The Co-Occurrence of Physiological and Epicotyl Physiological Dormancy in Three Desiccation-Sensitive Castanopsis (Fagaceae) Acorns from China with Specific Reference to the Embryonic Axis Position
Abstract
:1. Introduction
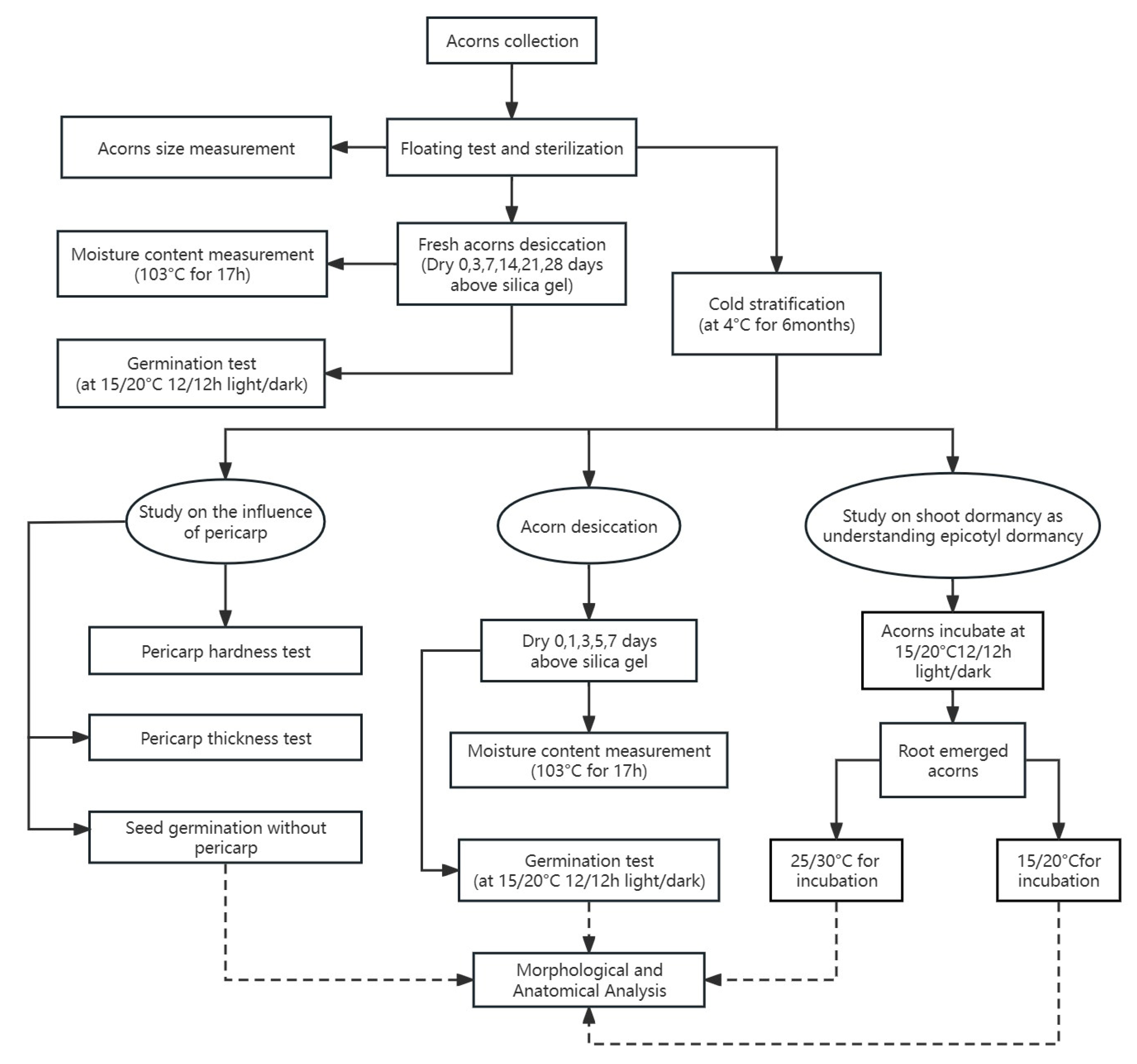
2. Materials and Methods
2.1. Sample Site and Acorn Collection
2.2. Basic Acorn Characteristics
2.3. Acorn Desiccation and Germination Tests
2.4. Dormancy Breaking Treatments
2.4.1. Effects of Cold Stratification (CS) on Fresh Acorns’ Physiological Dormancy (PD) Release and Desiccation Sensitivity
2.4.2. Effect of Warmer Temperature (Warm Stratification) on Shoot Dormancy Breaking in Root-Emerged Acorns
2.5. Morphological and Anatomical Analysis
2.6. Literature Review of Dormancy in Castanopsis
2.7. Statistical Analysis
3. Results
3.1. Acorn Basic Characteristics
3.2. Germination of Fresh and Cold-Stratified Acorns
4. Desiccation Response
4.1. Effects of Cold Stratification (CS) in Dormancy Break
4.2. Effect of Warm Stratification (WS) on Shoot Emergence
4.3. Acorn Morphology and Germination Type
4.4. Literature Review of Dormancy in Castanopsis
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kermode, A.; Finch-Savage, B. Desiccation sensitivity in orthodox and recalcitrant seeds in relation to development. In Desiccation and Survival in Plants Drying without Dying; CABI: Wallingford, UK, 2002; pp. 149–184. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Roberts, E.H. Predicting the Storage Life of Seeds. Seed Sci. Technol. 1973, 1, 499–514. [Google Scholar]
- Berjak, P.; Pammenter, N.W. From Avicennia to Zizania: Seed R ecalcitrance in Perspective. Ann. Bot. 2008, 101, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, H.W.; Daws, M.I.; Fletcher, B.J.; Gaméné, C.S.; Msanga, H.P.; Omondi, W. Ecological correlates of seed desiccation tolerance in tropical African dryland trees. Am. J. Bot. 2004, 91, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Tweddle, J.C.; Dickie, J.B.; Baskin, C.C.; Baskin, J.M. Ecological aspects of seed desiccation sensitivity. J. Ecol. 2003, 91, 294–304. [Google Scholar] [CrossRef]
