorporating intricate characteristics observed by electron microscopy. Under the pressure applied to the torus and margo, the margo stretching and the torus deflection were investigated. The simulation results facilitated comprehension of the torus–margo structure’s role in closing the pit chamber. However, the comprehensive exploration of the interaction between the torus and the pit border, which served as a crucial mechanism in the development of contact seals, remained not fully investigated.
To gain insights into the mechanical properties of pit aspiration, a mechanical model was developed. This model incorporated the elastic deformation of the torus–margo structure and the contact behavior between the torus and the pit border. The investigation unveiled the mechanical process by which the contact seal in aspirated bordered pits developed, under the influence of external pressure exerted on the torus and margo. This study aimed to examine the associations between the mechanical properties and the anatomical structure of bordered pits in the stem of Platycladus orientalis. The obtained results provided theoretical support for the capillary-seeding hypothesis and will be valuable for future investigations into the phenomenon of capillary-seeding failure among tracheids.
3. Results
The dataset consisted of 53 samples (
Figure 6a). The pit membrane diameter varied between 6.770 μm and 9.350 μm, with an average of 8.193 μm and a standard deviation of 0.680 μm. Similarly, the torus diameter ranged from 2.333 μm to 4.592 μm, with a mean of 3.405 μm and a standard deviation of 0.528 μm. A strong positive correlation was seen between the diameter of the pit membrane and the diameter of the torus (
p < 0.001). The depth of the pit varied between 1.401 μm and 2.106 μm, with an average depth of 1.785 μm and a standard deviation of 0.224 μm, as shown in
Figure 6b. The negative relationship between pit diameter and pit depth was not significant (
p = 0.1171).
The displacement of the torus exhibited a nonlinear increase when the applied pressure increased from 0 to 0.35 MPa, as shown in
Figure 7. Based on the analysis of the deflection gradient, the pit aspiration procedure can be divided into two distinct subprocesses. The initial subprocess involves the closure of the pit chamber by the torus, which was characterized by a high deflection gradient. Subsequently, the torus proceeds to seal the pit aperture, employing a lower deflection gradient. During the initial stage, the margo underwent elongation due to the exerted pressure, resulting in the displacement of the torus from its original centered position. As the torus was much thicker than the margo, the deformation of the margo was more significant than the torus deformation. With applied pressures ranging from 14.912 to 194.912 kPa (mean for the ten models of 61.874 kPa and a standard deviation of 59.4613 kPa), the torus closed the pit chamber and began to contact the pit borders with the center of the torus deflecting between 0.457 and 0.881 μm. Through the process of incorporating the exerted pressure onto the torus and margo surfaces, it was observed that the closure of the chamber necessitated forces within the range of 0.372 to 3.978 μN. The average force across the ten models was found to be 1.521 μN, with a standard deviation of 1.448 μN.
In the subsequent stage of the process, the interaction between the torus and the pit border provided support to the torus and hindered further deflection of the torus. The alteration of the torus shape played a significant role in augmenting the surface area of the contact between the torus and the pit border. The contact pressure saw a notable rise due to the limited contact area relative to the surface of the torus and the margo, where the external pressure was applied (
Figure 8). To effectively prevent the escape of air through the contact zone, it was necessary to apply a contact pressure that exceeded the external gas pressure. Additionally, the width of the contact region needed to be greater than the surface roughness on the pit border. To assess the minimal pressure necessary for effectively sealing bordered pits, a contact area width of more than 0.15 μm was used. This decision was based on the observation that the average diameter of warts typically seen on the pit border is less than 0.15 μm. Among the ten models examined, the sealing pressure exhibited a range of 42.412 to 275.912 kPa. The mean sealing pressure for these models was calculated to be 104.462 kPa, with a standard deviation of 70.504 kPa. Additionally, the closing-to-sealing pressure, defined as the difference between the sealing pressure and the closing pressure, varied from 10.5 to 81 kPa. The mean closing-to-sealing pressure for the ten models was determined to be 40.284 kPa, with a standard deviation of 21.879 kPa.
