Nutrient and Growth Response of Fagus sylvatica L. Saplings to Drought Is Modified by Fertilisation
Abstract
:1. Introduction
- I.
- Increase nutrient availability, partially mitigating the negative effects of drought on nutrition by maintaining adequate foliar concentrations of nitrogen, phosphorus, and potassium during drought.
- II.
- Alleviate the prolonged effect of drought on the foliar concentrations, growth, and biomass production of beech saplings by maintaining them at the level of regularly watered saplings.
2. Materials and Methods
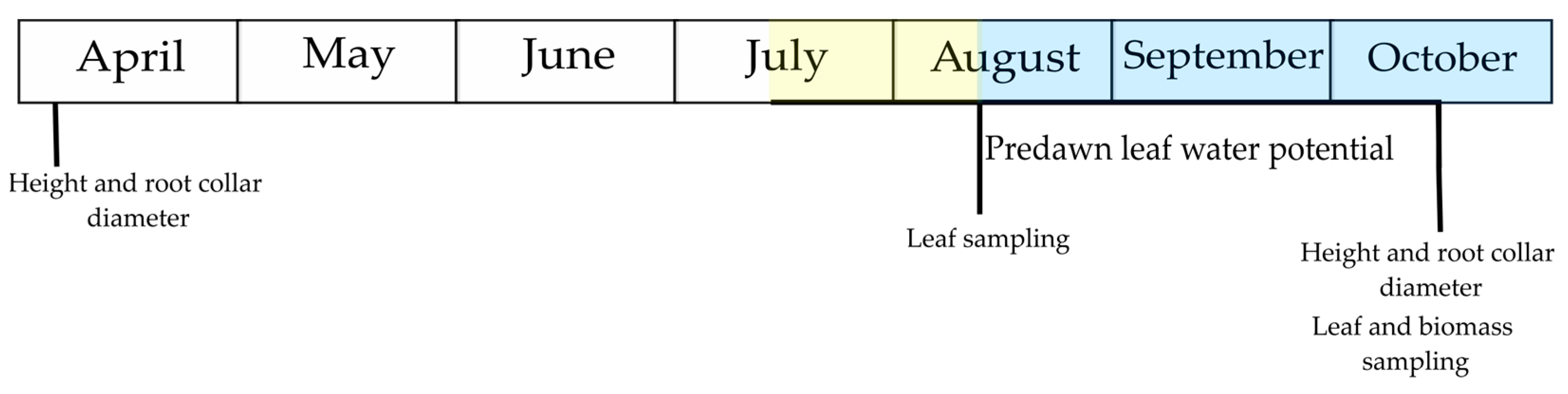
2.1. Experimental Design and Treatments
2.2. Response Parameters
2.3. Statistical Analysis
3. Results
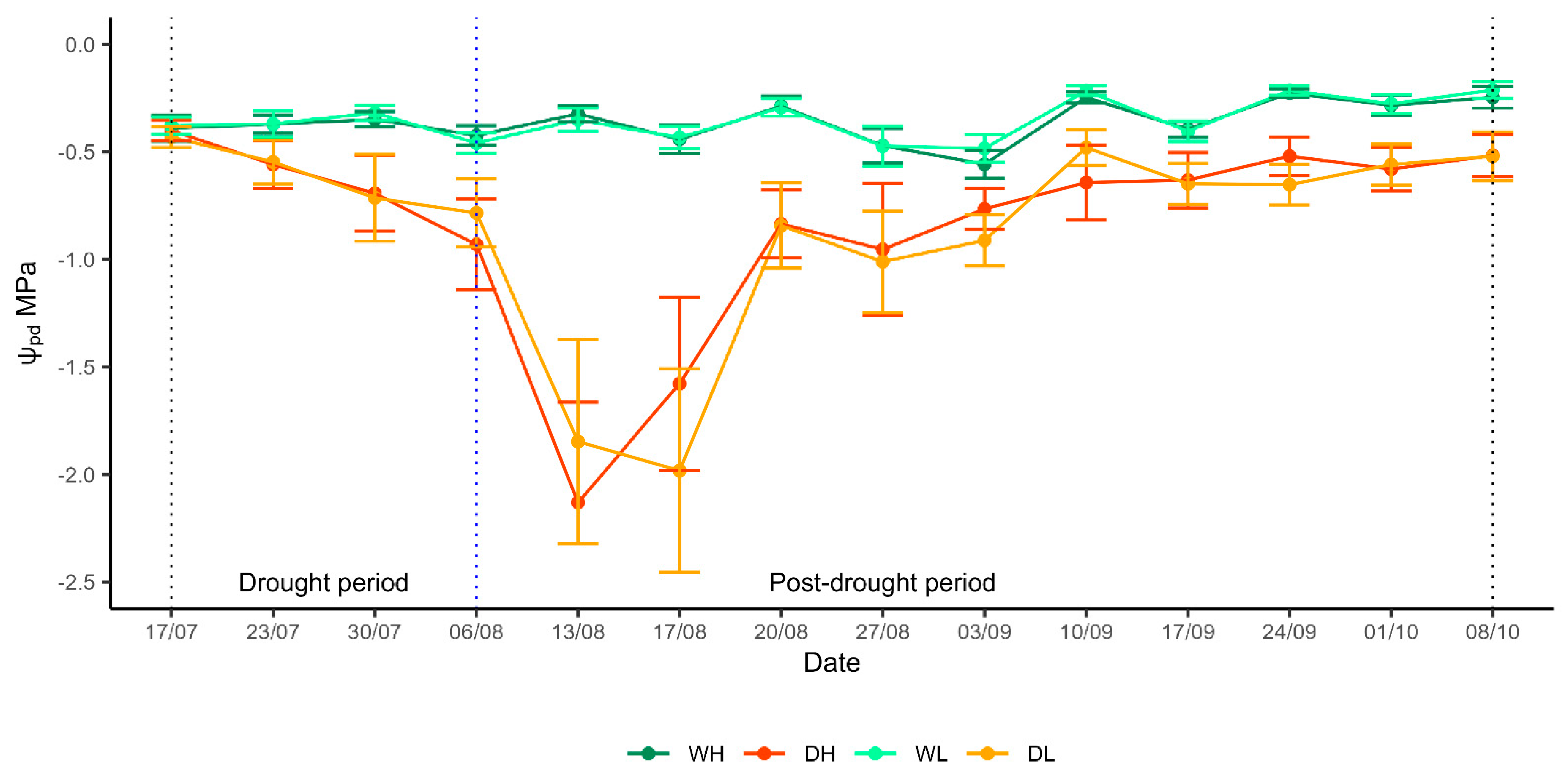
3.1. Water Status of Saplings
3.2. Nutritional Status of Saplings
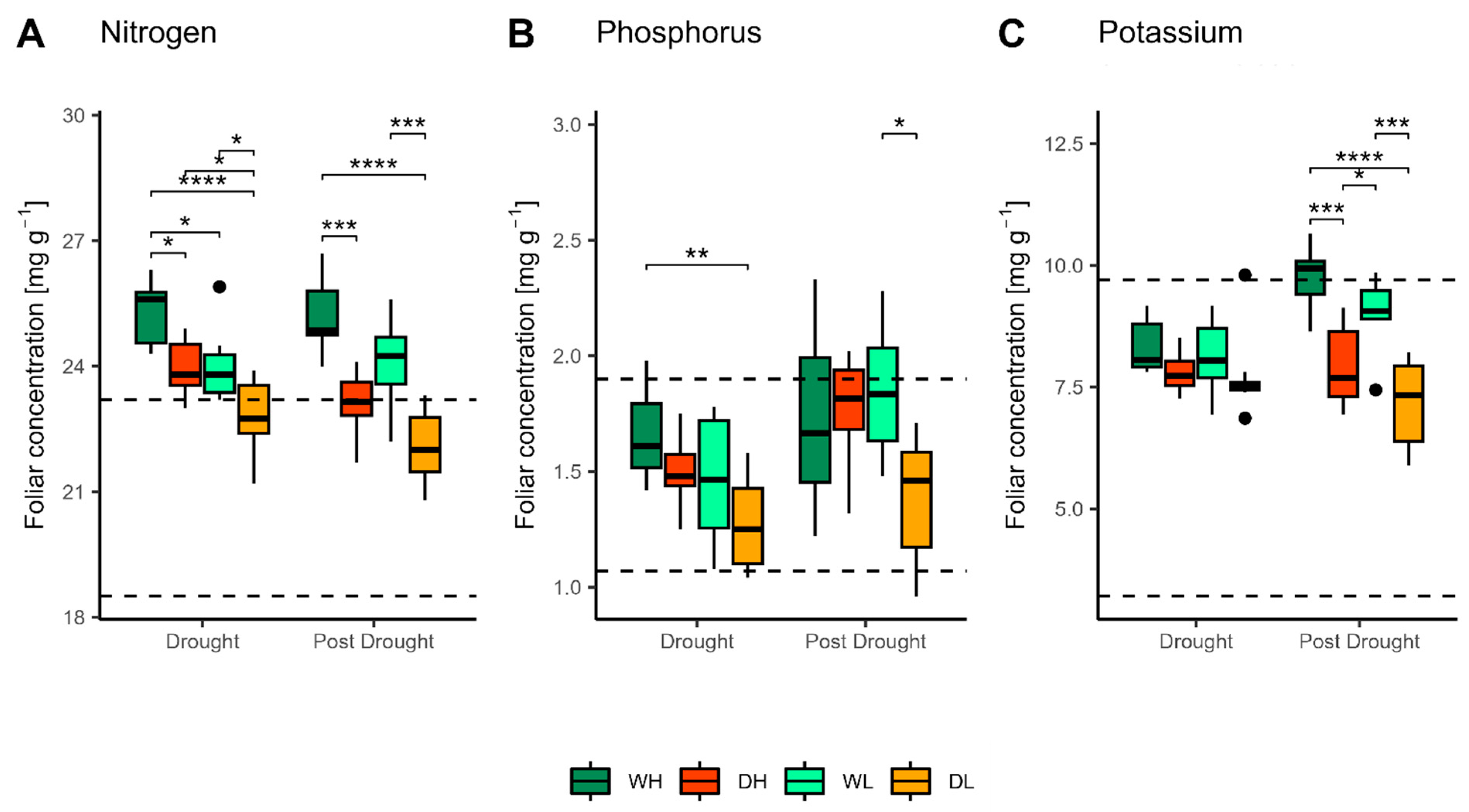
3.2.1. Foliar Concentrations
3.2.2. Fine-Root Nutrient Concentrations
3.3. Growth Responses
3.4. Biomass Responses
4. Discussion
4.1. Water Status of Saplings during Drought and in the Post-Drought Period
4.2. Nutritional Status of Saplings in the Drought Period
4.3. Nutritional Status of Saplings in the Post-Drought Period
4.4. Fine-Root Nutrient Concentrations
4.5. Effects of Drought and Fertilisation on Sapling Growth and Biomass Partitioning
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frei, E.R.; Gossner, M.M.; Vitasse, Y.; Queloz, V.; Dubach, V.; Gessler, A.; Ginzler, C.; Hagedorn, F.; Meusburger, K.; Moor, M.; et al. European Beech Dieback after Premature Leaf Senescence during the 2018 Drought in Northern Switzerland. Plant Biol. 2022, 24, 1132–1145. [Google Scholar] [CrossRef] [PubMed]
- Babst, F.; Bouriaud, O.; Poulter, B.; Trouet, V.; Girardin, M.P.; Frank, D.C. Twentieth Century Redistribution in Climatic Drivers of Global Tree Growth. Sci. Adv. 2019, 5, eaat4313. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A Global Overview of Drought and Heat-Induced Tree Mortality Reveals Emerging Climate Change Risks for Forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Hartmann, H.; Bastos, A.; Das, A.J.; Esquivel-Muelbert, A.; Hammond, W.M.; Martínez-Vilalta, J.; McDowell, N.G.; Powers, J.S.; Pugh, T.A.M.; Ruthrof, K.X.; et al. Climate Change Risks to Global Forest Health: Emergence of Unexpected Events of Elevated Tree Mortality Worldwide. Annu. Rev. Plant Biol. 2022, 73, 673–702. [Google Scholar] [CrossRef]
- Bredemeier, M. Forest Management and the Water Cycle: An Ecosystem-Based Approach; Bredemeier, M., Cohen, S., Godbold, D.L., Lode, E., Pichler, V., Schleppi, P., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2011; ISBN 978-90-481-9834-4. [Google Scholar]
- Glatthorn, J.; Annighöfer, P.; Balkenhol, N.; Leuschner, C.; Polle, A.; Scheu, S.; Schuldt, A.; Schuldt, B.; Ammer, C. An Interdisciplinary Framework to Describe and Evaluate the Functioning of Forest Ecosystems. Basic Appl. Ecol. 2021, 52, 1–14. [Google Scholar] [CrossRef]