- Jaganathan, G.K. Ecological insights into the coexistence of dormancy and desiccation-sensitivity in Arecaceae species. Ann. For. Sci. 2021, 78, 10. [Google Scholar] [CrossRef]
- Pritchard, H.W.; Tsan, F.Y.; Wen, B.; Jaganathan, G.K.; Calvi, G.; Pence, V.C.; Mattana, E.; Ferraz, I.D.; Seal, C.E. Regeneration in recalcitrant-seeded species and risks from climate change. In Plant Regeneration from Seeds: A Global Warming Perspective; Baskin, C.C., Baskin, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 259–273. [Google Scholar]
- Huang, C.J.; Zhang, Y.T.; Bartholomew, B. Fagaceae (Vol. 4). Flora of China (FOC); Science Press: Beijing, China, 1999. [Google Scholar]
- Manos, P.S.; Zhou, Z.C. Systematics of Fagaceae: Phylogenetic Tests of Reproductive Traitevolution. Int. J. Plant Sci. 2001, 162, 1361–1379. [Google Scholar] [CrossRef]
- Finch-Savage, W. Embryo water status and survival in the recalcitrant species Quercus robur L.: Evidence for a critical moisture content. J. Exp. Bot. 1992, 43, 663–669. [Google Scholar] [CrossRef]
- Bonner, F. Responses to Drying of Recalcitrant Seeds of Quercus nigra L. Ann. Bot. 1996, 78, 181–187. [Google Scholar] [CrossRef]
- Xia, K.; Daws, M.I.; Hay, F.R.; Chen, W.Y.; Zhou, Z.K.; Pritchard, H.W. A comparative study of desiccation responses of seeds of Asian evergreen oaks, Quercus subgenus Cyclobalanopsis and Quercus subgenus Quercus. S. Afr. J. Bot. 2012, 78, 47–54. [Google Scholar] [CrossRef]
- Suszka, B.; Zieta, L. A new presowing treatment for cold-stored beech (Fagus silvatica L.) seed. Arbor. Korn. 1977, 22, 237–255. [Google Scholar]
- POWO. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew; Published on the Internet. 2023. Available online: http://www.plantsoftheworldonline.org/ (accessed on 28 June 2023).
- Wu, J.-Y.; Ding, S.-T.; Li, Q.-J.; Zhao, Z.-R.; Dong, C.; Sun, B.-N. A new species of Castanopsis (Fagaceae) from the upper Pliocene of West Yunnan, China and its biogeographical implications. Palaeoworld 2014, 23, 370–382. [Google Scholar] [CrossRef]
- Society for Ecological Restoration, International Network for Seed Based Restoration and Royal Botanic Gardens Kew. Seed Information Database (SID). February 2023. Available online: https://ser-sid.org/ (accessed on 1 December 2022).