The statistical analysis between mechanics results and pit geometry presented in
Table 1 revealed significant dependencies on the pit diameter (
p < 0.05) and torus diameter (
p < 0.05) in terms of the pressure necessary to close the pit chamber. In comparison, the pit depth (
p < 0.1) exhibited a relatively weaker association. The results of the linear regression analysis (
Figure 9) demonstrated that the closing pressure had a negative correlation with the decrease in pit diameter (R = 0.424) and torus diameter (R = 0.400), as well as a positive relationship with the increase in pit depth (R = 0.356).
The pressure increase from the closing of the chamber to the sealing of the pit exhibited a significant dependence on the diameter of the torus (
p < 0.01), in comparison to the diameter of the pit (
p < 0.05). Nevertheless, the pit depth did not demonstrate statistical significance (
p > 0.1). The results of the linear regression analysis, as depicted in
Figure 10, suggested a negative relationship between the closing to sealing pressure and the pit diameter (R = 0.4719), as well as the torus diameter (R = 0.6105). Conversely, an increase in pit depth was associated with a positive but weak correlation with the closing to sealing pressure (R = 0.1689).
The evaluation of the effects of Young’s modulus and Poisson’s ratio on the closing pressure and the sealing pressure was conducted by implementing one out of ten models. The variation in the Young’s modulus value (
Figure 11a) ranging from 0.1 to 0.5 GPa exhibited a significant influence on the mechanical responses of the margo–torus structure. The relationship between the closure pressure and the sealing pressure exhibited a nearly proportionate trend with respect to Young’s modulus. Conversely, the impact of Poisson’s ratio on the pressures was found to be minimal, as indicated in
Figure 11b.
The analysis revealed a statistically significant positive connection (
p < 0.1) between the pit diameter and the mass of the torus and margo, as depicted in
Figure 12. In addition to the dimensions of pit diameter and pit depth, the dynamic modeling of the torus–margo structure was also affected by the inertia resulting from the mass of the torus and margo components. Under the applied pressure of 350 kPa, it was observed in
Figure 13 that the displacement of the torus exhibited a significant delay compared to the displacement of the margo, as the former possessed a significantly greater mass than the latter. For all the pit models, the duration for sealing pits was in the range of 2.68 × 10
−5 to 3.51 × 10
−5 s, with an average time of 3.05 × 10
−5 s and a standard deviation of 2.58 × 10
−6 s. The duration required for sealing pits was contingent upon the diameter of the pit (
p = 0.5148), the depth of the pit (
p = 0.3270), and the mass of the torus–margo structure (
p = 0.3394). The regression analysis conducted on the data (
Figure 14) revealed that there was a positive correlation between the time required for sealing pits and the decrease in pit diameter (R = 0.0629), as well as an increase in pit depth (R = 0.1369) and the mass of the torus and margo (R = 0.1305).
4. Discussion
The margo that elastically supported the torus underwent severe tensile deformation and caused a large deflection of the torus as a result of the external forces induced by the applied pressure. When the torus did not contact the pit border, the deformation of the torus was minimal compared to the margo. After the torus contacted the pit border, the applied pressure and the contact pressure drove the torus to distort, causing it to take on the profile of the pit border. The flexibility of the torus, which was also noted in micromechanical testing [
23], was crucial to creating the contact seal between the torus and the pit border, contrary to the assumption made in air-seeding models that the torus was rigid [
18,
21]. The contact pressure was greater than the external gas pressure inside the contact area. The bordered pit was able to stop the spread of the embolism due to the tight contact blocking the air leakage path in the interface. The simulation results supported the capillary seeding hypothesis that the torus’ adhesion to the pit border was the primary factor affecting cavitation resistance. The torus in
Pinus strobus L. observed using 4Pi and confocal laser scanning microscopy was a sandwich structure more complicated than the torus structure modeled in the present study, in which the dense and nonporous pectin layer on the surface encircled the inside cellulose layer [
14]. Although there may be differences in chemical composition and microstructure for the cellulose layer and the pectin layer across species, the presence of a sandwich structure has been consistently observed across several species [
40,
41]. Based on the empirical hypothesis that the pectin material was significantly softer than the cellulose material, it was advantageous for the torus sandwich structure that the inner cellulose layer was subject to external pressure load to prevent seal failure due to excessive deformation, while the soft pectin layer was able to fill and seal the roughness on the surface of the pit border under the squeezing forces in the contact area. The contact sealing mechanism between the torus and the pit border was substantiated by the link between the biological structure and the mechanical results.
