Abstract
Over the years, Eucalyptus plantations have rapidly expanded in Sarawak, Malaysia, accounting for 19% of the total forest plantation area. In a routine forest health surveillance conducted in 2022 at Sarawak, Malaysia, tree stands of Eucalyptus urograndis (Eucalyptus grandis × Eucalyptus urophylla hybrid) were detected with symptoms of stem canker disease caused by Chrysoporthe infection. Given the limited information on the chemical control of Chrysoporthe stem canker disease, there is a growing need to develop effective chemical control strategies to protect and cure Chrysoporthe infection on E. urograndis trees. Therefore, this study aimed to identify the causal pathogen of this stem canker disease in 7-year-old E. urograndis trees in Sarawak, Malaysia, and evaluate the efficacy of various fungicides as curative or protectant treatments on canker infection using artificial inoculation methods. Fungal isolates were first collected and subjected to molecular identification and pathogenicity analysis. Then, in vitro efficacy tests were evaluated using five licensed fungicides: thiram, prochloraz manganese chloride, copper hydroxide, dimethomorph, and mancozeb. Subsequently, the performance of these fungicides was assessed through preventive and curative field experiments on 10-year-old E. urograndis trees using the artificial inoculation technique. Based on the morphological and phylogenetic analysis of the ITS1/ITS4, β-tubulin 2 (BT2), and the combined ITS1/ITS4 and BT2 sequences extracted from 20 fungal isolates, Chrysoporthe deuterocubensis was identified as the causal pathogen of the canker disease, with isolate CHRY18 recording the highest virulence. The in vitro efficacy tests showed that prochloraz manganese chloride achieved 100% inhibition against C. deuterocubensis at 1.0 mg/mL. In the preventive experiment, thiram significantly inhibited C. deuterocubensis infection, yielding the shortest lesion length (19.40 mm) compared to the non-treated control (47.48 mm) at 20 weeks post-inoculation. In the curative experiment, a significant reduction of 54.7% in lesion length was observed in inoculated symptomatic trees after 20 weeks of post-fungicide treatment with copper hydroxide. In conclusion, this study demonstrated prochloraz manganese chloride, thiram, and copper hydroxide as effective chemical controls of C. deuterocubensis stem canker on E. urograndis.
1. Introduction
Malaysia has actively expanded its tree plantation area since 1996, with over 500,000 ha of new tree plantation area in the Eastern Territory of Sarawak. The State Government has planned to designate 1 million ha of tree plantation area by 2025 [1,2] as a long-term strategy to support its wood-based industry with a new source of wood material. The three primary plant species planted include Acacia mangium Willd., Falcataria moluccana (Miq.) Barneby & J.W. Grimes (batai), Fabaceae (alt. Leguminosae), and Eucalyptus pellita F. Muell [3]. Although A. mangium is the dominant species among the three, E. pellita and its hybrids are being planted more lately due to the reduced volume production of A. mangium following their rapid destructive stem-wilt canker disease caused by Ceratocystis spp. that severely deteriorates the health and vitality of the plant [4].
Additionally, planters in Sarawak are uncertain about the yield and sustainability of A. mangium with subsequent rotation, prompting them to replace the species with other alternative tree species, such as E. pellita and its hybrids and Acacia crassicarpa A. Cunn. ex Benth [5]. While a study in 2019 characterised the disease as having poor virulence and that the risk of virulence may increase over successive rotations, reports of Ceratocystis disease incidence in Malaysia remain relatively low at 7.5% [3], reflecting the limited critical evaluation of this disease in the country, especially in Sarawak.
Eucalyptus and its hybrids belong to the myrtle family [Myrtaceae] and are native to Australia. They are one of the most preferred fast-growing industrial forest tree species in the tropics, subtropical, and temperate regions, with over 22.57 million ha of planted areas across 95 countries, equivalent to 30% of the global forest plantation area [6,7]. One of the essential Eucalyptus hybrids in the world and Malaysia is Eucalyptus urograndis [8]. The tropical interspecies between Eucalyptus grandis × E. urophylla was first hybridised in Brazil in the 1980s, a nation known for its global standard for clonal forest production [9,10,11]. The hybrid species is ideal for various end-product utilisations, such as solid wood, charcoal, pulp and paper, given its advantageous properties, including rapid growth and yield that can exceed an average of >40 m3/ha/year, greater resistance to pest and disease attacks, exceptional rooting ability, and excellent wood quality [9,11].
Despite that Eucalyptus appears to be a promising alternative plantation tree, there were concerns raised regarding their susceptibility and vulnerability against disease threats, ranging from the most common leaf diseases caused by Mychosphaerella and Teratosphaeria species [12,13,14], Ceratocystis wilt disease caused by Ceratocystis species [15,16], Myrtle rust disease [17,18], and stem canker disease caused by Cryphonectriaceae [19,20,21] and Botryosphaericeae [15,22]. Chrysoporthe canker is an infection caused by the genus Crysoporthe Gryzenh. and M.J. Wingf, a vital genus in the family Cryphonectriaceae (Diaporthales, Ascomycota) [19,23,24]. This pathogenic disease is detrimental to young Eucalyptus spp. stands and weaken the wood qualities in stems of older trees, frequently leading to stem rupture with altered forms and reduced volume [23].
Recently, two Chrysoporthe species that have been found to infect Eucalyptus tree plantations worldwide were identified in Malaysia, namely Chrysoporthe deuterocubensis and its sibling species Chrysoporthe cubensis [19,20,23]. The basal cankers caused by C. deuterocubensis can spread several metres up the stem and are lethal to young and juvenile trees [3]. Based on a pathogenicity test on C. deuterocubensis isolated in Sabah, Malaysia, the species was pathogenic to E. grandis trees and Eucalyptus deglupta [21].
C. deuterocubensis is the causal pathogen for stem canker disease in E. urogandis. The current management controls for mitigating Chrysoporthe stem canker include excising the canker, chopping down and burning the trees, or simply leaving it alone, depending on the severity of the infection. To date, there is limited information on the chemical control of Chrysoporthe stem canker disease. Besides, in vivo studies on the efficacy of fungicides as protectants and curative treatment against these species are scarce, with most of the available research mainly focusing on characterising the species, such as taxonomy, host range, and geographic distribution [19,20,21,25]. Hence, the sustainability of Eucalyptus spp. and the overall forest plantation sector depends on recognising emerging infectious diseases and incorporating effective chemical management strategies with proper silviculture techniques.
In view of the lack of study on chemical control and the need to develop chemical strategies for protection and wound healing of E. urograndis trees from Chrysoporthe infection, this study aimed to determine the canker disease-causing pathogens and evaluate the efficacy of a range of fungicide products through curative or protectant treatments on canker infection of C. deuterocubensis using artificial inoculation methods. Hypothetically, at least one of the various currently available fungicides in Malaysia is significantly more effective than the others in inhibiting the growth of C. deuterocubensis on E. urograndis and could be applied in the long-term Integrated Disease Management (IDM) strategies of Eucalyptus and its hybrids in Malaysia.
2. Methods
2.1. Disease Symptoms and Sample Collection
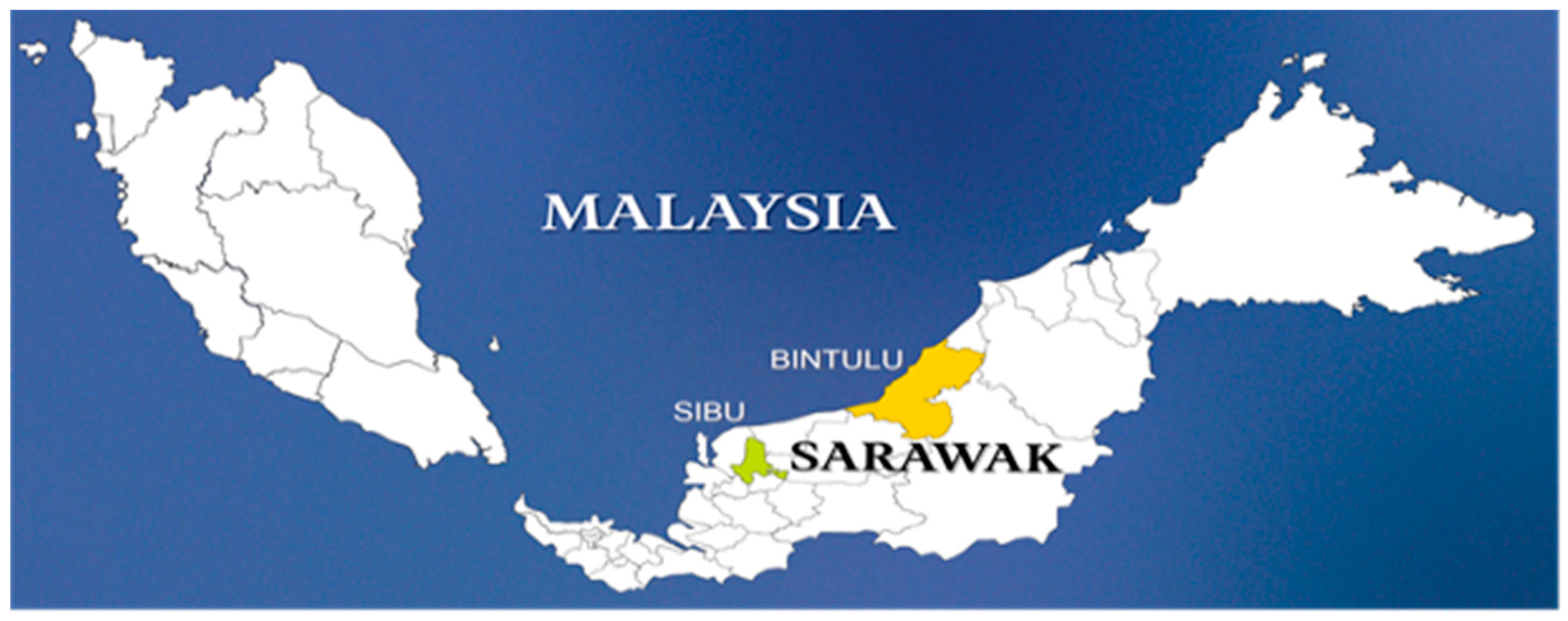
The disease survey was conducted in November 2022 by sampling a total of ten 7-year-old E. urograndis trees in Sibu and another 10 in Bintulu, Sarawak, Malaysia (Figure 1). Table 1 lists the relevant information on the sampling process. Fungal isolation was performed by collecting bark sections from the stem canker surface bearing fruiting structures resembling Chrysoporthe spp., including ascostromata and conidiomata. Subsequently, the samples were packed in large paper bags and labelled before being transported to the Forest Pathology Laboratory, Industrial Forest Research Centre (IFRC), Forest Department Sarawak in Kuching, Sarawak. The samples were then stored at 4 °C.
Figure 1.
Location of sampling sites in Bintulu and Sibu, Sarawak, Malaysia (less than 1000 m above sea level).

Table 1.
Details of the sampling sites.
In order to encourage spore formation from the fruiting bodies, the samples were first incubated in moist chambers for 1–3 days. During the isolation process, single ascospore and/or conidial masses from pycnidia [24] were transferred to sterile Petri dishes containing 2% (w/v) Difco® Malt Extract Agar (MEA) (BD Diagnostics, Franklin Lakes, NJ, USA) and incubated at 25 °C.
2.2. Identification of Isolated Fungal Pathogens
Morphological identification was conducted according to past literature [19,21,23] at the Forest Pathology Laboratory, IFRC, Forest Department Sarawak, Kuching. The workstation consisted of a Leica ICC50HD compound microscope and an Olympus SZ61 stereo microscope (SZ61TR2X-DI-TP-118000A-SET; Olympus Corporation, Tokyo, Japan) connected to a laptop for data and image acquisition.
All 20 individual fungal isolates were confirmed microscopically to be those of Chrysoporthe spp. and were then catalogued, as shown in Table 2. The samples were preserved in the culture collections of the IFRC and Laboratory of Forest Pathology and Tree Health, Department of Forestry Science and Biodiversity, Faculty of Forestry and Environment, University Putra Malaysia (UPM) Serdang Campus, Malaysia, for further analysis.

Table 2.
List of fungal isolates used for phylogenetic analysis and pathogenicity trials.