- Tian, M.; Tang, A. Seed Desiccation Sensitivity of Quercus fabri and Castanopsis fissa (Fagaceae). Seed Sci. Technol. 2010, 38, 225–230. [Google Scholar] [CrossRef]
- Xia, K.; Daws, M.I.; Stuppy, W.; Zhou, Z.K.; Pritchard, H.W. Rates of Water Loss and Uptake in Recalcitrant Fruits of Quercus Species are Determined by Pericarp Anatomy. PLoS ONE 2012, 7, e47368. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jaganathan, G.K.; Han, K.; Liu, B. Embryo position of Castanopsis sclerophylla (Fagaceae) seeds with recalcitrant storage behavior differs from Quercus genus but response to desiccation shows no difference. Botany 2022, 100, 401–407. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, L.; Li, Q. Effects of different mechanical treatments on Quercus variabilis, Q. wutaishanica and Q. robur acorn germination. Iforest-Biogeosci. For. 2015, 8, 728–734. [Google Scholar] [CrossRef]
- Yan, X.F.; Qiu, Z.H.; Zhang, Q.; Zhang, K.W.; Zhou, Y.F. Effects of coat and sowing depth on seed germination and early seedling growth of Quercus wutaishanica. Ying Yong Sheng Tai Xue Bao 2014, 25, 53–60. [Google Scholar]
- Hawkins, T.S. Regulating acorn germination and seedling emergence in Quercus pagoda (Raf.) as it relates to natural and artificial regeneration. New For. 2019, 50, 425–436. [Google Scholar] [CrossRef]
- Hawkins, T.S. Dormancy break and germination requirements in acorns of two bottomland Quercus species (Sect. Lobatae) of the eastern United States with references to ecology and phylogeny. Seed Sci. Res. 2020, 30, 199–205. [Google Scholar] [CrossRef]
- Farmer, R.E., Jr. Epicotyl Dormancy in White and Chestnut Oaks. For. Sci. 1977, 23, 329–332. [Google Scholar]
- Sun, X.; Song, Y.; Ge, B.; Dai, X.; Kozlowski, G. Intermediate epicotyl physiological dormancy in the recalcitrant seed of Quercus chungii F.P. Metcalf with the elongated cotyledonary petiole. Forests 2021, 12, 263. [Google Scholar] [CrossRef]
- Ng, F.S. Manual of forest fruits, seeds and seedlings. Malay. For. Rec. 1991, 2, 401–997. [Google Scholar]
- Huang, L.; Jin, C.; Zhou, L.; Song, K.; Qian, S.; Lin, D.; Zhao, L.; Chen, B.; Yan, E.; Michalet, R.; et al. Benefit versus sost trade-offs of masting across seed-to-seedling transition for a dominant subtropical forest species. J. Ecol. 2021, 109, 3087–3098. [Google Scholar] [CrossRef]
- Blakesley, D.; Elliott, S.; Kuarak, C.; Navakitbumrung, P.; Zangkum, S.; Anusarnsunthorn, V. Propagating framework tree species to restore seasonally dry tropical forest: Implications of seasonal seed dispersal and dormancy. For. Ecol. Manag. 2002, 164, 31–38. [Google Scholar] [CrossRef]
- Yu, Y.; Baskin, J.M.; Baskin, C.C.; Tang, Y.; Cao, M. Ecology of seed germination of eight non-pioneer tree species from a tropical seasonal rain forest in southwest China. Plant Ecol. 2008, 197, 1–16. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. The great diversity in kinds of seed dormancy: A revision of the Nikolaeva–Baskin classification system for primary seed dormancy. Seed Sci. Res. 2021, 31, 249–277. [Google Scholar] [CrossRef]
- Yang, Q.; Ye, W.; Zhang, Y. Eco-physiological Characteristics of Germination and Storage of Castanopsis chinensis Seeds. J. Beijing For. Univ. 2005, 27, 92–95. [Google Scholar]
- Guo, F.; Huang, M.; Lou, Z. Propositions on cultivation of precious tree resources in Ganzhou city. South China For. Sci. 2015, 43, 42–45. [Google Scholar]
- Liu, Z. Weather and Climate of Ganzhou: Climate Center of Jiangxi Province. 2020. Available online: https://www.ganzhou.gov.cn/gzszf/c100146/201206/3cd6f72317f64d508eb5b6db2d8c10b0.shtml2020-11-16 (accessed on 2 April 2023).
