Abstract
Stable carbon isotopes (δ13C) and elemental stoichiometry characteristics are important ways to research the water and nutrient use strategies of plants. Investigating the variation patterns inof δ13C and the major nutrient elements in different organs of plants and the correlation among them can reveal the ecological strategies of desert plants in extreme arid environments. In this study, two typical desert plants, Alhagi sparsifolia and Karelinia caspia, were studied in the Tarim Basin. By analyzing the changes in δ13C, carbon (C), nitrogen (N), and phosphorus (P) and the ecological stoichiometry of their roots, stems, and leaves, the distribution patterns among different organs and their correlation with soil environmental factors were revealed. The results showed the following: (1) The δ13C of the two plants differed significantly among different organs (p < 0.01). The root and stem of Alhagi sparsifolia had significantly greater δ13C than the leave, while the δ13C of Karelinia caspia showed a root > stem > leaf gradient; (2) the C content in the leaves of the two plants was significantly lower than that of the root (p < 0.01), whereas the N content showed the opposite trend (p < 0.01); (3) the average N:P of Alhagi sparsifolia was >16.00, indicating it was mainly limited by P elements, while the average N:P of Karelinia caspia was <14.00, suggesting it was mainly limited by N elements; (4) in the root, stem and leave of Alhagi sparsifolia and Karelinia caspia, the N content and C:N and the P content and C:P showed a significantly negative correlation (p < 0.01), and δ13C was negatively correlated with C:P; (5) soil total phosphorus (TP) is an important soil environmental factor affecting δ13C and the nutrient elements in Alhagi sparsifolia and Karelinia caspia. This study demonstrates that Alhagi sparsifolia and Karelinia caspia are able to effectively coordinate and regulate their water, N, and P use strategies in response to environmental stress. These results can provide scientific reference for the evaluation of plant physiological and ecological adaptations for ecological conservation in arid areas.
1. Introduction
The Tarim Basin in Xinjiang is a typical extreme arid region. The long-term effects of aridity, high temperatures, barrenness, and other stressors have resulted in plants in this region developing unique ecological adaptations to the extreme environment. Understanding the characteristics of plants in these arid desert areas is essential for the accurate prediction of fragile and sensitive ecosystems. Stable carbon isotopes (δ13C) are a crucial indicator for plant water use efficiency (WUE) [1], which to some extent represents plant photosynthetic and transpiration rates [2,3]. Under aridity stress, plants enhance their adaptation by increasing their WUE [4]. C, N, and P are structural elements necessary for plant growth and development [5]. They participate in many plant physiological and biochemical processes [6] and are closely related to the synthesis of enzymes, amino acids, and other substances [7]. The elemental stoichiometric ratios of C, N, and P can reflect the dynamics of C accumulation, the efficiency of N and P utilization, and the pattern of nutrient limitation in the habitat of plants [8]. C:N and C:P are closely related to plant growth rate, while N:P can reflect N or P limitations in plant growth, which assist in the determination of plant nutrient utilization strategies. In recent years, more studies have focused on revealing plant WUE and nutrient use strategies by analyzing the changing patterns of plant δ13C and nutrient elements [9,10,11]. The investigation of δ13C and major nutrient elements in different organs of plants can help to understand their own resource allocation and ecological adaptation strategies in varying environments. However, studies exploring the trade-offs among different plant organs by combining water and nutrient use strategies are still limited.
Numerous scholars have worked on the δ13C and nutrient characteristics of various research objects, including natural plants and cultivated plants [12,13,14,15]. Studies have verified that there are many factors affecting plants’ δ13C and nutrient elemental characteristics. The main factors are plant type, plant developmental stage, environmental changes, and human disturbances [16]. Diverse responses in the δ13C and nutrient element stoichiometric characteristics of plants are triggered by changes in environmental factors such as elevation, atmospheric pressure, temperature, precipitation, fertilization, and aridity. Studies have shown that plant δ13C is positively correlated with elevation [17] and negatively correlated with both precipitation and air temperature [18,19]; it also increases with increasing latitude [20]. At the species or community level, plant fine-root N and P contents are negatively correlated with mean annual temperature and annual precipitation [21]. Leaf N and P concentrations vary significantly among different life forms, usually showing a pattern of herb > shrub > tree, angiosperm > gymnosperm [22,23]. In arid desert regions, plants respond to water scarcity by altering their nutrient partitioning patterns.
Roots, stems, and leaves are key organs for the synthesis, uptake, transport, accumulation, and storage of nutrients in plants [24], and the ratio of nutrient distribution among them reflects the ability of plant resources to acquire, transport, and store nutrients [25]. Therefore, the distribution patterns of δ13C and major nutrient elements among different organs of plants, including plant nutrient organs (roots, stems, leaves, etc.) and reproductive organs (flowers, fruits, seeds, etc.), have been given attention [26]. Numerous studies have shown that δ13C in plant leaves is generally lower than in other organs. The N and P elements and their stoichiometric relationships in different plant organs depend critically on the growth and functional attributes of each organ. Due to the dilution of nutrients by water in the organs, N and P concentrations in plant stems and roots are lower than those in leaves and reproductive organs [27,28], which is confirmed by the global study of N and P’s stoichiometric relationships in plant roots (i.e., N and P contents in fine roots are generally lower than in leaves) [22]. However, by analyzing plant and soil N and P elements, some scholars found that the elemental relationships among different organs may be decoupled or unchanged with environmental changes [29]. In extreme arid environments, the study of the changes in δ13C and major nutrient content among different organs of desert plants can contribute to revealing their ecological adaptations.
Alhagi sparsifolia and Karelinia caspia are the dominant species in arid and semi-arid regions, with excellent tolerance to drought, salinity, and barrenness [30,31]. Previous studies mainly focused on their fixation of nutrient elements, the comparison of their ecological stoichiometric characteristics, and the changes in plant functional traits under adversity stress [32]. However, there are fewer studies on their δ13C and nutrient elemental characteristics and the variations among organs. In this study, the Tarim Basin was taken as the study area, and Alhagi sparsifolia and Karelinia caspia were chosen as the research objects. We analyzed the stoichiometric characteristics of major nutrient elements (C, N, and P) and stable carbon isotopes (δ13C) of plant roots, stems, and leaves and their correlations with soil physicochemical factors. The aim of this study is to enhance the understanding of the ecological adaptation strategies of plants in extreme environments, which could provide a theoretical basis for regional ecological environment protection and restoration.
2. Materials and Methods
2.1. Overview of the Study Area
The study area is located at the northern edge of the Taklamakan Desert in the Tarim Basin of Xinjiang, China, in the upper reaches of the Tarim River Basin. The region has a warm temperate continental arid climate characterized by perennial drought and low precipitation, with an average annual precipitation of only 75.00 mm. Moreover, it is prone to frequent wind and sandstorms. The temperature varies from a minimum of −25 °C to a maximum of 40 °C, and the frost-free period is 180−224 d. The main soil types are saline, wind−sand, brown desert, and scrub desert. There are a few vegetation types, dominated by arid desert plants such as Alhagi sparsifolia, Karelinia caspia, Tamarix chinensis, and Populus euphratica as the representative species. Alhagi sparsifolia and Karelinia caspia are perennial herbs of the Leguminosae and Asteraceae families, respectively. During the long evolutionary process, they have developed excellent resistance to drought, salinity, barrenness, wind, and sand. Therefore, Alhagi sparsifolia and Karelinia caspia are effective plants for desertification control, playing an important role in preventing sand erosion.