2.3. Pathogenicity Test
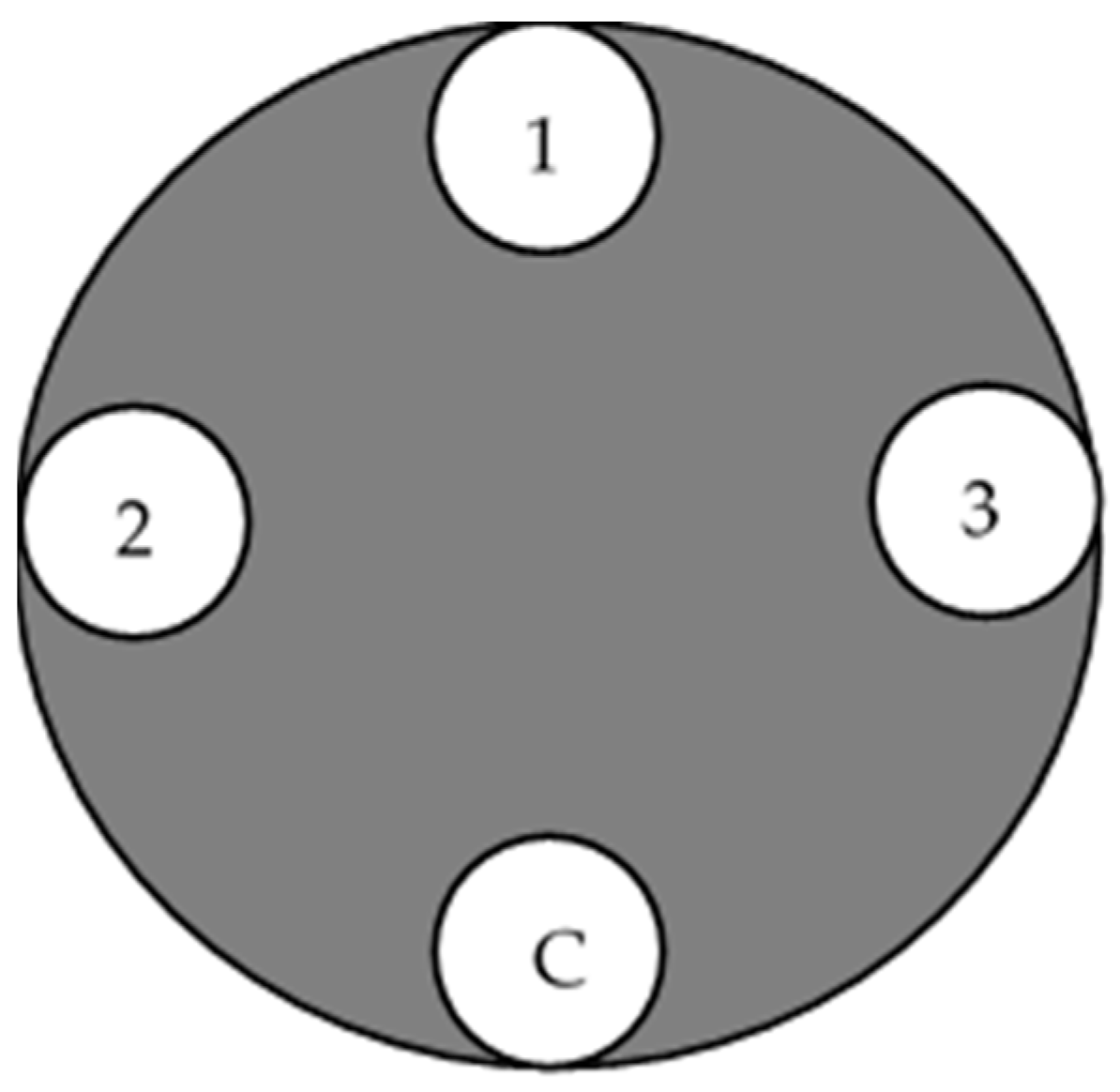
The pathogenicity test of the 20 isolated C. deuterocubensis was conducted on 40 healthy, undamaged, and disease-free 10-year-old E. urograndis trees from the same provenance grown in UPM Serdang Campus, Malaysia. Each isolate was replicated onto two individual trees, with three technical replicates (3 wound points) in each inoculated tree, and one wound point was designated as a negative control. Figure 2 shows the replication of the inoculated tree based on past procedures [25]. All the trees were arranged in a Completely Randomised Design (CRD).
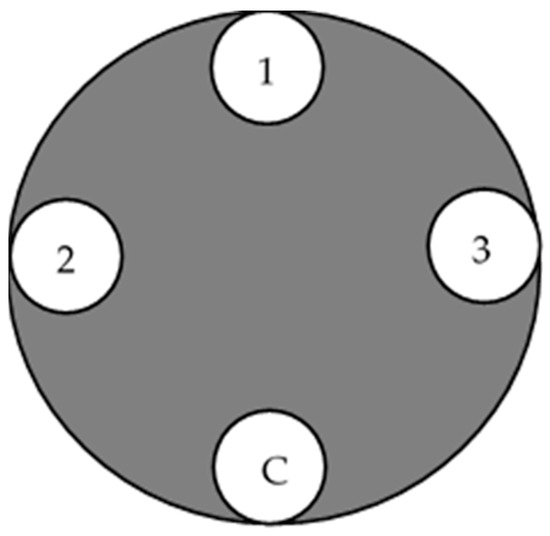
Figure 2.
Illustration of the inoculation points on the tree stand. (1) Inoculation point for replicate no. 1, (2) inoculation point for replicate no. 2, (3) inoculation point for replicate no. 3, and (C) uninoculated (negative) control.
A drill borer (3 mm diameter) was used to remove the bark, and the cambium was exposed for inoculation. The wound points were surface-sterilised using a cotton wool pad dipped in 75% ethanol. Then, a 4 mm disc of 7-day-old C. deuterocubensis was carefully placed onto the cut surface at the three wound points using a sterile scalpel tip and immediately sealed with parafilm (Sigma Aldrich, St. Louis, MO, USA) to prevent contamination and desiccation of the wound and the inoculum. Non-inoculated MEA was used as the control. The trees were observed for disease development once a week for over four weeks, and lesion development on the inoculated trees was evaluated by scraping the tree bark and measuring the lesion length at 20 weeks or 5 months. The mean lesion length of each isolate was statistically analysed using one-way Analysis of Variance (ANOVA) and compared with Tukey’s test (p ≤ 0.05) to determine the significant difference between the lesions associated with the various C. deuterocubensis isolates and the control sample.
2.4. DNA Extraction, PCR, Sequencing, and Phylogenetic Analysis
Fungal biomass was harvested from a 10-day actively growing C. deuterocubensis culture on MEA, which was macerated in liquid nitrogen, crushed, lyophilised, and ground into a powdery fine structure. Approximately 10 mg of the powdered samples were then subjected to DNA extraction using the FavorPrepTM Fungi/Yeast Genomic DNA Extraction Mini Kit according to the manufacturer’s protocol (Fisher Biotec, Wembley, WA, Australia). Nano-Drop 2000 Spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) was used to measure the concentration of the extracted DNA. Subsequently, about 50 μL of sample mixture was prepared for the Polymerase Chain Reaction (PCR) analysis, which comprised 5 μL of DNA template, 25 μL of exTEN PCR MasterMix, 1 μL of each primer (10 μM), and then topped up with nuclease-free water to 18 μL.
The Internal Transcribed Spacer (ITS) region of the ribosomal DNA (rDNA) gene of the fungal isolates was amplified with the ITS1 and ITS4 primers, while one region of the β-tubulin (BT) gene was amplified with the BT2a and BT2b primers [23]. PCR amplification was performed using a BioRad MyCycler Thermal Cycler (Marshall Scientific LLC, Hampton, NH, USA), with the following settings: 15 min at 96 °C; 35 cycles of 1 min at 94 °C, 1 min at 56.5 °C, and 2 min at 72 °C; and a final 5 min extension at 72 °C. After that, the PCR products were separated using a 1% agarose gel in 1× TAE buffer (90 mM Tris-acetate and 2 nM EDTA, pH 8), dyed with Florosafe, and analysed using FluroChemTM (Alpha Innotech, San Leandro, CA, USA). The PCR products were sent to Apical Scientific Sdn. Bhd., Malaysia, for sequencing using the Sanger sequencing technique. The sequences were deposited in NCBI GenBank and compared with those available in GenBank via BLAST searches.
The resulting DNA sequences were aligned using the MUSCLE program embedded in MEGA software version 10.1.8 [26], then manually trimmed and edited to obtain complete sequences. Homology searches were conducted using the BLAST program against the NCBI GenBank database (https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 12 July 2023). Additionally, a phylogenetic tree was constructed comprising the 30 ITS and BT2 sequences related to Chrysoporthe species from the NCBI GenBank (Supplementary Table S1), the 20 sequences from the fungal isolates in this study (Table 1), and two sequences (ITS and BT2) of Celoporthe dispersa (phyla Ascomycota, family Cryphonectriaceae), which were included as outgroups. A maximum likelihood tree was constructed using MEGA 11.0 software, which includes all positions containing gaps and missing data. The evolution model for the maximum likelihood (ML) analyses was determined by the best-fit substitution model [26,27]. Branch support was estimated using the bootstrap analysis based on 1000 bootstrap replicates.
2.5. In Vitro Evaluation of Chemical Fungicides against Chrysoporthe deuterocubensis
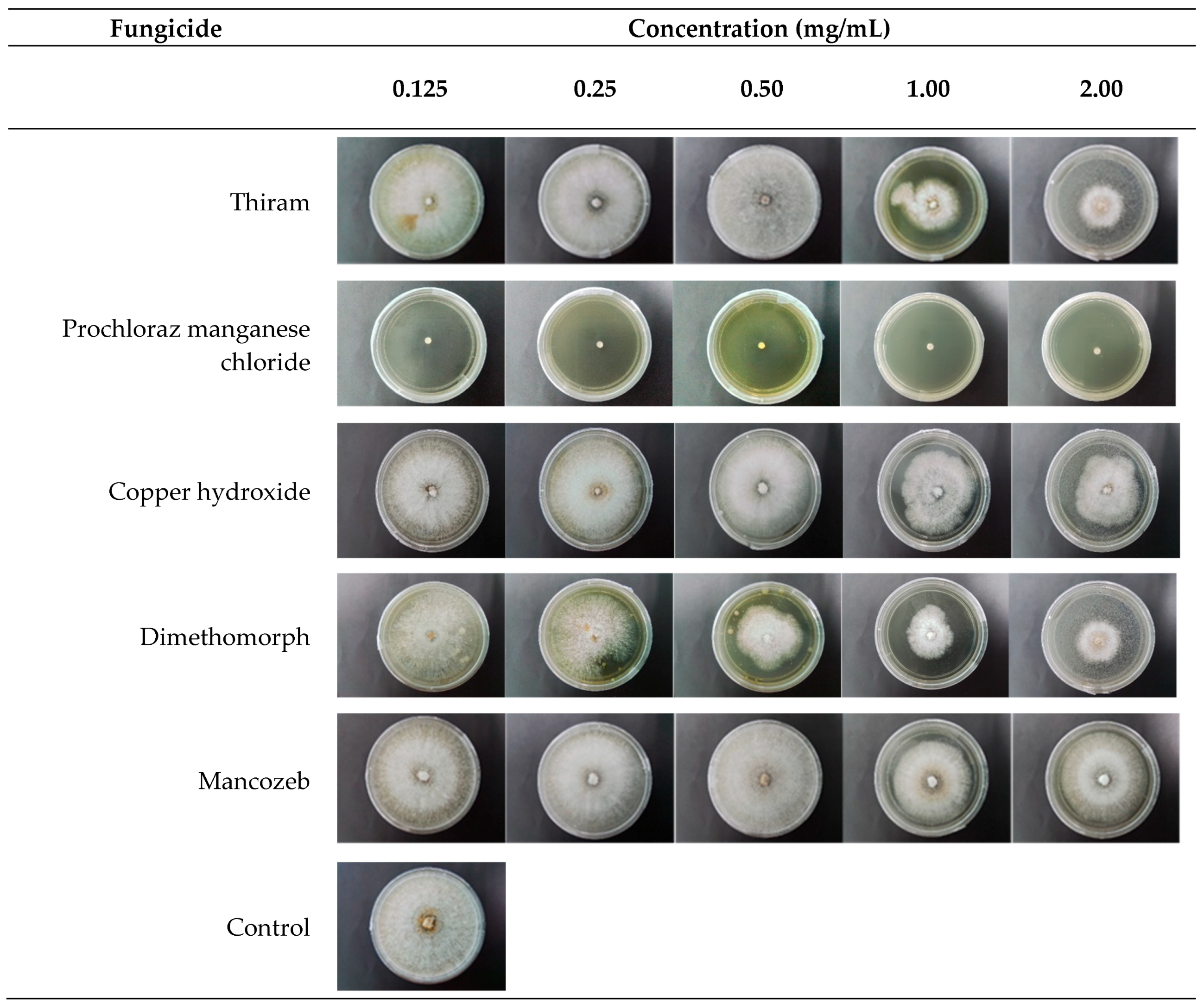
The poisoned food technique was performed in this study. C. deuterocubensis (CHRY18) was selected based on the pathogenicity test and assayed for the in vitro evaluation test. The commonly available fungicides detailed in Table 3 were selected from the local market.

