Natural Physiological Changes on Overwintering and Spring Recovery of Needles of Pinus densiflora Siebold & Zucc.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Photosynthesis Parameters
2.3. Photosynthetic Pigment Content and Protein Content
2.4. Water Content and Cell Sap Concentration
2.5. Soluble Sugar Content and MDA Content
2.6. Plasma Membrane Permeability
2.7. Data Processing
3. Results
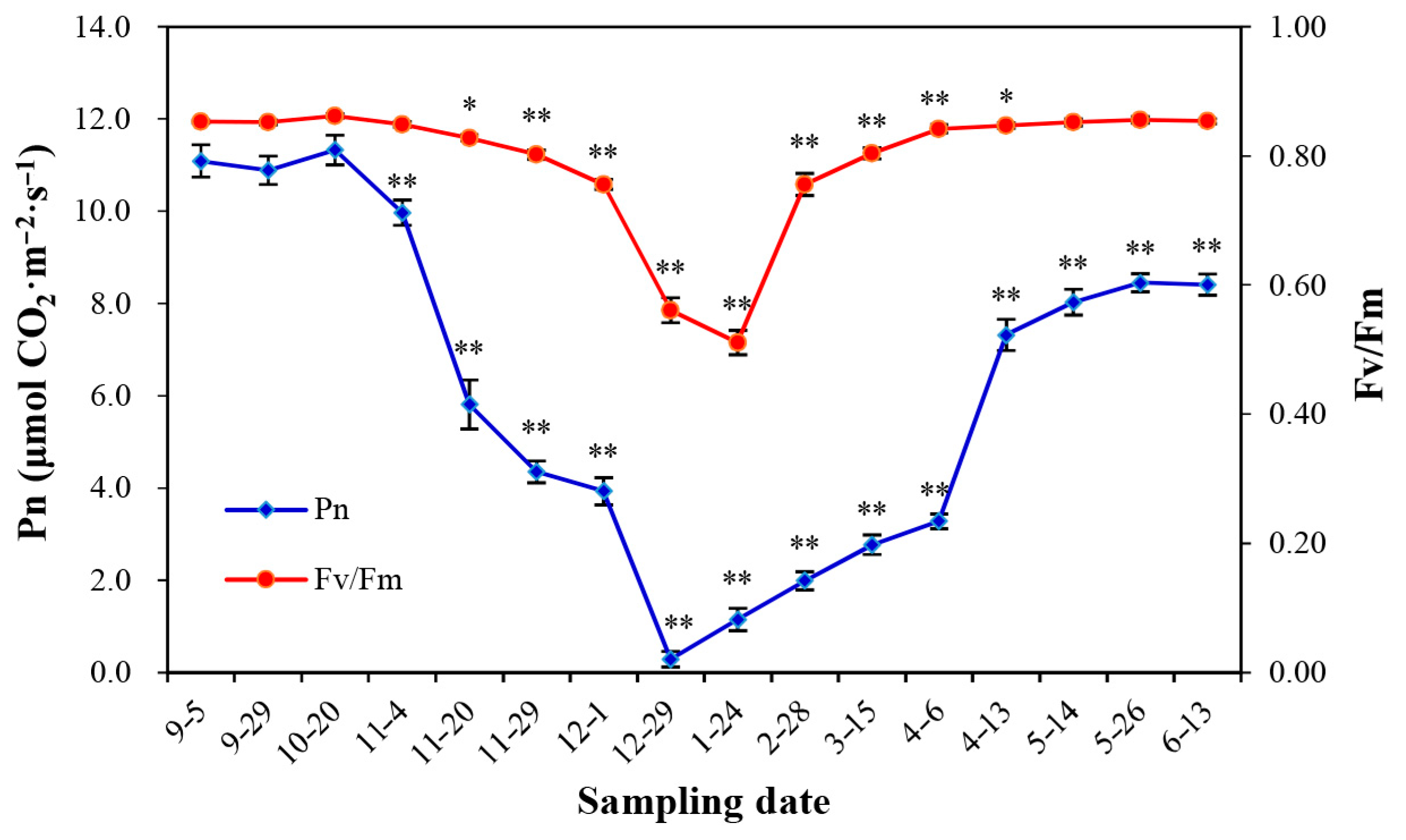
3.1. Natural Changes in Photosynthesis
3.2. Natural Changes in Photosynthetic Pigment Content
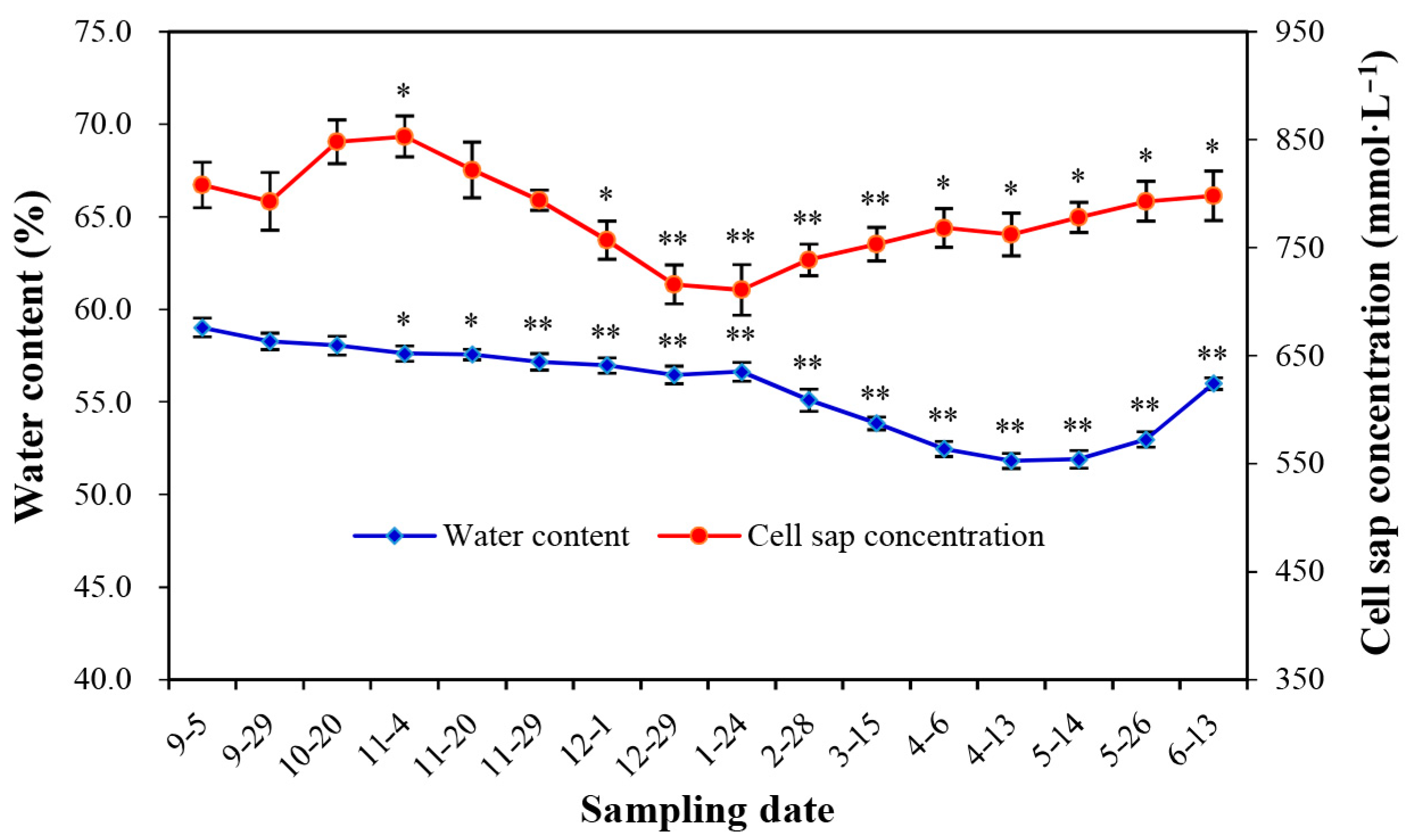
3.3. Natural Changes in Water Content
3.4. Injury and Recovery of Plasma Membranes
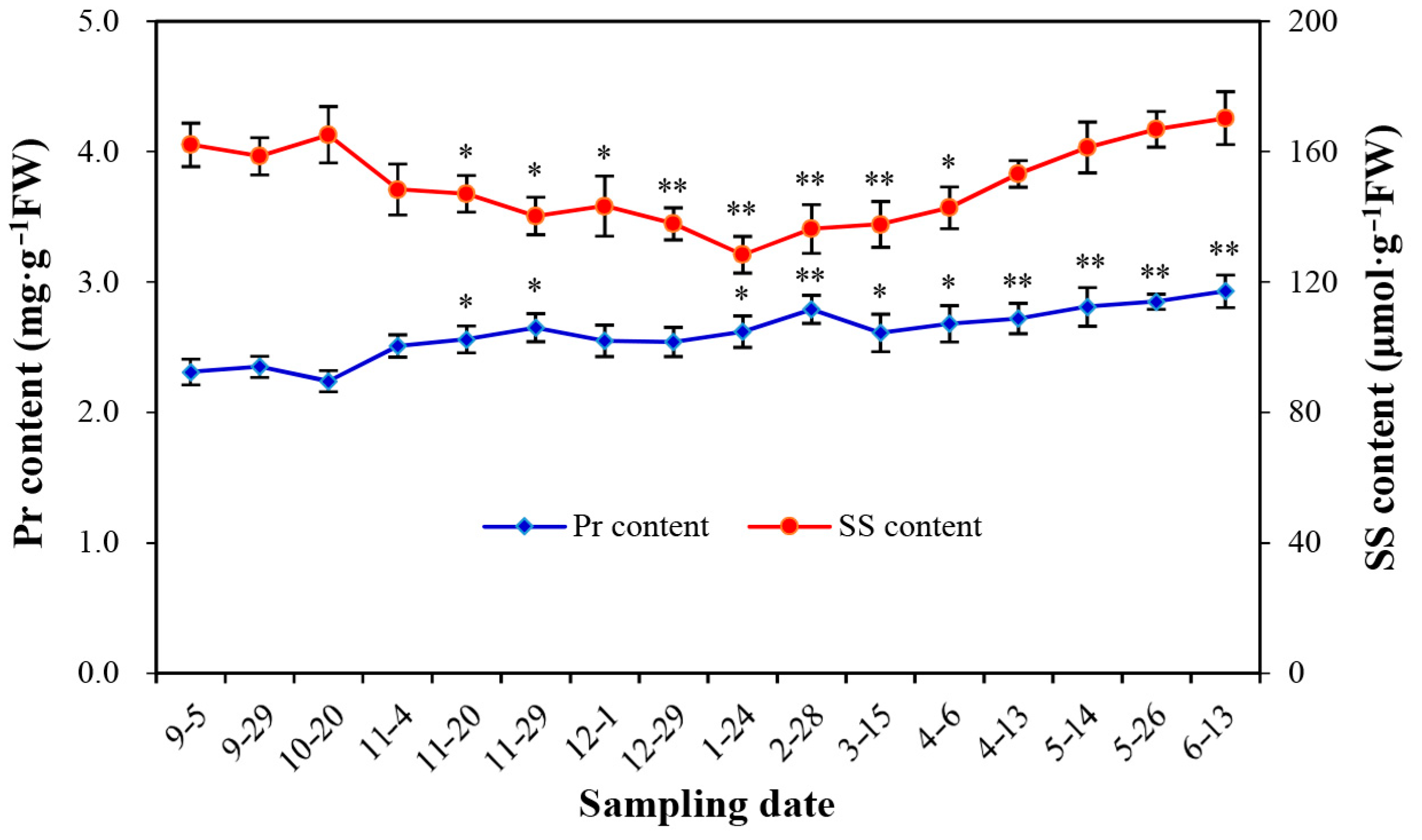
3.5. Natural Changes of Soluble Protein (Pr) Content and Soluble Sugar (SS) Content
4. Discussion
4.1. Damage and Recovery of P. densiflora Needles under Low Temperature
4.2. Defense Mechanism of P. densiflora against Low Temperature
4.3. Mechanism of Spring Recovery of Needles
4.4. Ecological Significance of Overwintering and Spring Recovery of P. densiflora
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, Q.; Kawasaki, T.; Nakano, T.; Chiba, Y. Leaf-age effects on seasonal variability in photosynthetic parameters and its relationships with leaf mass per area and leaf nitrogen concentration within a Pinus densiflora crown. Tree Physiol. 2008, 28, 551–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Hayes, D. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [Green Version]
- Pearce, R.S. Plant freezing and damage. Ann. Bot. 2001, 87, 417–424. [Google Scholar] [CrossRef]
- Thalhammer, A.; Bryant, G.; Sulpice, R.; Hincha, D.K. Disordered cold regulated 15 proteins protect chloroplast membranes during freezing through binding and folding, but do not stabilize chloroplast enzymes in vivo. Plant Physiol. 2014, 166, 190–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.; Shi, Y.; Yang, S. Advances and challenges in uncovering cold tolerance regulatory mechanisms in plants. New Phytol. 