Abstract
In soils, high aggregate stability often represents higher quality and anti-erosion ability; however, few studies have systematically analyzed how different forest stands affect soil aggregate stability. We selected five typical mixed forest stands on Jinyun Mountain in Chongqing, China, as research sites to evaluate soil aggregate stability. Within these sites, we analyzed the factors influencing soil aggregate stability in different stands by measuring soil characteristics and root traits. Soil aggregation stability, plant root traits, and soil properties varied among the mixed forest stands. The broadleaf tree mixed forest improved soil aggregate stability by 57%–103% over that of the Pinus massoniana mixed forest. The soil organic carbon, cation exchange capacity, Fe-Al oxides, and fine root proportion were positively correlated with soil aggregate stability. The specific root length and very fine root proportion were negatively correlated with soil aggregate stability, whereas the fine root proportion was positively correlated with this property. Specifically, we found that arbuscular mycorrhizal fungi did not affect soil aggregate stability in acid rain areas. Structural equation modeling indicated that soil aggregate stability was closely related to soil physicochemical properties and plant root characteristics. Predictive factors accounted for 69% of the variation in mean weight diameter, and plant root traits influenced soil aggregate stability by affecting soil organic matter, texture, and Fe-Al oxides. This study elucidated the impact of soil physicochemical properties and plant root characteristics on soil aggregate stability in different forest stand types, which has crucial implications for optimizing the management of various forest types.
1. Introduction
The stability of soil aggregates is a key factor affecting soil carbon storage and nutrient availability as well as an important indicator of soil quality and plant growth [1,2]. Soil aggregate stability also affects soil erosion processes, such as soil detachment, soil removal, and crust and water infiltration [3]. Therefore, high aggregate stability helps to maintain soil productivity and reduce soil erosion [4]. Many studies have investigated the mechanism of soil aggregate formation and stabilization, owing to the importance of aggregate stability [5]. However, most of these studies focused on different land-use types (such as farmland, orchard, grass, or forest) [6,7], tillage measures [8,9], and plant community levels [10,11] to determine the factors that affect aggregate stability but often ignored the plant–soil interactions of different tree species. Moreover, these focus areas cannot determine the effects of tree species or forest type on soil aggregates.
The factors affecting aggregate stability are attributed to intrinsic soil properties, such as soil type, soil organic carbon (SOC), clay mineralogy, Fe-Al oxides, and soil microbes, as well as extrinsic factors, such as climate, pedogenic processes, biological factors, and human land-use patterns [12,13]. In tropical and subtropical regions, Fe-Al oxides and SOC are considered the most important cementing materials for soil aggregates [6,14]. Regelink et al. [15] suggested that amorphous iron oxides (Feo) have a large specific surface area, which is conducive to the formation of stable aggregates. Duiker et al. [16] found that Feo were more effective at stabilizing aggregates than free iron oxides (Fed). Another study found that aluminum oxide cement aggregates have a greater effect at stabilizing aggregates [17]. Arbuscular mycorrhizal fungi (AMF) also affect the formation and stability of soil aggregates [18], mainly by producing extraradical hyphae and glomalin [19]. During plant growth, mycelia form microaggregates around plant roots through entanglement, and soil particles are aggregated and cemented with glomalin [20]. Although these studies provide insight into the complexity of soil aggregate stability, there is an urgent need to clarify the relationship between soil aggregate stability and soil properties.
Roots may improve aggregate stability through various physical, chemical, and biological methods, including winding fine particles into macroaggregates, releasing exudates to cement soil particles together, stimulating rhizosphere microbial activities, providing organic matter through metabolism, and absorbing water, which increases the frequency of wetting–drying cycles [21,22]. A positive correlation exists between root density and aggregate stability [23]. The fibrous root system is more effective in enhancing the state of soil aggregates and its anti-erosion capabilities than the tap root system [24]. However, the relationship between the root systems and aggregate stability at the community level remains unclear. Scholars have proposed that the relationship between roots and aggregates should be studied based on root morphological characteristics rather than root density [25]. Therefore, in this study, root morphology indicators will be used to explore the relationship with soil aggregate stability, such as root average diameter, specific root length, very fine roots, and fine roots.
The extensive degradation of evergreen broadleaf forests and the emergence of mixed coniferous forests in southern China have been primarily caused by human activities. Chongqing City is located in the Three Gorges Reservoir Area and exemplifies the subtropical monsoon climate typical of southern China, where evergreen broadleaf and mixed coniferous forests are key ecosystems. Forest lands are characterized as most erosion protected [26]. Although the factors influencing soil aggregate stability, such as soil mineral composition and microbiology, have been explored, only limited studies have explored the potential variations in soil aggregate stability among different forest stand types. This study focused on various forest stand types within the subtropical forest ecosystem of Jinyun Mountain in Chongqing City and aimed to uncover the relationships between soil characteristics and root properties with that of soil aggregate stability across these stand types. Specifically, the objectives of this study were to address (1) whether substantial differences in soil aggregate stability exist among different forest stand types and (2) which factors, either root properties or soil characteristics, predominantly influence soil aggregate stability. We hypothesized that (1) notable disparities in soil aggregate stability occur among different forest stand types, potentially attributed to variations in tree species composition and that (2) root properties play a pivotal role in governing soil aggregate stability within diverse forest stands.
2. Materials and Methods
2.1. Study Area
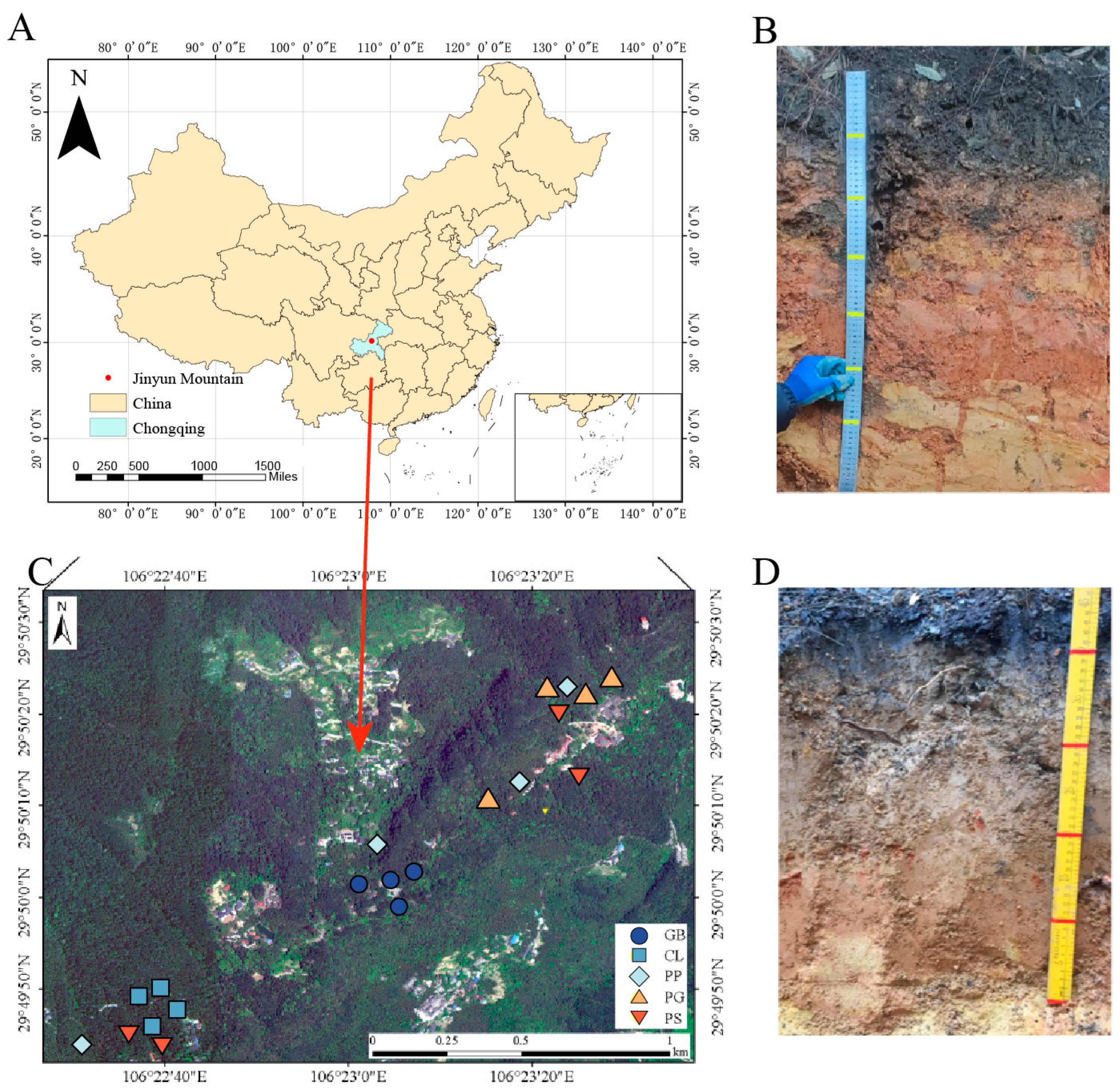
The study site was located in the mid-latitude region in the Jinyun Mountain Nature Reserve in Chongqing, China (106°17′–106°24′ E, 29°41′–29°52′ N, 170–950 m), in the Three Gorges Reservoir Area. The Chongqing Three Gorges Reservoir is situated at the confluence of three major tectonic units: the Daba Mountain Fold, East Sichuan Fold Belt, and Sichuan–Eastern Hunan–Guizhou Uplift Fold Belts. This site has a typical subtropical monsoon climate with an average annual temperature of 13.6 °C and an annual precipitation of 1611.8 mm. The rainy season extends from April to September, during which precipitation accounts for approximately 77.2% of the annual precipitation volume. The average annual evaporation capacity is 777 mm, and the average annual sunshine time is 1293.9 h. The soil of Jinyun Mountain is often called yellow soil (Figure 1) and is composed of thick quartz sandstone, gray shale, and argillaceous shale. According to the World Reference Base, the soil type in the area is acrisol. The forested history of Jinyun Mountain is storied, with documented forest coverage spanning an impressive two to three centuries. In addition, Jinyun Mountain boasts numerous centenarian trees, with the arboreal denizens within our sample plots averaging 60 years of age. Upon its designation as a national-level nature reserve in China, the forests within Jinyun Mountain’s boundaries have enjoyed exceptional preservation. Jinyun Mountain has a single soil type and abundant tree species, which were key to the development of our experiment.
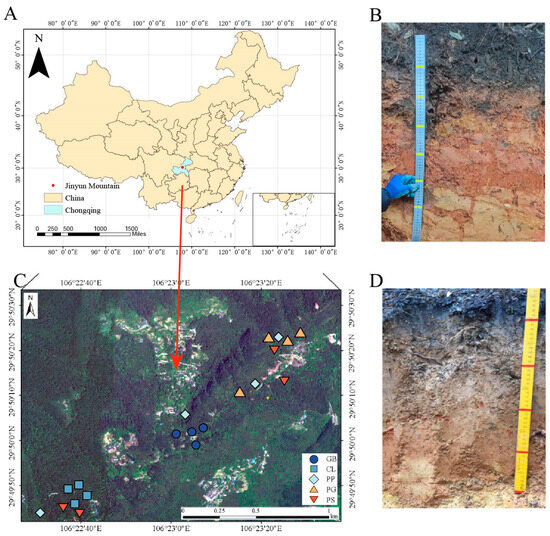
Figure 1.