- ISTA. The International Seed Testing Association; ISTA: Bassersdorf, Switzerland, 2020. [Google Scholar]
- Dussert, S.; Chabrillange, N.; Engelmann, F.; Hamon, S. Quantitative estimation of seed desiccation sensitivity using a quantal response model: Application to nine species of the genus Coffea L. Seed Sci. Res. 1999, 9, 135–144. [Google Scholar] [CrossRef]
- Pasquini, S.; Mizzau, M.; Petrussa, E.; Braidot, E.; Patui, S.; Gorian, F.; Lambardi, M.; Vianello, A. Seed storage in polyethylene bags of a recalcitrant species (Quercus ilex): Analysis of some bio-energetic and oxidative parameters. Acta Physiol. Plant. 2012, 34, 1963–1974. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. A Classification System for Seed Dormancy. Seed Sci. Res. 2004, 14, 1–16. [Google Scholar] [CrossRef]
- Feng, D.; Zhang, L.; Huang, Z.; Chen, J.; Zhu, X. Effects of gap size on the seed germination and seedling growth of Castanopsis carlesii and Castanopsis fargesii. J. Northwest A&F Univ. Nat. Sci. Ed. 2013, 41, 61–66. [Google Scholar]
- Du, Y.; Huang, Z. Effects of seed mass and emergence time on seedling performance in Castanopsis chinensis. For. Ecol. Manag. 2008, 255, 2495–2501. [Google Scholar] [CrossRef]
- Chen, Z.; Peng, J.; Zhang, D.; Zhao, J. Seed germination and storage of woody species in the lower subtropical forest. J. Integr. Plant Biol. 2002, 44, 1469–1476. [Google Scholar]
- Chen, J. Practical cultivation techniques of Castanopsis fargesii. Beijing Agric. 2015, 23, 84–85. [Google Scholar]
- Gao, X.; Huang, L.; Yang, C.; Yang, Y.; Yuan, X. Seed Germination Characteristics of the Dominate Tree Castanopsis fargesii in Evergreen Broadleaved Forest on Mt.Jinyun. J. Chongqing Norm. Univ. (Nat. Sci.) 2016, 33, 127–133. [Google Scholar]
- Qiu, F.; Yang, L.; Xiao, F.; Cheng, Q. Experimental research on seed storage and germination of Castanopsis fargesii. For. Sci. Technol. 2015, 11, 30–33. [Google Scholar]
- Liu, J.; Zhong, Z. Nature of seed sain, the seed bank and regeneration of a Castanopsis fargesii community on Fanjing mountain. Acta Phytoecol. Sin. 2000, 24, 402–407. [Google Scholar]
- Cornelissen, J.; Zhong, Z.; Nelaan, S. Timing of germination in the subtropical Chinese tree Castanopsis fargesii. J. Southwest China Norm. Univ. 1994, 19, 419–430. [Google Scholar]
- Chen, B.; Da, L.; Song, Y. Seed Germination and Seedling Growth of Castanopsis fargesii in Evergreen Broadleaved Forest and in Gaps. J. Trop. Subtrop. Bot. 2002, 10, 207–214. [Google Scholar]
- Wang, J.; Wang, Z.; Yang, L.; Ren, H. Effects of Litter Coverage and Watering Frequency on Seed Germination and Seedling Survival of Castanopsis fissa. Chin. J. Appl. Ecol. 2008, 19, 2097–2102. [Google Scholar]
- Chen, W. The Seed Emergence of Castanopsis hystrix Affected by Forest Gap and Undergrowth Environment. J. Fujian For. Sci. Technol. 2007, 34, 40–41. [Google Scholar]
- Feng, T. The experiment on seedling raising of Castanopsis hystrix with concentrated sulfuric acid. Agric. Res. Appl. 2015, 6, 9–11. [Google Scholar]
- Hasnat, G.N.T.; Hossain, M.A.; Hossain, M.K.; Uddin, M.M. Effect of Pre-Sowing Treatments on Germination and Initial Seedling Growth of Castanopsis Indica—An Endangered Tree Species in Bangladesh. J. For. Environ. Sci. 2019, 35, 223–231. [Google Scholar]