Table 3.
Details of fungicides used for in vitro evaluation against C. deuterocubensis.
The respective fungicide was placed in flasks and dissolved with sterile distilled water to yield different fungicide concentrations (0.125, 0.25, 0.5, 1.0, and 2.0 mg/mL). Then, the fungicide suspension was added with autoclaved MEA (OxoidTM) media and mixed well in the flask, while flasks without fungicide were designated as control treatments. Next, 15 mL of the poisoned fungicide media were poured into sterilised petri plates and left for 24 h to observe contamination. Subsequently, a 5 mm mycelial disc was incised from the edge of a 7-day fungal culture and inoculated at the centre of the plates. The inoculated plates were then incubated at room temperature for 7 days. Plates without fungicides were used as a control treatment. Three replicate plates were prepared for each fungicide treatment, and the experiment was repeated twice.
Once the growth on the control plates had completely covered the plate after 7 days of incubation, the radial colony diameter was measured from the underside of the plate in two perpendicular directions, with the mean of the two measurements used to calculate the growth. Fungal radial growth was then measured based on [28,29,30]. In addition, the Relative Mycelial Growth (RMG) was used to determine sensitivity or resistance by comparing with baseline populations. The percentage growth inhibition (%) was calculated using the following equation [28,31,32]:
where D = Percentage inhibition (%); D1 = Average increase in diameter of the fungal colony with control; D2 = Average increase in diameter of the fungal colony in treatment.
2.6. Field Efficiency of Fungicides Applied for Preventive and Curative Activity against Chrysoporthe deuterocubensis
After the initial in vitro evaluation, the efficiency of the five fungicides (thiram, prochloraz manganese chloride, copper hydroxide, dimethomorph, and mancozeb) was subjected to field efficiency analysis to determine their preventive and curative activity against C. deuterocubensis using an artificial inoculation protocol. Isolate CHRY18 was selected based on its high virulence from the pathogenicity test. Each fungicide’s concentration was prepared per the manufacturer’s recommendation, as shown in Table 4. Research plots were then established in a high number of healthy 10-year-old E. urograndis tree stands planted in UPM Serdang Campus, Malaysia.

Table 4.
Details of the five fungicides for the preventive and curative activity against C. deuterocubensis.
The preparation for the preventive and curative fungicide experiments followed similar procedures. Basically, the experimental plots were designed in randomised blocks with five plots (or replicates) and nine trees for each treatment. The plot was treated with the five tested fungicides, including one untreated control block, making nine trees (nine replicate trees) per fungicide treatment. Technical replicates comprising four boreholes in each tree were created at Diameter Breast Height (DBH) with a 3 mm drill borer head, and the cambium section was reached after drilling to that depth.
The preventive fungicide experiment was conducted from January 2023 to May 2023. Initially, the inoculum was prepared from a 10-day-old active fungal culture of CHRY18 isolate grown on MEA in the dark at 25 °C. The fungicides were applied to the borehole wounds using hand-spray bottles until runoff or 5 mL/wound and left for 24 h. Subsequently, 5 mm mycelial inoculum disc plugs were artificially inoculated into the borehole wounds using a sterile scalpel tip with the mycelium facing the cambium. The process was repeated for the negative control trees and immediately sealed with parafilm (Sigma Aldrich, St. Louis, MO, USA) to reduce contamination and desiccation. The severity of the infection in the negative control trees and fungicide-treated trees was measured by assessing the length of the xylem lesion after 20 weeks post-inoculation.
Meanwhile, the curative fungicide experiment was conducted from November 2022 to May 2023. Isolate CHRY18 was initially inoculated in 45 healthy trees. After significant pathogen growth was observed at 8 weeks, the lesion length in all four borehole wounds was measured. Subsequently, the fungicide treatment was applied to the boreholes. Negative control trees were inoculated with the same CHRY18 isolate using the same procedures as the wounded fungicide-treated plots but without fungicide application. The severity of the infection in the negative control trees and fungicide-treated trees was evaluated by measuring the length of the xylem lesion after 20 weeks post-fungicide treatment. The percentage reduction in the lesion length was subsequently calculated using the following formula:
where: R = Reduction length (%); R1 = Average lesion length at 8 weeks before fungicide treatment; R2 = Average lesion length treatment at 20 weeks post-fungicide treatment.
2.7. Statistical Analysis
The SAS/STAT 14.2 (SAS Institute, Inc., Cary, NC, USA) software was used for statistical analysis. Data analysis regarding fungal growth and mycelium growth inhibition after 7 days of inoculation was determined using ANOVA and Standard Error (SE). The Tukey method was used to detect any significant differences (p ≤ 0.05). Subsequently, the mean percentage inhibition of radial growth was plotted using Microsoft Excel.
For the preventive fungicide experiment, the length of bark lesions in all four boreholes on each tree for each fungicide treatment was calculated and analysed using ANOVA with mean values compared among them and with the controls by Tukey’s test (p ≤ 0.05). In addition, the normality test was performed using Kolmogorov–Smirnov, Shapiro–Wilk, Anderson–Darling, and Cramér–von Mises tests (p ≥ 0.05). The ANOVA and Tukey tests were also performed to study the effects of “fungicide treatment” and their interaction on the reduction of lesion size. The mean values were compared equally among the fungicides and negative controls using Tukey’s test (p ≤ 0.05).
3. Results
3.1. Collection of Fungal Isolates and Their General Morphological Characterisation
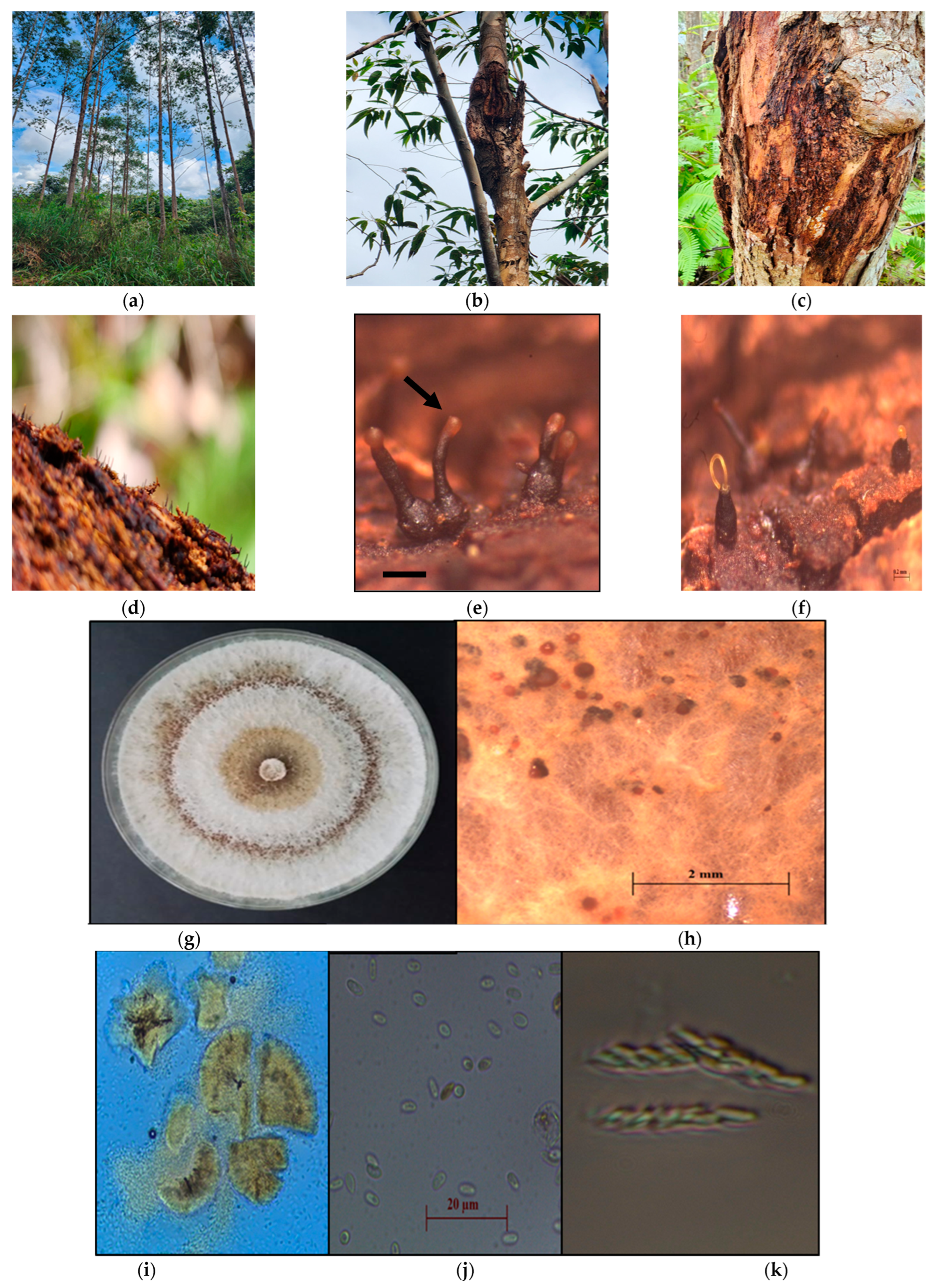
The 20 fungal isolates collected from the bark-covering cankers of infected E. urograndis exhibited similar morphology to Chrysoporthe spp., including a protuberant neck of perithecia embedded in pseudosomal tissues and fuscous black perithecia [21,23]. Figure 3 compiles the images of the symptoms associated with Chrysoporthe stem canker on infected E. urograndis stands, as observed through the naked eye (Figure 3a–g) and microscopic examination (Figure 3h–k). Figure 3a displays the typical fungal structures of Chrysoporthe spp. on infected E. urograndis tree stands grown on the plantation. The main symptoms were cankers at lower stems and bark cracking (Figure 3b,c) with perithecia and pycnidia (Figure 3d) of the pathogen on the bark of the infected trees. Perithecial bases were encased in a cinnamon-to-orange fungal tissue, occasionally visible above the bark surface (Figure 3e).

Figure 3.
Symptoms associated with Chrysoporthe stem canker on infected E. urograndis tree stands. (a) Stem canker infecting 4-year-old E. urograndis tree stands, (b) Multiple canker formation on the upper tree stem with swelling, fissured, and cracked bark, (c) Cancerous necrotic lesion with gummosis in the lower stem, (d) Blackish hairy-like fruiting bodies of conidioma on the bark, (e) Ascostroma on the bark with golden spore mass at the apex, (f) Long golden-coloured cirrhi fruiting bodies of C. deuterocubensis, (g) Culture grown at room temperature in the dark for 7 days on MEA, (h) Asexual fruiting structures of C. deuterocubensis in grown culture, (i) Conidiomata tissue, (j) Ascospores (3.1) 3.3–4.5 (4.7) × (1.5) 1.7–2.2 (2.5) µm, n = 20, and (k) Clavate-shaped ascus. Scale bar: (e,f): 0.2 μm; (i) = 100 μm; (j) = 20 μm; (k) = 10 μm.
Meanwhile, the whitish-coloured mycelia culture grown on 2% MEA in the juvenile state turned yellowish–brownish when matured (Figure 3g). Conidiomata embedded on the surface or some as separated structures with tendrils of salmon-orange colour conidia masses, pyriform-to-clavate-shaped, and having a smooth cell wall. Ascus also appeared clavate-shaped. The ascospores could be described as fusoid-to-oval forms with tapered apices, hyaline ascospores with a single septum anywhere in the spore but are often in the centre, (3.2) 3.3–4.9 (5.4) × (1.6) 1.64–2.1 (2.2) µm.