Distribution of sampling sites and soil profiles. (A) Location of sample sites in China, (B,D) soil profile excavated during sample collection, and (C) detailed distribution of sample plots.
2.2. Selection of Sample Plots and Vegetation Characteristics
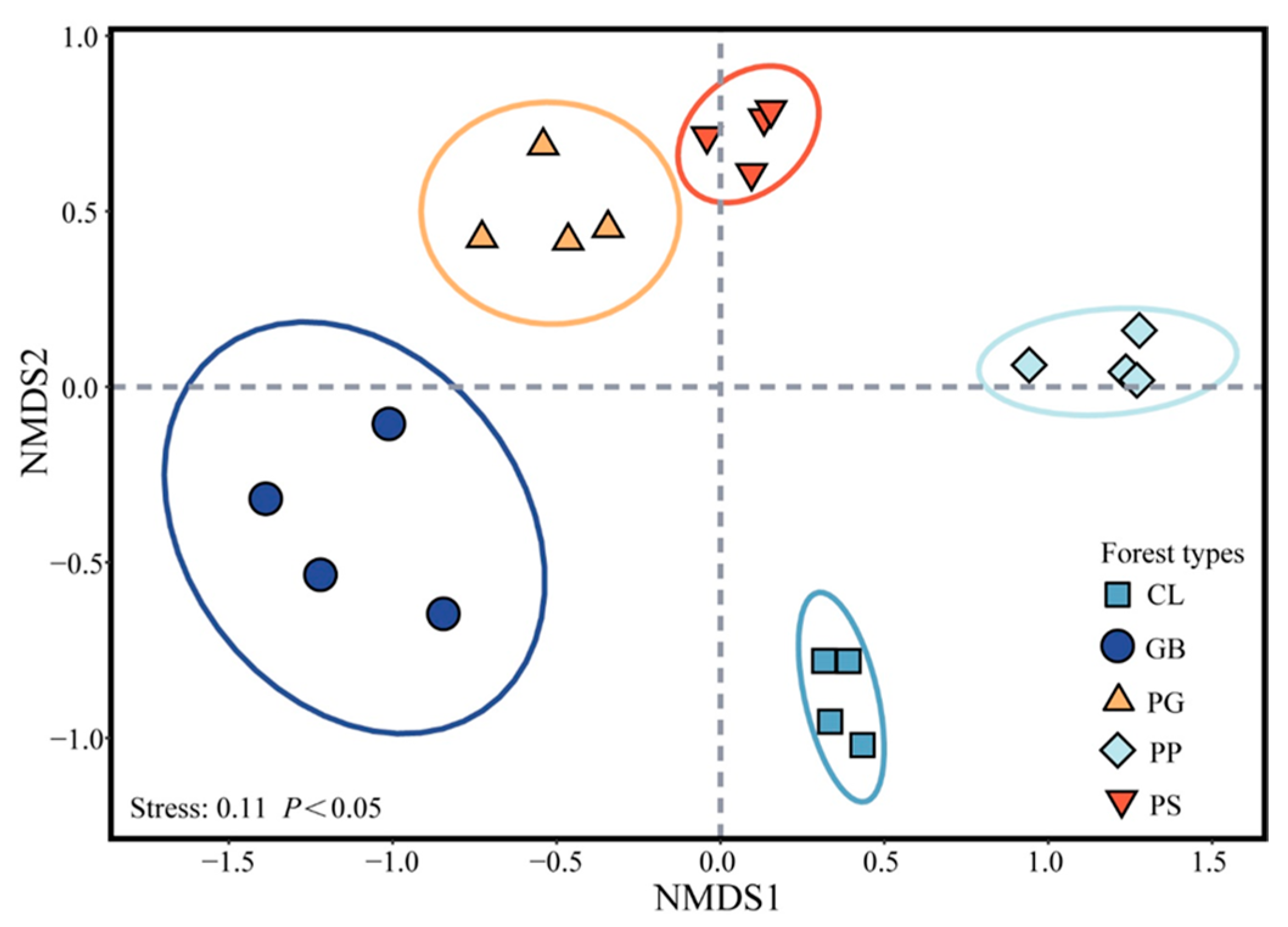
Five typical stands in Jinyun Mountain were selected as the research objects (Table S1): Gordonia axillaris and other broad-leaved tree mixed forest (GB), Cunninghamia lanceolata and Lindera kwangtungensis mixed forest (CL), Phyllostachys pubescens mixed forest (PP), Pinus massoniana and G. axillaris mixed forest (PG), and P. massoniana and Symplocos sumuntia mixed forest (PS). Four plots were selected for sampling within each stand for a total of 20 plots (Figure 1). When selecting sample plots, we ensured that the soil types were identical. The distance between plots was greater than 100 m, and the slope of the sample plots was less than 30°. The elevation of the plots was 750–860 m above sea level. We first conducted a stand investigation to obtain all the plant species in the plots, and then the plant species in the plots were arranged using nonmetric multidimensional scaling analysis to ensure that the four samples from the same forest stands could be used as replicates (Figure 2).
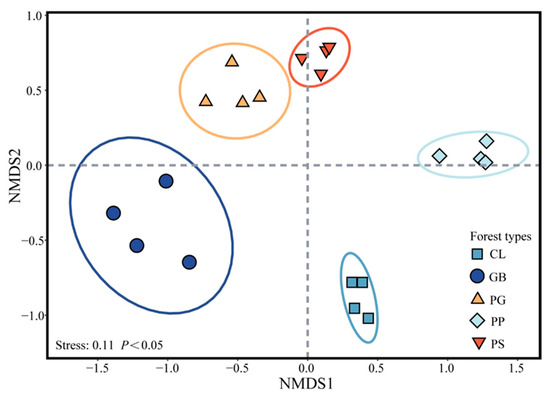
Figure 2.
Ordination of the forest communities. Confidence ellipse p < 0.05. GB means Gorodnia axillaris and other broad-leaved tree mixed forest, CL means Cunninghamina lanceolata and Lindera kwangtungensis mixed forest, PP means Phyllostachys pubescens mixed forest, PG means Pinus massoniana and G. axillaris mixed forest, and PS means P. massoniana and Symplocos sumuntia mixed forest.
2.3. Characterization of Soil Aggregate Stability
A cylindrical core (6.4 cm × 15 cm, diameter × length) was used to collect soil samples from five sites in each of the four sample plots within each stand. We used surface soil (0–15 cm) because it is the main soil layer affected by rainfall erosion. The selection of sample sites was not randomized because we wanted to highlight the effects of the different stands on soil properties. Therefore, we set all sample sites under the canopy of the major tree species in the stand to increase the probability of rhizosphere soil collection. The influence of different tree species on soil properties under the canopy sites is more pronounced than that outside the canopy [27].
Soil aggregate stability was determined by following the ISO 10930 [28] method derived from Le Bissonnais [29], which can be used to determine aggregate stability under different breakdown mechanisms. We used the fast-wetting disruptive test to determine the ability of aggregates to resist slaking, because slaking is the best representative of the mechanism of aggregate breakdown under heavy rainfall in areas with a subtropical monsoon climate. Soil samples from the five sample points in each plot were mixed and dried in the laboratory for 1 week at room temperature in the air. Thereafter, approximately 20 g of soil aggregates (5 g from each site repeated three times), 3–5 mm in thickness, was gently isolated using tweezers and subjected to a fast-wetting disruptive test. The remaining soil samples were used for further characterization. The selected soil aggregates were placed in an oven and dried at 40 °C for 24 h. Approximately 5 g of each sample was weighed and immersed in a beaker (250 mL) containing 50 mL of deionized water for 10 min. The water in the beaker was then extracted using a pipette, and the remaining soil aggregates were filtered using a 0.05 mm sieve that had been cleaned with 95% alcohol. The sieve was gently stirred by hand in a circular motion five times (amplitude 4 cm, frequency 1 s, once), and the remaining aggregates on the sieve were collected and dried at 40 °C for 48 h. A column with six sieves (2.00, 1.00, 0.50, 0.20, 0.10, and 0.05 mm) was used for drying the aggregates. The mean weight diameter (MWD) was calculated to determine aggregate stability as follows:
where Di is the mean diameter of each size class within each of the seven diameter classes (i = 1–7), with D1 (minimum) = 0.025 mm and D7 (maximum) = 3.50 mm, and Mi (g) is the mass of stable aggregates within the corresponding size class. The accuracy of the data was assessed as follows: “Stable aggregates” above the 2 mm sieve were broken down, and the remaining coarse material (gravel > 2 mm) that was retained in the sieve was weighed. M7 was corrected by removing the mass of the gravel. When the weight of the stones was greater than 2 g (40% of 5 g), the data were considered invalid, and the samples were measured again.
Soil texture (e.g., sand, silt, and clay percentage) was determined via laser diffraction using a Bettersize 2600 (Bettersize Instruments, Dandong, China). Soil organic matter (SOM) was determined via oxidation with potassium dichromate in a heated oil bath. Cation exchange capacity (CEC) was assessed by treating the sample with ammonium acetate buffer (pH 7.0), as previously described [30]. Soil pH was measured in 1:2.5 m/v soil:water samples using a glass electrode after shaking the samples for ~30 min [31]. Free (Fed and Ald) and amorphous (Feo and Alo) iron and aluminum were extracted via citrate bicarbonate-dithionite treatment [32] and ammonium oxalate treatment, respectively. The Fe and Al concentrations in the extracts were determined using inductively coupled plasma optical emission spectrometry after dilution. Each index test was repeated three times.
2.4. Measurements of Root Traits
To characterize root traits, six sampling points were selected within each plot. Two of the six samples were randomly selected for one replicate (soil volume, 966 cm3), with three such replicates per sample plot. The samples were washed using 4 and 1 mm sieves, and roots < 2 mm in diameter were carefully collected. The collected fine roots were stored in distilled water at 4 °C and warmed to room temperature (approximately 20 °C) before being stained with methyl blue (0.5 g L−1) for 2 min to evaluate root morphological characteristics. The roots were placed on a transparent plate without overlap and scanned at 400 DPI using a root scanner (Epson Expression 10000XL; Epson, Tokyo, Japan). Multiple batches were scanned when the root samples were complex and they interfered with the imaging. The resulting images were processed using image analysis software (Winrhizo, version 2020 Pro, Regent Instrument, Quebec, Canada) to determine total root length (cm), fine (diameter 0.21–1.0 mm) and very fine (diameter < 0.2 mm) root length, and root average diameter (mm). After scanning, the roots were oven-dried at 60 °C and weighed to determine dry mass (mg). Root mass density, root length density, specific root length, percentage of very fine roots, and percentage of fine roots were calculated using the following formulae [33]:
where denotes root dry mass (mg), is the total root length (mm), V is the soil volume (cm−3), and and denote very fine and fine root length (mm), respectively. RMD is root mass density (kg m−3), RLD is root length density (km m−3), SRL is specific root length (m g−1), VFR is percentage of very fine roots (%), and FR is percentage of fine roots (%).