- Wang, Z.; Lan, Y.; He, Z.; Liu, J.; Xing, C.; Zhu, J.; Wang, X.; Shi, Y.; Shen, C. Effects of Castanopsis kawakamii Forest Litter Extract on its Seed Germination and Radicle Growth. J. Fujian Agric. For. Univ. (Nat. Sci. Ed.) 2020, 49, 51–58. [Google Scholar]
- Huang, J. The Seedling Nursing Technology of Castanopsis kawakamii. For. Environ. Sci. 2009, 25, 91–92. [Google Scholar]
- He, Z.; Liu, J.; Hong, W.; Zheng, S.; Wu, C.; Wu, Z.; Lin, Y.; Su, S. Effects of dfferent treatments on seed germination of Castanopsis kawakamii. J. Beijing For. Univ. 2012, 34, 66–70. [Google Scholar]
- Qiu, S. Effects of different treatments on seed germination of Castanopsis lamontii. Sci. Technol. Qinghai Agric. For. 2018, 4, 12–15. [Google Scholar]
- Le, X.; Wang, Z.; Ning, X.; Sun, X.; Song, Y. The Germination Characteristics and Dormancy Type of the Seed of Castanopsis sclerophylla. Bull. Bot. Res. 2022, 42, 688–693. [Google Scholar]
- Junwen, Y. An Analysis of Variation in Traits of Seeds and Seedlings and Genetic Diversity in Castanopsis Tibetana of Zhejiang and Anhui. Master’s Thesis, Zhejiang A&F University, Hangzhou, China, 2017. [Google Scholar]
- Bonner, F.; Vozzo, J. Seed Biology and Technology of Quercus; US Department of Agriculture, Forest Service, Southern Forest Experiment Station: New Orleans, LA, USA, 1987.
- Kang, H.; Jaganathan, G.K.; Han, Y.; Li, J.; Liu, B. Revisiting the pericarp as a barrier restricting water entry/loss from cotyledons and embryonic axis of temperate desiccation-sensitive Quercus acorns. Planta 2023, 257, 33. [Google Scholar] [CrossRef] [PubMed]
- Bonner, F. Water uptake and germination of red oak acorns. Bot. Gaz. 1968, 129, 83–85. [Google Scholar] [CrossRef]
- Nikolić, N.P.; Merkulov, L.S.; Krstić, B.Đ.; Pajević, S.P.; Borišev, M.K.; Orlović, S.S. Variability of acorn anatomical characteristics in Quercus robur L. genotypes. Zb. Matice Srp. Za Prir. Nauk. 2010, 2010, 47–58. [Google Scholar] [CrossRef]
- Joët, T.; Ourcival, J.-M.; Dussert, S. Ecological significance of seed desiccation sensitivity in Quercus ilex. Ann. Bot. 2013, 111, 693–701. [Google Scholar] [CrossRef]
- Finchsavage, W.E. Seed Development in the Recalcitrant Species Quercus robur L.: Germinability and Desiccation Tolerance. Seed Sci. Res. 1992, 2, 17–22. [Google Scholar] [CrossRef]
- Amimi, N.; Dussert, S.; Vaissayre, V.; Ghouil, H.; Doulbeau, S.; Costantini, C.; Ammari, Y.; Joët, T. Variation in seed traits among Mediterranean oaks in Tunisia and their ecological significance. Ann. Bot. 2020, 125, 891–904. [Google Scholar] [CrossRef]
- Peterson, J.K. Mechanisms Involved in Delayed Germination of Quercus nigra L. Seeds. Ann. Bot. 1983, 52, 81–92. [Google Scholar] [CrossRef]
- Xia, K.; Daws, M.I.; Peng, L.L. Climate drives patterns of seed traits in Quercus species across China. New Phytol. 2022, 234, 1629–1638. [Google Scholar] [CrossRef]
- Wigston, D. Epicotyl dormancy in Quercus robur L. Q. J. For. 1987, 81, 10–12. [Google Scholar]
- McCartan, S.A.; Jinks, R.L.; Barsoum, N. Using thermal time models to predict the impact of assisted migration on the synchronization of germination and shoot emergence of oak (Quercus robur L.). Ann. For. Sci. 2015, 72, 479–487. [Google Scholar] [CrossRef]