3.2. Pathogenicity Test on Eucalyptus urograndis
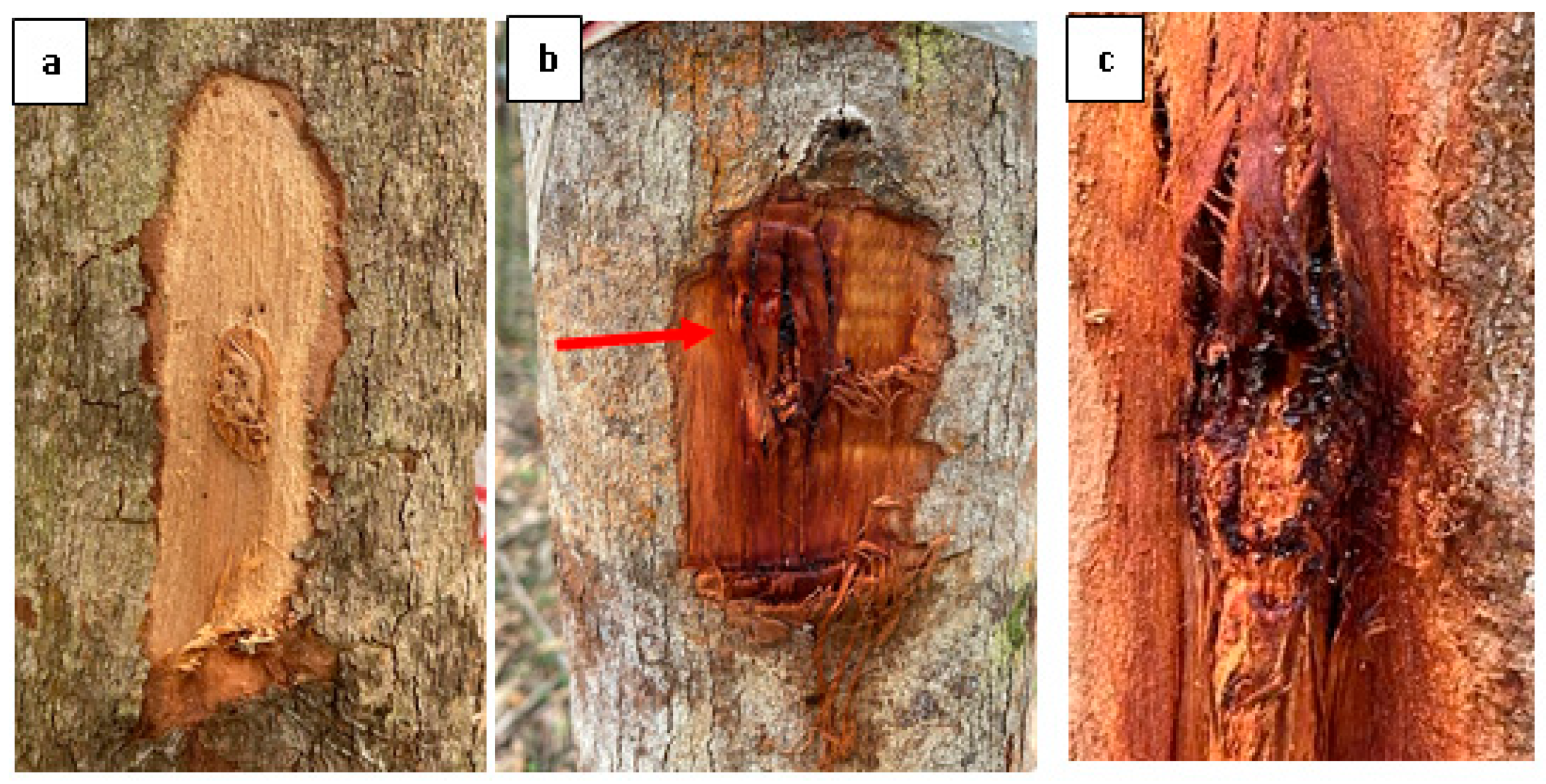
Figure 4 depicts the overall observation of the 10-year-old E. urograndis trees infected with the pathogenic isolates. The control trees did not exhibit any lesions and remained healthy till the end of the experiment (Figure 4a). However, signs and symptoms of Chrysoporthe infection in terms of lesion response and pathogenic infection symptoms appeared at 20 weeks post-inoculation in infected E. urograndis stands (Figure 4b,c). Chrysoporthe species with similar characteristics to those of the originally inoculated fungi were successfully re-isolated from the diseased bark, fulfilling the requirements of Koch’s postulates.
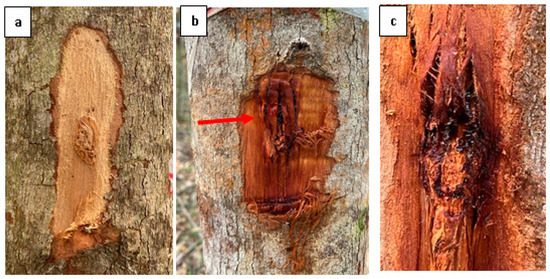
Figure 4.
Symptoms associated with Chrysoporthe stem canker on E. urograndis stand at 20 weeks post-inoculation. (a) Negative control inoculation on E. urograndis showing no formation of lesions, (b) Red arrow: Sunken discoloured lesion area on inoculated E. urograndis with fresh and dried kino/gummosis, and (c) Close up of fresh and dried blackish kino/gummosis.
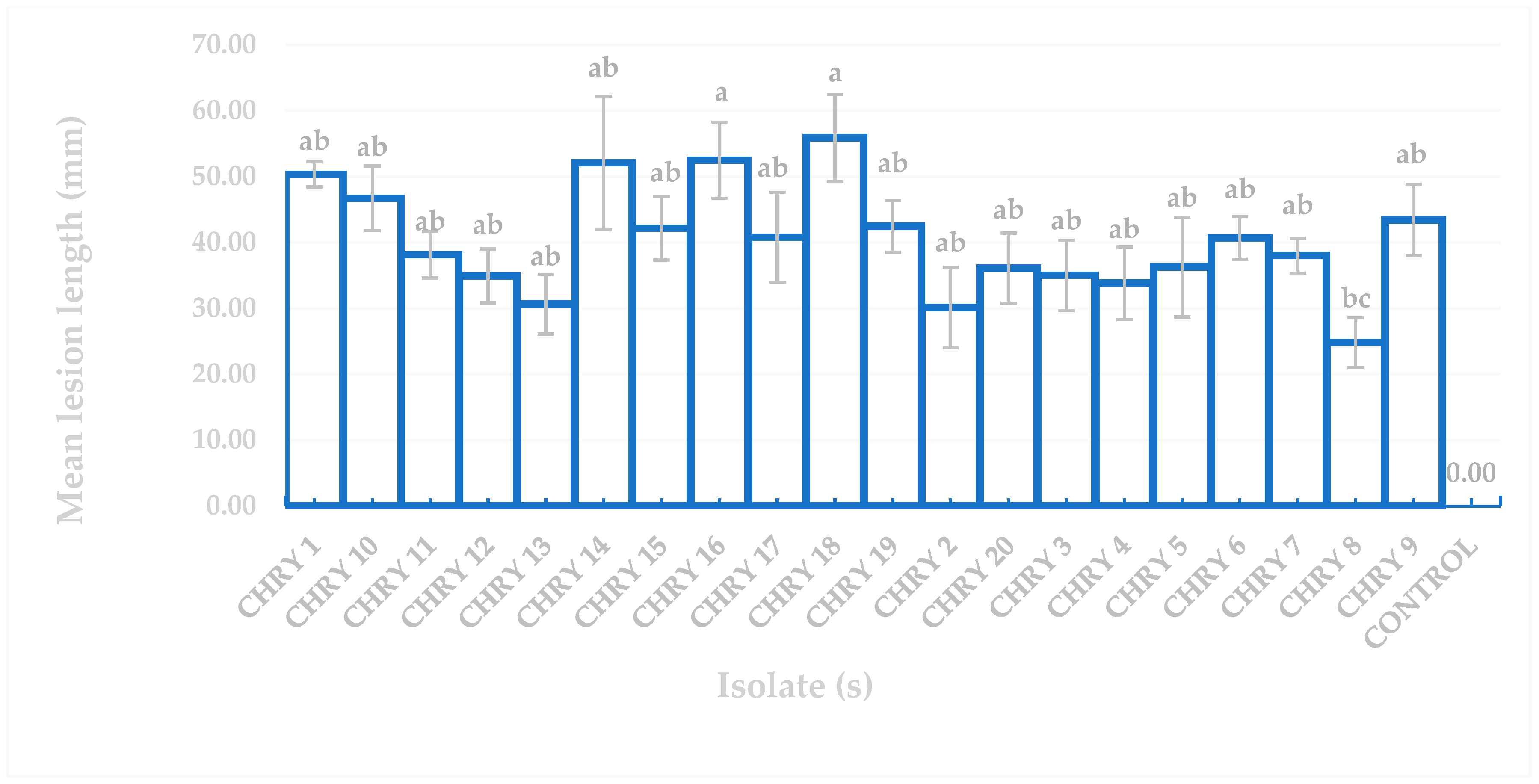
The ANOVA results for the pathogenicity test in Figure 5 confirmed that all the fungal isolates produced lesions that differed significantly from the uninoculated controls based on Tukey’s difference test (p ≤ 0.05). Figure 5 also demonstrates that the longest and shortest mean lesion lengths induced by C. deuterocubensis were 55.87 mm (CHRY18) and 13.64 mm (CHRY8), respectively. Although isolate CHRY18 recorded the longest mean lesion length of 55.87 mm, no significant differences were observed compared to isolate CHRY16 (mean lesion length of 52.48 mm).
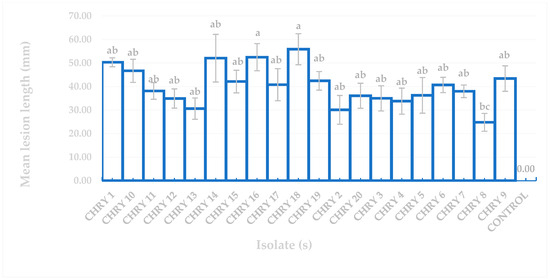
Figure 5.
Comparison of mean lesion length of E. urograndis inoculated with different C. deuterocubensis isolates. The mean length of the lesion is shown with SE. Means followed by the same letter are insignificantly different according to Tukey’s test (p = 0.05).
3.3. Molecular Phylogenetic Analyses
All the ITS and BT2 sequences of the Chrysoporthe isolates from this study exhibited high similarity with existing sequences in the NCBI database. Both ITS and BT2 sequences of all isolates showed 100% query cover (E value = 0.0) with C. deuterocubensis, MN263600 and MN263694, respectively. The ITS and BT2 sequences obtained in the present work were successfully deposited in the NCBI GenBank (ITS: OR234820–OR234839; BT2:OR262469–OR262488) for future reference.