2.5. Measurement of Arbuscular Mycorrhizal Fungi
Small portions of fresh soil samples, which were used to determine aggregate stability, were randomly selected after collection and storage at −80 °C to determine the soil AMF content. The abundance of AMF was determined using the phospholipid fatty acid (PLFA) method, adopted from Frostegard et al. [34], using the PLFA module of the Sherlock MIDI Microbial Identification System Platform. Hydrogen was used as the carrier gas. An Agilent 7890 meteorological chromatogram and flame ionization detector were used as hardware platforms. An Agilent 19091B-102E (25 m × 200 m × 0.33 m) chromatographic column was used with a 2 L sample volume. According to the MIDI platform specifications, all tests were calibrated using 0.5 mg mL−1 methyl nonadecanoate fatty acid (19:0). Fatty acids were quantitatively characterized by peak area and internal standard curves (nmol/g). The AMF were characterized using the phospholipid fatty acid 16:1ω5c [35].
2.6. Data Analysis
Data analysis used the mean values for each plot. One-way analysis of variance (ANOVA) and Tukey HSD post hoc tests were used to analyze the differences in soil characteristics, root traits, and AMF in the five forest stands. When the data did not meet normal distribution or homogeneity of variance, log-transformation was performed. Principal component analysis (PCA) was used to analyze the relationships between soil characteristics, root traits, and AMF abundance in the different plots (n = 20). The effects of soil physical and chemical properties, root traits, and AMF abundance on aggregate stability were analyzed using single linear regression. We then selected factors that significantly affected MWD to construct a piecewise structural equation model (SEM) for evaluating the direct and indirect effects of plant roots, SOC, soil texture, and soil colloids on MWD. We performed a PCA to reduce collinearity among multiple variables. Plant roots included specific root length, fine roots, and very fine roots. The first component (PC1) explained 65.72%–98.37% of the total variance in each group (Tables S2 and S3), suggesting that it is credible to use PC1 as a new variable to represent combined attributes in the SEM analysis. SEM images was constructed using the “piecewise SEM” software package (version 2.3.0). All data analyses were performed using the R software (version 4.0.2).
3. Results
3.1. Soil Characteristics, Root Traits, and AMF Abundance in Different Forest Stands
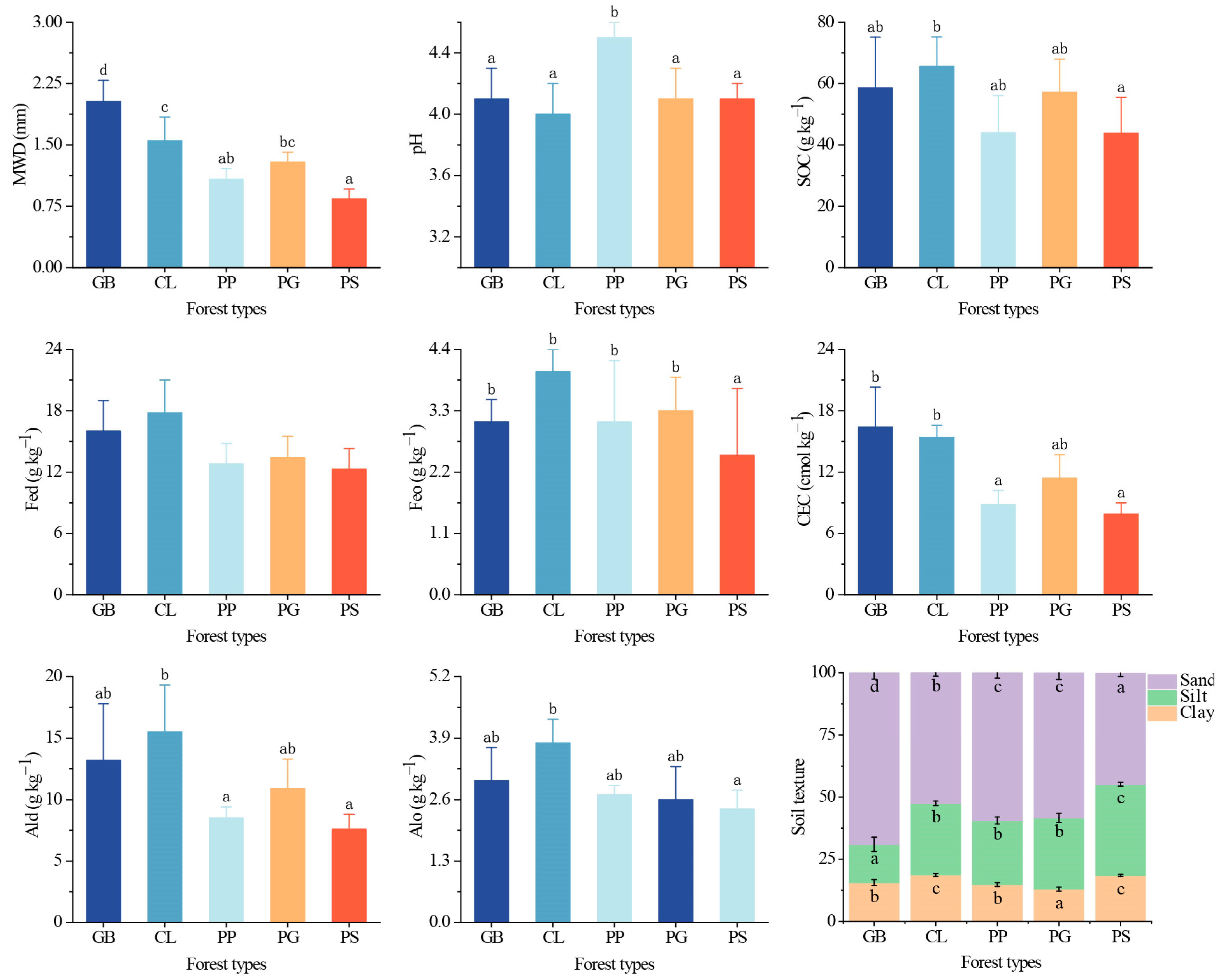
Based on the MWD classification described [29], the GB aggregates were considered very stable, the CL aggregates were stable, and the aggregates of the other three stand types were moderately stable (Figure 3), indicating that the aggregate stability differed significantly among stands (p < 0.05). The soil aggregate stability of GB was improved by 57% and 103% compared to PG and PS, respectively. The SOC concentrations in all stands were very high; PS had the lowest SOC concentration, whereas CL had the highest, and the difference between them was significant (p < 0.05). An ANOVA revealed significant differences in Feo, Ald, and Alo content among the different plots. The Feo content in PS was significantly lower than that in the other stands, while the Ald content of PP and PS was significantly lower than that of CL. The difference in Alo content among the stands was similar to that of the SOC concentration. The difference in the CEC among the stands was significant (p < 0.05), with that in GB and CL being significantly higher than that in PP and PS. Within the study region, the soil texture of each forest differed significantly (p < 0.05); GB had the highest silt and sand content, whereas those of PS were the lowest.
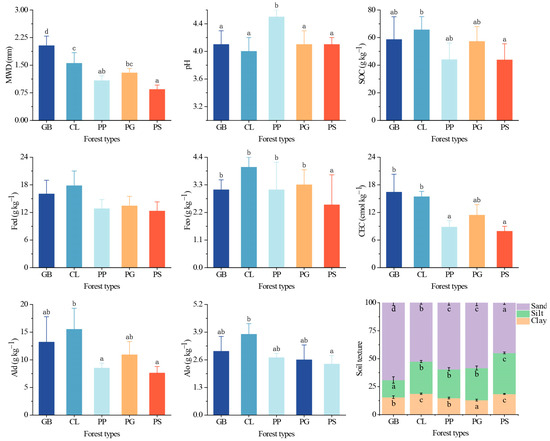
Figure 3.
Soil indicators for the different forest stand types. Values represent the mean ± SD (n = 4). The letters indicate significant differences among different forests for the same index based on the Tukey HSD post hoc test (p < 0.05). MWD, mean weight diameter; SOC, soil organic carbon; Fed, free iron; Feo, amorphous iron; Ald, free aluminum; Alo, amorphous aluminum; CEC, cation exchange capacity; sand, 2–0.05 mm; silt, 0.05–0.002 mm; clay, <0.002 mm. GB means Gorodnia axillaris and other broad-leaved tree mixed forest, CL means Cunninghamina lanceolata and Lindera kwangtungensis mixed forest, PP means Phyllostachys pubescens mixed forest, PG means Pinus massoniana and G. axillaris mixed forest, and PS means P. massoniana and Symplocos sumuntia mixed forest.
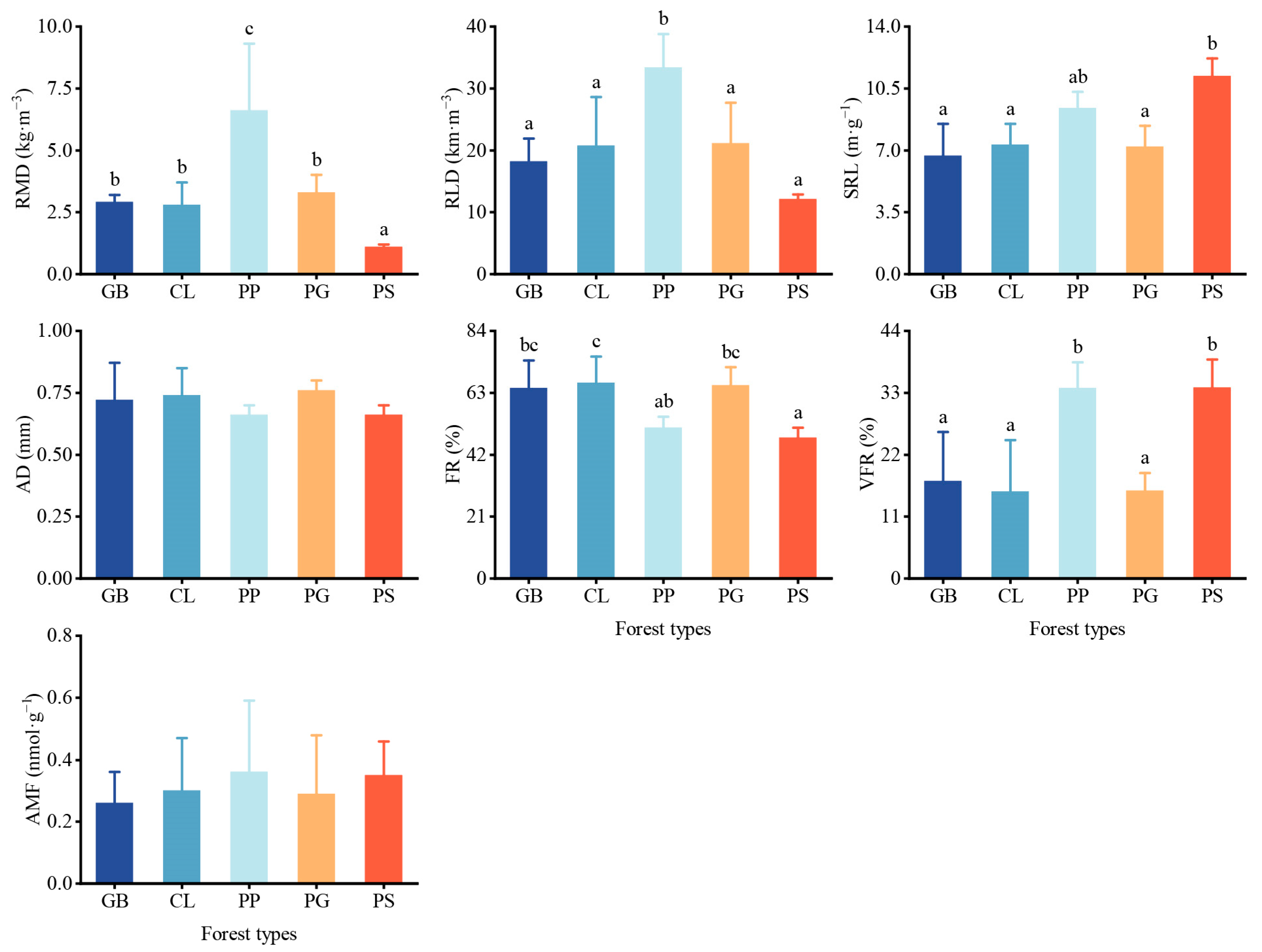
The root characteristics also differed among the stands. The root mass density and root length density were significantly higher in PP than in the other stands (Figure 4), except for the root mass density in PS, which was only 1.1 ± 0.1 kg m−3, significantly lower than that of the other stands. No significant differences in specific root length were observed among the stands, except for PS. The very fine roots in PS and PP were significantly higher than those in the other stands, while the fine roots in PS were significantly lower than those in the other three stands. The standard deviation of AMF in each stand was relatively large, and no differences in AMF were observed among the stands.