- Brunet, J.; Fritz, Ö.; Richnau, G. Biodiversity in European Beech Forests—A Review with Recommendations for Sustainable Forest Management. Ecol. Bull. 2010, 53, 77–94. [Google Scholar]
- Leuschner, C.; Ellenberg, H. Ecology of Central European Forests; Springer: Cham, Switzerland, 2017; Volume I, ISBN 9783319430409. [Google Scholar]
- Wang, F.; Israel, D.; Ramírez-Valiente, J.A.; Sánchez-Gómez, D.; Aranda, I.; Aphalo, P.J.; Robson, T.M. Seedlings from Marginal and Core Populations of European Beech (Fagus sylvatica L.) Respond Differently to Imposed Drought and Shade. Trees Struct. Funct. 2020, 35, 53–67. [Google Scholar] [CrossRef]
- Stojnić, S.; Orlović, S.; Miljković, D.; Galić, Z.; Kebert, M.; von Wuehlisch, G. Provenance Plasticity of European Beech Leaf Traits under Differing Environmental Conditions at Two Serbian Common Garden Sites. Eur. J. For. Res. 2015, 134, 1109–1125. [Google Scholar] [CrossRef]
- Seletković, Z.; Tikvić, I.; Prpić, B. Ekološka Konstitucija Obične Bukve. In Obična Bukva (Fagus sylvatica L.) u Hrvatskoj; Matić, S., Ed.; Akademija šumarskih znanosti: Zagreb, Republic of Croatia, 2003. [Google Scholar]
- Pretzsch, H.; Hilmers, T.; Uhl, E.; Bielak, K.; Bosela, M.; del Rio, M.; Dobor, L.; Forrester, D.I.; Nagel, T.A.; Pach, M.; et al. European Beech Stem Diameter Grows Better in Mixed than in Mono-Specific Stands at the Edge of Its Distribution in Mountain Forests. Eur. J. For. Res. 2021, 140, 127–145. [Google Scholar] [CrossRef]
- Bolte, A.; Czajkowski, T.; Cocozza, C.; Tognetti, R.; De Miguel, M.; Pšidová, E.; Ditmarová, L.; Dinca, L.; Delzon, S.; Cochard, H.; et al. Desiccation and Mortality Dynamics in Seedlings of Different European Beech (Fagus sylvatica L.) Populations under Extreme Drought Conditions. Front. Plant Sci. 2016, 7, 751. [Google Scholar] [CrossRef]
- Gessler, A.; Keitel, C.; Kreuzwieser, J.; Matyssek, R.; Seiler, W.; Rennenberg, H. Potential Risks for European Beech (Fagus sylvatica L.) in a Changing Climate. Trees Struct. Funct. 2007, 21, 1–11. [Google Scholar] [CrossRef]
- Meier, I.C.; Leuschner, C. Belowground Drought Response of European Beech: Fine Root Biomass and Carbon Partitioning in 14 Mature Stands across a Precipitation Gradient. Glob. Chang. Biol. 2008, 14, 2081–2095. [Google Scholar] [CrossRef]
- Leuschner, C. Drought Response of European Beech (Fagus sylvatica L.)—A Review. Perspect. Plant Ecol. Evol. Syst. 2020, 47, 125576. [Google Scholar] [CrossRef]
- Schuldt, B.; Ruehr, N.K. Responses of European Forests to Global Change-Type Droughts. Plant Biol. 2022, 24, 1093–1097. [Google Scholar] [CrossRef] [PubMed]
- Walthert, L.; Ganthaler, A.; Mayr, S.; Saurer, M.; Waldner, P.; Walser, M.; Zweifel, R.; von Arx, G. From the Comfort Zone to Crown Dieback: Sequence of Physiological Stress Thresholds in Mature European Beech Trees across Progressive Drought. Sci. Total Environ. 2021, 753, 141792. [Google Scholar] [CrossRef] [PubMed]
- Martinez del Castillo, E.; Zang, C.S.; Buras, A.; Hacket-Pain, A.; Esper, J.; Serrano-Notivoli, R.; Hartl, C.; Weigel, R.; Klesse, S.; Resco de Dios, V.; et al. Climate-Change-Driven Growth Decline of European Beech Forests. Commun. Biol. 2022, 5, 163. [Google Scholar] [CrossRef] [PubMed]