2.2. Sample Collection and Measurement
2.2.1. Sample Survey
In June and July 2021, during the plant-growing season, representative sample sites where there was less human influence and more vegetation cover were selected through investigation of the study area. Six 1.00 × 1.00 m herbaceous sample plots were set up with a distance of 20.00 m between different plots at each sample site. The number of Alhagi sparsifolia and Karelinia caspia plants in each sample plot was counted, and the plant height, crown width, and basal diameter were accurately measured and recorded. The specific information is shown in Table 1.

Table 1.
Alhagi sparsifolia and Karelinia caspia parameters of plant height, crown width, basal diameter, and density (mean ± SE).
2.2.2. Sample Collection
Within each sample plot, Alhagi sparsifolia and Karelinia caspia individuals of similar morphology, well established, and without obvious gnawing marks were selected.
Healthy and mature leaves of the two studied species from different orientations were collected and mixed, then put into kraft envelopes. One-year-old stems were cut using fruit pruning shears, stripped of leaves, and packed into kraft envelopes. A number of fine roots (diameter ranging from 0.05 to 0.30 cm and length of around 7.00 cm) were obtained by digging, then shaken off the soil on the root surface and put into kraft envelopes. In the center of each sample plot, soil samples ranging from 0 to 100.00 cm were collected according to the five-point sampling method (the soil depth included 0–20.00 cm, 20.00–40.00 cm, 40.00–60.00 cm, 60.00–80.00 cm, and 80.00–100.00 cm). After removing other plant roots, stones, and other debris, the collected soil samples were mixed evenly and divided into two portions. One portion was put into sample bags for determining the soil physicochemical properties, and the other portion was put into an aluminum box for determining the soil water content. Finally, all the collected samples were kept in a cryogenic ice box and brought back to the laboratory.
2.3. Sample Determination
2.3.1. Sample Processing
Collected plant roots, stems, and leaves were brought back to the laboratory and washed thoroughly, then placed into an oven, killed out at 105 °C for 15 min, and dried at 75 °C for 48 h until reaching a constant weight state. The dried plants were ground using a multifunctional pulverizer (NM200, Retsch, Haan, Germany) and passed through a 0.15 mm sieve, then put into sample bags for measurement. After the soil samples were brought back, one part of the samples was placed in an oven at 105 °C for 24 h to measure the soil moisture content, and the other part was air-dried in a cool place, ground, sieved using a 0.30 mm mesh sieve, and put into sample bags for measurement [33].
2.3.2. Stable Carbon Isotope Composition Determination (δ13C)
The dried plants were ground and passed through a 0.15 mm sieve, then measured with a stable-isotope-ratio mass spectrometer (DELTA A V Advantage, Waltham, MA, USA). The δ13C value of the sample was calculated according to the following formula:
δ13C (‰) = [(Rsample − Rstandard) ÷ Rstandard] × 1000
In the formula, Rstandard and Rsample are the 13C/12C standard sample and the 13C/12C sample, respectively; Rstandard is the adopted international standard substance, Vienna Pee Dee Belemnite (VPDB) [16].
2.3.3. Plant Nutrient Element Determination
Determination of plant C content was carried out using the potassium dichromate volumetric method (external heating method); determination of N content was performed using a fully automated Kjeldahl nitrogen analyzer (The Swedish FOSS, KjeltecTM8400, Copenhagen, Denmark); and P was determined using the aqua regia microwave-assisted digestion ICP−AES method.
2.3.4. Determination of Physical and Chemical Properties of Soil Samples
In the soil environment factors, the measurement characteristics included soil organic carbon (SOC), total nitrogen (TN) and total phosphorus (TP) elemental content (g·kg−1), alkaline nitrogen content (AN, mg·kg−1), quick-acting phosphorus content (AP, mg·kg−1), soil water content (SWC, %), electrical conductivity (EC, µS·cm−1), and pH. The SOC, TN, and TP contents of the soil were determined using the potassium dichromate volumetric method, Kjeldahl nitrogen determination, and molybdenum antimony colorimetric method, respectively. The soil AN and AP contents were determined using the alkaline diffusion method and sodium bicarbonate method, respectively. Soil SWC was determined using the drying method. Soil EC was determined using the electrical conductivity method (DDS−307, Shanghai Yidian Scientific Instrument Co., Ltd., Shanghai, China). Soil pH was determined using a pH meter (PHS−3C, Shanghai Yidian Scientific Instrument Co., Ltd., Shanghai, China). Specific experimental methods are described in the literature [34].
2.4. Data Processing
Differences in δ13C and nutrient contents among different organs of Alhagi sparsifolia and Karelinia caspia were examined using One-Way ANOVA and the Multiple Comparison Tukey method. Pearson analysis was used to analyze the correlation between δ13C and nutrient element contents across different organs of the two desert plants. Redundancy Analysis (RDA) was used to study the relationships among the whole plant δ13C, nutrient element contents, and soil environmental factors. Data processing, statistical analysis, and drawing were completed using SPSS 26 (IBM, Chicago, IL, USA), Canoco 5 (CanocoLab, Microsoft, Redmond, Washington, DC, USA), and Origin 2021 (OriginLab, Nothampton, MA, USA).
3. Results and Analysis
3.1. Characteristics of δ13C and Major Nutrient Elements in Alhagi sparsifolia and Karelinia caspia
As shown in Table 2, except for the P content, the average δ13C and major nutrient element contents of Karelinia caspia were greater than those of Alhagi sparsifolia. The stoichiometric ratios showed that the C:P and N:P of Karelinia caspia were greater than those of Alhagi sparsifolia, while C:N was lower than that of Alhagi sparsifolia. The coefficients of variation in δ13C and the C content were within 6.00% for both plants, which was low; the coefficients of variation in N, P, C:N, C:P, and N:P in Karelinia caspia and P and C:P in Alhagi sparsifolia ranged from 20.00% to 40.00%, which was relatively stable. However, the coefficients of variation in N, C:N, and N:P in Karelinia caspia ranged from 50.00% to 60.00%, showing relatively high variability.

Table 2.
Characteristics of δ13C and major nutrient element contents of Alhagi sparsifolia and Karelinia caspia.