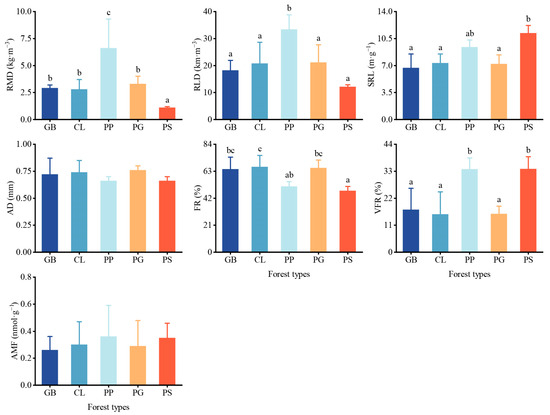
Figure 4.
Root traits and arbuscular mycorrhizal fungi present in different forest stand types. Values represent the mean ± SD (n = 4). The letters indicate significant differences among different forests for the same index using the Tukey HSD post hoc test (p < 0.05). RMD, root mass density; RLD, root length density; SRL, specific root length; AD, root average diameter; FR, fine roots; VFR, very fine roots; AMF, arbuscular mycorrhizal fungi. GB means Gorodnia axillaris and other broad-leaved tree mixed forest, CL means Cunninghamina lanceolata and Lindera kwangtungensis mixed forest, PP means Phyllostachys pubescens mixed forest, PG means Pinus massoniana and G. axillaris mixed forest, and PS means P. massoniana and Symplocos sumuntia mixed forest.
3.2. Relationships among Soil Characteristics, Root Traits, and AMF Abundance in Different Forest Stands
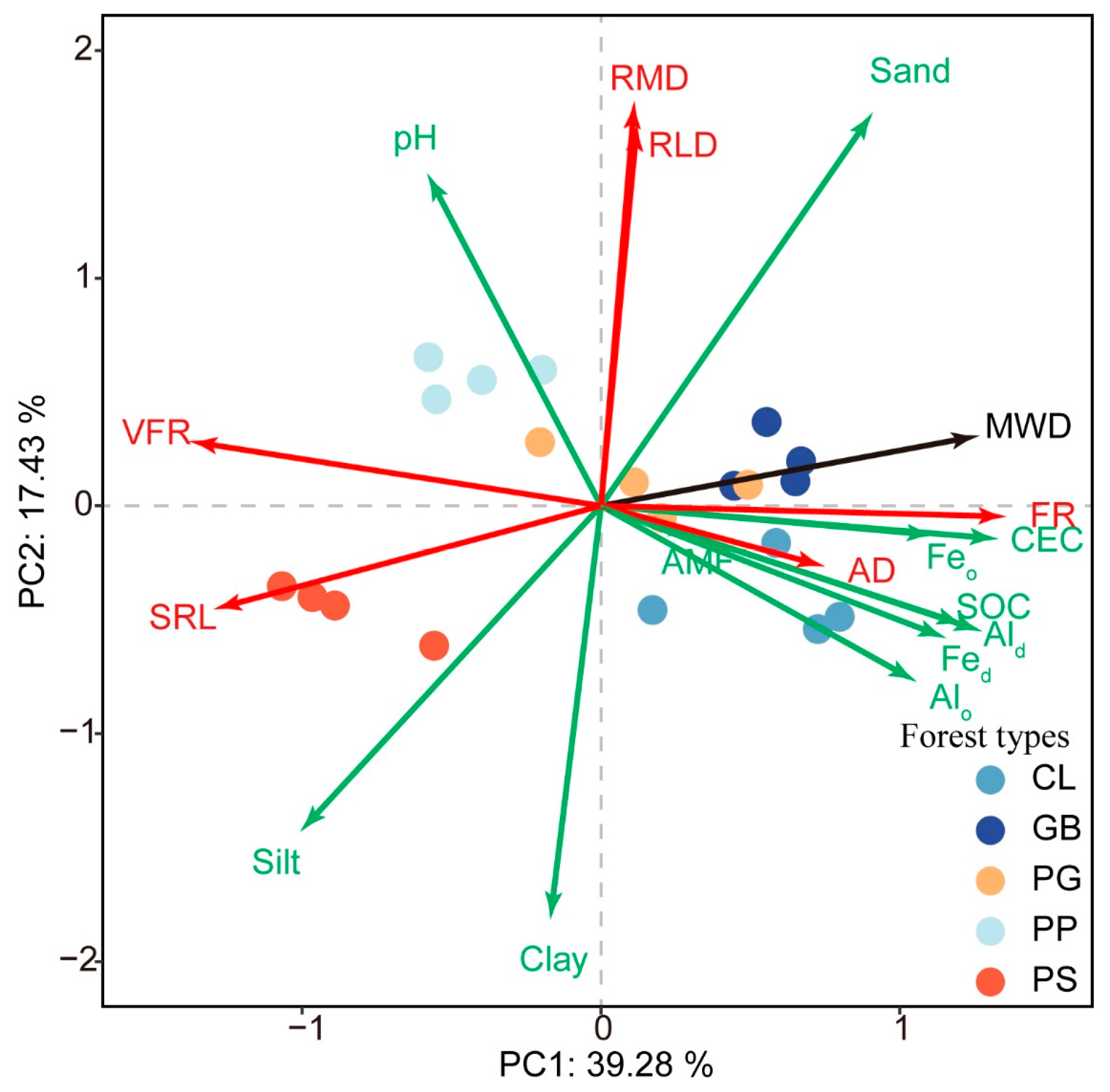
PCA revealed that the variability rates that explained the first and second principal components were 39.28% and 17.43%, respectively (Figure 5). Axis 1 was positively correlated with fine roots, CEC, MWD, SOC, Fe-Al oxide, and sand but negatively correlated with very fine roots, specific root length, and silt. Axis 2 was positively correlated with root mass density, sand, RLD, and pH but negatively correlated with clay and silt. PS and PP were distributed on the right side of Axis 1 because their fine roots, CEC, MWD, SOC, and Fe-Al oxide content were lower, whereas their very fine roots and specific root length were higher than those of the other stands. PP was characterized by a high pH and large root mass density and root length density. Hence, it was mainly distributed on the upper left side of the PCA chart. In comparison, PS was characterized by high silt and clay content but low sand content. Thus, it was distributed on the lower left side of the PCA chart. The soil chemical properties and root characteristics in GB and CL were similar, but there were substantial differences in soil texture between these stands, with the sand content of GB considerably higher than that of CL. In contrast, the clay and silt contents of CL were higher than those of GB. These results placed GB on the upper right side and CL on the lower right side of the PCA chart. No prominent index was found in PG, positioning it closer than the other stands to the origin of the chart.
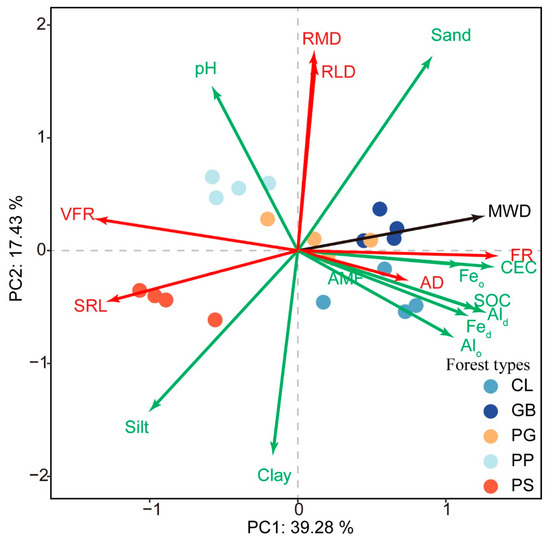
Figure 5.
Principal component analysis of soil characteristics and root traits for the different forest stand types. MWD, mean weight diameter; SOC, soil organic carbon; Fed, free iron; Feo, amorphous iron; Ald, free aluminum; Alo, amorphous aluminum; CEC, cation exchange capacity; sand, 2–0.05 mm; silt, 0.05–0.002 mm; clay, <0.002 mm. RMD, root mass density; RLD, root length density; SRL, specific root length; AD, root average diameter; FR, fine roots; VFR, very fine roots; AMF, arbuscular mycorrhizal fungi. GB means Gorodnia axillaris and other broad-leaved tree mixed forest, CL means Cunninghamina lanceolata and Lindera kwangtungensis mixed forest, PP means Phyllostachys pubescens mixed forest, PG means Pinus massoniana and G. axillaris mixed forest, and PS means P. massoniana and Symplocos sumuntia mixed forest.
