Transcriptome Analysis of 5-Aminolevulinic Acid Contributing to Cold Tolerance in Tea Leaves (Camellia sinensis L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Collection
2.2. Measurement of Soluble Sugar, Soluble Protein, and Chlorophyll Contents
2.3. RNA Extraction, Library Construction, and RNA Sequencing
2.4. RNA-Seq Data Analysis
2.5. GO and KEGG Analysis
2.6. RT-PCR Analysis
2.7. Statistical Analyses
3. Results
3.1. Biochemical Attributes Respond Differently under T-A-4 Treatment and T-4 Treatment
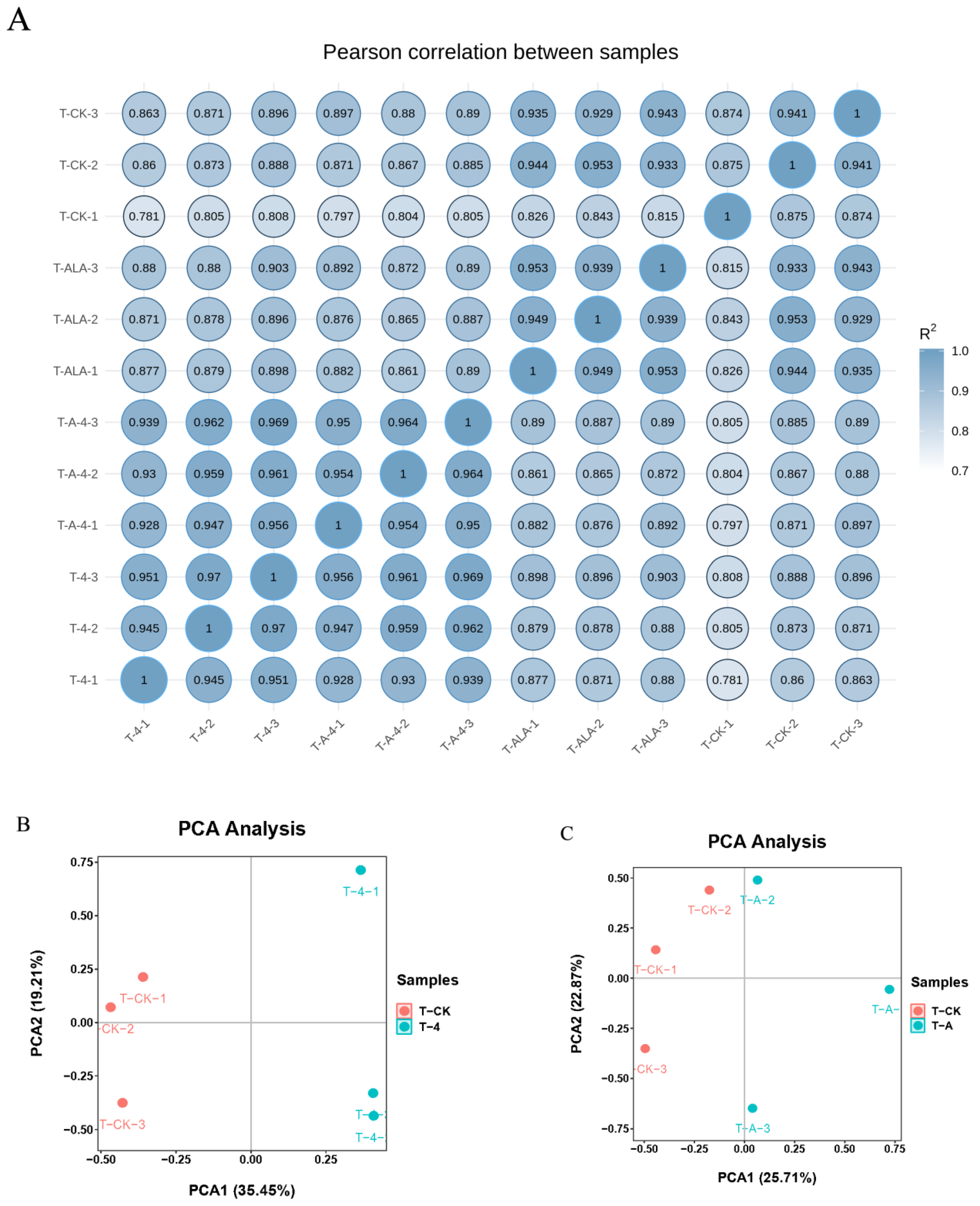
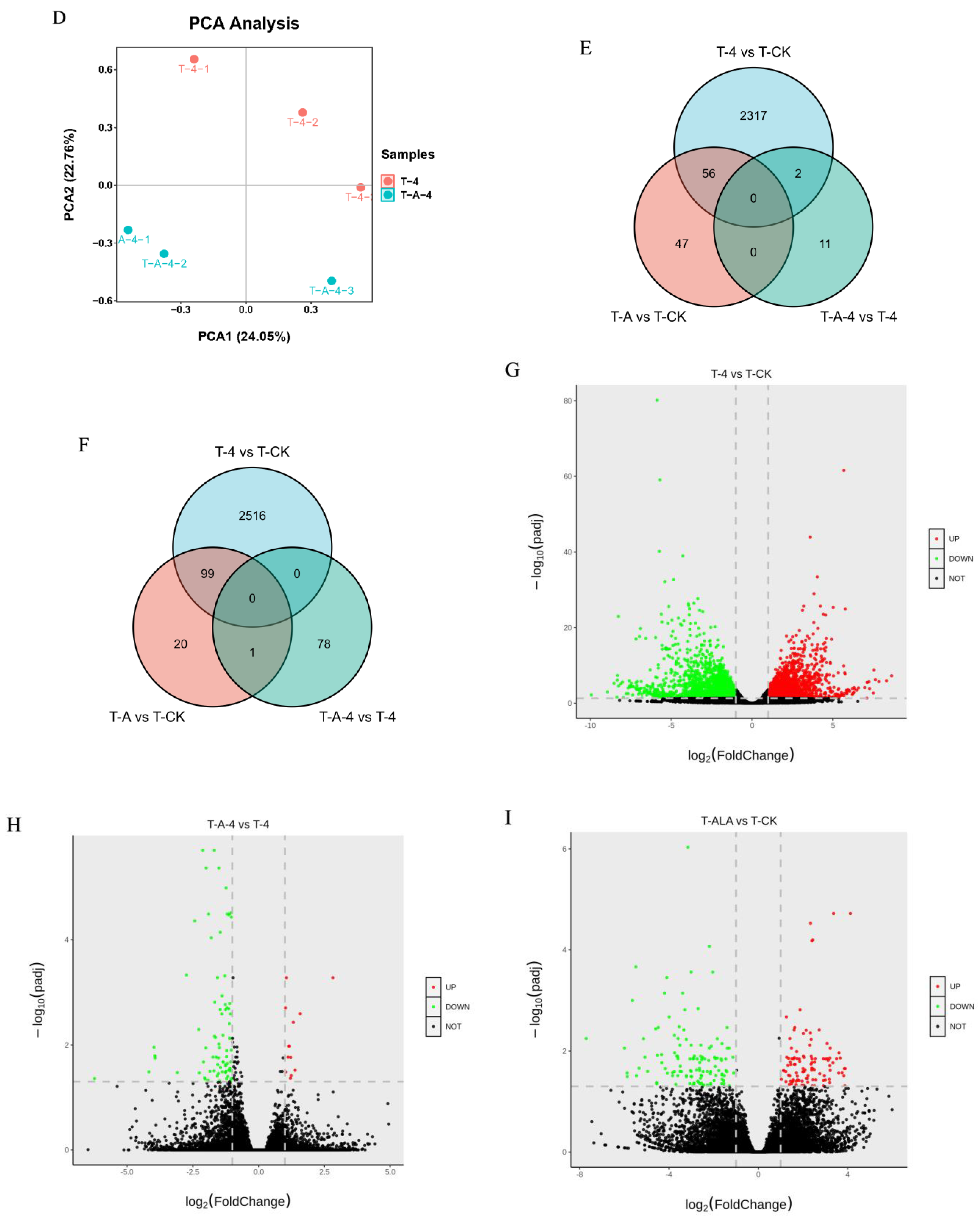