3.2. Characteristics of δ13C and Major Nutrient Elements in Different Organs of Alhagi sparsifolia and Karelinia caspia
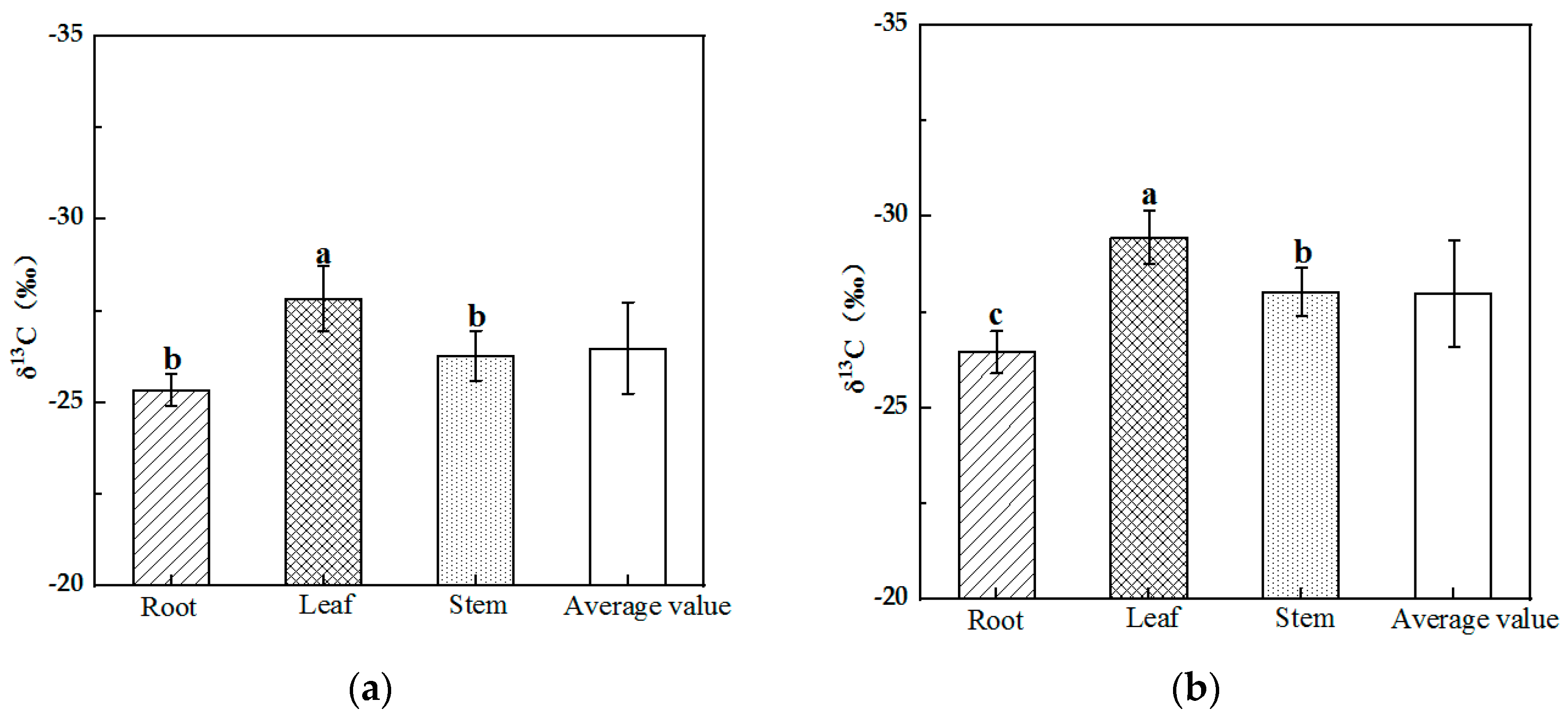
The δ13C values ranged from −30.00 to −25.00‰ for Alhagi sparsifolia and Karelinia caspia, and the mean δ13C values for the two plants were −26.49‰ and −27.98‰, respectively. As shown in Figure 1, the results of the One-Way ANOVA indicated that the difference in δ13C among different organs for Alhagi sparsifolia and Karelinia caspia was significant (p < 0.05). The δ13C of Alhagi sparsifolia showed a pattern of root > stem > leaf, with both root and stem δ13C values significantly higher than that of the leaf. The δ13C of Karelinia caspia showed a root > stem > leaf pattern; the δ13C of the root was significantly higher than that of the stem, and the δ13C of the stem was significantly higher than that of the leaf.
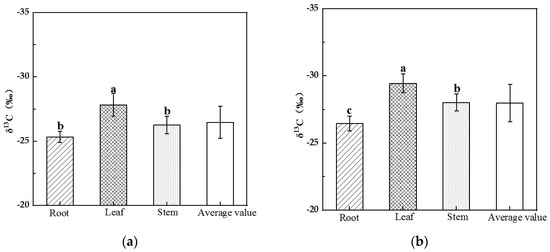
Figure 1.
Characteristics of δ13C in different organs of Alhagi sparsifolia (a) and Karelinia caspia (b) (mean ± SE). Note: Different lowercase letters indicate a significant difference (p < 0.01).
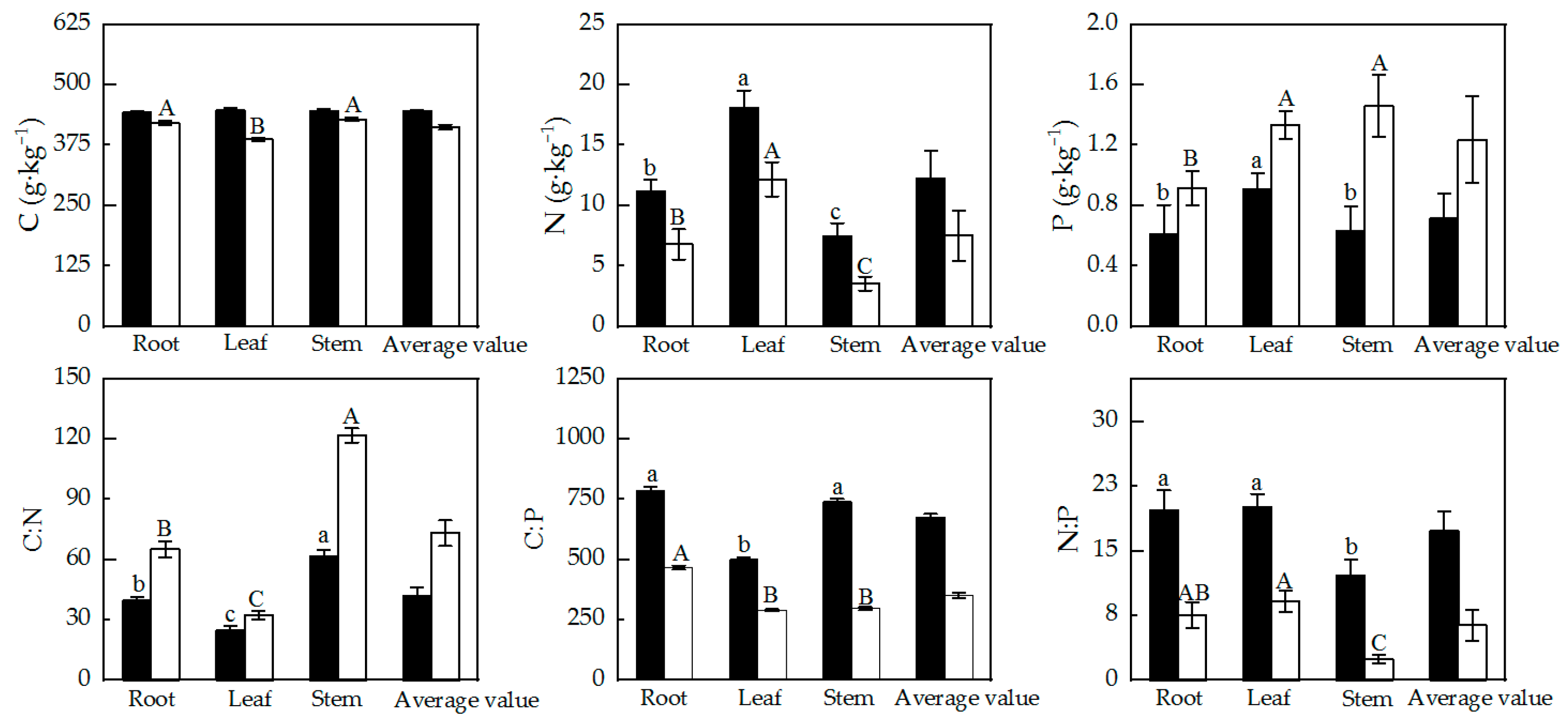
The major nutrient contents and stoichiometric ratio characteristics across different organs of Alhagi sparsifolia and Karelinia caspia are shown in Figure 2. Except for the C content in Alhagi sparsifolia, the other nutrient contents and stoichiometry of the two studied species were significantly different among the root, stem, and leaf. For Karelinia caspia, the C contents of the root and stem were significantly higher than that of the leaf. The N content in all organs of Alhagi sparsifolia and Karelinia caspia showed a pattern of leaf > root > stem. The leaf P content in Alhagi sparsifolia was significantly higher than in the root and stem, whereas the P element content in Karelinia caspia was much higher in the stem and leaf compared to the root (p < 0.01). In addition, the variation patterns in C:N among organs for both Alhagi sparsifolia and Karelinia caspia were consistent (stem > root > leaf (p < 0.01)). The C:P values in the root and stem were significantly higher than in the leaf in Alhagi sparsifolia, and the C:P value in the root was significantly higher than in the leaf and stem in Karelinia caspia (p < 0.01). For Alhagi sparsifolia, leaf N:P was higher than root N:P, and root N:P was significantly higher than stem N:P (p < 0.05).