2019, 222, 1690–1704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bremer, A.; Kent, B.; Hauss, T.; Thalhammer, A.; Yepuri, N.R.; Darwish, T.A.; Garvey, C.J.; Bryant, G.; Hincha, D.K. Intrinsically disordered stress protein COR15A resides at the membrane surface during dehydration. Biophys. J. 2017, 113, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, N.; Farres, J.; Bailey, J.E.; Kallio, P.T. Targeted expression of a synthetic codon optimized gene, encoding the spruce budworm antifreeze protein, leads to accumulation of antifreeze activity in the apoplasts of transgenic tobacco. Gene 2001, 275, 115–124. [Google Scholar] [CrossRef]
- Zhang, C.Z.; Fei, S.Z.; Arora, R.; Hannapel, D.J. Ice recrystallization inhibition proteins of perennial ryegrass enhance freezing tolerance. Planta 2010, 232, 155–164. [Google Scholar] [CrossRef]
- Davies, P.L. Ice-binding proteins: A remarkable diversity of structures for stop-ping and starting ice growth. Trends Biochem. Sci. 2014, 39, 548–555. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Bucci, S.J.; Arias, N.S.; Scholz, F.G.; Hao, G.Y.; Cao, K.F.; Goldstein, G. Freezing resistance in Patagonian woody shrubs: The role of cell wall elasticity and stem vessel size. Tree Physiol. 2016, 36, 1007–1018. [Google Scholar] [CrossRef]
- Ployet, R.; Soler, M.; Carocha, V.; Ladouce, N.; Alves, A.; Rodrigues, J.; Harvengt, L.; Marque, C.; Teulières, C.; Grima-Pettenati, J.; et al. Long cold exposure induces transcriptional and biochemical remodelling of xylem secondary cell wall in Eucalyptus. Tree Physiol. 2017, 38, 409–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willick, I.R.; Takahashi, D.; Fowler, D.B.; Uemura, M.; Tanino, K.K. Tissue-specific changes in apoplastic proteins and cell wall structure during cold acclimation of winter wheat crowns. J. Exp. Bot. 2018, 69, 1221–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ensminger, I.; Busch, F.; Huner, N.P.A. Photostasis and cold acclimation: Sensing low temperature through photosynthesis. Physiol. Plant. 2006, 126, 28–44. [Google Scholar] [CrossRef]
- Wang, F.; Chen, S.; Liang, D.; Qu, G.Z.; Chen, S.; Zhao, X. Transcriptomic analyses of Pinus koraiensis under different cold stresses. BMC Genom. 2020, 21, 10. [Google Scholar] [CrossRef] [Green Version]