- Calme, S.; Bigras, F.J.; Margolis, H.A.; Hebert, C. Frost tolerance and bud dormancy of container-grown yellow birch, red oak and sugar maple seedlings. Tree Physiol. 1994, 14, 1313–1325. [Google Scholar] [CrossRef]
- Falusi, M.; Calamassi, R. Dormancy of Fagus sylvatica L. buds III. Temperature and hormones in the evolution of dormancy in one-node cuttings. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2003, 137, 185–191. [Google Scholar]
- Bronnum, P. Assessment of seedling storability of Quercus robur and Pinus sylvestris. Scand. J. For. Res. 2005, 20, 26–35. [Google Scholar] [CrossRef]
- Harper, R.C.; O’Reilly, C. Stress resistance levels at the time of lifting affect post-planting performance of Quercus robur seedlings. Plant Biosyst. 2013, 147, 583–592. [Google Scholar] [CrossRef]
- Merouani, H.; Branco, C.; Almeida, M.H.; Pereira, J.S. Effects of acorn storage duration and parental tree on emergence and physiological status of Cork oak (Quercus suber L.) seedlings. Ann. For. Sci. 2001, 58, 543–554. [Google Scholar] [CrossRef]
- Tilki, F.; Alptekin, C.U. Germination and seedling growth of Quercus vulcanica: Effects of stratification, desiccation, radicle pruning, and season of sowing. New For. 2006, 32, 243–251. [Google Scholar] [CrossRef]
- Jastrzębowski, S.; Ukalska, J.; Walck, J.L. Does the lag time between radicle and epicotyl emergences in acorns of pedunculate oak (Quercus robur L.) depend on the duration of cold stratification and post-stratification temperatures? Modelling with the sigmoidal growth curves approach. Seed Sci. Res. 2021, 31, 105–115. [Google Scholar] [CrossRef]
- Yi, X.; Bartlow, A.W.; Curtis, R.; Agosta, S.J.; Steele, M.A. Responses of seedling growth and survival to post-germination cotyledon removal: An investigation among seven oak species. J. Ecol. 2019, 107, 1817–1827. [Google Scholar] [CrossRef]






| Species | C. chinensis | C. purpurella | C. sclerophylla |
|---|---|---|---|
| 100 acorn mass (g) | 56.10 ± 0.64 a | 96.93 ± 1.17 b | 99.23 ± 1.26 b |
| Initial moisture content (% FWB) | 32.69 ± 0.73 a | 35.49 ± 2.21 ab | 38.01 ± 1.33 b |
| Height (mm) | 10.38 ± 0.83 a | 13.20 ± 1.06 b | 13.12 ± 1.05 b |
| Width (mm) | 9.90 ± 0.56 a | 11.84 ± 1.00 b | 11.96 ± 0.95 b |
| Scar diameter (mm) | 8.09 ± 0.77 a | 10.59 ± 1.25 b | 10.70 ± 1.14 b |
| Ratio of scar diameter to width | 0.817 ± 0.056 a | 0.893 ± 0.057 b | 0.888 ± 0.052 b |
| Scar thickness (mm) | 0.837 ± 0.151 a | 0.717 ± 0.138 b | 0.651 ± 0.143 b |
| Main pericarp thickness (mm) | 0.620 ± 0.073 a | 0.537 ± 0.104 b | 0.465 ± 0.080 c |
| Scar hardness (N) | 20.564 ± 5.46 a | 11.011 ± 3.63 b | 7.266 ± 2.36 c |
| Main pericarp hardness (N) | 20.769 ± 5.10 a | 10.154 ± 3.34 b | 6.241 ± 1.89 c |
| Natural dispersal time | September–December | September–December | October–December |
| Vi | R2 | WC50 (FWB) | b | ||
|---|---|---|---|---|---|
| Fresh acorns | C. chinensis | 94.67 | 1 | 22.627 ± 0.001 | 0.87509 |
| C. purpurella | 85.33 | 0.99776 | 27.848 ± 0.214 | 0.50127 | |
| C. sclerophylla | 82.67 | 0.99952 | 28.594 ± 0.090 | 0.44457 | |
| CS acorns | C. chinensis | 85.33 | 0.99880 | 24.413 ± 0.077 | 0.73186 |
| C. purpurella | 61.33 | 0.79398 | 27.347 ± 1.333 | 0.44289 | |