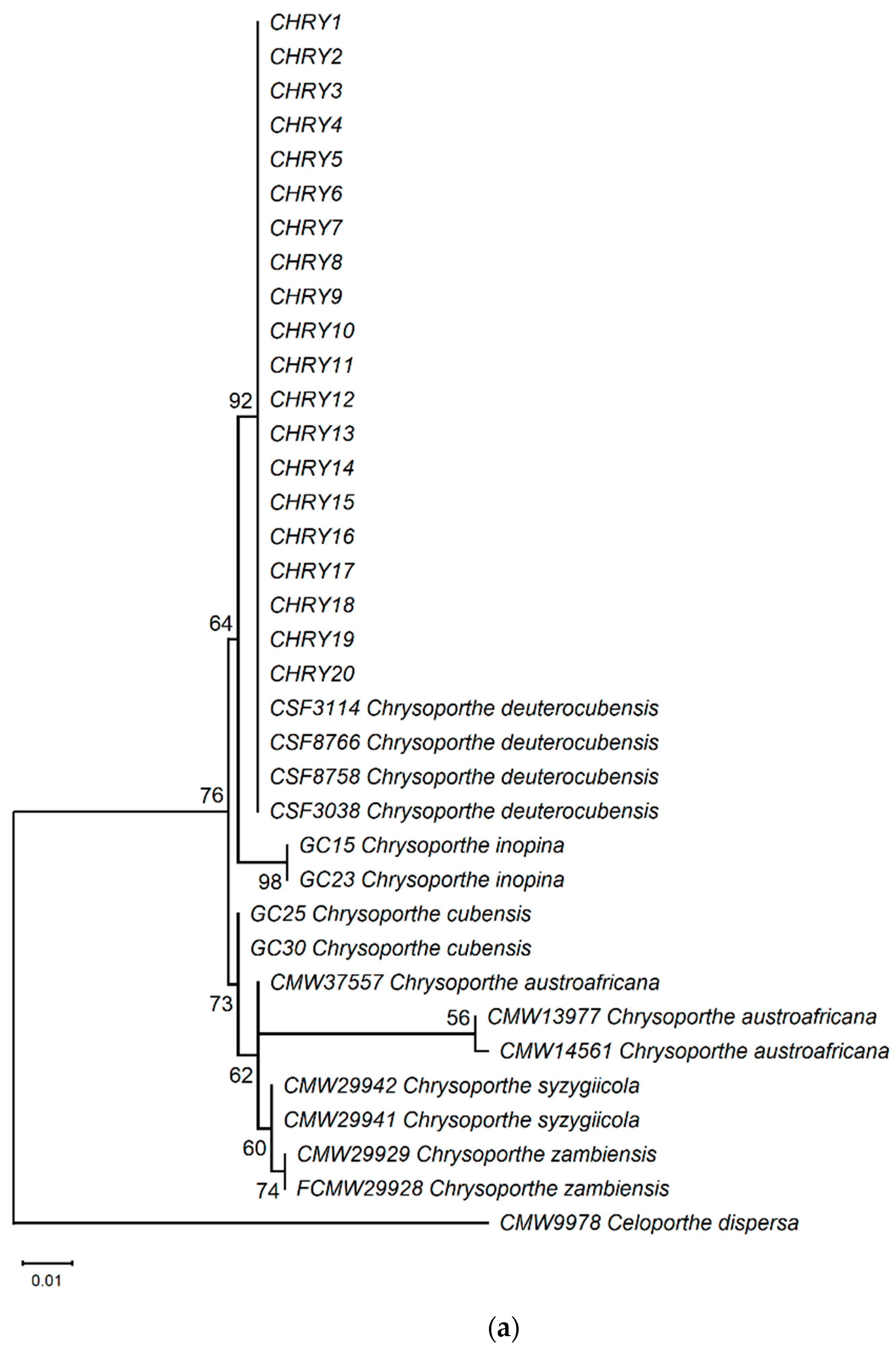
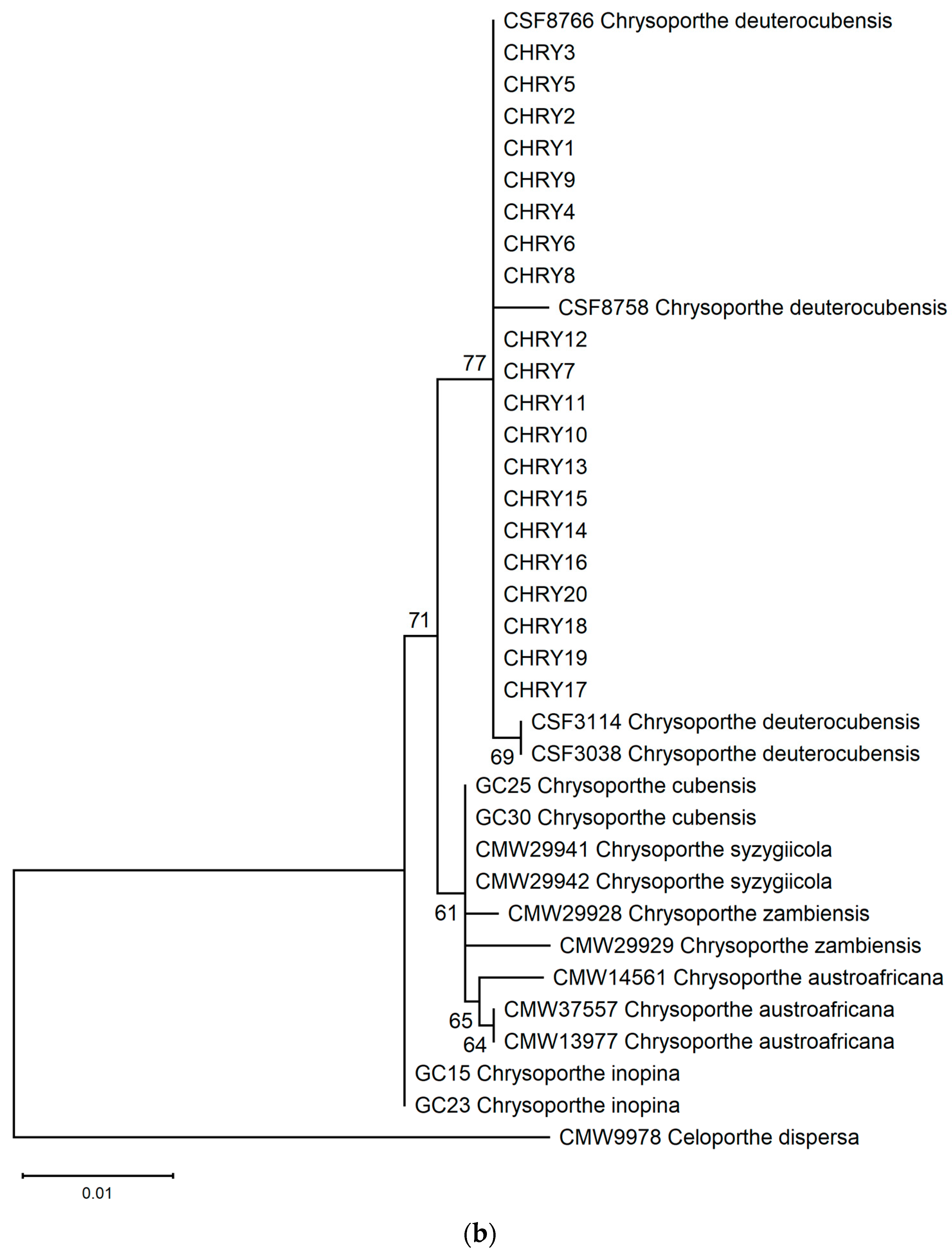
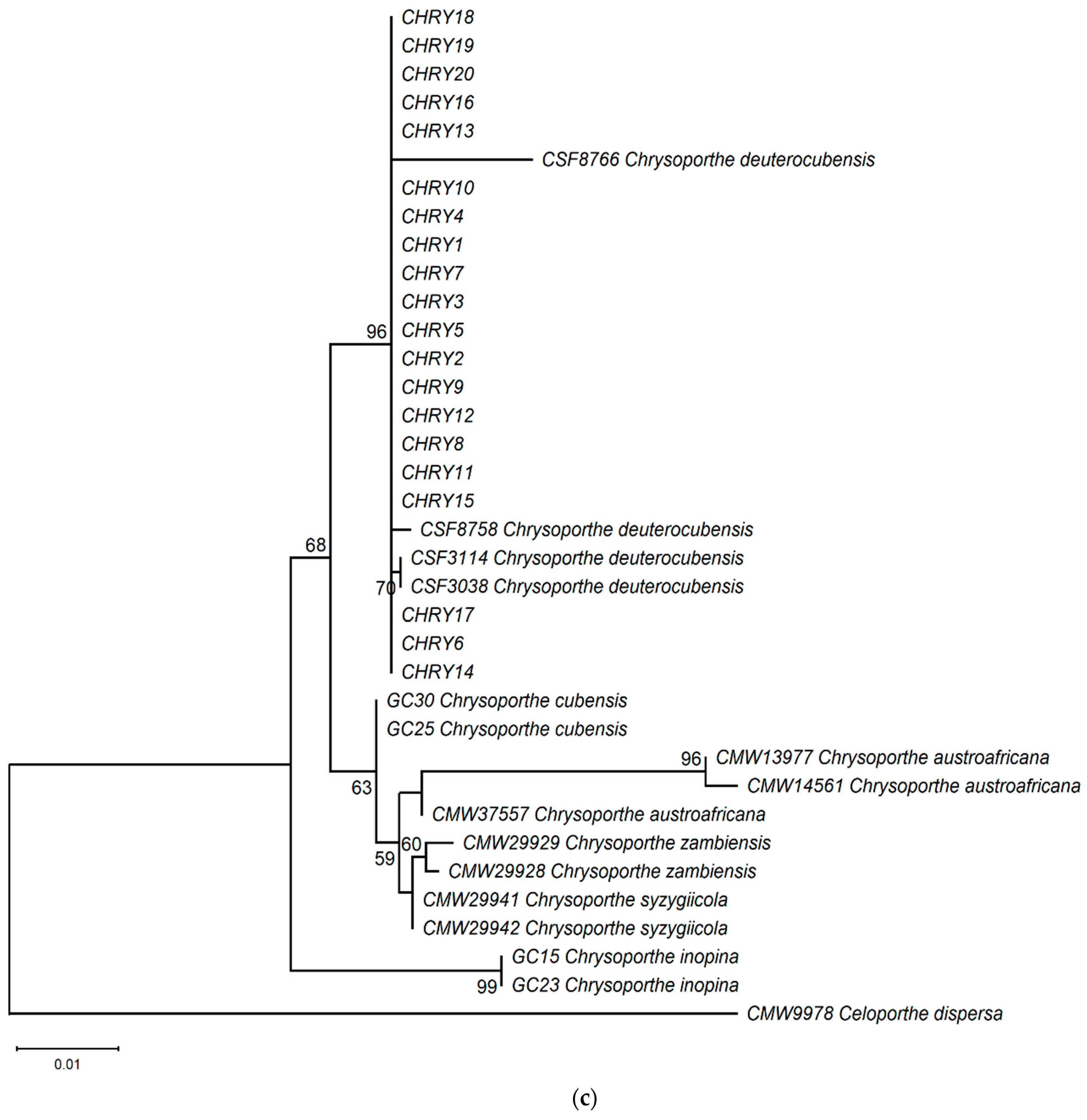
The ML phylogeny in Figure 6 shows that the 20 isolates (CHRY1–CHRY20) were grouped in the same clade with C. deuterocubensis (CSF3038, CSF3114, CSF8756, and CSF8766) in all three phylogenies. For the ITS phylogeny (Figure 6a), C. deuterocubensis was separated as a sister clade to other species, including Chrysoporthe cubensis, Chrysoporthe syzgiicola, Chrysoporthe zambiensis, and Chrysoporthe austroafricana (71% bootstrap value). In contrast, the BT2 phylogeny (Figure 6b) showed that C. deuterocubensis formed a sister group with Chrysoporthe inopina and separated from other species (76% bootstrap value). Comparatively, a multilocus phylogeny (Figure 6c) from the combined ITS and BT2 sequences recorded a similar pattern as the individual isolates and was grouped with C. deuterocubensis and separated from other species (68% bootstrap value). The BLASTn results of the ITS and BT2 sequences in the NCBI database and phylogeny trees confirmed that all the Chrysoporthe isolates from this study were identified as C. deuterocubensis.