The study’s predictions for the closure pressures were consistent with the pressure differential (between 33 and 470 kPa) calculated from measurements of hydraulic conductivity [
24,
42]. The margo membrane was extremely thin and possessed many pores, which made it highly compliant. The mean force required to close the pit (lower than 1.521 μN) also agreed with the force (much lower than 3 μN) measured in the test [
23]. The torus–margo structure needed more external pressure to seal bordered pits once the torus came into contact with the pit border. For ideally smooth surfaces, the sealing condition was that the two sealing surfaces had to come into contact with one another. Given that the pit border’s surface was rough, it was conservatively suggested that the sealing requirement for bordered pits be that the contact pressure in the contact region should be higher than the external gas pressure and the contact breadth should be greater than the surface roughness. It was crucial that both the contact pressure and the width of the contact zone grew when the applied pressure increased and the torus made contact with the pit border. This could be the cause of the conifers’ capacity to resist air-seeding spread in the tracheids’ water transportation system.
There was a complex link between the torus–margo structure and the mechanical reactions to the external pressure for all of the pits represented in the study, as evidenced by the variations in the closure pressure and the closing-to-sealing pressure. The margo flexibility, the external force, and the pit depth were the primary factors in closing pits. By increasing the margo’s surface area, the increase in pit diameter decreased the margo’s stiffness. The torus diameter increased and the pit depth reduced as the pit diameter rose, according to the positive connection between the torus diameter and the pit diameter, and the negative connection between the pit depth and the pit diameter, respectively. The increase in the pit diameter strengthened the structural flexibility, increased the external force on the torus–margo structure, and reduced the torus displacement required to close the pit chamber. As a result, a negative association was observed between the pit diameter and the closing pressure. Once the torus made contact with the pit border, the torus in the cross-section resembled that of a beam supported by two endpoints within the contact region. The augmentation of the torus diameter resulted in enhanced torus flexibility and facilitated greater interaction between the torus and the pit border. Simultaneously, the augmentation of the torus diameter resulted in the amplification of the external force. Hence, a negative association was seen between the diameter of the torus and the closing to sealing pressure. The findings of this study demonstrated that the geometric characteristics of the torus–margo structure play a significant role in influencing the safety valve effects during tracheid cavitation. According to the research in [
20], there were notable variations in the geometry of bordered pits across different species. Therefore, it was imperative to conduct a comparative analysis of the mechanical properties among the various conifer species.
The model results were significantly influenced by material parameters, such as the Young’s modulus. In line with previous studies on the mechanical analysis of the pit membrane [
28,
31], our model solution demonstrates a positive correlation between Young’s modulus values and both the closing pressure and sealing pressure. Despite the emphasis placed on the significance of material properties [
28,
31], the availability of experimental data for the pit membrane remained limited due to the inherent challenges associated with measuring these variables. Despite the presence of uncertainties in Young’s modulus values, the mechanical behavior in the model solutions remained reliable as long as the material properties between the margo and the torus did not exhibit considerable differences. To mitigate the occurrence of air-seeding, it was hypothesized that the Young’s modulus of the margo and the torus materials should not exhibit excessively high values. Otherwise, the torus–margo structure will be unable to effectively seal the bordered pit as a result of insufficient pressure differential between neighboring tracheids. To enhance the sealing capacity, it was important for species that exhibited resistance to cavitation to possess a margo with high flexibility. However, the deflection of the torus caused by a highly flexible margo under the pressure difference between functional tracheids would increase flow resistance [
18]. The margo flexibility was supposed as a viable indicator for assessing the trade-off qualities between hydraulic efficiency and safety in the xylem. On the other hand, many investigations have expressed concerns regarding the potential for the torus to be extracted through the pit aperture due to the significant flexibility of the margo [
18]. In the statistical analysis conducted on 115 conifer species [
20], it was consistently observed that the diameter of the torus exceeded the diameter of the pit aperture. This finding underscores the significance of the torus’s structural-load-carrying capacity in preventing torus prolapse. Given the assumption that the external force (about 60 μN) that pushed the torus out of the pit aperture was uniformly distributed across the surface area of the pit membrane [
23], the calculated pressure with the pit models in the study was between 2.02 and 2.94 MPa. The findings suggested that the torus touching the pit border exhibited a robust ability to withstand external pressure.