- Öquist, G.; Hunerm, N.P.A. Photosynthesis of overwintering evergreen plants. Annu. Rev. Plant Biol. 2003, 54, 329–355. [Google Scholar] [CrossRef]
- Verhoeven, A. Sustained energy dissipation in winter evergreens. New Phytol. 2013, 201, 57–65. [Google Scholar] [CrossRef]
- Grebe, S.; Trotta, A.; Bajwa, A.A.; Mancini, I.; Aro, E.M. Specific thylakoid protein phosphorylations are prerequisites for overwintering of Norway spruce (Picea abies) photosynthesis. Proc. Natl. Acad. Sci. USA 2020, 117, 17499–17509. [Google Scholar] [CrossRef]
- Mandre, M.; Tullus, H.; Klõšeiko, J. Partitioning of carbohydrates and biomass of needles in Scots pine canopy. Z. Naturforsch. C 2002, 57, 296–302. [Google Scholar] [CrossRef]
- Henson, C.A.; Duke, S.H.; Livingston, D.P. Metabolic changes in Avena sativa crowns recovering from freezing. PLoS ONE 2014, 9, e93085. [Google Scholar] [CrossRef]
- Vyse, K.; Penzlin, J.; Sergeant, K.; Hincha, D.K.; Arora, R.; Zuther, E. Repair of sub-lethal freezing damage in leaves of Arabidopsis thaliana. BMC Plant Biol. 2020, 20, 35. [Google Scholar] [CrossRef]
- Bag, P.; Chukhutsina, V.; Zhang, Z.; Paul, S.; Jansson, S. Direct energy transfer from photosystem II to photosystem I confers winter sustainability in Scots pine. Nat. Commun. 2020, 11, 6388. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Arora, R. Understanding the cellular mechanism of recovery from freeze–thaw injury in spinach: Possible role of aquaporins, heat shock proteins, dehydrin and antioxidant system. Physiol. Plant. 2014, 150, 374–387. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.Y.S.; Gamon, J.A. The photochemical reflectance index provides an optical indicator of spring photosynthetic activation in evergreen conifers. New Phytol. 2015, 206, 196–208. [Google Scholar] [CrossRef]
- Wong, C.Y.S.; D’Odorico, P.; Bhathena, Y.; Arain, M.A.; Ensminger, I. Carotenoid based vegetation indices for accurate monitoring of the phenology of photosynthesis at the leaf-scale in deciduous and evergreen trees. Remote Sens. Environ. 2019, 233, 111407. [Google Scholar] [CrossRef]