- Rukh, S.; Sanders, T.G.M.; Krüger, I.; Schad, T.; Bolte, A. Distinct Responses of European Beech (Fagus sylvatica L.) to Drought Intensity and Length—A Review of the Impacts of the 2003 and 2018–2019 Drought Events in Central Europe. Forests 2023, 14, 248. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Klein, T.; Bartlett, M.; Sack, L.; Pellegrini, A.F.A.; Choat, B.; Jansen, S. Meta-Analysis Reveals That Hydraulic Traits Explain Cross-Species Patterns of Drought-Induced Tree Mortality across the Globe. Proc. Natl. Acad. Sci. USA 2016, 113, 5024–5029. [Google Scholar] [CrossRef]
- Zang, U.; Goisser, M.; Grams, T.E.E.; Häberle, K.H.; Matyssek, R.; Matzner, E.; Borken, W.; Epron, D. Fate of Recently Fixed Carbon in European Beech (Fagus sylvatica) Saplings during Drought and Subsequent Recovery. Tree Physiol. 2014, 34, 29–38. [Google Scholar] [CrossRef]
- Arend, M.; Sever, K.; Pflug, E.; Gessler, A.; Schaub, M. Seasonal Photosynthetic Response of European Beech to Severe Summer Drought: Limitation, Recovery and Post-Drought Stimulation. Agric. For. Meteorol. 2016, 220, 83–89. [Google Scholar] [CrossRef]
- Rennenberg, H.; Loreto, F.; Polle, A.; Brilli, F.; Fares, S.; Beniwal, R.S.; Gessler, A. Physiological Responses of Forest Trees to Heat and Drought. Plant Biol. 2006, 8, 556–571. [Google Scholar] [CrossRef]
- Bhusal, N.; Lee, M.; Reum Han, A.; Han, A.; Kim, H.S. Responses to Drought Stress in Prunus sargentii and Larix kaempferi Seedlings Using Morphological and Physiological Parameters. For. Ecol. Manag. 2020, 465, 118099. [Google Scholar] [CrossRef]
- Gessler, A.; Keitel, C.; Nahm, M.; Rennenberg, H.; Geßler, A.; Keitel, C.; Nahm, M.; Rennenberg, H. Water Shortage Affects the Water and Nitrogen Balance in Central European Beech Forests. Plant Biol. 2004, 6, 289–298. [Google Scholar] [CrossRef]
- Salehi, M.; Walthert, L.; Zimmermann, S.; Waldner, P.; Schmitt, M.; Schleppi, P.; Liechti, K.; Ahmadi, M.; Zahedi Amiri, G.; Brunner, I.; et al. Leaf Morphological Traits and Leaf Nutrient Concentrations of European Beech Across a Water Availability Gradient in Switzerland. Front. For. Glob. Chang. 2020, 3, 19. [Google Scholar] [CrossRef]
- Kreuzwieser, J.; Gessler, A. Global Climate Change and Tree Nutrition: Influence of Water Availability. Tree Physiol. 2010, 30, 1221–1234. [Google Scholar] [CrossRef] [PubMed]
- Peuke, A.D.; Rennenberg, H. Impacts of Drought on Mineral Macro- and Microelements in Provenances of Beech (Fagus sylvatica L.) Seedlings. Tree Physiol. 2011, 31, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Gessler, A.; Schaub, M.; McDowell, N.G. The Role of Nutrients in Drought-Induced Tree Mortality and Recovery. New Phytol. 2016, 214, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Schönbeck, L.; Li, M.H.; Lehmann, M.M.; Rigling, A.; Schaub, M.; Hoch, G.; Kahmen, A.; Gessler, A. Soil Nutrient Availability Alters Tree Carbon Allocation Dynamics during Drought. Tree Physiol. 2021, 41, 697–707. [Google Scholar] [CrossRef]