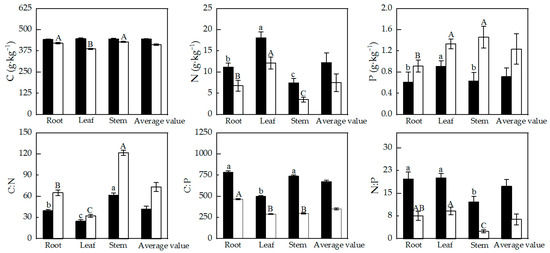
Figure 2.
Characteristics of main nutrient element contents and stoichiometric ratios in different organs of Alhagi sparsifolia and Karelinia caspia (mean ± SE). Note: Black columns represent Alhagi sparsifolia; white columns represent Karelinia caspia. Note that lowercase letters indicate a significant difference in Alhagi sparsifolia, while capital letters indicate a significant difference in Karelinia caspia.
3.3. Correlation between δ13C and Major Nutrient Elements in Different Organs of Alhagi sparsifolia and Karelinia caspia
As shown in Table 3, only Alhagi sparsifolia’s leaf δ13C showed a significant positive correlation with the C content (p < 0.05). There was no significant correlation between δ13C and major nutrient elements in other organs. However, the major nutrient elements and their stoichiometric ratios exhibited close relationships, as the N content and C:N and the P content and C:P of the three organs of Alhagi sparsifolia all showed extremely significant negative correlations. There was also an extremely significant negative correlation between root P content and N:P, and an extremely significant positive correlation between root C:P and N:P (p < 0.01).

Table 3.
Correlation between δ13C and major nutrient elements in different organs of Alhagi sparsifolia.
Based on Table 4, it can be found that the stem δ13C of Karelinia caspia showed an extremely significant positive correlation with the N content (p < 0.01), but the relationship with C:N showed an extremely significant negative correlation. The N content and C:N and the P content and C:P of the three organs all showed significantly negative correlations. Furthermore, the C:N and N:P of the root and leaf and the N and C:P of the stem also displayed significantly negative correlations (p < 0.05). There was a significantly positive correlation observed between N:P and P in the root and leaf.

Table 4.
Correlation between δ13C and major nutrient elements in different organs of Karelinia caspia.
3.4. Relationships among δ13C, Major Nutrient Elements, and Soil Physicochemical Factors in Alhagi sparsifolia and Karelinia caspia
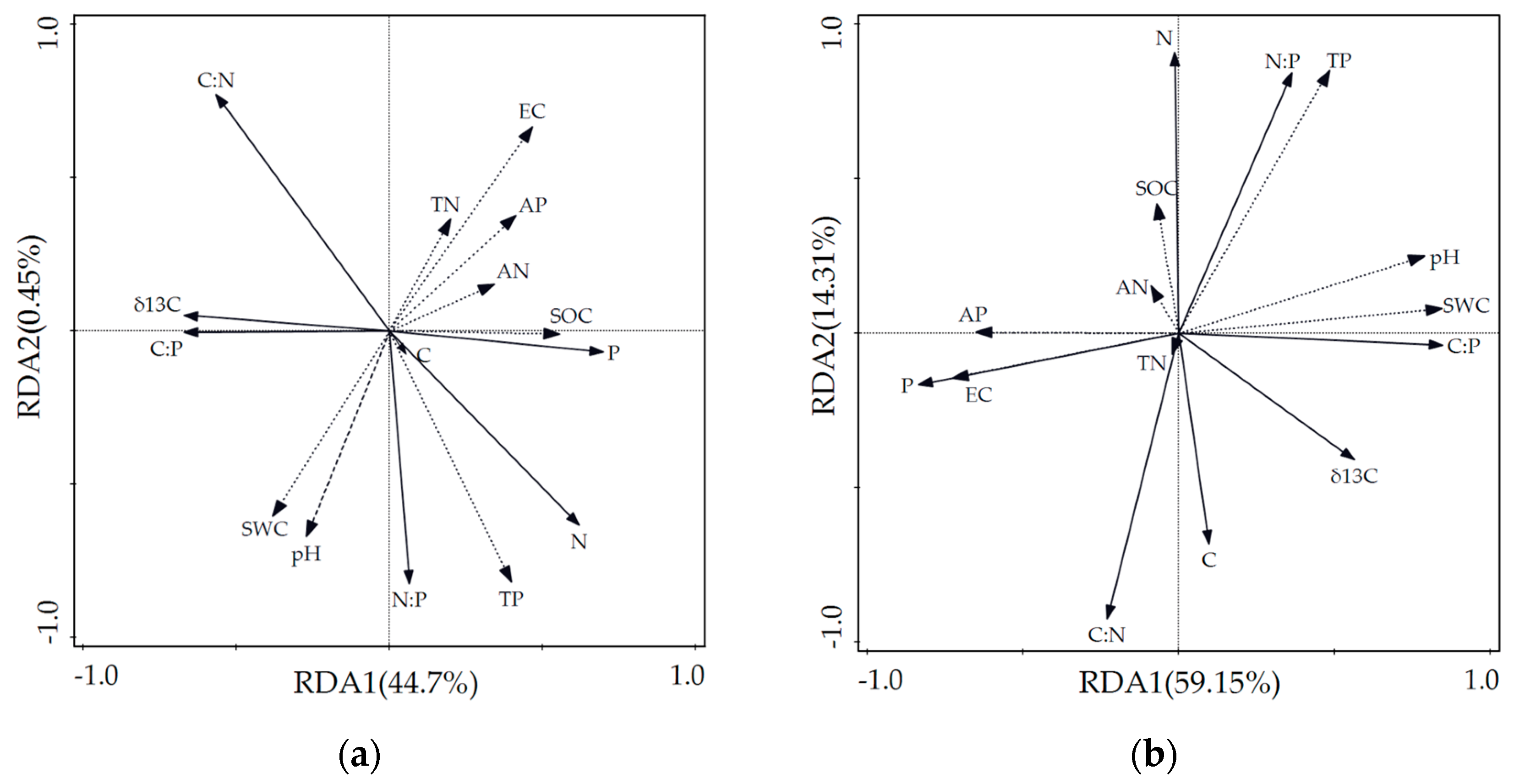
According to the results of the RDA, the interpretation amounts of soil environmental factors to the variations in δ13C, major nutrient elements, and stoichiometric ratios of Alhagi sparsifolia in axes I and II were 44.47% and 0.45%, respectively. Those of Karelinia caspia were explained at 59.15% and 14.31%, respectively. The sum of the explanations of the two axes was 44.93% and 73.46% for Alhagi sparsifolia and Karelinia caspia, respectively. Therefore, the first two axes could effectively reflect the relationship between δ13C, the major nutrient elements, and the soil physicochemical factors, with axis I playing the more important role.