| C. sclerophylla | 94.67 | 0.96791 | 27.450 ± 0.625 | 0.43443 |
| Species | Germination Time (Days) | Drying Period | F Ratio | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| 0 (Day) | 1 (Days) | 3 (Days) | 5 (Days) | 7 (Days) | ||||
| C. chinensis | 0–30 | 30.67 ± 0.47 Aa | 45.33 ± 1.70 Ab | 14.67 ± 1.25 Ac | 4.00 ± 0.00 Acd | 0.00 ± 0.00 Ad | 48.024 | 0.000 |
| 30–60 | 36.00 ± 1.25 ABa | 10.67 ± 0.00 Bb | 0.00 ± 0.00 Bb | 0.00 ± 0.00 Ab | 0.00 ± 0.00 Ab | 42.813 | 0.000 | |
| 60–90 | 18.67 ± 1.25 Ba | 2.67 ± 0.00 Bb | 0.00 ± 0.00 Bb | 0.00 ± 0.00 Ab | 0.00 ± 0.00 Ab | 9.300 | 0.002 | |
| F ratio | 6.650 | 28.933 | 17.286 | 1.750 | ||||
| p-Level | 0.030 | 0.001 | 0.003 | 0.252 | ||||
| C. purpurella | 0–30 | 9.33 ± 1.25 Aa | 37.33 ± 1.25Ab | 9.33 ± 2.05 Aa | 4.00 ± 0.82 Aa | 0.00 ± 0.00 Aa | 16.875 | 0.000 |
| 30–60 | 10.67 ± 2.45 Aab | 14.67 ± 1.41 Ba | 8.00 ± 1.89 Aab | 1.33 ± 1.25 Ab | 0.00 ± 0.00 Ab | 7.233 | 0.005 | |
| 60–90 | 17.33 ± 1.25 Aa | 9.33 ± 0.47 Bab | 2.67 ± 2.16 Ab | 0.00 ± 0.00 Ab | 0.00 ± 0.00 Ab | 10.433 | 0.001 | |
| F ratio | 1.476 | 20.722 | 0.913 | 1.750 | ||||
| p-Level | 0.301 | 0.002 | 0.451 | 0.252 | ||||
| C. sclerophylla | 0–30 | 41.33 ± 0.94 Aa | 50.67 ± 2.49 Aa | 28.00 ± 2.83 Aab | 16.00 ± 1.41 Abc | 0.00 ± 0.00 Ac | 14.760 | 0.000 |
| 30–60 | 32.00 ± 0.94 Ba | 29.33 ± 1.63 ABa | 22.67 ± 1.70 Aa | 1.33 ± 1.70 Bb | 2.67 ± 1.70 Ab | 46.423 | 0.000 | |
| 60–90 | 12.00 ± 0.94 Cab | 14.67 ± 0.47 Ba | 12.00 ± 0.47 Aab | 1.33 ± 1.70 Bab | 0.00 ± 0.00 Ab | 5.000 | 0.018 | |
| F ratio | 94.750 | 12.289 | 2.036 | 11.000 | 4.000 | |||
| p-Level | 0.000 | 0.008 | 0.211 | 0.010 | 0.079 | |||
| Species | Temperature (°C) | Germination Time (days) | Germination (%) | Seed Collection Time | Time of Initiation of Germination | Incubation Environment | Pre-Sowing Treatment | Dormancy Type | Germination Judgment | Collection Site (Province) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| C. carlesii | >150 | 55 | December | May–June | Soil burial test | PD 3 + ePD | Radicle/seedling emergence | Chongqing | [39] | ||
| C. chinensis | 90–120 | 24–66 | December | Mid March | Nursery (shade house) | PD 3 + ePD | Seedling emergence | Guangdong | [40] | ||
| C. chinensis | 25/20 | 27 | Chamber | Soak in water for 12 h | ND | Guangdong | [41] | ||||
| C. chinensis | 20/35, 18~32 | 3–7 in incubator, 60-180 in field | >60 | November | Chamber/soil burial test | ePD | Radicle/seedling emergence | Guangdong | [32] | ||
| C. fargesii | Late October–early November | April–May | Nursery | CS in wet sand; Soak in water for 3–5 d | PD * | Seedling emergence | Chongqing | [42] | |||
| C. fargesii | 23/13 | 4–20 | 85–64 | November | March | Chamber | Storage in wet sand for 4 months | PD 3 | Radicle emergence | Chongqing | [43] |
| C. fargesii | 25 | 6–20 | 95 | December | February | Chamber | Storage for winter and soak in cold water for 24 h | PD * | Radicle emergence | Jiangxi | [44] |
| C. fargesii | 60–90 | 11 seeds, 45 seedlings | December | February | Soil seed bank | PD 2 | Seedling emergence | Guizhou | [45] | ||
| C. fargesii | >150 | 59 | December | May–June | Soil burial test | PD 3 + ePD | Radicle/seedling emergence | Chongqing | [39] | ||