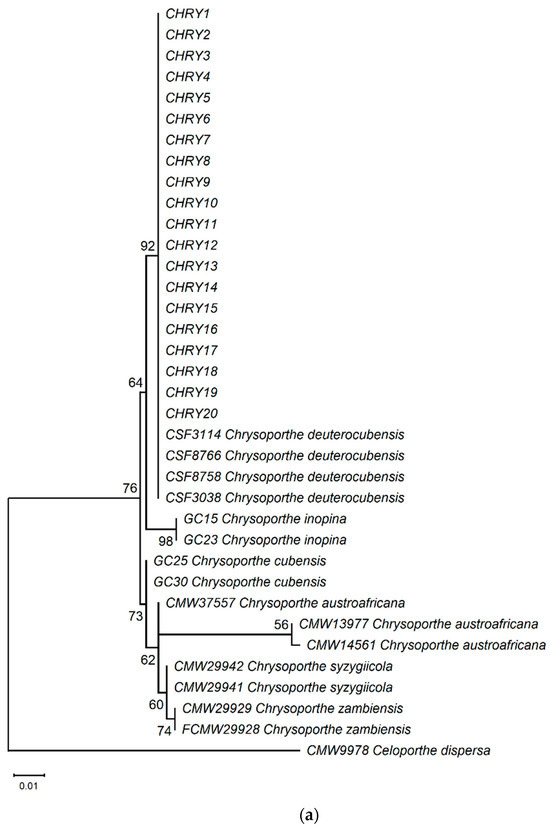
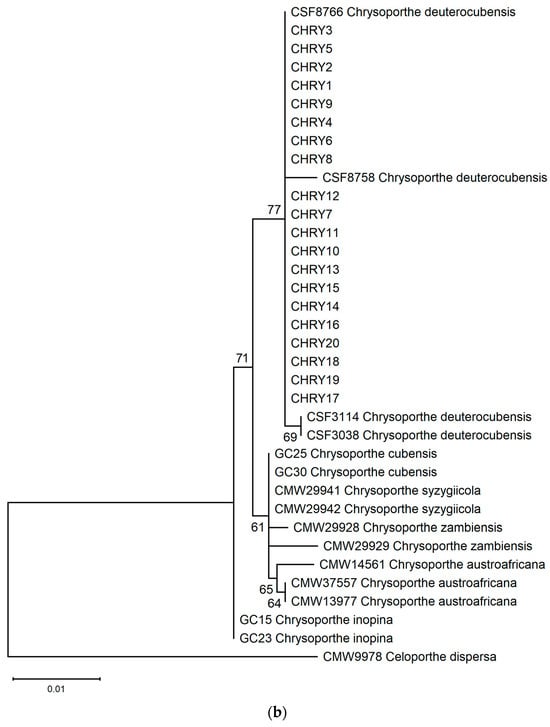
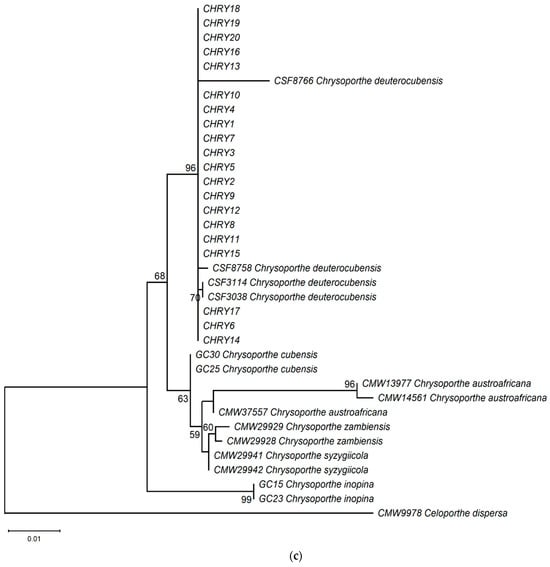
Figure 6.
Maximum-likelihood (ML) phylogenies were constructed from (a) ITS gene sequences, (b) BT2 gene sequences, and (c) the multilocus phylogeny combining the ITS and BT2 sequences. The phylogenies were rooted with the outgroup Celoportha dispersa. Bootstrap values above 50% (1000 replicates) are indicated at each branch.
3.4. In Vitro Evaluation of Chemical Fungicides against Chrysorpothe deuterocubensis
The data analysis in Table 5 and Figure 7 demonstrates that prochloraz manganese chloride exhibited the most significant inhibiting effect against C. deuterocubensis growth at all concentrations. Even at the lowest concentration of 0.125 mg/mL, the fungicide produced a 91.1% Radial Growth Inhibition (RGI) while completely inhibiting the mycelial growth of C. deuterocubensis at 1.0 mg/mL. Dimethomorph fungicide recorded the second-most significant inhibition effect with 57.6% RGI at 2.0 mg/mL concentration, followed by thiram with 42.3% RGI at 2.0 mg/mL concentration. Oppositely, mancozeb showed no inhibition ability against the pathogen, even at the highest concentration of 2.0 mg/mL.

Table 5.
Mean percentage of in vitro RGI of the five tested fungicides against C. deuterocubensis.
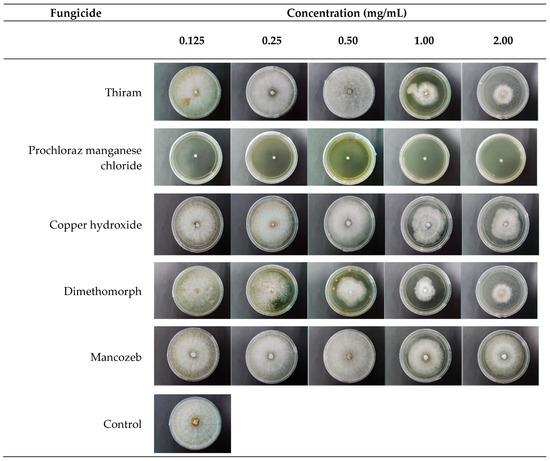
Figure 7.
In Vitro fungicides evaluation test. Anterior view of colonies of C. deuterocubensis response to different types of fungicides at 6 days of inoculation.
3.5. Field Efficiency of Fungicides Applied for Preventive and Curative Activity against Chrysoporthe deuterocubensis
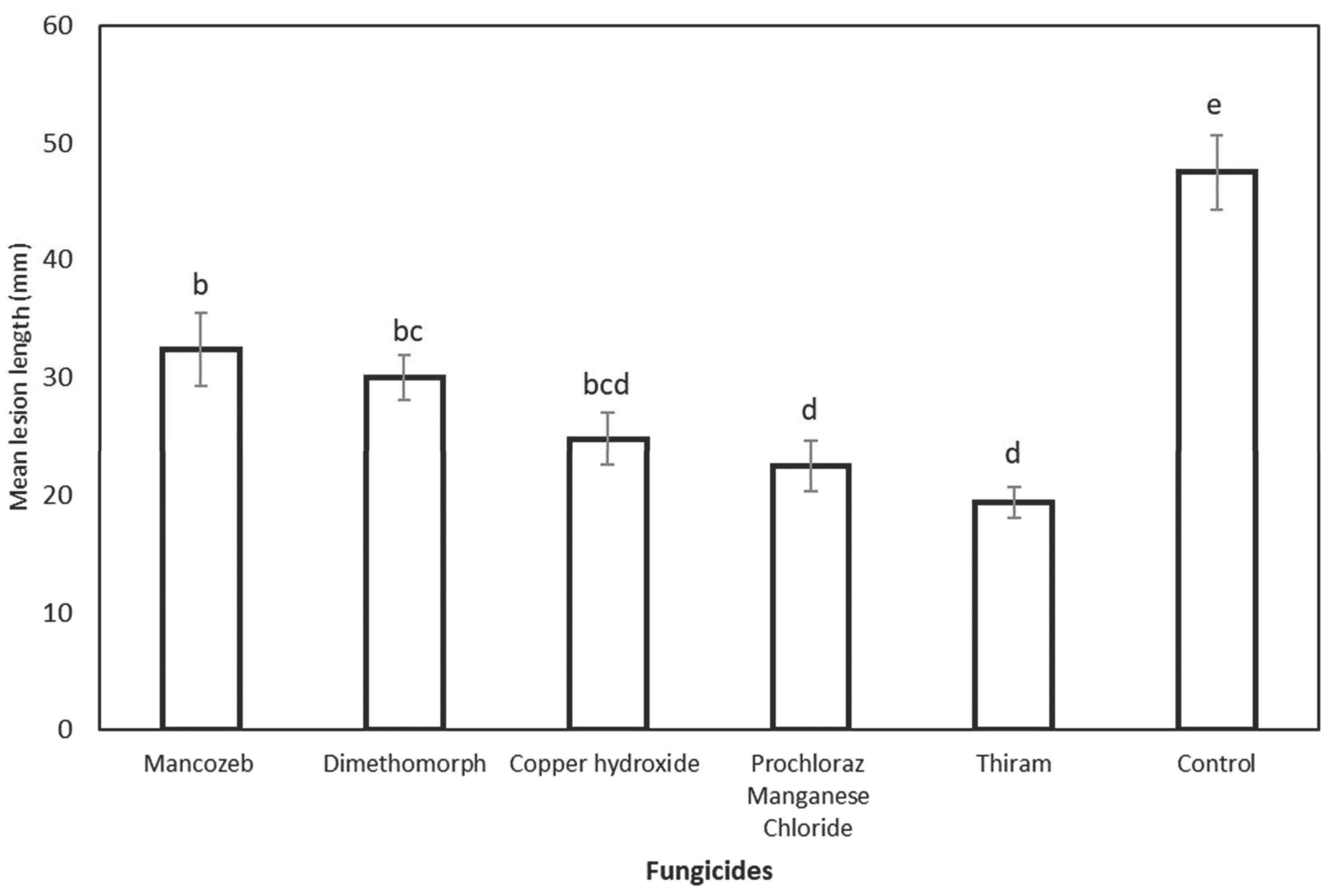
Based on the preventive fungicide experiment results, all the tested fungicides induced shorter necrotic lesion lengths than the more prominent lesion developed in non-treated control wound points (47.48 mm). Statistical analysis of the lesion lengths of all tested fungicides also showed significant differences compared to the non-treated control (Figure 8). The inoculated wound points treated with thiram yielded the shortest mean lesion length (19.40 mm), although they were insignificantly different compared to the wound points treated with prochloraz manganese chloride (22.47 mm) and copper hydroxide (24.77 mm). The wound points treated with mancozeb (32.38 cm) produced the longest lesion length but were insignificantly different from those treated with dimethomorph (29.98 mm).
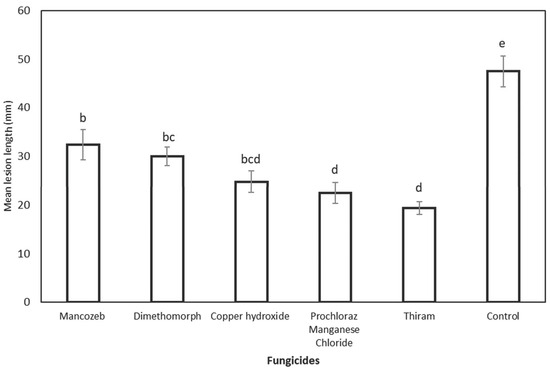
Figure 8.
Preventive effect of fungicide on E. urograndis trees inoculated with C. deuterocubensis at 20 weeks post-inoculation followed by fungicide treatment application. Bars represent the SE of the means. Mean values and SE obtained from the lesion length of cankers followed by different letters indicate a significant difference according to Tukey’s difference test (p ≤ 0.05).