3.3. Influence of Soil Characteristics, Root Traits, and AMF on Soil Aggregate Stability
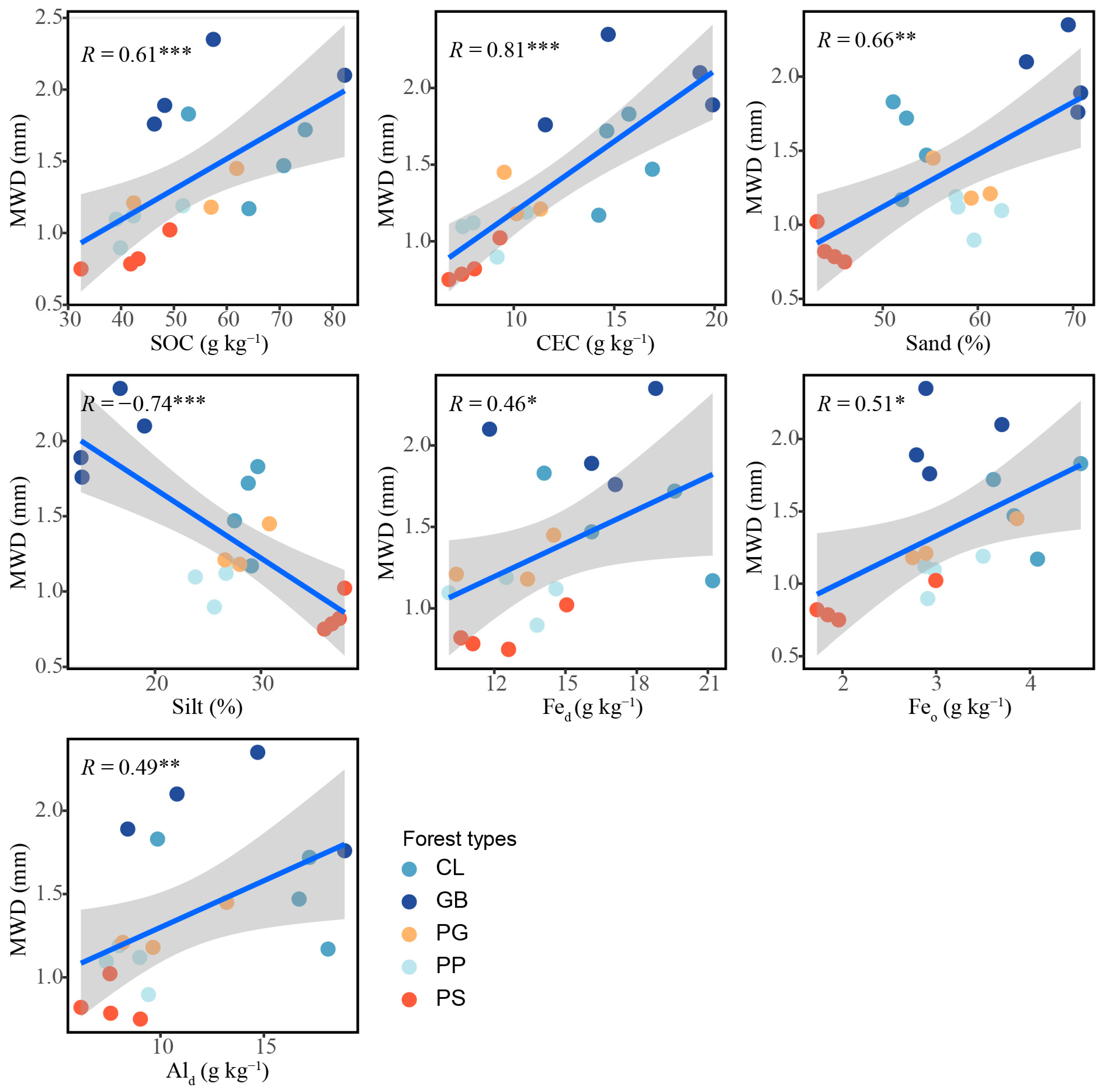
The results of the general linear regression analysis showed that the chemical properties of the soil, including the CEC, SOC, Feo, Ald, and Fed, influenced aggregate stability (p < 0.05; Figure 6). The sand content was positively correlated with aggregate stability (p < 0.01), whereas the silt content was negatively correlated with aggregate stability (p < 0.001).
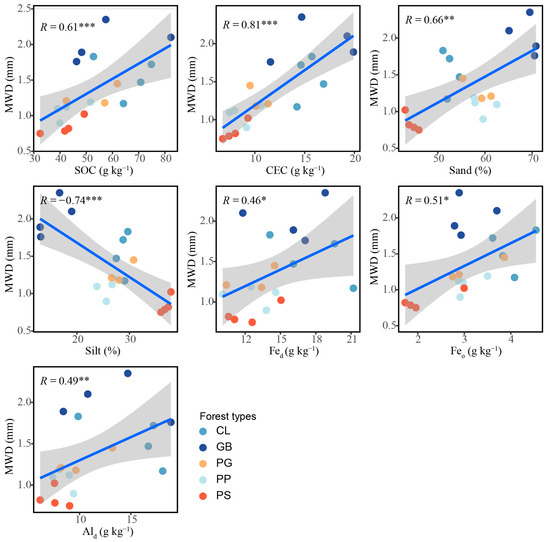
Figure 6.
Influence of soil characteristics on aggregate stability in the different forest stand types. *: p < 0.05; **: p < 0.01; ***: p < 0.001. The blue linear line symbolizes a linear fitting, while the grey area delineates the 95% confidence interval. MWD, mean weight diameter; SOC, soil organic carbon; Fed, free iron; Feo, amorphous iron; Ald, free aluminum; CEC, cation exchange capacity; sand, 2–0.05 mm; silt, 0.05–0.002 mm; GB means Gorodnia axillaris and other broad-leaved tree mixed forest, CL means Cunninghamina lanceolata and Lindera kwangtungensis mixed forest, PP means Phyllostachys pubescens mixed forest, PG means Pinus massoniana and G. axillaris mixed forest, and PS means P. massoniana and Symplocos sumuntia mixed forest.
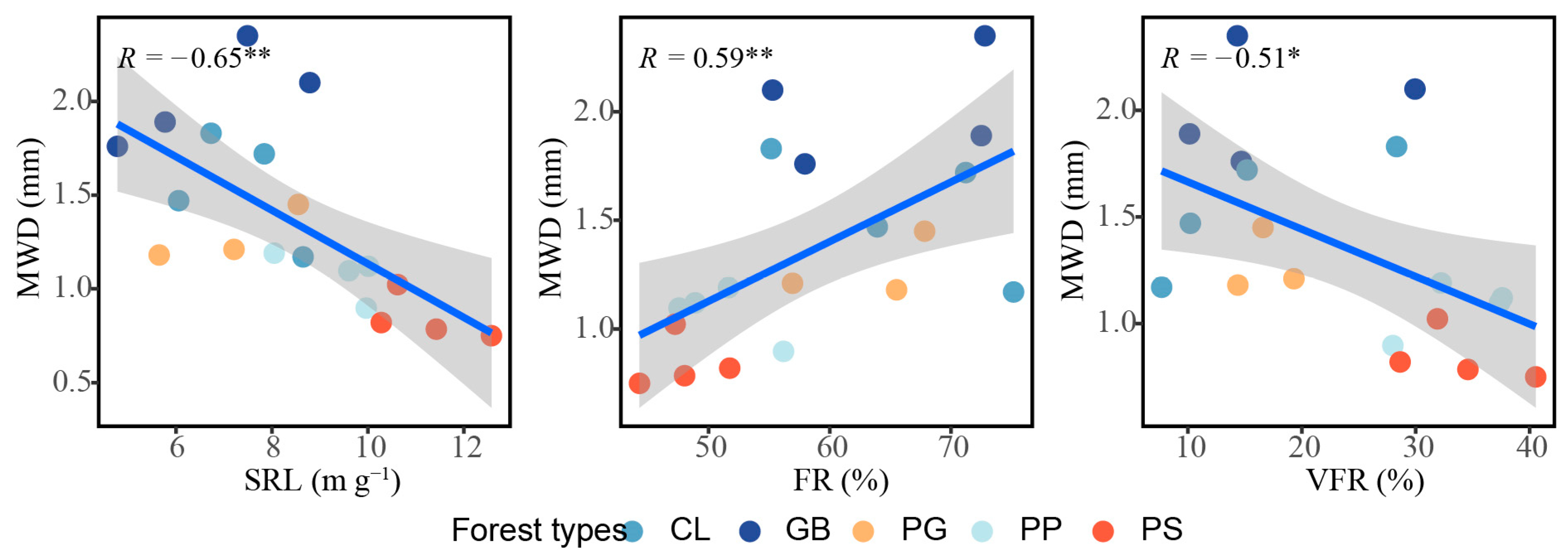
The root density indices, root mass density and root length density, did not correlate with aggregate stability; however, root morphology was closely associated with aggregate stability (Figure 7). The specific root length (p < 0.01) and very fine roots (p < 0.05) were significantly negatively correlated with aggregate stability, while fine roots were significantly positively correlated with aggregate stability (p < 0.01). No relationship was observed between the other indicators measured and aggregate stability (Figures S1 and S2).
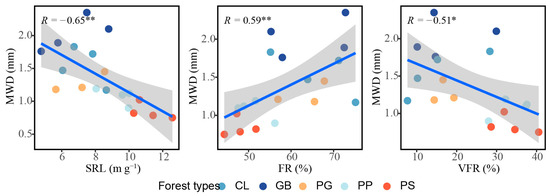
Figure 7.
Influence of root traits on aggregate stability in the different forest stand types. *: p < 0.05; **: p < 0.01. The blue linear line symbolizes a linear fitting, while the grey area delineates the 95% confidence interval. MWD, mean weight diameter; SRL, specific root length; FR, fine roots; VFR, very fine roots; GB means Gorodnia axillaris and other broad-leaved tree mixed forest, CL means Cunninghamina lanceolata and Lindera kwangtungensis mixed forest, PP means Phyllostachys pubescens mixed forest, PG means Pinus massoniana and G. axillaris mixed forest, and PS means P. massoniana and Symplocos sumuntia mixed forest.