- Thiel, D.; Kreyling, J.; Backhaus, S.; Beierkuhnlein, C.; Buhk, C.; Egen, K.; Huber, G.; Konnert, M.; Nagy, L.; Jentsch, A. Different Reactions of Central and Marginal Provenances of Fagus sylvatica to Experimental Drought. Eur. J. For. Res. 2014, 133, 247–260. [Google Scholar] [CrossRef]
- Leuschner, C.; Backes, K.; Hertel, D.; Schipka, F.; Schmitt, U.; Terborg, O.; Runge, M. Drought Responses at Leaf, Stem and Fine Root Levels of Competitive Fagus sylvatica L. and Quercus petraea (Matt.) Liebl. Trees in Dry and Wet Years. For. Ecol. Manag. 2001, 149, 33–46. [Google Scholar] [CrossRef]
- Ruehr, N.K.; Offermann, C.A.; Gessler, A.; Winkler, J.B.; Ferrio, J.P.; Buchmann, N.; Barnard, R.L. Drought Effects on Allocation of Recent Carbon: From Beech Leaves to Soil CO2 Efflux. New Phytol. 2009, 184, 950–961. [Google Scholar] [CrossRef] [PubMed]
- Zang, U.; Goisser, M.; Häberle, K.H.; Matyssek, R.; Matzner, E.; Borken, W. Effects of Drought Stress on Photosynthesis, Rhizosphere Respiration, and Fine-Root Characteristics of Beech Saplings: A Rhizotron Field Study. J. Plant Nutr. Soil Sci. 2014, 177, 168–177. [Google Scholar] [CrossRef]
- Bista, D.R.; Heckathorn, S.A.; Jayawardena, D.M.; Mishra, S.; Boldt, J.K. Effects of Drought on Nutrient Uptake and the Levels of Nutrient-Uptake Proteins in Roots of Drought-Sensitive and -Tolerant Grasses. Plants 2018, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Ciais, P.; Reichstein, M.; Viovy, N.; Granier, A.; Ogée, J.; Allard, V.; Aubinet, M.; Buchmann, N.; Bernhofer, C.; Carrara, A.; et al. Europe-Wide Reduction in Primary Productivity Caused by the Heat and Drought in 2003. Nature 2005, 437, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Varsamis, G.; Papageorgiou, A.C.; Merou, T.; Takos, I.; Malesios, C.; Manolis, A.; Tsiripidis, I.; Gailing, O. Adaptive Diversity of Beech Seedlings under Climate Change Scenarios. Front. Plant Sci. 2019, 9, 1918. [Google Scholar] [CrossRef]
- Gessler, A.; Bottero, A.; Marshall, J.; Arend, M. The Way Back: Recovery of Trees from Drought and Its Implication for Acclimation. New Phytol. 2020, 228, 1704–1709. [Google Scholar] [CrossRef]
- González de Andrés, E.; Rosas, T.; Camarero, J.J.; Martínez-Vilalta, J. The Intraspecific Variation of Functional Traits Modulates Drought Resilience of European Beech and Pubescent Oak. J. Ecol. 2021, 109, 3652–3669. [Google Scholar] [CrossRef]
- Zang, U.; Goisser, M.; Meyer, N.; Häberle, K.H.; Borken, W. Chemical and Morphological Response of Beech Saplings (Fagus sylvatica L.) to an Experimental Soil Drought Gradient. For. Ecol. Manag. 2021, 498, 119569. [Google Scholar] [CrossRef]
- Coomes, D.A.; Jenkins, K.L.; Cole, L.E.S. Scaling of Tree Vascular Transport Systems along Gradients of Nutrient Supply and Altitude. Biol. Lett. 2007, 3, 86–90. [Google Scholar] [CrossRef]