- Ensminger, I.; Schmidt, L.; Lloyd, J. Soil temperature and intermittent frost modulate the rate of recovery of photosynthesis in Scots pine under simulated spring conditions. New Phytol. 2008, 177, 428–442. [Google Scholar] [CrossRef] [Green Version]
- Wallin, G.; Hall, M.; Slaney, M.; Räntfors, M.; Medhurst, J.; Linder, S. Spring photosynthetic recovery of boreal Norway spruce under conditions of elevated [CO2] and air temperature. Tree Physiol. 2013, 33, 1177–1191. [Google Scholar] [CrossRef] [Green Version]
- Porra, R.J. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth. Res. 2002, 73, 149–156. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Q.; Zhang, M.; Guo, Z.; Yang, J.; Mo, J.; Cui, J.; Hu, H.; Xu, J. Individual Cryptomeria fortunei hooibrenk clones show varying degrees of chilling stress resistance. Forests 2020, 11, 189. [Google Scholar] [CrossRef] [Green Version]
- Thomashow, M.F. Plant cold acclimation: Freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 571–599. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, H.; Yang, J.; Xue, J.; Xu, J. Physiological, transcriptomic and metabolomic analyses of overwintering Cryptomeria fortunei needles. Forests 2022, 13, 1249. [Google Scholar] [CrossRef]
- Lintunen, A.; Hölttä, T.; Kulmala, M. Anatomical regulation of ice nucleation and cavitation helps trees to survive freezing and drought stress. Sci. Rep. 2013, 3, 2031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willick, I.R.; Gusta, L.V.; Fowler, D.B.; Tanino, K.K. Ice segregation in the crown of winter cereals: Evidence for extraorgan and extratissue freezing. Plant Cell Environ. 2019, 42, 701–716. [Google Scholar] [CrossRef] [PubMed]
- Pagter, M.; Jensen, C.R.; Petersen, K.K.; Liu, F.; Arora, R. Changes in carbohydrates, ABA and bark proteins during seasonal cold acclimation and deacclimation in Hydrangea species differing in cold hardiness. Physiol. Plant. 2008, 134, 473–485. [Google Scholar] [CrossRef]
- Poirier, M.; Lacointe, A.; Améglio, T. A semi-physiological model of cold hardening and dehardening in walnut stem. Tree Physiol. 2010, 30, 1555–1569. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Yu, D.J.; Kim, S.J.; Choi, D.; Lee, H.J. Intraspecies differences in cold hardiness, carbohydrate content and β-amylase gene expression of Vaccinium corymbosum during cold acclimation and deacclimation. Tree Physiol. 2012, 32, 1533–1540. [Google Scholar] [CrossRef] [Green Version]