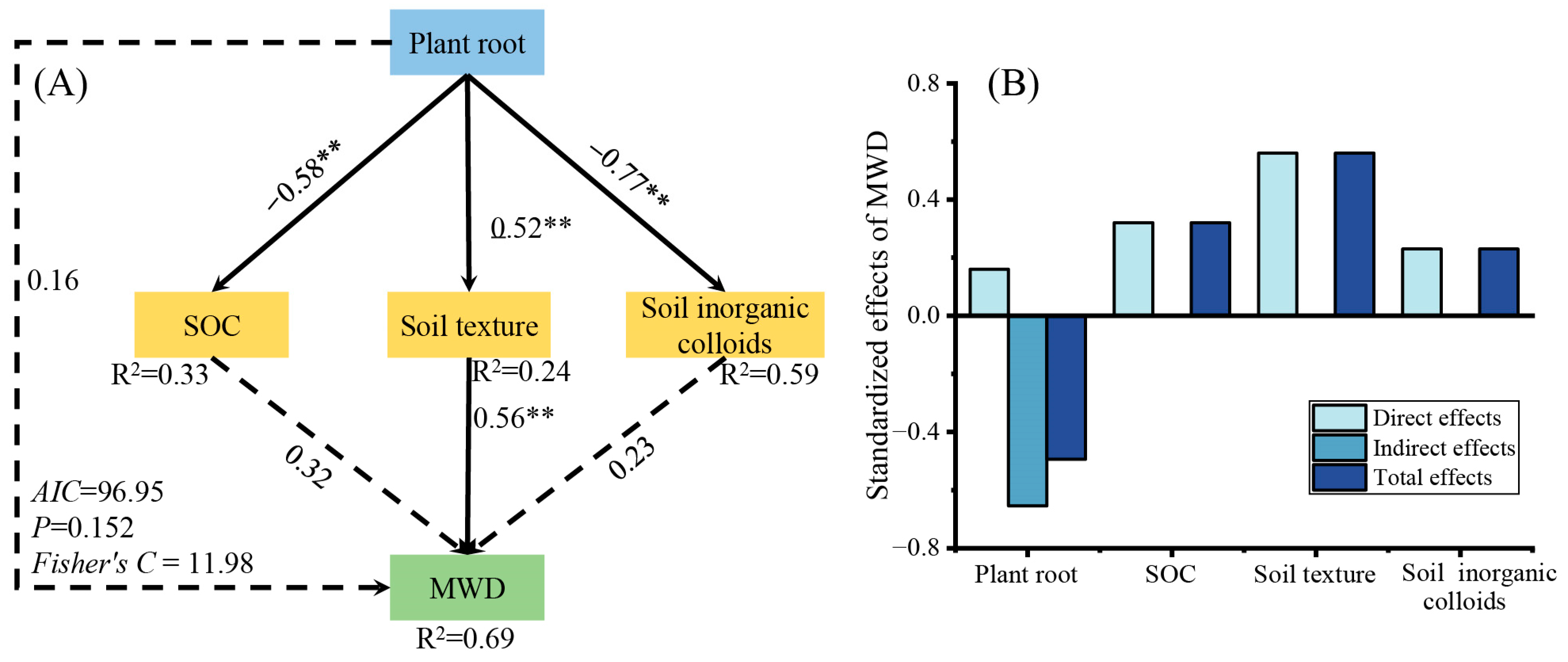
Variables with a significant effect on the MWD in the linear regression were selected as predictors to generate segmented SEMs and identify key drivers of MWD. The results showed that the predictors explained 69% (R2) of the variation in MWD (Figure 8A). Plant roots, SOC, and soil texture directly contributed to soil colloids, and plant roots were also indirectly related to soil colloids and MWD through SOC and soil texture. Negative values on the SEM path indicated negative effects; for example, the path coefficient between SOC and MWD was 0.32, which indicated that an increase in SOC directly increased the MWD. The histogram shows the direct, indirect, and total effects of each factor on MWD (Figure 8B). The total effect of plant roots on the MWD was −0.49. In addition, stand type directly and indirectly affected the MWD through plant roots, SOC, soil texture, and soil colloids.
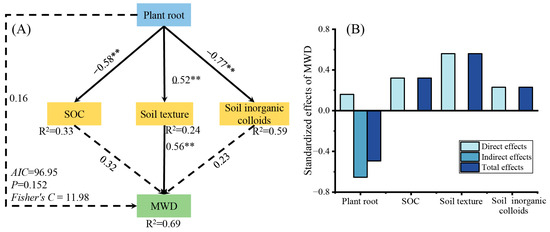
Figure 8.
Piecewise structural equation model describing soil chemical properties and characteristics versus mean weight diameter for a typical forest stand. (A) Path coefficients between indicators. (B) Standardized effect of indicators on MWD. The numbers on the arrows are the path coefficients that indicate the strength of influence one index (inside the box) has on the other. Numbers on arrows indicate significant normalized path coefficients. The R2 values represent the variance in the dependent variable explained using the internal model. SOC, soil organic carbon; plant root includes specific root length, fine roots, and very fine roots; soil texture includes sand and silt. Soil inorganic colloids include cation exchange capacity, free iron, amorphous iron, and free aluminum. ** p < 0.01.
4. Discussion
4.1. Stability Characteristics of Soil Aggregates in Different Forest Stands
We observed that soil aggregate stability differed significantly between broadleaf mixed forest (GB) and coniferous and broadleaf mixed forest (CL, PS, and PG), and PS and PG were significantly different from CL in aggregate stability. This verifies our first hypothesis. This divergence arose from the varying proportions of broad- and needle-leaved trees in the forests. Broadleaf trees constituted only 30% of the trees in PG and PS, whereas in CL, broadleaf trees accounted for as much as 60% (Table S1). Evidently, as the proportion of broadleaf trees within a stand increases, soil aggregate stability also rises [36]. This phenomenon can be attributed to substances found in needle-leaved forest litter that inhibit bacterial activity, resulting in fewer fungal hyphae in the soil [37] and less promotion of the formation of large soil aggregates by the action of fungal hyphae [38]. Additionally, P. massoniana litter, which is primarily composed of pine needles rich in tannins, resins, lignin, and other recalcitrant compounds, restricts the release of nutrients from the litter into the soil [39]. These known actions most likely contributed to the relatively low SOC content in the PG and PS forest soils. The binding of organic matter plays a significant role in aggregate formation [40]. Through long-term interactions between trees and soil, distinct soil aggregate differences ultimately manifest among different forest stand types.
Significant differences were observed in soil properties and root characteristics among the different forest stands; however, no notable differences in AMF were observed among the tested forest stand types (Figure 4). The soil of the evergreen broadleaf forests was characterized by increased levels of sand, Fe-Al oxide concentration, CEC, and fine roots indices but lower specific root length and very fine root indices. In contrast, the mixed coniferous forests demonstrated contrasting soil properties with respect to these parameters. The impact of vegetation on intrinsic soil properties is complex; litter fall and the decomposition processes of different plant species can lead to discrepancies in the SOC, pH, Fe-Al oxides, and soil water content [41,42]. Correlation analysis revealed that the specific root length was significantly negatively correlated with the CEC in the tested forest stands. In addition, very fine roots and fine roots were strongly correlated with the SOC, Fed, Ald, and CEC in our forest stand samples (Figure 3). This phenomenon was caused by differences in the nutrient absorption and utilization patterns of vegetation roots that affect soil aggregate stability. The root system also affects soil properties to a certain extent, causing differences in the SOM content, CEC, and Fe–Al oxides in the rhizosphere of different species [22]. Plant associations can also affect the amount and chemical composition of throughfall and stemflow [43], which have been shown to change soil properties [44,45].
4.2. Relationship among Plant Root Traits and Soil Aggregate Stability
The SEM showed that plant root traits influenced soil aggregate stability by affecting the SOM, texture, and Fe-Al oxides. This also validates our second hypothesis. The shape of the plant root system is a crucial driving factor that influences soil aggregate stability. The specific root length and very fine roots were negatively correlated with aggregate stability, whereas fine roots were positively correlated with aggregate stability, consistent with results from the literature [37]. Plant root systems exhibit both direct and indirect influences on the stability of soil aggregates. This is exemplified by the impact of extremely fine roots on soil aggregate stability, a phenomenon predominantly facilitated through indirect modulation involving mycorrhizal fungi [46]. Mycorrhizal fungi form a mutualistic relationship with plant roots, where the fungi help the plant acquire nutrients from the soil while receiving carbohydrates from the plant in return. This fungal network can enhance soil structure and stability in several ways [11]. Root exudates from fine roots can directly act as binding agents of soil particles, decompose SOM, enhance the nutrient supply, and stimulate the stability of soil aggregates through interactions with the soil microbial community [36,47]. In this study, the specific root length and very fine roots of the PP and PS forest stands were significantly higher than those of the other stands, which could be attributed to the lower proportions of shrubs and herbaceous plants in these stands. No significant correlations were found among root mass density, root length density, and soil aggregate stability. The PP stand exhibited the highest root mass density and root length density, whereas the PS group exhibited the lowest values. The differences in MWD between the two stand types were not statistically significant (Figure 3). The relative importance of root density to soil aggregate stability varied in the different stands. When the soil aggregate stability was relatively high (MWD > 2.5), the contribution of organic carbon provided by root metabolism appeared to be less significant. In this study, although soil aggregate stability was not high, the overall SOM content was relatively high, which might have mitigated the impact of root-derived organic carbon on soil aggregate stability. In mature broadleaf mixed forests, plants allocate less carbon to transport roots and direct a relatively higher proportion of carbon to absorptive roots in response to higher nutrient availability [48,49]. Six et al. [50] reported that the decomposition of dead roots affected aggregate stability; however, the amount of root exudates and the stimulation of soil microorganisms are related to the plant species [51]. This also explains the significant differences in soil aggregate stability observed among different forest stand types.
Plant root characteristics are closely related to soil properties. The SEM results demonstrated that plant roots significantly influenced the SOC, soil texture, CEC, and Fe-Al oxide content (Figure 8). A high diversity in the shape of the plant root system is associated with differences in tissue chemistry and decomposition rates, which provide a wide range of ecological niches for root-associated microorganisms [52]. Through their activities, these microorganisms modify soil physicochemical properties [53]. In different forest stand types, the continual mechanical and chemical interactions of the roots with soil properties ultimately lead to variations in soil characteristics among distinct forest stands [54,55]. AMF are closely associated with roots [42], making it difficult to distinguish the effects of roots on aggregate stability from those of arbuscular fungi [56,57]. Accordingly, we attempted to directly measure the amount of AMF to determine their influence on aggregate stability. Unfortunately, the experimental results had a high amount of variation, which made it difficult to interpret. A meta-analysis revealed that the impact of arbuscular fungi on aggregate stability depends on the environmental and experimental conditions [58]. Compared with flowerpot experiments that use sterilized experimental soil and set the soil pH to neutral, field experiments use natural soil that is generally more acidic. Hence, measuring the effects of AMF on aggregate stability in the field is difficult.
4.3. Relationship between Soil Characteristics and Soil Aggregate Stability
The SEM showed that the physical and chemical properties of soil directly affect the stability of soil aggregates. Mineral particles cemented to the SOC enhance aggregate stability, and the SOC may also reduce the wettability of aggregates, thereby reducing damage caused by slaking [56]. Adding organic matter to soils with low organic carbon content can considerably improve the aggregate stability [40]. Although organic matter was found to influence aggregate stability in the present study, it was not the most important factor affecting this property (Figure 8), similar to a previous study [6]. Our research focus was forest soil with a high SOM content, and the difference among the stands was relatively small. Moreover, under high organic matter conditions, the effect of organic matter on aggregate stability is not obvious [59]. Furthermore, owing to differences in the formation of various aggregate components, the combination of organic matter, clay minerals, and structure can vary, as can the microbial-mediated decomposition of organic carbon within different aggregate fractions [60]. Hence, the relationships between soil aggregate stability and factors such as organic carbon content often change with regional and forest stand variations.