Figure 3a,b show the two-dimensional ordination maps of RDA for Alhagi sparsifolia and Karelinia caspia. The solid lines represent δ13C, the major nutrient elements, and the ecological stoichiometry ratios; the dotted lines represent the soil physicochemical factors of their habitats. The length of the arrow line indicates the strength of the correlation, with a longer arrow representing a greater correlation and vice versa. The angle of the arrow to the sorting axis indicates a positive or negative correlation, with an acute or obtuse angle representing a positive or negative correlation, respectively. In the figure, the length of the arrows of the TP content in the soil physicochemical factors was the longest, indicating that soil TP played a crucial role in explaining the δ13C, major nutrient elements, and stoichiometric variability in the two studied species. Soil TP was negatively correlated with the δ13C values of Alhagi sparsifolia and Karelinia caspia and most highly correlated with N, C:N, and N:P.
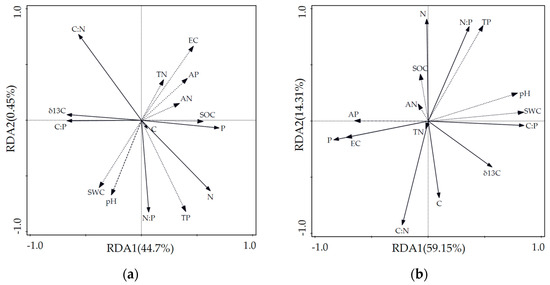
Figure 3.
RDA two-dimensional ordination plots of soil physicochemical factors for δ13C and major nutrient elements in Alhagi sparsifolia and Karelinia caspia. (a) Correlation plots for δ13C, major nutrient elements, and soil physicochemical factors in Alhagi sparsifolia. (b) Correlation plots for δ13C, major nutrient elements, and soil physicochemical factors in Karelinia caspia. Note: Solid lines indicate plants. Dashed lines indicate soil physicochemical factors.
4. Discussion
4.1. Variation in δ13C Characteristics among Organs of Alhagi sparsifolia and Karelinia caspia
Water use is a crucial process for photosynthetic carbon sequestration in plants. Its efficiency reflects the plant’s ability to adapt to stressful environments. Traditional instantaneous WUE refers to the ratio of photosynthetic rate (A) to transpiration rate (E), while δ13C is a key indicator of long-term WUE in plants, which is important for revealing the relationship between plants and their long-term role in the environment. Due to the fractionation effect of δ13C, carbon isotope discrimination (Δ) in plant tissue was developed as a method for determining transpiration efficiency. It has been verified that it is negatively correlated with A/E at the leaf level [35]. By reviewing numerous studies, it was found that the most important factor affecting δ13C characteristics is the type of plant photosynthesis (C3, C4, and CAM). In terrestrial ecosystems, the δ13C of C3, C4, and CAM plants ranges from −34.00% to −21.00‰, −19.00% to −9.00‰, and −38.00% to −1.30‰, respectively [36]. In the current study, both Alhagi sparsifolia and Karelinia caspia were identified as C3 plants. Their average δ13C values were −26.49‰ and −27.98‰, respectively, which are relatively higher among C3 plants. This may be due to the fact that they are distributed in the extreme arid zone, where maximum temperatures can reach 39 °C and annual precipitation is less than 100.00 mm, with evaporation exceeding 2500.00 mm. These higher temperatures and scarcer precipitation resulted in relatively high δ13C values in Alhagi sparsifolia and Karelinia caspia (Table 2).
Previous studies have verified that δ13C content tends to differ among plant organs. The δ13C values across different organs of plants can reveal the physiological and ecological characteristics of plants and their responses to environmental changes (e.g., climate, history, geography, etc.) [37,38]. A study of 10 Glycine max by Feng et al. found that δ13C presented a pattern of roots > stems > seeds > leaves [39]. The δ13C pattern of different organs in Populus tomentosa was root > twig > leaf [40]. The current study showed that the δ13C of the root and stem of Alhagi sparsifolia was significantly greater than that of the leaf, while the δ13C of Karelinia caspia displayed a trend of root > stem > leaf, which is consistent with most scholars’ findings. There may be several explanations for the discrepancy among different organs. Firstly, their chemical and physiological properties vary greatly [41]. Secondly, since water metabolism in plants begins with water uptake by the root system, soil water, atmospheric precipitation, surface runoff, or groundwater can enter a plant’s roots under the combined effect of root pressure and transpiration tension. Then, it is transported upwards along the ducts or tubular cells to the leaves and other tissues. On the one hand, it finally dissipates through transpiration or exhalation. On the other hand, it is involved in life activities such as respiration and photosynthesis in the cells of various tissues. These complex processes could lead to potential fractionation during water transport in plants. Thirdly, under arid conditions, dry matter accumulation is reduced to varying degrees in all plant organs, with leaves being more susceptible to stress than stems or roots [42]. Finally, as 12C is a lighter isotope than 13C, leaves and stems are more likely to uptake 12C during growth. However, the heavier 13C is enriched in its position closer to the soil, thus resulting in a maximum δ13C value in the roots [43,44,45].
4.2. Variation in Major Nutrient Element Content and Stoichiometric Ratio among Organs of Alhagi sparsifolia and Karelinia caspia
Plant growth and development are closely related to the environment in which they are located. Changes in elemental content and its stoichiometry could reflect the ability of plants to absorb and accumulate soil nutrients. Hence, they can characterize their response to changes in environmental conditions [46,47,48]. Therefore, investigating the ecological stoichiometric characteristics of different organs can provide novel insight into the distribution of the C, N, and P elements in desert plants and the ability of plants to regulate their own nutrients in order to adapt to the environment [49]. Alhagi sparsifolia and Karelinia caspia are dominant species in arid desert areas with infertile soils, scarce water resources, and severe saline intrusion. Their capacity to sustain the relative stability of chemical composition plays a determining role, and they have developed unique nutrient utilization strategies that are compatible with arid desert environments [50,51].
Through the analysis of the major nutrient element contents in different organs of the two studied species, we found that the leaf C content in Karelinia caspia was significantly lower than in the root and stem. The leaf N content in the two species was significantly higher than in the root and stem, and the leaf P content was significantly higher than in the root (p < 0.01). The main reason for this phenomenon may be that plants absorb or transport C, N, and P in different ways. The C element is mainly fixed into the plant by the leaves through photosynthesis, while N and P are mainly absorbed from the soil through the fine roots [52]. Secondly, desert plants are susceptible to water stress, which contributes to the increase in free amino acids in the leaves to maintain intracellular osmotic balance [53]; therefore, the leaf N content increases. This is a sign of the active adaptation employed by plants to promote photosynthesis and a response to prevent the loss of organic C due to salt stress [12]. Meanwhile, the higher P content in the leaf relative to the root may be due to the strategy adopted by plants to cope with high soil salinity and low water availability [54]. According to the nutrient limitation theory proposed by Koerselman et al. [55], the average N:P of Alhagi sparsifolia was greater than 16, suggesting it is mainly limited by the P element. The probable reason for this is that the sampling occurred during the peak plant growth season, when the uptake of nutrients by the plant was not as fast as the rate of cell expansion, resulting in an insufficient supply of the P element [56]. In contrast, the average N:P in Karelinia caspia was below 14, indicating that it was mainly N-limited. This may be because water stress significantly reduces nitrate reductase activity, slowing down nitrate reduction and ammonia assimilation. The uptake of nitrate N by Karelinia caspia’s roots is reduced [54,57]. Meanwhile, as Alhagi sparsifolia belongs to the leguminous family, Koerselman et al.’s study reported that dryland legumes may enhance their adaptability to saline environments by both building symbiotic associations with rhizobium and utilizing readily available N when salt stress is moderate. It was proven that non-leguminous plants could also obtain nitrogen through their interaction with leguminous plants, but nitrogen mobility was generally low [31,58]. Therefore, Karelinia caspia, the non-leguminous plant, is limited by the N element.