- Mitchell, P.J.; O’Grady, A.P.; Tissue, D.T.; White, D.A.; Ottenschlaeger, M.L.; Pinkard, E.A. Drought Response Strategies Define the Relative Contributions of Hydraulic Dysfunction and Carbohydrate Depletion during Tree Mortality. New Phytol. 2013, 197, 862–872. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of Plant Survival and Mortality during Drought: Why Do Some Plants Survive While Others Succumb to Drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- Scoffoni, C.; Albuquerque, C.; Brodersen, C.R.; Townes, S.V.; John, G.P.; Cochard, H.; Buckley, T.N.; McElrone, A.J.; Sack, L. Leaf Vein Xylem Conduit Diameter Influences Susceptibility to Embolism and Hydraulic Decline. New Phytol. 2017, 213, 1076–1092. [Google Scholar] [CrossRef]
- Geremew, A.; Carson, L.; Woldesenbet, S.; Carpenter, C.; Peace, E.; Weerasooriya, A. Interactive Effects of Organic Fertilizers and Drought Stress on Growth and Nutrient Content of Brassica juncea at Vegetative Stage. Sustainability 2021, 13, 13948. [Google Scholar] [CrossRef]
- Schönbeck, L.; Gessler, A.; Schaub, M.; Rigling, A.; Hoch, G.; Kahmen, A.; Li, M.-H. Soil Nutrients and Lowered Source:Sink Ratio Mitigate Effects of Mild but Not of Extreme Drought in Trees. Environ. Exp. Bot. 2020, 169, 103905. [Google Scholar] [CrossRef]
- Ouyang, S.-N.; Gessler, A.; Saurer, M.; Hagedorn, F.; Gao, D.-C.; Wang, X.-Y.; Schaub, M.; Li, M.-H.; Shen, W.-J.; Schönbeck, L. Root Carbon and Nutrient Homeostasis Determines Downy Oak Sapling Survival and Recovery from Drought. Tree Physiol. 2021, 41, 1400–1412. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ouyang, S.; Gessler, A.; Wang, X.; Na, R.; He, H.S.; Wu, Z.; Li, M.H. Root Carbon Resources Determine Survival and Growth of Young Trees Under Long Drought in Combination with Fertilization. Front. Plant Sci. 2022, 13, 929855. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.F.; Mateus, N.S.; Rosário, M.O.; Garcez, T.B.; Mazzafera, P.; Lavres, J. Enhancing Potassium Content in Leaves and Stems Improves Drought Tolerance of Eucalyptus Clones. Physiol. Plant. 2021, 172, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Potočić, N.; Seletković, I.; Čater, M.; Ćosić, T.; Mario, Š.; Vedriš, M.; Šango, M.; Vedriš, M. Ekofiziološki Odziv Suncu Izloženih Sadnica Obične Bukve (Fagus sylvatica L.) Pri Različitim Razinama Gnojidbe. Sumar. List 2009, 133, 289–300. [Google Scholar]
- Rautio, P.; Fürst, A.; Stefan, K.; Raitio, H.; Bartels, U. Part XII: Sampling and Analysis of Needles and Leaves. In UNECE ICP Forests Programme Co-Ordinating Centre (ed.): Manual on Methods and Criteria for Harmonized Sampling, Assessment, Monitoring and Analysis of the Effects of Air Pollution on Forests; Thünen Institute of ForestEcosystems: Eberswalde, Germany, 2022; p. 19 + Annex. [Google Scholar]
- Mellert, K.H.; Göttlein, A. Comparison of New Foliar Nutrient Thresholds Derived from van Den Burg’s Literature Compilation with Established Central European References. Eur. J. For. Res. 2012, 131, 1461–1472. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 12 December 2022).