Soil particle size distribution is also related to soil aggregate stability. We found that silt content was negatively correlated with aggregate stability, which was consistent with a previous report [11]. Although clay content was not correlated with aggregate stability, sand content was positively correlated. These results were inconsistent with those of previous studies that reported that clay content was positively correlated and sand content was negatively correlated with aggregate stability [4,61]. This difference may be related to the clay content of the forest plots used between studies (Figure 3). The significant correlations among sand, silt, and soil aggregate stability (Figure 6) could be associated with variations in the composition of tree species among the different forest stand types. In broadleaf mixed forests, higher sand and lower silt content were indicative of more stable and developed vegetation cover, which was linked to higher soil aggregation stability. In coarse sandy soils, fine roots can grow into pores and bind sand grains as the primary stabilization mechanism. Barthès et al. [17] highlighted that the relationship between clay content and aggregate stability is usually established over a small area, which was similar to the findings in this study. However, no relationship was observed between the clay content and aggregate stability. Another study found that clay content can reduce aggregate stability [62]; therefore, we speculate that the relationship between clay particles and aggregate stability is determined by their content and type. Erktan et al. [11] evaluated the stability of aggregates under different succession gradients and found that this property was positively correlated with fine sand (0.05–1 mm), similar to our observations. The frequent dry–wet alternations on Jinyun Mountain reduced the ability of aggregates to resist slaking [63]. Sand particles are insensitive to changes in the external environment [64], indicating that the sand content has a positive correlation with aggregate stability.
Inorganic soil colloids [1,5] were also investigated, which revealed that Fed, Feo, and Ald were positively correlated with aggregate stability. Numerous studies have demonstrated that Fe-Al oxides are important factors affecting aggregate stability. The inner-sphere complexes between Fe-Al oxides and phosphates or silicates introduce a net negative charge to the surface of Fe-Al oxides, stimulating their aggregation in some oxide soils [65]. Additionally, poorly crystalline silicates act as bridges between Fe-Al oxides and the quartz surfaces of sandy particles, facilitating the formation of inner-sphere complexes by organic ions on the surface of Fe-Al oxides that alter their charge [16,66]. Polyvalent cations in the soil can form ionic bond bridges, helping to polymerize clay particles and organic matter and improving aggregate stability.
5. Conclusions
In this study, five representative mixed forest stands at the end of the Three Gorges Reservoir Area were used to investigate the relationships between soil physicochemical properties, plant root system shape, and soil aggregate stability. Unfortunately, the results did not provide evidence of a connection between AMF and soil aggregate stability; however, through an analysis of soil aggregate stability in five typical forest stands, we discovered that among the different stand characteristics, the plant root system shape indirectly influenced soil aggregate stability by affecting soil properties. Given the strategic geographical importance of the Three Gorges Reservoir Area in China and the complex human–environment interactions in this region, a scientific understanding of the driving factors behind soil aggregate stability is very important for the region’s ecological health in the context of climate change.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f14122402/s1, Table S1: Dominant tree species in the tested forest stands and sample site base information. Table S2: Total variance interpretation of soil and plant root indicators. Table S3: First principal component factor score coefficients for soil and plant root indicators. Figure S1: Influence of soil characteristics on aggregate stability in the different forest stand types. MWD, mean weight diameter; clay, <0.002 mm. Alo, amorphous aluminum; clay, <0.002 mm; AFM, arbuscular mycorrhizal fungi. GB mean Gorodnia axillaris and other broad-leaved tree mixed forest, CL mean Cunninghamina lanceolata and Lindera kwangtungensis mixed forest, PP mean Phyllostachys pubescens pure forest, PG mean Pinus massoniana and G. axillaris mixed forest, PS mean P. massoniana and Symplocos sumuntia mixed forest. Figure S2: Influence of root traits on aggregate stability in the different forest stand types. MWD, mean weight diameter; RMD, root mass density; RLD, root length density; AD, Root average diameter; GB mean Gorodnia axillaris and other broad-leaved tree mixed forest, CL mean Cunninghamina lanceolata and Lindera kwangtungensis mixed forest, PP mean Phyllostachys pubescens pure forest, PG mean Pinus massoniana and G. axillaris mixed forest, PS mean P. massoniana and Symplocos sumuntia mixed forest.
Author Contributions
Y.Z. (Yonglin Zheng) generated the initial idea, which was developed together with Y.W. (Yunqi Wang), Y.Z. (Yuxuan Zhang) and J.Z. (Jialiang Zhang). J.Z. (Jialiang Zhang) and J.Z. (Junlin Zhu) provided the soil samples. Y.W. (Yunqi Wang) and Y.W. (Yujie Wang) led the writing of this paper, with all authors contributing to manuscript development. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (No. 32371963).
Data Availability Statement
The data are not publicly available due to the policy of the institute.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- Six, J.; Elliott, E.T.; Paustian, K. Soil Structure and Soil Organic Matter: II. A normalized stability index and the effect of mineralogy. Soil Sci. Soc. Am. J. 2000, 64, 1042–1049. [Google Scholar] [CrossRef]
- Wang, X.; Yost, R.S.; Linquist, B.A. Soil aggregate size affects phosphorus desorption from highly weathered soils and plant growth. Soil Sci. Soc. Am. J. 2001, 65, 139–146. [Google Scholar] [CrossRef]
- Somasundaram, J.; Chaudhary, R.S.; Awanish, K.D.; Biswas, A.K.; Sinha, N.K.; Mohanty, M.; Chaudhari, S.K. Effect of contrasting tillage and crop systems on soil aggregation, carbon pools and aggregate associated carbon in rainfed Vertisols. Eur. J. Soil Sci. 2018, 69, 879–891. [Google Scholar] [CrossRef]
- Pohl, M.; Alig, D.; Körner, C.; Rixen, C. Higher plant diversity enhances soil stability in disturbed alpine ecosystems. Plant Soil 2009, 324, 91–102. [Google Scholar] [CrossRef]
- Wu, X.; Cai, C.; Wang, J.; Wei, Y.; Wang, S. Spatial variations of aggregate stability in relation to sesquioxides for zonal soils, South-central China. Soil Tillage Res. 2016, 157, 11–22. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, S.; Hu, R.; Li, Y. Aggregate stability and size distribution of red soils under different land uses integrally regulated by soil organic matter, and iron and aluminum oxides. Soil Tillage Res. 2017, 167, 73–79. [Google Scholar] [CrossRef]
- Le Bissonnais, Y.; Prieto, I.; Roumet, C.; Nespoulous, J.; Metayer, J.; Huon, S.; Villatoro, M.; Stokes, A. Soil aggregate stability in Mediterranean and tropical agro-ecosystems: Effect of plant roots and soil characteristics. Plant Soil 2018, 424, 303–317. [Google Scholar] [CrossRef]
- Kasper, M.; Buchan, G.D.; Mentler, A.; Blum, W.E.H. Influence of soil tillage systems on aggregate stability and the distribution of C and N in different aggregate fractions. Soil Tillage Res. 2009, 105, 192–199. [Google Scholar] [CrossRef]
- Bartlová, J.; Badalíková, B.; Pospíšilová, L.; Pokorný, E.; Šarapatka, B. Water stability of soil aggregates in different systems of Tillage. Soil Water Res. 2015, 10, 147–154. [Google Scholar] [CrossRef]
- Amézketa, E. Soil aggregate stability: A review. J. Sustain. Agric. 1999, 14, 83–151. [Google Scholar] [CrossRef]
- Erktan, A.; Cécillon, L.; Graf, F.; Roumet, C.; Legout, C.; Rey, F. Increase in soil aggregate stability along a Mediterranean successional gradient in severely eroded gully bed ecosystems: Combined effects of soil, root traits and plant community characteristics. Plant Soil 2016, 398, 121–137. [Google Scholar] [CrossRef]
- Deviren, S.S.; Cornelis, W.M.; Erpul, G.; Gabriels, D. Comparison of different aggregate stability approaches for loamy sand soils. Appl. Soil Ecol. 2012, 54, 1–6. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, M.; Xiong, H.; Li, Y.; Zhang, Y.; Huang, X.; Yang, Y.; Zhu, H.; Jiang, T. Influence of long-term fertilization on soil aggregates stability and organic carbon occurrence characteristics in karst yellow soil of Southwest China. Front. Plant Sci. 2023, 14, 1126150. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wei, Y.; Wang, J.; Di, W.; Cai, C. Effects of soil physicochemical properties on aggregate stability along a weathering gradient. Catena 2017, 156, 205–215. [Google Scholar] [CrossRef]
- Regelink, I.C.; Stoof, C.R.; Rousseva, S.; Weng, L.; Lair, G.J.; Kram, P.; Nikolaidis, N.P.; Kercheva, M.; Banwart, S.; Comans, R. Linkages between aggregate formation, porosity and soil chemical properties. Geoderma 2015, 247–248, 24–37. [Google Scholar] [CrossRef]
- Duiker, S.W.; Rhoton, F.E.; Torrent, J.; Smeck, N.E.; Lal, R. Iron (hydr) oxide crystallinity effects on soil aggregation. Soil Sci. Soc. Am. J. 2003, 67, 606–611. [Google Scholar] [CrossRef]
- Barthès, B.G.; Kouakoua, E.; Larré-Larrouy, M.C.; De Luca, E.F.; Azontonde, A.; Neves, C.; De Freitas, P.L.; Feller, C. Texture and sesquioxide effects on water-stable aggregates and organic matter in some tropical soils. Geoderma 2008, 143, 14–25. [Google Scholar] [CrossRef]
- Lehmann, A.; Leifheit, E.F.; Rillig, M.C. Mycorrhizas and Soil Aggregation; Elsevier: New York, NY, USA, 2017. [Google Scholar]
- Rillig, M.C.; Muller, L.A.; Lehmann, A. Soil aggregates as massively concurrent evolutionary incubators. ISME J. 2017, 11, 1943–1948. [Google Scholar] [CrossRef]
- Barbosa, M.V.; Pedroso, D.D.F.; Curi, N.; Carneiro, M.A.C. Do different arbuscular mycorrhizal fungi affect the formation and stability of soil aggregates? Ciênc. Agrotec. 2019, 43, e003519. [Google Scholar] [CrossRef]
- Gould, I.J.; Quinton, J.N.; Weigelt, A.; De Deyn, G.B.; Bardgett, R.D. Plant diversity and root traits benefit physical properties key to soil function in grasslands. Ecol. Lett. 2016, 19, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Demenois, J.; Rey, F.; Ibanez, T.; Stokes, A.; Carriconde, F. Linkages between root traits, soil fungi and aggregate stability in tropical plant communities along a successional vegetation gradient. Plant Soil 2018, 424, 319–334. [Google Scholar] [CrossRef]
- Ali, H.E.; Reineking, B.; Münkemüller, T. Effects of plant functional traits on soil stability: Intraspecific variability matters. Plant Soil 2017, 411, 359–375. [Google Scholar] [CrossRef]
- Stokes, A.; Atger, C.; Bengough, A.G.; Fourcaud, T.; Sidle, R.C. Desirable plant root traits for protecting natural and engineered slopes against landslides. Plant Soil 2009, 324, 1–30. [Google Scholar] [CrossRef]
- Rillig, M.C.; Aguilar-Trigueros, C.A.; Bergmann, J.; Verbruggen, E.; Veresoglou, S.D.; Lehmann, A. Plant root and mycorrhizal fungal traits for understanding soil aggregation. New Phytol. 2015, 205, 1385–1388. [Google Scholar] [CrossRef] [PubMed]
- Komissarov, M.; Ogura, S. Siltation and radiocesium pollution of small lakes in different catchment types far from the Fukushima Daiichi nuclear power plant accident site. Int. Soil Water Conserv. Res. 2020, 8, 56–65. [Google Scholar] [CrossRef]
- Ushio, M.; Kitayama, K.; Balser, T.C. Tree species-mediated spatial patchiness of the composition of microbial community and physicochemical properties in the topsoils of a tropical montane forest. Soil Biol. Biochem. 2010, 42, 1588–1595. [Google Scholar] [CrossRef]
- ISO Standard 10930; Soil Quality—Measurement of the Stability of Soil Aggregates Subjected to the Action of Water. International Organization for Standardization: Geneva, Switzerland, 2012. Available online: http://www.iso.org/standard/46433 (accessed on 15 August 2023).