4.3. Relationships among δ13C, Major Nutrient Elements, and Soil Physicochemical Factors in Alhagi sparsifolia and Karelinia caspia
The study of plant–soil correlations is conducive to reducing ecological niche overlap and rationalizing species allocation. The results of the RDA indicated that soil TP content had the greatest effect on δ13C, major nutrient elements, and stoichiometry in the two studied plants; thus, it was the main driver affecting δ13C and the major nutrient elements of Alhagi sparsifolia and Karelinia caspia. In summarizing a large number of studies, we found that the relationships between plant δ13C and soil TP were inconsistent. Harris et al. found a negative correlation between δ13C and soil TP in the vegetation of Xilingol grassland; they thought the uptake of soil P by plants was related to the mass-flow effect [59]. But some scholars, through experiments, found a positive correlation between plant δ13C and soil P content under conditions of severe water deficit and the addition of different N sources [60]. The uptake of P mainly comes from the transpiration force, which drives soil-soluble P to the root surface. The greater the transpiration accompanied by lower WUE, the lower the corresponding δ13C value; thus, there is an inverse relationship between δ13C and soil P content. Our results were in line with this conclusion. The average annual precipitation in the study area is within 75.00 mm, but the annual evaporation exceeds 2500.00 mm, which indicates that moisture is a critical limiting factor for plant growth and survival in this area. Affected by strong transpiration, δ13C was negatively correlated with soil TP content in Alhagi sparsifolia and Karelinia caspia. However, plant δ13C and soil TP content are not necessarily negatively correlated in high-salt areas. This is because salinity changes plant δ13C mainly by affecting stomatal conductance, photosynthesis, and other physiological activities in C3 plant leaves [61]. The process of photosynthetic product synthesis in the saline environment is affected by P, which in turn affects leaf δ13C. High-salt environments reduce soil-soluble P, resulting in the inability of plants to enrich more P by increasing transpiration.
5. Conclusions
This study has revealed that the δ13C values in the organs of Alhagi sparsifolia and Karelinia caspia show a varying pattern of root > stem > leaf. The N and P contents in the leaf were higher than those in the root. The results showed that the δ13C of the two species was negatively correlated with C:P and N:P, indicating that they may promote C and N fixation under conditions of low water-use efficiency by increasing the efficiency of P utilization. Aridity and infertility are the main environmental factors that limit plant growth and vegetation recovery in the Tarim Basin. Alhagi sparsifolia and Karelinia caspia have developed adaptive strategies that involve coordinating water use efficiency and nutrient use efficiency. The findings of this study could provide a theoretical basis for the protection and restoration of vegetation in desert ecosystems.
Author Contributions
Conceptualization, Z.Z. and X.W.; methodology, Z.Z. and X.W.; software, Z.Z., X.Z. and Z.L.; validation, Z.Z.; formal analysis, Z.Z.; investigation, X.W., R.L., Y.L. and X.Z.; resources, Z.Z., X.W., R.L. and X.Z.; data curation, Z.Z.; writing—original draft preparation, Z.Z.; writing—review and editing, Z.Z., X.W. and L.G.; visualization, Z.Z.; supervision, X.W.; project administration, Z.Z. and X.W.; funding acquisition, X.W. All authors have read and agreed to the published version of the manuscript.
Funding
National Natural Sciences Foundation of China (No. 32001145); Xinjiang Uygur Autonomous Region Education Department Basic Scientific Project (No. XJEDU2023P005); Xinjiang Uygur Autonomous Region Education Department Tianchi Doctoral Research Project (No. TCBS202054); Xinjiang University Doctoral Science Foundation (No. 2020670018); and Sciences Foundation of Xinjiang Uygur Autonomous Region (No. 2020D01C053).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shi, R.L.; Zhang, Q.F.; Li, M.; Li, Q.Q.; Zhang, M.M. Application of Plant Carbon Isotope Fractionation in the Study of Water Use Efficiency. Chin. Agric. Sci. Bull. 2022, 38, 15–20. [Google Scholar] [CrossRef]
- Gavito, M.E.; Jakobsen, I.; Mikkelsen, T.N.; Mora, F. Direct evidence for modulation of photosynthesis by an arbuscular mycorrhiza-induced carbon sink strength. New Phytol. 2019, 223, 896–907. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhang, J.S.; Meng, P.; He, C.X.; Jia, C.X.; Li, J.Z. Feasibility analysis on the determination of WUE by stable carbon isotope: Cassia obtusifolia L. as an example. Acta Ecol. Sin. 2014, 34, 5453–5459. [Google Scholar] [CrossRef][Green Version]
- Deng, X.X.; Shi, Z.; Zeng, L.X.; Lei, L.; Pei, X.X.; Wu, S.; Xiao, W.F. Effects of drought and shading on instantaneous water use efficiency and δ13C of Pinus massoniana seedlings. Chin. J. Ecol. 2023, 40, 2735–2742. [Google Scholar]
- Warren, C.R.; Adams, M.A.; Chen, Z.L. Is photosynthesis related to concentrations of nitrogen and Rubisco in leaves of Australian native plants? Funct. Plant Biol. 2000, 27, 407–416. [Google Scholar] [CrossRef]
- Ma, F.; Liang, W.Y.; Zhou, Z.N.; Xiao, G.J.; Liu, J.L.; He, J.; Jiao, B.Z.; Xu, T.T. Spatial Variation in Leaf Stable Carbon Isotope Composition of Three Caragana Species in Northern China. Forests 2018, 9, 297. [Google Scholar] [CrossRef]
- Walker, A.P.; Beckerman, A.P.; Gu, L.H.; Kattge, J.; Cernusak, L.A.; Domingues, T.F.; Scales, J.C.; Wohlfahrt, G.; Wullschleger, S.D.; Woodward, F.I. The relationship of leaf photosynthetic traits—Vcmax and Jmax—To leaf nitrogen, leaf phosphorus, and specific leaf area: A meta-analysis and modeling study. Ecol. Evol. 2014, 4, 3218–3235. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, F.A.; Carrillo, Y.; Aspinwall, M.J.; Maier, C.; Canarini, A.; Tahaei, H.; Choat, B.; Tissue, D.T. Water, nitrogen and phosphorus use efficiencies of four tree species in response to variable water and nutrient supply. Plant Soil 2016, 406, 187–199. [Google Scholar] [CrossRef]