- Pflug, E.E.; Buchmann, N.; Siegwolf, R.T.W.; Schaub, M.; Rigling, A.; Arend, M. Resilient Leaf Physiological Response of European Beech (Fagus sylvatica L.) to Summer Drought and Drought Release. Front. Plant Sci. 2018, 9, 187. [Google Scholar] [CrossRef]
- Tognetti, R.; Johnson, J.D.; Michelozzi, M. The Response of European Beech (Fagus sylvatica L.) Seedlings from Two Italian Populations to Drought and Recovery. Trees 1995, 9, 348–354. [Google Scholar] [CrossRef]
- Johnson, K.M.; Jordan, G.J.; Brodribb, T.J. Wheat Leaves Embolized by Water Stress Do Not Recover Function upon Rewatering. Plant. Cell Environ. 2018, 41, 2704–2714. [Google Scholar] [CrossRef]
- Tomasella, M.; Nardini, A.; Hesse, B.D.; MacHlet, A.; Matyssek, R.; Häberle, K.H. Close to the Edge: Effects of Repeated Severe Drought on Stem Hydraulics and Non-Structural Carbohydrates in European Beech Saplings. Tree Physiol. 2019, 39, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.A.; Batz, T.A.; McAdam, S.A.M. Xylem Embolism Resistance Determines Leaf Mortality during Drought in Persea Americana. Plant Physiol. 2020, 182, 547. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.O.; An, J.Y.; Combalicer, M.S.; Chun, J.P.; Oh, S.K.; Park, B.B. Morpho-Anatomical Traits and Soluble Sugar Concentration Largely Explain the Responses of Three Deciduous Tree Species to Progressive Water Stress. Front. Plant Sci. 2021, 12, 738301. [Google Scholar] [CrossRef]
- Hesse, B.D.; Gebhardt, T.; Hafner, B.D.; Hikino, K.; Reitsam, A.; Gigl, M.; Dawid, C.; Häberle, K.-H.; Grams, T.E.E. Physiological Recovery of Tree Water Relations upon Drought Release—Response of Mature Beech and Spruce after Five Years of Recurrent Summer Drought. Tree Physiol. 2023, 43, 522–538. [Google Scholar] [CrossRef] [PubMed]
- Arend, M.; Link, R.M.; Zahnd, C.; Hoch, G.; Schuldt, B.; Kahmen, A. Lack of Hydraulic Recovery as a Cause of Post-Drought Foliage Reduction and Canopy Decline in European Beech. New Phytol. 2022, 234, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- García-Plazaola, J.I.; Becerril, J.M. Effects of Drought on Photoprotective Mechanisms in European Beech (Fagus sylvatica L.) Seedlings from Different Provenances. Trees Struct. Funct. 2000, 14, 485–490. [Google Scholar] [CrossRef]
- Netzer, F.; Thöm, C.; Celepirovic, N.; Ivankovic, M.; Alfarraj, S.; Dounavi, A.; Simon, J.; Herschbach, C.; Rennenberg, H. Drought Effects on C, N, and P Nutrition and the Antioxidative System of Beech Seedlings Depend on Geographic Origin. J. Plant Nutr. Soil Sci. 2016, 179, 136–150. [Google Scholar] [CrossRef]
- Villar-Salvador, P.; Uscola, M.; Jacobs, D.F. The Role of Stored Carbohydrates and Nitrogen in the Growth and Stress Tolerance of Planted Forest Trees. New For. 2015, 46, 813–839. [Google Scholar] [CrossRef]
- da Silva, E.; Nogueira, R.; da Silva, M.; de Albuquerque, M. Drought Stress and Plant Nutrition. Plant Stress 2011, 5, 32–41. [Google Scholar]
- Saud, S.; Fahad, S.; Yajun, C.; Ihsan, M.Z.; Hammad, H.M.; Nasim, W.; Amanullah; Arif, M.; Alharby, H. Effects of Nitrogen Supply on Water Stress and Recovery Mechanisms in Kentucky Bluegrass Plants. Front. Plant Sci. 2017, 8, 983. [Google Scholar] [CrossRef] [PubMed]
- Nye, P.H.; Tinker, P.B. Solute Movement in the Soil-Root System; Studies in Ecology; University of California Press: Oakland, CA, USA, 1977; ISBN 9780520034518. [Google Scholar]
- Peuke, A.D.; Rennenberg, H. Carbon, Nitrogen, Phosphorus, and Sulphur Concentration and Partitioning in Beech Ecotypes (Fagus sylvatica L.): Phosphorus Most Affected by Drought. Trees 2004, 18, 639–648. [Google Scholar] [CrossRef]