| C. fargesii | 51.9–82.5 | 66.6–81.0 | November | May | Soil burial test | Storage in wet sand, fridge (3 °C), and wet soil for 3 months | PD 3 | Chongqing | [46] | ||
| C. fargesii | 120 | 31.7–33.3 | November–December | April | Soil burial test | PD 3 + ePD | Radicle/seedling emergence | Zhejiang | [47] | ||
| C. fargesii | >150 germination, >210 seedling | October–November | Soil seed bank | PD 3 + ePD | Seedling emergence | Chongqing | [28] | ||||
| C. fissa | 31.5 | 4–60 | 30–35 | December | March | Greenhouse | Storage at 4 °C for 3 months | PD 2, PD 3 | Guangdong | [48] | |
| C. fissa | 25/20 | 10 | Chamber | Soak in water for 12 h | ND | Guangdong | [41] | ||||
| C. purpurella | 30 | 6–20 | 97 in incubator, 25–59 in the field | September–November | Chamber/shade house | ND | Yunan | [30] | |||
| C. purpurella | 120 | 7.2 seedlings | May–June | Soil burial test | PD 3 + ePD | Seedling emergence | Fujian | [49] | |||
| C. purpurella A. DC | 68–98 | Nursery | Soak in 98% concentrated sulphuric acid for 15 to 30 min | PD * | Guangdong | [50] | |||||
| C. indica | 174.6 | 25–67 | August | October | Nursery | Control; sandpaper rubbing | PD 3 + ePD | Seedling emergence | Bangladesh | [51] | |
| C. kawakamii | 25/15 | 57 | November–December | Chamber | Storage at 5 °C for 60–80 d, 50 mg/L GA3 24 h, manual scratching of seed coat | PD 2 | Fujian | [52] | |||
| C. kawakamii | 15~20 | 20 | September–November | Soil burial test | Storage in wet sand for 60–80 d, 300–500 mg/L GA3 24 h | PD 2 | Guangdong | [53] | |||
| C. kawakamii Hayata | 25 | 13.54 | 63–78 | October | Artificial climate chamber | Storage in wet sand at 5 °C for 60–80 d; 10–50 mg/L GA3 24 h; remove the seed coat; incubation at 40–50 °C for 2.5 h | PD 2 | Radicle emergence | Fujian | [54] | |
| C. lamontii | 30–20 | 82 | October | Artificial climate chamber | Soak in water at 40 °C for 24 h, 50 mg/L GA3 24 h | PD 1 | Fujian | [55] | |||
| C. sclerophylla | 15–35 | 16 | 100 without pericarp | October | Chamber | Storage at 4 °C, remove the pericarp | PD * | Jiangxi | [56] | ||
| C. tibetana Hance | 28/20 | 39 | late October | April | Artificial climate chamber | Storage in wet sand; soak in water for 24 h | PD * | Zhejiang, Anhui | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Jaganathan, G.K.; Kang, H.; Liu, B. The Co-Occurrence of Physiological and Epicotyl Physiological Dormancy in Three Desiccation-Sensitive Castanopsis (Fagaceae) Acorns from China with Specific Reference to the Embryonic Axis Position. Forests 2023, 14, 2330. https://doi.org/10.3390/f14122330
Li J, Jaganathan GK, Kang H, Liu B. The Co-Occurrence of Physiological and Epicotyl Physiological Dormancy in Three Desiccation-Sensitive Castanopsis (Fagaceae) Acorns from China with Specific Reference to the Embryonic Axis Position. Forests. 2023; 14(12):2330. https://doi.org/10.3390/f14122330
Chicago/Turabian StyleLi, Jiajin, Ganesh K. Jaganathan, Han Kang, and Baolin Liu. 2023. "The Co-Occurrence of Physiological and Epicotyl Physiological Dormancy in Three Desiccation-Sensitive Castanopsis (Fagaceae) Acorns from China with Specific Reference to the Embryonic Axis Position" Forests 14, no. 12: 2330. https://doi.org/10.3390/f14122330