- Xia, D.J.; Liu, Q.R.; Zou, L.L.; Ge, Z.W.; Xue, X.H.; Peng, S.L. Foliar δ13C correlates with elemental stoichiometey in halophytes of coastal wetlands. Acta Ecol. Sin. 2020, 40, 2215–2224. [Google Scholar]
- Knelman, J.E.; Schmidt, S.K.; Lynch, R.C.; Darcy, J.L.; Castle, S.C.; Cleveland, C.C.; Nemergut, D.R.; Anthony, G.J. Nutrient Addition Dramatically Accelerates Microbial Community Succession. PLoS ONE 2014, 9, e102609. [Google Scholar] [CrossRef]
- Castle, S.C.; Sullivan, B.W.; Knelman, J.; Hood, E.; Nemergut, D.R.; Schmidt, S.K.; Cleveland, C.C. Nutrient limitation of soil microbial activity during the earliest stages of ecosystem development. Oecologia 2017, 185, 429–511. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.H.; Song, X.Q.; Zheng, J.W.; Zhang, Z.H.; Tang, Z.H. Ecological Stoichiometric Characteristics of C, N and P and Their Relationship with Soil Factors from Different Organs of the Halophytic Chenopodiaceae Plants in Hulunbuir. Bull. Bot. Res. 2022, 42, 910–920. [Google Scholar] [CrossRef]
- Li, W.Z.; Gao, Y.; Yang, L.; Jiang, Z.Y.; Wang, X.W. Carbon, nitrogen, and phosphorus stoichiometry of recently senesced larch leaves in response to environmental factors across an entire growing season. Chin. J. Ecol. 2020, 39, 2832–2841. [Google Scholar] [CrossRef]
- Wang, R.Z.; Luo, L.Y.; Sun, J.W.; Gu, H.B.; Wang, G.J. Seasonal dynamics of leaf, branch and root C:N:P ecological stoichiometry of mature Cunninghamia lanceolata in Huitong. J. Cent. South Univ. For. Technol. 2020, 40, 64–71. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, B.R.; An, S.S. Ecological stoichiometry in leaves, roots, litters and soil among different plant communities in a desertified region of Northern China. Catena 2018, 166, 328–338. [Google Scholar] [CrossRef]
- Liu, Y.; Li, P.; Shen, B.; Feng, C.H.; Liu, Q.; Zhang, Y. Effects of drought stress on Bothriochloa ischaemum water-use efficiency based on stable carbon isotope. Acta Ecol. Sin. 2017, 37, 3055–3064. [Google Scholar] [CrossRef][Green Version]
- Zhou, C.L.; Li, Y.K.; Cao, G.M.; Peng, C.J.; Song, M.H.; Xu, X.L.; Zhou, H.K.; Lin, L. Carbon and nitrogen stable isotopes technology in the researches on alpine meadow ecosystem in Qinghai-Tibet Plateau: Progress and prospect. Chin. J. Appl. Ecol. 2020, 31, 3568–3578. [Google Scholar] [CrossRef]
- Li, J.Z. Stable Carbon Isotope Compositions in C3, C4 Herbaceous Plants and Their Responce to Changing Temperature. Master’s Thesis, Ludong University, Yantai, China, 2009. [Google Scholar]
- Yang, S.Y.; Zhao, X.N.; Gao, X.D.; Yu, L.Y. Difference of Water Use Efficiency Between Ecological and Economic Forest and Its Response to Environment Using Carbon Isotopeithe Loess Plateau of China. J. Soil Water Conserv. 2022, 36, 7. [Google Scholar] [CrossRef]
- Haliguli, A.; Yiliminuer; Guan, W.K.; Abudourexii, R. Resposeof Leaf δ13C and δ15N Environmental Factorsin Different Habitats of Populus euphraticai. Acta Bot. Boreal. Occident. Sin. 2020, 40, 1031–1042. [Google Scholar] [CrossRef]
- Ma, Y.Z.; Zhong, Q.L.; Jin, B.J.; Lu, H.D.; Guo, B.Q.; Zheng, Y.; Li, M.; Cheng, D.L. Spatial changes and influencing factors of fine root carbon, nitrogen and phosphorus stoichiometry of plants in China. Chin. J. Plant Ecol. 2015, 39, 159–166. [Google Scholar] [CrossRef]
- Tian, D.; Yan, Z.B.; Niklas, K.J.; Han, W.X.; Kattge, J.; Reich, P.B.; Luo, Y.K.; Chen, Y.H.; Tang, Z.Y.; Hu, H.F. Global leaf nitrogen and phosphorus stoichiometry and their scaling exponent. Natl. Sci. Rev. 2017, 5, 728–739. [Google Scholar] [CrossRef]
- Tian, D.; Yan, Z.B.; Fang, J.Y. Review on characteristics and main hypotheses of plant ecological stoichiometry. Chin. J. Plant Ecol. 2021, 45, 682–713. [Google Scholar] [CrossRef]
- Iii, F.; Mooney, S. The Ecology and Economics of Storage in Plants—Annual Review of Ecology and Systematics. Annu. Rev. Ecol. Syst. 1990, 21, 423–447. [Google Scholar]
- Jiang, G.F.; Li, S.Y.; Li, Y.C.; Roddy, A.B. Coordination of hydraulic thresholds across roots, stems, and leaves of two co-occurring mangrove species. Plant Physiol. 2023, 4, 4. [Google Scholar] [CrossRef]
- Badeck, F.W.; Tcherkez, G.; Salvador, N.; Clément, P.; Ghashghaie, J. Post-photosynthetic fractionation of stable carbon isotopes between plant organs—A widespread phenomenon. Rapid Commun. Mass Spectrom. 2005, 19, 1381–1391. [Google Scholar] [CrossRef]
- Tang, Z.Y.; Xu, W.T.; Zhou, G.Y.; Bai, Y.F.; Li, J.X.; Tang, X.L.; Chen, D.M.; Liu, Q.; Ma, W.H.; Xiong, G.M.; et al. Patterns of plant carbon, nitrogen, and phosphorus concentration in relation to productivity in China’s terrestrial ecosystems. Proc. Natl. Acad. Sci. USA 2018, 115, 4033. [Google Scholar] [CrossRef]
- Zhao, N.; Yu, G.R.; He, N.P.; Xia, F.C.; Wang, Q.F.; Wang, R.L.; Xu, Z.W.; Jia, Y.L. Invariant allometric scaling of nitrogen and phosphorus in leaves, stems, and fine roots of woody plants along an altitudinal gradient. J. Plant Res. 2016, 129, 647–657. [Google Scholar] [CrossRef]
- He, M.Z.; Dijkstra, F.A.; Zhang, K.; Li, X.R.; Tan, H.J.; Gao, Y.H.; Li, G. Leaf nitrogen and phosphorus of temperate desert plants in response to climate and soil nutrient availability. Sci. Rep. 2014, 4, 6932. [Google Scholar] [CrossRef]
- Xing, R.X.; Zhang, B.; Guo, P.L.; Zhang, Z.H.; Huang, C.B.; Zeng, F.J. The ecological stoichiometric characteristics of Alhagi sparsifolia and Karelinia caspia in different habitats. Chin. J. Ecol. 2020, 39, 733–740. [Google Scholar] [CrossRef]
- Guo, P.L.; Liu, B.; Zhang, Z.H.; Xing, R.X.; Zhang, B.; Zeng, F.J. Effects of interaction between Alhagi sparsifolia and Karelinia caspia on nitrogen fixation and rhizosphere microorganisms. Acta Ecol. Sin. 2020, 40, 6632–6643. [Google Scholar]
- Zhang, L. Mechanism of the effects of typical temperate desert plant diversity on ecosystem function. Master’s Thesis, Xingjiang University, Urumqi, China, 2019. [Google Scholar]