- Dhuli, P.; Rohloff, J.; Strimbeck, G.R. Metabolite changes in conifer buds and needles during forced bud break in Norway spruce (Picea abies) and European silver fir (Abies alba). Front. Plant Sci. 2014, 5, 706. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.; Oh, Y.; Kim, D. Differences in cold hardiness, carbohydrates, dehydrins and related gene expressions under an experimental deacclimation and reacclimation in Prunus persica. Physiol. Plant. 2015, 154, 485–499. [Google Scholar] [CrossRef]
- Andersen, U.B.; Kjaer, K.H.; Erban, A.; Alpers, J.; Hincha, D.K.; Kopka, J.; Zuther, E.; Pagter, M. Impact of seasonal warming on overwintering and spring phenology of blackcurrant. Environ. Exp. Bot. 2017, 140, 96–109. [Google Scholar] [CrossRef]
- Mayr, S.; Schmid, P.; Rosner, S. Winter embolism and recovery in the conifer shrub Pinus mugo L. Forests 2019, 10, 941. [Google Scholar] [CrossRef]
- Jankowski, A.; Wyka, T.P.; Zytkowiak, R.; Nihlgard, B.; Reich, P.B.; Oleksyn, J. Cold adaptation drives variability in needle structure and anatomy in Pinus sylvestris L. along a 1900 km temperate-boreal transect. Funct. Ecol. 2017, 31, 2212–2223. [Google Scholar] [CrossRef] [Green Version]
- Jankowski, A.; Wyka, T.P.; Zytkowiak, R.; Danusevicius, D.; Oleksyn, J. Does climate-related in situ variability of Scots pine (Pinus sylvestris L.) needles have a genetic basis? Evidence from common garden experiments. Tree Physiol. 2021, 39, 573–589. [Google Scholar] [CrossRef] [PubMed]
- Holliday, J.A.; Ralph, S.G.; White, R.; Bohlmann, J.; Aitken, S.N. Global monitoring of autumn gene expression within and among phenotypically divergent populations of Sitka spruce (Picea sitchensis). New Phytol. 2010, 178, 103–122. [Google Scholar] [CrossRef] [PubMed]