- Le Bissonnais, Y. Aggregate stability and assessment of soil crustability and erodibility: I. theory and methodology. Eur. J. Soil Sci. 1996, 47, 425–437. [Google Scholar] [CrossRef]
- Bao, S. Soil and Agricultural Chemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Dick, W.A.; Cheng, L.; Wang, P. Soil acid and alkaline phosphatase activity as pH adjustment indicators. Soil Biol. Biochem. 2000, 32, 1915–1919. [Google Scholar] [CrossRef]
- Mehra, O.P.; Jackson, M.L. Iron oxide removal from soils and clays by a dithionite citrate system buffered with sodium bicarbonate. Clays Clay Miner. 1958, 7, 313–317. [Google Scholar] [CrossRef]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Díaz, S.; Buchmann, N.; Gurvich, D.E. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335. [Google Scholar] [CrossRef]
- Frostegård, Å.; Tunlid, A.; Bååth, E. Microbial biomass measured as total lipid phosphate in soils of different organic content. J. Microbiol. Meth. 1991, 14, 151–163. [Google Scholar] [CrossRef]
- Guo, X.; Chen, H.Y.; Meng, M.; Biswas, S.R.; Ye, L.; Zhang, J. Effects ofland use change on the composition of soil microbial communities in a managed subtropical forest. Forest Ecol. Manag. 2016, 373, 93–99. [Google Scholar] [CrossRef]
- Kemner, J.E.; Adams, M.B.; McDonald, L.M.; Peterjohn, W.T.; Kelly, C.N. Fertilization and tree species influence on stable aggregates in forest soil. Forests 2020, 12, 39. [Google Scholar] [CrossRef]
- Garcia-Franco, N.; Martínez-Mena, M.; Goberna, M.; Albaladejo, J. Changes in soil aggregation and microbial community structure control carbon sequestration after afforestation of semiarid shrublands. Soil Biol. Biochem. 2015, 87, 110–121. [Google Scholar] [CrossRef]
- Piotrowski, J.S.; Denich, T.; Klironomos, J.N.; Graham, J.M.; Rillig, M.C. The effects of arbuscular mycorrhizas on soil aggregation depend on the interaction between plant and fungal species. New Phytol. 2004, 164, 365–373. [Google Scholar] [CrossRef]
- Shen, Y.; Cheng, R.; Xiao, W.; Yang, S. Effects of understory removal and thinning on soil aggregation, and organic carbon distribution in Pinus massoniana plantations in the three Gorges Reservoir area. Ecol. Indic. 2021, 123, 107323. [Google Scholar] [CrossRef]
- Yoo, G.; Yang, X.; Wander, M.M. Influence of soil aggregation on SOC sequestration: A preliminary model of SOC protection by aggregate dynamics. Ecol. Eng. 2011, 37, 487–495. [Google Scholar] [CrossRef]
- Yadav, R.S.; Yadav, B.L.; Chhipa, B.R. Litter dynamics and soil properties under different tree species in a semi-arid region of Rajasthan, India. Agroforest. Syst. 2008, 73, 1–12. [Google Scholar] [CrossRef]
- Ullah, S.; Muhammad, B.; Amin, R.; Abbas, H.; Muneer, M.A. Sensitivity of arbuscular mycorrhizal fungi in old-growth forests: Direct effect on growth and soil carbon storage. Appl. Ecol. Env. Res. 2019, 6, 13749–13758. [Google Scholar] [CrossRef]
- Crockford, R.H.; Richardson, D.P. Partitioning of rainfall into throughfall, stemflow and interception: Effect of forest type, ground cover and climate. Hydrol. Process. 2000, 14, 2903–2920. [Google Scholar] [CrossRef]
- Gersper, P.L.; Holowaychuk, N. Effects of Stemflow Water on a Miami Soil Under a Beech Tree: II. Chemical Properties. Soil Sci. Soc. Am. J. 1970, 34, 786–794. [Google Scholar] [CrossRef]
- Corti, G.; Agnelli, A.; Cocco, S.; Cardelli, V.; Masse, J.; Courchesne, F. Soil affects throughfall and stemflow under Turkey oak (Quercus cerris L.). Geoderma 2019, 333, 43–56. [Google Scholar] [CrossRef]
- Gupta, V.V.; Germida, J.J. Soil aggregation: Influence on microbial biomass and implications for biological processes. Soil Biol. Biochem. 2015, 80, A3–A9. [Google Scholar] [CrossRef]
- Bai, T.; Wang, P.; Ye, C.; Hu, S. Form of nitrogen input dominates N effects on root growth and soil aggregation: A meta-analysis. Soil Biol. Biochem. 2021, 157, 108251. [Google Scholar] [CrossRef]
- Liu, R.; Zhou, X.; Wang, J.; Shao, J.; Fu, Y.; Liang, C.; Bai, S.H. Differential magnitude of rhizosphere effects on soil aggregation at three stages of subtropical secondary forest successions. Plant Soil 2019, 436, 365–380. [Google Scholar] [CrossRef]
- Hou, W.; Xu, Y.; Xue, S.; Li, J.; Yang, Y.; Yi, Z.; Fu, T. Effects of soil physics, chemistry, and microbiology on soil carbon sequestration in infertile red soils after long-term cultivation of perennial grasses. GCB Bioenergy 2023, 15, 239–253. [Google Scholar] [CrossRef]
- Six, J.; Bossuyt, H.; Degryze, S.; Denef, K. A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics. Soil Tillage Res. 2004, 79, 7–31. [Google Scholar] [CrossRef]
- Hütsch, B.W.; Augustin, J.; Merbach, W. Plant rhizodeposition—An important source for carbon turnover in soils. J. Plant Nutr. Soil Sci. 2002, 165, 397–407. [Google Scholar] [CrossRef]
- Li, C.; Yu, Z.; Lin, J.; Meng, M.; Zhao, Y.; Jia, Z.; Peng, X.; Liu, X.; Zhang, J. Forest conversion and soil depth can modify the contributions of organic and inorganic colloids to the stability of soil aggregates. Forests 2022, 13, 546. [Google Scholar] [CrossRef]
- Lu, J.Z.; Scheu, S. Response of soil microbial communities to mixed beech-conifer forests varies with site conditions. Soil Biol. Biochem. 2021, 155, 108155. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Yang, L. A long-term effect of Larix monocultures on soil physicochemical properties and microbes in northeast China. Eur. J. Soil Biol. 2020, 96, 103149. [Google Scholar] [CrossRef]
- Woś, B.; Józefowska, A.; Likus-Cieślik, J.; Chodak, M.; Pietrzykowski, M. Effect of tree species and soil texture on the carbon stock, macronutrient content, and physicochemical properties of regenerated postfire forest soils. Land Degrad. Dev. 2021, 32, 5227–5240. [Google Scholar] [CrossRef]
- Edlinger, A.; Garland, G.; Banerjee, S.; Degrune, F.; García-Palacios, P.; Herzog, C.; Pescador, D.S.; Romdhane, S.; Ryo, M.; Saghaï, A.; et al. The impact of agricultural management on soil aggregation and carbon storage is regulated by climatic thresholds across a 3000 km European gradient. Glob. Chang. Biol. 2023, 29, 3177–3192. [Google Scholar] [CrossRef] [PubMed]
- Van Der Heijden, M.G.A.; Streitwolf-Engel, R.; Riedl, R.; Siegrist, S.; Neudecker, A.; Ineichen, K.; Boller, T.; Wiemken, A.; Sanders, I.R. The mycorrhizal contribution to plant productivity, plant nutrition and soil structure in experimental grassland. New Phytol. 2006, 172, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Leifheit, E.F.; Veresoglou, S.D.; Lehmann, A.; Morris, E.K.; Rillig, M.C. Multiple factors influence the role of arbuscular mycorrhizal fungi in soil aggregation—A meta-analysis. Plant Soil 2014, 374, 523–537. [Google Scholar] [CrossRef]
- Chenu, C.; Le Bissonnais, Y.; Arrouays, D. Organic matter influence on clay wettability and soil aggregate stability. Soil Sci. Soc. Am. J. 2000, 64, 1479–1486. [Google Scholar] [CrossRef]
- Sun, D.; Li, K.; Bi, Q.; Zhu, J.; Zhang, Q.; Jin, C.; Lu, L.; Lin, X. Effects of organic amendment on soil aggregation and microbial community composition during drying-rewetting alternation. Sci. Total Environ. 2017, 574, 735–743. [Google Scholar] [CrossRef]
- Barto, E.K.; Alt, F.; Oelmann, Y.; Wilcke, W.; Rillig, M.C. Contributions of biotic and abiotic factors to soil aggregation across a land use gradient. Soil Biol. Biochem. 2010, 42, 2316–2324. [Google Scholar] [CrossRef]
- Ternan, J.L.; Elmes, A.; Williams, A.G.; Hartley, R. Aggregate stability of soils in central spain and the role of land management. Proc. Land. 1996, 21, 181–193. [Google Scholar] [CrossRef]
- Hu, B.; Wang, Y.; Wang, B.; Zhang, H.; Guo, P.; Liu, C. Effects of simulated nitrogen deposition on soil acidification and soil buffering capacity. Res. Environ. Sci. 2015, 28, 418–424. [Google Scholar] [CrossRef]
- Denef, K.; Six, J.; Bossuyt, H.; Frey, S.D.; Elliott, E.T.; Merckx, R.; Paustian, K. Influence of dry-wet cycles on the interrelationship between aggregate, particulate organic matter, and microbial community dynamics. Soil Biol. Biochem. 2001, 33, 1599–1611. [Google Scholar] [CrossRef]
- Li, M.; You, Y.; Tan, X.; Wen, Y.; Yu, S.; Xiao, N.; Shen, W.; Huang, X. Mixture of N2-fixing tree species promotes organic phosphorus accumulation and transformation in topsoil aggregates in a degraded karst region of subtropical China. Geoderma 2022, 413, 115752. [Google Scholar] [CrossRef]
- Saidi, D. Importance and Role of Cation Exchange Capacity on the Physicals Properties of the Cheliff Saline Soils (Algeria). Procedia Eng. 2012, 33, 435–449. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).