- Gang, C.; Sheng, L. Plant Physiology Lab; Higher Education Press (HEP): Beijing, China, 2016. [Google Scholar]
- Dong, M. Observation and Analysis of Terrestrial Biocommunities; Standards Press of China: Beijing, China, 1997. [Google Scholar]
- Lin, Z.; Xing, X. Review on influential factors of plant water use efficiency. Agric. Res. Arid Areas 2005, 23, 208–213. [Google Scholar] [CrossRef]
- Zhou, R.C.; Zhang, W.B.; Cheng, X.L.; Xu, X.Y. A Review on the Responses of Plant and Soil Carbon Stable Isotope. Composition to Environmental Change. Environ. Sci. 2019, 32, 565–572. [Google Scholar] [CrossRef]
- Ge, T.D.; Wang, D.D.; Zhu, Z.K.; Wei, L.; Wei, X.M.; Wu, J.S. Tracing technology of carbon isotope and its applications to studies of carbon cycling in terrestrial ecosystem. Chin. J. Plant Ecol. 2020, 44, 360–372. [Google Scholar] [CrossRef]
- Feng, Q.H.; Shi, Z.M.; Dong, L.L. Response of Plant Functional Traits to Environment and Its Application. Acta Ecol. Sin. 2008, 44, 125–131. [Google Scholar] [CrossRef]
- Feng, H.Y.; Chen, T.; Xu, S.J.; An, L.Z.; Wang, X.L. Effect of enhanced UV-B radiation on growth, yield and stable carbon isotope composition in Glycine max cultivars (SCI). Acta Bot. Sin. 2001, 43, 709–713. [Google Scholar] [CrossRef]
- Fang, X.J.; Li, J.Y.; Nie, L.B.; Shen, Y.B.; Zhang, Z.Y. The characteristics of stable carbon isotope and water use efficiency for Populus tomentosa hybrid clones. Ecol. Environ. Ences 2009, 18, 2267–2271. [Google Scholar] [CrossRef]
- Greitner, C.S.; Winner, W.E. Increases in δ13 values of radish and soybean plants caused by ozone. New Phytol. 2010, 108, 489–494. [Google Scholar] [CrossRef]
- Li, Y.Y. Application of carbon isotope technique on the study of water water use efficiency of crops. Acta Agric. Nucl. Sin. 2000, 14, 115–121. [Google Scholar] [CrossRef]
- Quan, X.L.; Duan, Z.H.; Qiao, Y.M.; Pei, H.K.; Chen, M.C.; He, G.F. Variations in soil carbon and nitrogen stable isotopes and densitu among different alpine meadows. Acta Pratac. Sin. 2016, 25, 27–34. [Google Scholar] [CrossRef]
- Li, M.C.; Liu, H.H.; Yi, X.F.; Li, L.X. Characterization of photosynthetic pathway of plant species growing in the eastern Tibetan plateau using stable carbon isotope composition. Photosynthetica 2006, 44, 102–108. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Sun, X.Y.; Zhang, L.; Li, S.Y. A Study on the Characteristics of Soil Stable Carbon Isotope Composition in Different Types of Grassland in Northwest China. Chin. J. Soil Sci. 2013, 44, 348–354. [Google Scholar] [CrossRef]
- Sun, L.; Gong, L.; Zhu, M.L.; Xie, L.N.; Li, H.L.; Luo, Y. Leaf stoichiometric characteristics of typical desert plants and their relationships to soil environmental factors in the northern margin of the Tarin Basin. Chin. J. Ecol. 2017, 36, 1208–1214. [Google Scholar] [CrossRef]
- Wang, Z.N.; Yang, H.M. Response of ecological stoichiometry of carbon, nitrigen and phosphorus in plants to abiotic envrionmental factors. Pratac. Sci. 2013, 30, 927–934. [Google Scholar]
- Abliz, A.; Lü, G.H.; Zhang, X.N.; Gong, Y.M. Carbon, nitrogen and phosphorus stoichiometry of photosynthetic organs across Ebinur Lake Wetland Natural Reserve of Xinjiang. Chin. J. Ecol. 2015, 34, 2123–2130. [Google Scholar] [CrossRef]
- Wang, S.Q.; Yu, G.R. Ecological stoichiometry characteristics of ecosystem carbon, nitrogen and phosphorus elements. Acta Ecol. Sin. 2008, 28, 3937–3947. [Google Scholar] [CrossRef]
- Drenovsky, R.E.; James, J.J.; Richards, J.H. Variation in nutrient resorption by desert shrubs. J. Arid. Environ. 2010, 74, 1564–1568. [Google Scholar] [CrossRef]
- Xiang, X.M.; De, K.J.; Feng, T.X.; Lin, W.S.; Qian, S.W.; Wei, X.J.; Wang, W.; Xu, C.T.; Zhang, L.; Geng, X.P. Effect of Exogenous Nitrogen Addition on inter-monthly Variation of Plant-soil Nutrients in Alpine Meadow. Acta Agrestia Sin. 2022, 30, 1836–1845. [Google Scholar] [CrossRef]
- Zhang, J.F.; Zhang, X.D.; Zhou, J.X.; Franz, M. Effects of salinity stress on poplars seedling growth and soil enzyme activity. Chin. J. Appl. Ecol. 2005, 16, 426–430. [Google Scholar]
- Zhang, J.X.; Ge, S.F.; Wu, Y.H.; Yang, Y.F.; Xu, G.D.; Liu, P. Effects of Drought Stress on Carbon and Nitrogen Metabolism of Ardisia japonical Leaves. J. Soil Water Conserv. 2015, 29, 278–282. [Google Scholar] [CrossRef]
- Zhang, J.; Qi, X.X.; Liu, D.; Zhao, H.Y.; Xie, H.J.; Cao, J.J. Stoichiometric responses of Phragmites australis organs to soil factors in a wetland from arid area. Chin. J. Ecol. 2021, 40, 701–711. [Google Scholar] [CrossRef]
- Koerselman, W.; Meuleman, A.F. The Vegetation N:P Ratio: A New Tool to Detect the Nature of Nutrient Limitation. J. Appl. Ecol. 1996, 33, 1441. [Google Scholar] [CrossRef]
- Li, H.L.; Gong, L.; Hong, Y. Seasonal variations in C, N, and P stoichiometry of roots, stems, and leaves of Phragmites australis in the Keriya Oasis. Acta Ecol. Sin. 2016, 36, 6547–6555. [Google Scholar] [CrossRef]
- Su, Y.H.; Song, X.Q.; Zheng, J.W.; Zhang, Z.H.; Tang, Z.H. Comparison of stoichiometric characteristics of main nutrient elements in different organs from four Chenopodiaceae species. Chin. J. Ecol. 2022, 42, 1–12. [Google Scholar] [CrossRef]
- Li, M.M.; Petrie, M.D.; Tariq, A.; Zeng, F.J. Response of nodulation, nitrogen fixation to salt stress in a desert legume Alhagi sparsifolia. Environ. Exp. Botany 2021, 183, 104348. [Google Scholar] [CrossRef]
- Zhou, Y.C.; Cheng, X.L.; Fan, J.W.; Harris, W. Relationships between foliar carbon isotope composition and elements of C-3 species in grasslands of Inner Mongolia, China. Plant Ecol. 2016, 217, 883–897. [Google Scholar] [CrossRef]
- Chen, K.L. Soil phosphorus levels in relation to crop stable carbon isotope fractionation and biological yield. For. Pract. Technol. 2003, 6, 15–16. [Google Scholar] [CrossRef]
- Wang, W.W. Summary on Relationship Between Stable Carbon Isotope Composition of Plants and Soil Salinity. J. Anhui Agric. Sci. 2012, 40, 431–436. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).