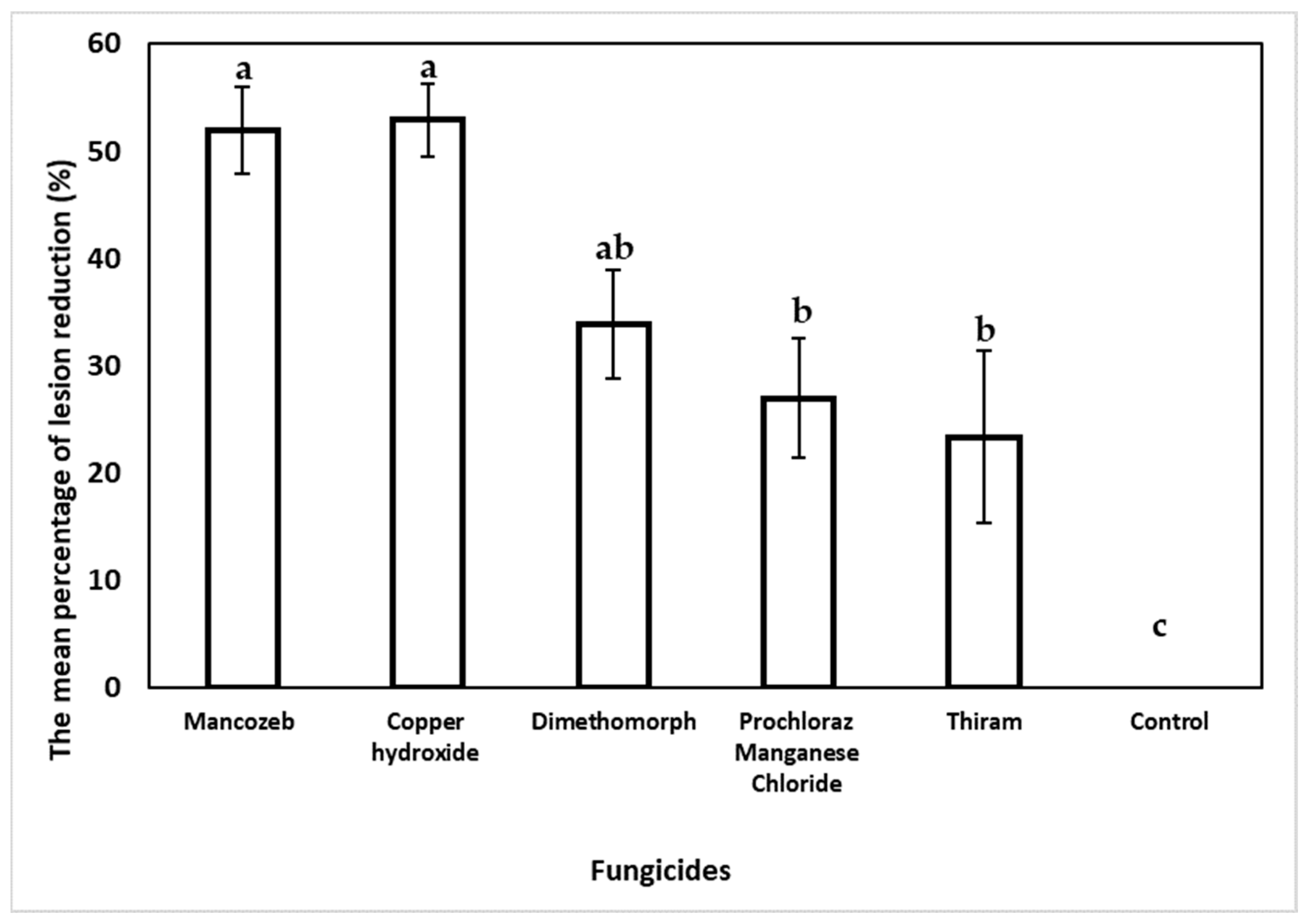
In the curative fungicide experiment, the statistical analysis showed a reduced necrotic lesion length for all tested fungicides compared to the non-treated control wound points (Figure 9). Inoculated trees treated with copper hydroxide recorded the highest reduction in the final lesion length by 52.7% but was statistically insignificant compared to the necrotic lesions treated with mancozeb (51.93%). Dimethomorph reduced the initial necrotic lesion length by 33.87%, followed by prochloraz manganese chloride (26.96%) and thiram (23.34%). On the contrary, the non-treated control inoculated necrotic points showed an increase in lesion length by 19.21%.
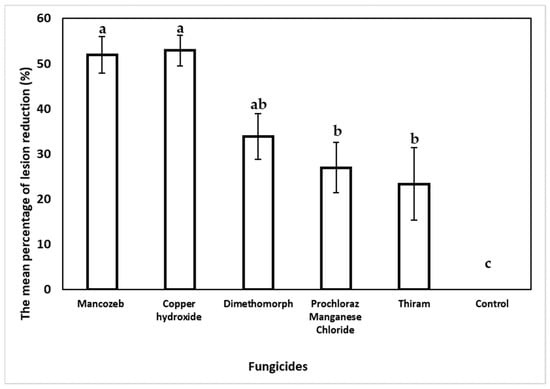
Figure 9.
Curative effect of fungicide on E. urograndis trees inoculated with C. deuterocubensis at 20 weeks post-fungicide application. Bars represent the SE of the means. Percentage reduction (%) in lesion length with SE obtained from lesion length of cankers followed by different letters indicates a significant difference according to Tukey’s difference test (p ≤ 0.05).
4. Discussion
Results of this study confirmed the presence of C. deuterocubensis infection on E. urograndis plants in Sarawak, Malaysia. This pathogen was first described in Sabah, Malaysia, isolated from E. grandis and also pathogenic to E. deglupta [21]. This study also signifies the first step towards using fungicides to control the canker disease of E. urograndis caused by C. deuterocubensis.
The efficiency of fungicides through in vitro screening revealed that C. deuterocubensis was highly sensitive to prochloraz manganese chloride. The fungicide inhibited the mycelium growth of the pathogen (91.1% RGI) at the lowest concentration of 0.125 mg/mL while completely inhibited the growth (100%) at 1.0 mg/mL. Previous in vitro screening studies also recorded the ability of prochloraz manganese chloride to inhibit pathogenic species of Botryosphaeriaceae that caused grapevine trunk disease in South Africa [33,34]. Moreover, this fungicide effectively inhibited the growth of C. deuterocubensis in both preventive and curative field tests.
Prior to this study, no research had been conducted or published on the use of thiram in controlling Eucalyptus-related diseases. Based on the present findings, the in vitro screening experiments showed that thiram reduced the mycelia growth of C. deuterocubensis by 57.6% RGI compared to the control (no thiram) at 2.0 mg/mL. Thiram was also proven effective in the preventive experiment against the pathogen by yielding the shortest mean lesion length (19.40 mm). However, it did not yield the same promising results as the other tested fungicides in the curative experiment. As a non-systematic dimethyl dithiocarbamate fungicide, thiram demonstrated effective in vitro antifungal response against Ganoderma boninense, a fungal pathogen of Basal Stem Rot (BSR) in oil palm trees in Malaysia [35]. It is also predominantly utilised to protect field crops, vegetables, ornamental plants, and fruits from pathogenic Botrytis species, rust, scab, and storage diseases [36,37]. Besides, thiram is typically used in forest plantation trees as a seed protector or seed coating [38] and to control the damping of disease in pine tree seedling stock in nurseries against pathogens [39]. Applying fungicides before the infection occurs will eliminate fungal spores or suppress any growth of fungal structures on the plant [40].
Although mancozeb recorded a relatively poor inhibition against the mycelium growth of C. deuterocubensis in the in vitro screening study, the fungicide was effective in the curative experiment with a reduced final necrotic lesion length of 51.93%. Mancozeb is a protective, non-systemic dithiocarbamate fungicide that has been on the market since 1962 and has an established reputation for controlling 400 different diseases infecting 70 crops [41,42]. In the forest plantation sector, this fungicide has been used to control Eucalyptus rust caused by Puccinia psidii in Brazil [43] and manage stem cankers disease in Protea magnifica caused by Botryosphaeria based on a field trial [44,45]. According to an artificial inoculation experiment on excised leaves and one-year-old seedlings, mancozeb has shown the potential to control and mitigate wilt disease of A. mangium caused by Ceratocystis manginecans [45].
Comparatively, copper hydroxide yielded the highest percentage of the final reduction length (52.7%) in the curative experiment but was insignificantly different from mancozeb. Past field trials have demonstrated that copper hydroxide was consistently efficacious in preventing new infections of European canker caused by Neonectria ditissima [46] and was potentially effective in controlling citrus canker caused by wild-type Escherichia coli [47]. Despite their effectiveness, curative fungicides require direct contact with the fungal pathogen’s vegetative or generative structure inside the plant, and the effects of post-fungicide application are assessed by the progression of the disease symptoms or the decrease of spore counts [40,48].
Cultural practices and breeding plant-resistant material are the primary modes of controlling insect pests and pathogens in forest tree plantations [49]. Breeding for disease-resistant cultivars is notably recommended for long-term strategies for canker disease management [9,17]. However, this approach involves a long-term commitment, is costly, and consumes lengthy response time to new pests and disease threats [50]. While the deployment of pesticides through drone technology has been extensively applied in the oil palm industry [51], spraying fungicides on forest tree plantations may only be feasible for foliar disease control but relatively unsustainable to control stem and root diseases. Thus, other management approaches should be explored to minimise the impact of this disease and to reduce further losses.
In the absence of disease-resistance genotypes, the chemical control approach can be seen as an initial primary strategy in the Integrated Pest Management (IPM) for plant disease. The use of chemical agents is considered an essential tool for managing plant disease [49], and in forestry, it is mainly applied at the nursery, where the impact of pathogens is largely destructive to small tree seedlings. Regardless of the inconsistent effectiveness of the five fungicides in all evaluated experiments in this study, they displayed their potential as effective chemical agents to control Chrysoporthe stem canker disease. Only one single concentration and application rate were tested in preventive and curative field experiments.
The efficiency of fungicides must consider the season, frequency, application method, and potential for resistance to develop [45,50,52]. The preventive and curative field experiments on the 10-year-old trees were conducted from January to May 2023. Generally, this period in Malaysia is relatively wet, as the northern monsoon usually commences in early November and ends in March. As a recommendation for future studies, the effectiveness of the formulation with a combination of different fungicides should be conducted in various weather seasons and concentrations. Another factor to be considered is the cost-effectiveness of these fungicides for large-scale forest plantations.
5. Conclusions
This study showed that prochloraz manganese chloride, thiram, and copper hydroxide were highly inhibitory against C. deuterocubensis infection on 10-year-old E. urograndis trees. Apart from the in vitro screening, the preventive and curative field experiments highlighted that the three fungicides are promising chemical agents to control C. deuterocubensis stem canker on Eucalyptus spp. These findings would eventually complement the formulation and implementation of long-term Integrated Disease Management (IDM) strategies that combine cultural, chemical, biological control, and resistant cultivars of Eucalyptus and its hybrids in Malaysia.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f14122337/s1, Table S1: Isolates of Chrysoporthe sp. used in the phylogenetic studies, and their GenBank accession numbers for the ITS and β-tubulin 2 sequences.
Author Contributions
Conceptualization, R.T.; Methodology, A.A., A.B.P.R., N.A.S.K., A.M.M.P., W.M.A.W.A., N.H.A. and R.T.; Formal analysis, A.M.M.P., W.M.A.W.A. and R.T.; Investigation, A.A., A.B.P.R., N.A.S.K. and R.T.; Resources, J.L. and R.T.; Data curation, A.A., A.B.P.R., N.A.S.K. and R.T.; Writing—original draft, A.A.; Writing—review & editing, R.T.; Supervision, R.T.; Funding acquisition, R.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Sarawak Forest Research Project Phase 1 (Projek Rakyat).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
The authors would like to acknowledge Datu Haji Hamden bin Haji Mohammad, the Director of Forest, Forest Department Sarawak and Pang Shek Ling, Head of the Centre of Industrial Forest Research Centre (IFRC), together with all collaborating License Planted Forest (LPF) holders of Sarawak for their continuous support. Special thanks to the Universiti Putra Malaysia (UPM), Serdang Campus, Selangor, for granting access to the Eucalyptus forest plantation plot. The authors also wish to thank Tuan Haji Happysupina Sait and Ms Zarina Shebli for contributing to the management of this project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Science, E.; Hamid, H.A.; Abiri, R. The way forward of Forest Plantation in Malaysia. IOP Conf. Ser. Earth Environ. Sci. 2022, 18, 959. [Google Scholar]
- Ratnasingam, J.; Latib, H.A.; Paramjothy, N.; Liat, L.C.; Nadarajah, M.; Ioras, F. Plantation Forestry in Malaysia: An Evaluation of its Successes and Failures Since the 1970. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 1789–1801. [Google Scholar] [CrossRef]
- Ambrose, A.; Liam, J.; Terhem, R. New and Emerging Disease Threats to Forest Plantations in Sarawak Borneo, Malaysia. In Current and Emerging Challenges in the Diseases of Trees; Bellé, D.C., Ed.; IntechOpen: Rijeka, Croatia, 2022; p. 112. [Google Scholar] [CrossRef]
- Tarigan, M.; Roux, J.; Wyk, M.V.; Tjahjono, B.; Wingfield, M.J. A new wilt and die-back disease of Acacia mangium associated with A new wilt and die-back disease of Acacia mangium associated with Ceratocystis manginecans and C. acaciivora sp. nov. in Indonesia. S. Afr. J. Bot. 2011, 77, 292–304. [Google Scholar] [CrossRef]
- Ss, L.; Lee, S.S. Observations on the successes and failures of acacia plantations in sabah and sarawak and the way forward. J. Trop. For. Sci. 2018, 30, 468–475. [Google Scholar]
- Zhang, Y.X.; Wang, X.J. Geographical spatial distribution and productivity dynamic change of eucalyptus plantations in China. Sci. Rep. 2021, 11, 19764. [Google Scholar] [CrossRef]
- Naidoo, S.; Külheim, C.; Zwart, L.; Mangwanda, R.; Oates, C.N.; Visser, E.A.; Wilken, F.E.; Mamni, T.B.; Myburg, A.A. Uncovering the defence responses of eucalyptus to pests and pathogens in the genomics age. Tree Physiol. 2014, 34, 931–943. [Google Scholar] [CrossRef]
- Zuhaidi Yahya, A. Planting of Eucalyptus in Malaysia. Acta Sci. Agric. 2020, 4, 139–140. [Google Scholar] [CrossRef]
- De Oliveira Castro, C.A.; dos Santos, G.A.; Takahashi, E.K.; Pires Nunes, A.C.; Souza, G.A.; de Resende, M.D.V. Accelerating Eucalyptus breeding strategies through top grafting applied to young seedlings. Ind. Crops Prod. 2021, 171, 113906. [Google Scholar] [CrossRef]
- Colodette, J.L.; Gomes, C.M.; Gomes, F.J.; Cabral, C.P. The Brazilian wood biomass supply and utilisation focusing on eucalypt. Chem. Biol. Technol. Agric. 2014, 1, 25. [Google Scholar] [CrossRef]
- Rezende, G.D.S.P.; de Resende, M.D.V.; de Assis, T.F. Eucalyptus Breeding for Clonal Forestry. In Challenges and Opportunities for the World’s Forests in the 21st Century; Springer: Berlin/Heidelberg, Germany, 2014; pp. 393–424. [Google Scholar]
- Hunter, G.C.; Crous, P.W.; Carnegie, A.J.; Burgess, T.I.; Wingfield, M.J. Mycosphaerella and Teratosphaeria diseases of Eucalyptus; easily confused and with serious consequences. Fungal Divers. 2011, 50, 145–166. [Google Scholar] [CrossRef]
- Wingfield, M.J. Pathology | Disease Affecting Exotic Plantation Species. Encycl. For. Sci. 2004, 816–822. Available online: https://www.sciencedirect.com/science/article/pii/B0121451607000624 (accessed on 12 July 2023).