When air-seeding occurred within a tracheid, the sap within the tracheid underwent quick gasification due to the negative pressure. To prevent the spread of air-seeding through bordered pits, it is advisable to perform the torus aspiration in a swift manner. The analysis of the model solution revealed that the torus aspiration, when subjected to a pressure of 350 kPa, required around 10
−5 s to effectively seal bordered pits. According to the study [
43], it was found that the negative pressure, which was influenced by the height of conifers, may reach values lower than −2 MPa. Consequently, the duration required for the sealing pits could be significantly reduced. The structural inertia of the torus–margo structure, as determined by its mass in Equation (2), impeded the torus from sealing the bordered pit under cavitation pressure forces. Hence, the reduction in torus–margo mass resulted in a decrease in the duration needed for pit sealing. Based on the static results, it was found that an increase in the diameter of the pit led to an improvement in the flexibility of the margo, resulting in a reduction in the time required for sealing pits. The augmentation of the depth of the pit necessitated a greater degree of torus deflection in order to effectively seal the pits, resulting in a lengthier duration for the pit-sealing process. While the statistical associations between the pit width, pit depth, and mass of the torus–margo structure with the sealing time were found to be non-significant, the linear regression analysis provided support for the mechanical findings on the impacts of the torus–margo structure. In contrast to the static simulation, the dynamic reactions in the transient aspiration were influenced by a greater number of parameters. The increase in pit diameter resulted in contrasting impacts on the sealing time due to the combined influence of the enhanced margo flexibility and the increased torus and margo mass. The observed discrepancy between the relevance of the pit diameter and the anticipated value derived from the static simulation may be attributed to this factor. The complex relationship between the torus–margo structure and its dynamic responses was revealed through the investigation of the structure’s behavior under applied pressure. Hence, it was possible that the linear regression analysis did not adequately capture the relationship between the torus–margo structure and the sealing time. In comparison with the static simulation focusing on the sealing mechanism in aspirated bordered pits, the dynamic analysis preferred simulating transient responses during pit aspiration. The findings from both the static and dynamic analyses suggested that the aspiration of the bordered pit in conifers was a complex mechanical process that relied on the specific structural and material characteristics of the torus and margo.
The numerical results of the mechanical model originally elucidated the contact sealing process and unveiled the effects of the torus–margo structure. The findings suggested that employing a numerical simulation grounded in reliable mechanics theory could serve as a viable method for studying aspiration in bordered pits. This approach was particularly useful in investigating mesoscopic structures such as torus and margo, where performing experiments could be challenging. When compared to the study in [
31], the p-value obtained from the correlation analysis in the present study was found to be lower. In contrast to the analysis for the elastic deformation of the torus and margo structure in the study, the contact behavior in the present study exhibited a pronounced nonlinearity that was closely associated with the surface profile of the torus, margo, and pit border. Due to the presence of notable variations in the surface profile throughout each pit, the inevitable differences were detrimental to the effectiveness of the correlation analysis. To mitigate random error in the correlation analysis, it would be necessary to augment the dataset of pit borders.
To enhance the accuracy of the mechanical model, it was recommended that three key areas of investigation should be undertaken. The first involved the reconstruction of a more intricate sandwich configuration of the torus, accompanied by thorough observation [
14], as the presence of soft pectin surrounding the torus would substantially enhance the contact regions inside the interface between the torus and the pit border. The second was quantifying and characterizing the material properties of the torus and margo, specifically focusing on factors such as the orientation of the fibrils and the anisotropic nature [
44]. The final was to enhance the precision of fluid pressure exerted on the torus and margo, as it was observed to be non-uniform over the surface of the torus–margo [
45], and exhibited variations according to the deflection of the torus. The integration of fluid dynamics and solid mechanics in a coupling study was a viable and practical technique. Furthermore, the mechanical model that has been provided, incorporating intricate user-defined programming, could be utilized to conduct further investigations into the failure of the contact seal resulting from pressure overload and material yield.