- Koag, M.C.; Fenton, R.D.; Wilkens, S.; Close, T.J. The binding of maize DHN1 to lipid vesicles. Gain of structure and lipid specificity. Plant. Physiol. 2003, 131, 309–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bremer, A.; Wolff, M.; Thalhammer, A.; Hincha, D.K. Folding of intrinsically disordered plant LEA proteins is driven by glycerol-induced crowding and the presence of membranes. FEBS J. 2017, 284, 919–936. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.Y.; Bräutigam, K.; Hüner, N.P.A.; Ensminger, I. Champions of winter survival: Cold acclimation and molecular regulation of cold hardiness in evergreen conifers. New Phytol. 2021, 229, 675–691. [Google Scholar] [CrossRef]
- Bigras, F.J.; Ryyppo, A.; Lindstrom, A.; Stattin, E. Cold acclimaion and deacclimation of shoots and roots of conifer seedlings. In Conifer Cold Hardiness; Bigras, F.J., Colombo, S.J., Eds.; Kluwer Academic: Dordrecht, The Netherlands, 2001; pp. 57–88. [Google Scholar] [CrossRef]
- Ensminger, I.; Sveshnikov, D.; Campbell, D.A.; Funk, C.; Jansson, S.; Lloyd, J.; Shibistova, O.; Öquist, G. Intermittent low temperatures constrain spring recovery of photosynthesis in boreal Scots pine forests. Glob. Chang. Biol. 2004, 10, 995–1008. [Google Scholar] [CrossRef]
- Bigras, F.J.; Bertrand, A. Responses of Picea mariana to elevated CO2 concentration during growth, cold hardening and dehardening: Phenology, cold tolerance, photosynthesis and growth. Tree Physiol. 2006, 26, 875–888. [Google Scholar] [CrossRef] [Green Version]
- Adams, W.W.; Zarter, C.R.; Ebbert, V.; Demmig-Adams, B. Photoprotective strategies of overwintering evergreens. BioScience 2004, 54, 41–49. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Cohu, C.M.; Muller, O.; Adams, W.W. Modulation of photosynthetic energy conversion efficiency in nature: From seconds to seasons. Photosynth. Res. 2012, 113, 75–88. [Google Scholar] [CrossRef]
- Talts, P.; Pärnik, T.; Gardeström, P.; Keerberg, O. Respiratory acclimation in Arabidopsis thaliana leaves at low temperature. J. Plant Physiol. 2004, 161, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Arora, R. Mechanism of freeze-thaw injury and recovery: A cool retrospective and warming up to new ideas. Plant Sci. 2018, 270, 301–313. [Google Scholar] [CrossRef]