- Tariq, A.; Pan, K.; Olatunji, O.A.; Graciano, C.; Li, Z.; Sun, F.; Zhang, L.; Wu, X.; Chen, W.; Song, D.; et al. Phosphorous Fertilization Alleviates Drought Effects on Alnus Cremastogyne by Regulating Its Antioxidant and Osmotic Potential. Sci. Rep. 2018, 8, 5644. [Google Scholar] [CrossRef] [PubMed]
- Rausch, C.; Bucher, M. Molecular Mechanisms of Phosphate Transport in Plants. Planta 2002, 216, 23–37. [Google Scholar] [CrossRef]
- Goll, D.S.; Joetzjer, E.; Huang, M.; Ciais, P. Low Phosphorus Availability Decreases Susceptibility of Tropical Primary Productivity to Droughts. Geophys. Res. Lett. 2018, 45, 8231–8240. [Google Scholar] [CrossRef]
- Grossman, A.R.; Takahashi, H. Macronutrient Utilization by Photosynthetic Eukaryotes and the Fabric of Interactions. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 163–210. [Google Scholar] [CrossRef]
- Grzebisz, W.; Gransee, A.; Szczepaniak, W.; Diatta, J. The Effects of Potassium Fertilization on Water-Use Efficiency in Crop Plants. J. Plant Nutr. Soil Sci. 2013, 176, 355–374. [Google Scholar] [CrossRef]
- Martineau, E.; Domec, J.C.; Bosc, A.; Denoroy, P.; Fandino, V.A.; Lavres, J.; Jordan-Meille, L. The Effects of Potassium Nutrition on Water Use in Field-Grown Maize (Zea mays L.). Environ. Exp. Bot. 2017, 134, 62–71. [Google Scholar] [CrossRef]
- Leberecht, M.; Dannenmann, M.; Tejedor, J.; Simon, J.; Rennenberg, H.; Polle, A. Segregation of Nitrogen Use between Ammonium and Nitrate of Ectomycorrhizas and Beech Trees. Plant. Cell Environ. 2016, 39, 2691–2700. [Google Scholar] [CrossRef]
- Nikolova, P.S.; Bauerle, T.L.; Häberle, K.H.; Blaschke, H.; Brunner, I.; Matyssek, R. Fine-Root Traits Reveal Contrasting Ecological Strategies in European Beech and Norway Spruce During Extreme Drought. Front. Plant Sci. 2020, 11, 1211. [Google Scholar] [CrossRef] [PubMed]
- Bagherzadeh, A.; Brumme, R.; Beese, F. Biomass and Nutrients Allocation in Pot Cultured Beech Seedlings: Influence of Nitrogen Fertilizer. J. For. Res. 2008, 19, 263–270. [Google Scholar] [CrossRef]
- Jacobs, D.F.; Salifu, K.F.; Seifert, J.R. Relative Contribution of Initial Root and Shoot Morphology in Predicting Field Performance of Hardwood Seedlings. New For. 2005, 30, 235–251. [Google Scholar] [CrossRef]
- Hagedorn, F.; Joseph, J.; Peter, M.; Luster, J.; Pritsch, K.; Geppert, U.; Kerner, R.; Molinier, V.; Egli, S.; Schaub, M.; et al. Recovery of Trees from Drought Depends on Belowground Sink Control. Nat. Plants 2016, 2, 16111. [Google Scholar] [CrossRef] [PubMed]
- Brunner, I.; Herzog, C.; Dawes, M.A.; Arend, M.; Sperisen, C. How Tree Roots Respond to Drought. Front. Plant Sci. 2015, 6, 547. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Wahid, A.; Siddique, K. Drought Stress in Plants: An Overview. In Plant Responses to Drought Stress; From Morphological to Molecular Features; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–33. ISBN 978-3-642-32652-3. [Google Scholar]
- Lauri, P.-É.; Marceron, A.; Normand, F.; Dambreville, A.; Regnard, J.-L. Soil Water Deficit Decreases Xylem Conductance Efficiency Relative to Leaf Area and Mass in the Apple. J. Plant Hydraul. 2014, 1, e0003. [Google Scholar] [CrossRef]
- Chen, Z.; Li, S.; Wan, X.; Liu, S. Strategies of Tree Species to Adapt to Drought from Leaf Stomatal Regulation and Stem Embolism Resistance to Root Properties. Front. Plant Sci. 2022, 13, 926535. [Google Scholar] [CrossRef] [PubMed]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK, 2012; ISBN 9780123849052. [Google Scholar]
- Guasconi, D.; Manzoni, S.; Hugelius, G. Climate-Dependent Responses of Root and Shoot Biomass to Drought Duration and Intensity in Grasslands–a Meta-Analysis. Sci. Total Environ. 2023, 903, 166209. [Google Scholar] [CrossRef]
- Greenwood, D.J. Quantitative Theory and the Control of Soil Fertility. New Phytol. 1983, 94, 1–18. [Google Scholar] [CrossRef]