- Silva, X.; Asiegbu, F.O. Chapter 15—Eucalyptus fungal diseases. In Forest Microbiology; Asiegbu, F.O., Kovalchuk, A., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 313–337. [Google Scholar]
- Chen, S.; Van Wyk, M.; Roux, J.; Wingfield, M.J.; Xie, Y.; Zhou, X. Taxonomy and pathogenicity of Ceratocystis species on Eucalyptus trees in South China, including C. chinaeucensis sp. nov. Fungal Divers. 2013, 58, 267–279. [Google Scholar] [CrossRef]
- Roux, J.; Wingfield, M.J.; Fourie, A.; Noeth, K.; Barnes, I. Ceratocystis wilt on Eucalyptus: First record from South Africa. South. For. J. For. Sci. 2020, 82, 24–31. [Google Scholar] [CrossRef]
- Yong, W.T.L.; Ades, P.K.; Runa, F.A.; Bossinger, G.; Sandhu, K.S.; Potts, B.M.; Tibbits, J.F.G. Genome-wide association study of myrtle rust (Austropuccinia psidii) resistance in Eucalyptus obliqua (subgenus Eucalyptus). Tree Genet. Genomes 2021, 17, 31. [Google Scholar] [CrossRef]
- Carnegie, A.J. First Report of Puccinia psidii (Myrtle Rust) in Eucalyptus Plantations in Australia. Plant Dis. 2015, 99, 161. [Google Scholar] [CrossRef] [PubMed]
- Gryzenhout, M.; Myburg, H.; Van Der Merwe, N.A.; Wingfield, B.D.; Wingfield, M.J. Chrysoporthe, a new genus to accommodate Cryphonectria cubensis. Stud. Mycol. 2004, 50, 119–142. [Google Scholar]
- Pegg, G.S.; Gryzenhout, M.; O’Dwyer, C.; Drenth, A.; Wingfield, M.J. The Eucalyptus canker pathogen Chrysoporthe cubensis discovered in eastern Australia. Australas. Plant Pathol. 2010, 39, 343–349. [Google Scholar] [CrossRef]
- Rauf, M.R.B.A.; McTaggart, A.R.; Marincowitz, S.; Barnes, I.; Japarudin, Y.; Wingfield, M.J. Pathogenicity of Chrysoporthe deuterocubensis and Myrtoporthe bodenii gen. et sp. nov. on Eucalyptus in Sabah, Malaysia. Australas. Plant Pathol. 2020, 49, 53–64. [Google Scholar] [CrossRef]
- Li, G.; Chen, W.; Jie, F.; Chen, S. Selection of tolerant Eucalyptus genotypes to Botryosphaeriaceae species in southern China. J. Plant Pathol. 2022, 104, 527–535. [Google Scholar] [CrossRef]
- Chen, S.F.; Gryzenhout, M.; Roux, J.; Xie, Y.J.; Wingfield, M.J.; Zhou, X.D. Identification and pathogenicity of Chrysoporthe cubensis on Eucalyptus and Syzygium spp. in South China. Plant Dis. 2010, 94, 1143–1150. [Google Scholar] [CrossRef]
- Soares, T.P.F.; Ferreira, M.A.; Mafia, R.G.; Oliveira, L.S.S.; Hodges, C.S.; Alfenas, A.C. Canker disease caused by Chrysoporthe doradensis and C. cubensis on Eucalyptus sp. and Tibouchina spp. in Brazil. Trop. Plant Pathol. 2018, 43, 314–322. [Google Scholar] [CrossRef]
- Awing, N.H.; Ambrose, A.; Abdu, A.; Hassan, A.; Terhem, R. Characterisation of Chrysoporthe cubensis and Chrysoporthe deuterocubensis, the Stem Canker Diseases of Eucalyptus spp. in a Forest Plantation in Malaysia. Forests 2023, 14, 1660. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Rokas, A.; Charlesworth, D.; Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics. By M. Nei and S. Kumar. Oxford University Press. 2000. ISBN: 0-19-513584-9 (hbk); 0-19-513585-7 (pbk). xiv+ 333 pages. Price: £ 65 (hbk); £ 32.50 (pbk). Genet. Res. 2001, 77, 117–120. [Google Scholar] [CrossRef]
- Malipatil, R.; Yenjerappa, S.T.; Amaresh, Y.S.; Sreedevi, S.C.; Jaiprakash Narayan, R.P. Efficacy of different fungicides by in vitro against Colletotrichum gloeosporioides, the causal agent of mango anthracnose. Int. J. Chem. Stud. 2021, 9, 3408–3412. [Google Scholar] [CrossRef]
- Musdalifa, A.A.; Rosmana, A. The response of different fungicides against Lasiodiplodia pseudotheobromae causing dieback disease of cocoa through in vitro test. IOP Conf. Ser. Earth Environ. Sci. 2021, 807, 22091. [Google Scholar] [CrossRef]
- Ullah, S. In Vitro evaluation of commonly available fungicides against three fungal isolates. Plant Pathol. Q. 2018, 8, 67–77. [Google Scholar] [CrossRef]
- Dahal, N.; Shrestha, R.K. Evaluation of Efficacy of Fungicides Against Fusarium oxysporum f. sp. lentis In Vitro at Lamjung, Nepal. J. Inst. Agric. Anim. Sci. 2018, 35, 105–112. [Google Scholar] [CrossRef]
- Admasu, W.; Sintayehu, A.; Gezahgne, A.; Terefework, Z. In Vitro bioefficacy of Trichoderma species against two Botryosphaeriaceae fungi causing Eucalyptus stem canker disease in Ethiopia. J. Nat. Pestic. Res. 2023, 4, 100037. [Google Scholar] [CrossRef]
- Bester, W.; Crous, P.W.; Fourie, P.H. Evaluation of fungicides as potential grapevine pruning wound protectants against Botryosphaeria species. Australas. Plant Pathol. 2007, 36, 73–77. [Google Scholar] [CrossRef]
- Halleen, F.; Fourie, P.H.; Crous, P.W. Control of black foot disease in grapevine nurseries. Plant Pathol. 2007, 56, 637–645. [Google Scholar] [CrossRef]
- Yuli, E.; Dayou, J.; Chong, K.P. In Vitro Antifungal Activity of Thiram against Ganoderma boninense. Trans. Sci. Technol. 2020, 7, 159–164. [Google Scholar]
- Pohanish, R.P.T. Sittig’s Handbook of Pesticides and Agricultural Chemicals, 2nd ed.; William Andrew Publishing: Oxford, UK, 2015; pp. 769–838. [Google Scholar]
- Picone, A.L.; Rizzato, M.L.; Lusi, A.R.; Romano, R.M. Stamplike flexible SERS substrate for in-situ rapid detection of thiram residues in fruits and vegetables. Food Chem. 2022, 373, 131570. [Google Scholar] [CrossRef]
- Kaushik, P.; Shakil, N.A.; Kumar, J.; Singh, M.K.; Singh, M.K.; Yadav, S.K. Development of controlled release formulations of thiram employing amphiphilic polymers and their bioefficacy evaluation in seed quality enhancement studies. J. Environ. Sci. Health Part B 2013, 48, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Belcher, E.W.; Waldrip, B.T. Effect of thiram on seed mold and germination of slash pine seed. Proc. Assoc. Off. Seed Anal. 1972, 62, 91–93. [Google Scholar]
- Ivić, D. Curative and Eradicative Effects of Fungicides. In Fungicides; IntechOpen: London, UK, 2010. [Google Scholar]
- Gullino, M.L.; Tinivella, F.; Garibaldi, A.; Kemmitt, G.M.; Bacci, L.; Sheppard, B. History and Role of Mancozeb in Disease Management. Plant Dis. 1963, 94, 1076–1087. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wang, P.; Pu, Z.; Lu, L.; Chen, G.; Hu, X.; Fayyaz, A.; Gai, Y. Effects of mancozeb on citrus rhizosphere bacterial community. Microb. Pathog. 2021, 154, 104845. [Google Scholar] [CrossRef]
- Vinicius, M.; Bucker, W.; Luiz, E.; Masson, M.V.; Moraes, W.B.; Furtado, E.L. Chemical Control of Eucalyptus Rust: Brazilian Experiences. In Fungicides—Showcases of Integrated Plant Disease Management from Around the World; InTech: London, UK, 2013. [Google Scholar]
- Crous, P.W.; Wingfield, M.J.; Denman, S.; Sadie, A.; Crous, P.W.; Sadie, A.; Wingfield, M.J. Evaluation of fungicides for the control of Botryosphaeria protearum on Protea magnifica in the Western Cape Province of South Africa. Australas. Plant Pathol. 2004, 33, 97. [Google Scholar]
- Tran, T.T.T.; Pham, T.Q.; Barber, P.A.; Nguyen, C.M. Control of Ceratocystis manginecans causing wilt disease on Acacia mangium seedlings. Australas. Plant Pathol. 2018, 47, 579–586. [Google Scholar] [CrossRef]
- Weber, R.W.S.; Børve, J. Infection biology as the basis of integrated control of apple canker (Neonectria ditissima) in Northern Europe. CABI Agric. Biosci. 2021, 2, 5. [Google Scholar] [CrossRef]
- Narciso, J.A.; Ference, C.M.; Ritenour, M.A.; Widmer, W.W. Effect of copper hydroxide sprays for citrus canker control on wild-type Escherichia coli. Lett. Appl. Microbiol. 2012, 54, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Kleczewski, N.M.; Butts-Willmsmeyer, C.; Scanlan, C. Assessing the Curative and Protective Impacts of Select Fungicides for Control of Powdery Mildew of Wheat. Plant Dis. 2020, 104, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Prospero, S.; Botella, L.; Santini, A.; Robin, C. Biological control of emerging forest diseases: How can we move from dreams to reality? For. Ecol. Manag. 2021, 496, 119377. [Google Scholar] [CrossRef]
- Rolando, C.A.; Dick, M.A.; Gardner, J.; Bader, M.K.F.; Williams, N.M. Chemical control of two Phytophthora species infecting the canopy of Monterey pine (Pinus radiata). For. Pathol. 2017, 47, e12327. [Google Scholar] [CrossRef]
- Khuzaimah, Z.; Nawi, N.M.; Adam, S.N.; Kalantar, B.; Emeka, O.J.; Ueda, N. Application and Potential of Drone Technology in Oil Palm Plantation: Potential and Limitations. J. Sens. 2022, 2022, 5385505. [Google Scholar] [CrossRef]
- Chi, N.M.; Thu, P.Q.; Nam, H.B.; Quang, D.Q.; Phong, L.V.; Van, N.D.; Trang, T.T.; Kien, T.T.; Tam, T.T.T.; Dell, B. Management of Phytophthora palmivora disease in Citrus reticulata with chemical fungicides. J. Gen. Plant Pathol. 2020, 86, 494–502. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).