- Merry, R.; Jerrard, J.; Frebault, J.; Verhoeven, A. A comparison of pine and spruce in recovery from winter stress; changes in recovery kinetics, and the abundance and phosphorylation status of photosynthetic proteins during winter. Tree Physiol. 2018, 37, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Piao, S.; Friedlingstein, P.; Ciais, P.; Viovy, N.; Demarty, J. Growing season extension and its impact on terrestrial carbon cycle in the Northern Hemisphere over the past 2 decades. Glob. Biogeochem. Cycles 2007, 21, GB3018. [Google Scholar] [CrossRef]
- Gamon, J.A.; Huemmrich, K.F.; Wong, C. A remotely sensed pigment index reveals photosynthetic phenology in evergreen conifers. Proc. Natl. Acad. Sci. USA 2016, 113, 13087–13092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanja, S.; Berninger, F.; Vesala, T.; Markkanen, T.; Hari, P.; Mäkelä, A.; Lloyd, J. Air temperature triggers the recovery of evergreen boreal forest photosynthesis in spring. Glob. Chang. Biol. 2003, 9, 1410–1426. [Google Scholar] [CrossRef]
- Sevanto, S.; Suni, T.; Pumpanen, J.; Grönholm; Kolari, P.; Nikinmaa, E.; Hari, P.; Vesala, T. Wintertime photosynthesis and water uptake in a boreal forest. Tree Physiol. 2006, 26, 749–757. [Google Scholar] [CrossRef] [Green Version]
- Randerson, J.T.; Field, C.B.; Fung, I.Y.; Tans, P.P. Increases in early season ecosystem uptake explain recent changes in the seasonal cycle of atmospheric CO2 at high northern latitudes. Geophys. Res. Lett. 1999, 26, 2765–2768. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, D.; Chao, E.; Deng, Y.; Chen, K.; Wang, Z.; Qiu, N.; Zhang, H. Natural Physiological Changes on Overwintering and Spring Recovery of Needles of Pinus densiflora Siebold & Zucc. Forests 2023, 14, 168. https://doi.org/10.3390/f14020168
Yue D, Chao E, Deng Y, Chen K, Wang Z, Qiu N, Zhang H. Natural Physiological Changes on Overwintering and Spring Recovery of Needles of Pinus densiflora Siebold & Zucc. Forests. 2023; 14(2):168. https://doi.org/10.3390/f14020168
Chicago/Turabian StyleYue, Dongxue, Erkun Chao, Yiheng Deng, Kerui Chen, Zhengning Wang, Nianwei Qiu, and Hongxia Zhang. 2023. "Natural Physiological Changes on Overwintering and Spring Recovery of Needles of Pinus densiflora Siebold & Zucc." Forests 14, no. 2: 168. https://doi.org/10.3390/f14020168