- van Hees, A. Growth and Morphology of Pedunculate Oak (Quercus robur L.) and Beech (Fagus sylvatica L.) Seedlings in Relation to Shading and Drought. Ann. Sci. For. 1997, 54, 9–18. [Google Scholar] [CrossRef]

| N | P | K | |
|---|---|---|---|
| WH | −0.64 * | 0.20 ns | 1.36 ** |
| DH | −1.04 *** | 0.14 ns | −0.10 ns |
| WL | 0.59 * | 0.25 * | 0.98 * |
| DL | −0.54 ns | 0.24 * | −0.34 ns |
| WH | DH | WL | DL | |
|---|---|---|---|---|
| N (mg/g) | 16.71 ± 0.53 a | 17.66 ± 0.53 a | 14.73 ± 0.42 b | 14.80 ± 0.42 b |
| P (mg/g) | 2.09 ± 0.11 a | 1.93 ± 0.09 ab | 1.75 ± 0.08 bc | 1.45 ± 0.06 c |
| K (mg/g) | 7.91 ± 0.22 a | 7.74 ± 0.23 a | 7.41 ± 0.25 a | 6.48 ± 0.18 b |
| WH | DH | WL | DL | |
|---|---|---|---|---|
| hstart (cm) | 16.17 ± 3.28 a | 16.71 ± 4.06 ab | 17.26 ± 3.89 b | 16.80 ± 3.34 ab |
| dstart (mm) | 3.46 ± 0.82 a | 3.56 ± 0.91 ab | 3.69 ± 0.86 bc | 3.68 ± 0.80 c |
| hend (cm) | 52.22 ± 16.86 a | 46.89 ± 13.71 b | 50.84 ± 16.17 a | 45.27 ± 12.20 b |
| dend (mm) | 8.62 ± 1.89 a | 7.80 ± 1.36 bc | 8.21 ± 1.77 b | 7.56 ± 1.31 c |
| Parameters | Irrigation | Fertilisation | Irrigation × Fertilisation | |
|---|---|---|---|---|
| Dry biomass | TB | 7.45 ** | 0.75 ns | 5.25 * |
| AGB | 12.44 *** | 3.02 *** | 6.66 * | |
| LB | 6.27 * | 2.11 ns | 11.23 ** | |
| SB | 14.14 *** | 3.10 ns | 4.42 * | |
| BGB | 3.23 ns | 0.06 ns | 3.69 ns | |
| CRB | 4.78 * | 0.01 ns | 3.82 ns | |
| FRB | 0.04 ns | 0.26 ns | 0.90 ns | |
| Allometric relationships | BGB/AGB | 9.35 ** | 9.35 ** | 0.25 ns |
| CRB/SB | 5.55 * | 6.02 * | 0.46 ns | |
| FRB/LB | 5.48 * | 5.84 * | 8.30 ** | |
| Parameters | WH | DH | WL | DL | |
|---|---|---|---|---|---|
| Dry biomass | TB | 27.30 ± 1.68 a | 19.7 ± 1.07 b | 22.5 ± 1.72 ab | 21.8 ± 1.5 ab |
| AGB | 14.80 ± 0.89 a | 9.9 ± 0.51 b | 11.3 ± 0.87 b | 10.5 ± 0.88 b | |
| LB | 4.5 ± 0.22 a | 3.0 ± 0.21 b | 3.3 ± 0.28 b | 3.5 ± 0.28 b | |
| SB | 10.03 ± 0.70 a | 6.8 ± 0.35 b | 8 ± 0.61 b | 7 ± 0.63 b | |
| BGB | 12.50 ± 0.90 a | 9.8 ± 0.65 b | 11.2 ± 0.9 ab | 11.3 ± 0.71 ab | |
| CRB | 8.6 ± 0.69 a | 6.2 ± 0.37 b | 7.4 ± 0.67 ab | 7.3 ± 0.46 ab | |
| FRB | 3.90 ± 0.29 a | 3.6 ± 0.31 a | 3.8 ± 0.3 a | 4 ± 0.31 a | |
| Allometric relationship | BGB/AGB | 0.86 ± 0.22 a | 1.02 ± 0.26 ab | 1.02 ± 0.17 ab | 1.11 ± 0.24 b |
| CRB/SB | 0.84 ± 0.25 a | 0.94 ± 0.25 ab | 0.94 ± 0.24 ab | 1.11 ± 0.32 b | |
| FRB/LB | 0.88 ± 0.28 a | 1.21 ± 0.34 b | 1.21 ± 0.34 b | 1.18 ± 0.25 c | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marušić, M.; Seletković, I.; Ognjenović, M.; Jonard, M.; Sever, K.; Schaub, M.; Gessler, A.; Šango, M.; Sirovica, I.; Zegnal, I.; et al. Nutrient and Growth Response of Fagus sylvatica L. Saplings to Drought Is Modified by Fertilisation. Forests 2023, 14, 2445. https://doi.org/10.3390/f14122445
Marušić M, Seletković I, Ognjenović M, Jonard M, Sever K, Schaub M, Gessler A, Šango M, Sirovica I, Zegnal I, et al. Nutrient and Growth Response of Fagus sylvatica L. Saplings to Drought Is Modified by Fertilisation. Forests. 2023; 14(12):2445. https://doi.org/10.3390/f14122445
Chicago/Turabian StyleMarušić, Mia, Ivan Seletković, Mladen Ognjenović, Mathieu Jonard, Krunoslav Sever, Marcus Schaub, Arthur Gessler, Mario Šango, Ivana Sirovica, Ivana Zegnal, and et al. 2023. "Nutrient and Growth Response of Fagus sylvatica L. Saplings to Drought Is Modified by Fertilisation" Forests 14, no. 12: 2445. https://doi.org/10.3390/f14122445
APA StyleMarušić, M., Seletković, I., Ognjenović, M., Jonard, M., Sever, K., Schaub, M., Gessler, A., Šango, M., Sirovica, I., Zegnal, I., Bogdanić, R., & Potočić, N. (2023). Nutrient and Growth Response of Fagus sylvatica L. Saplings to Drought Is Modified by Fertilisation. Forests, 14(12), 2445. https://doi.org/10.3390/f14122445