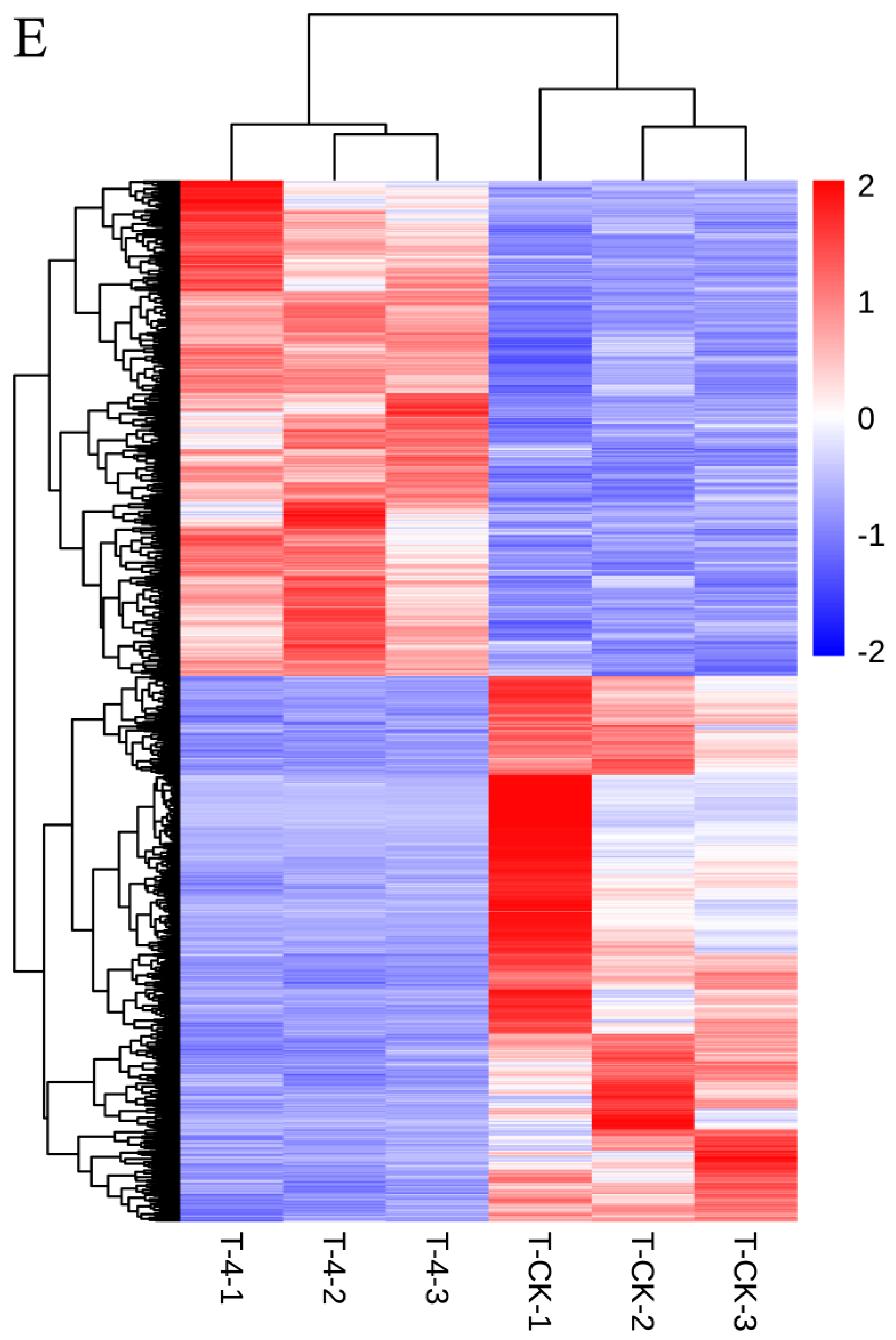
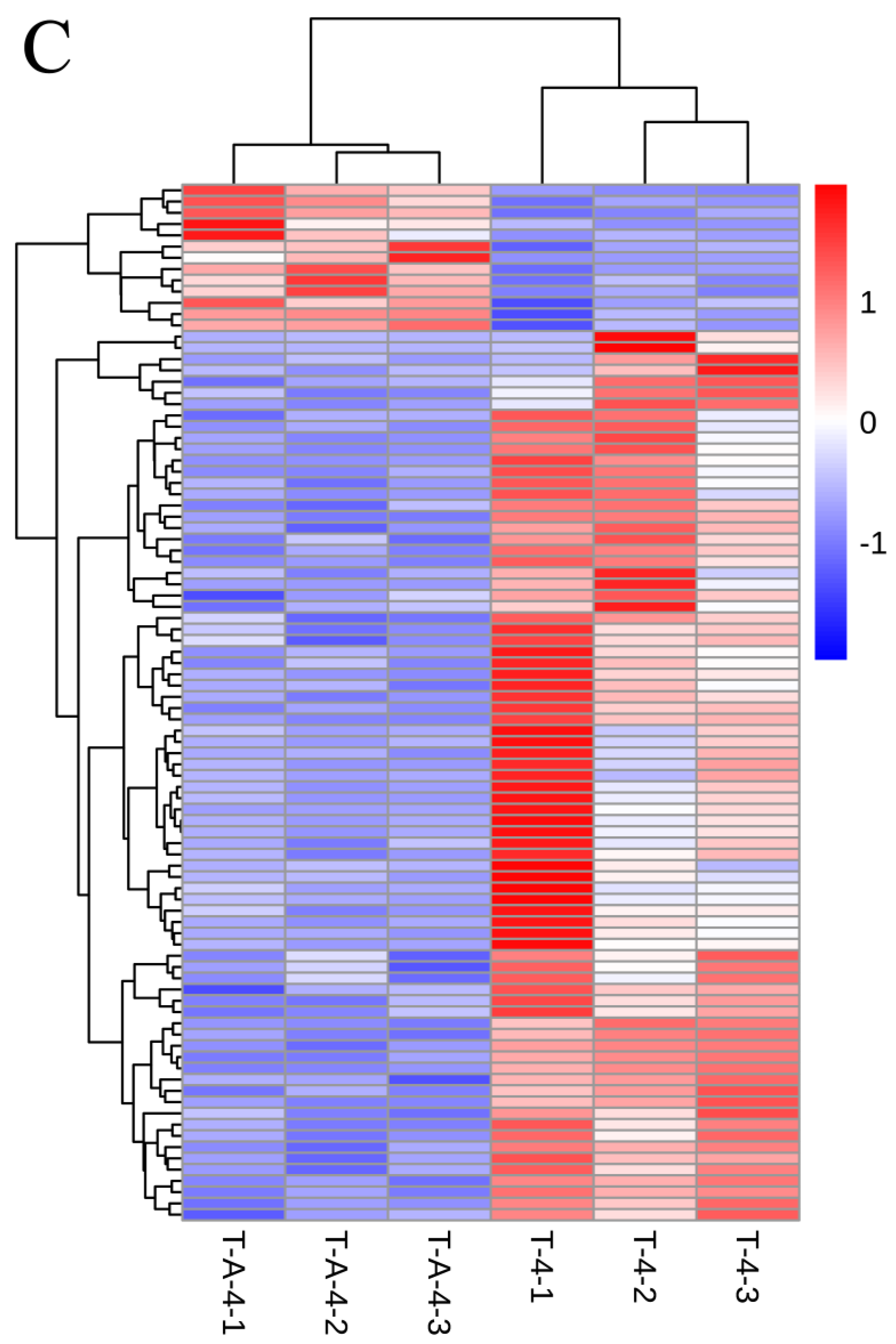
3.2. T-4 and T-A Treatment Induced Dramatical Variation in the Transcriptomic Patterns of Tea Leaves
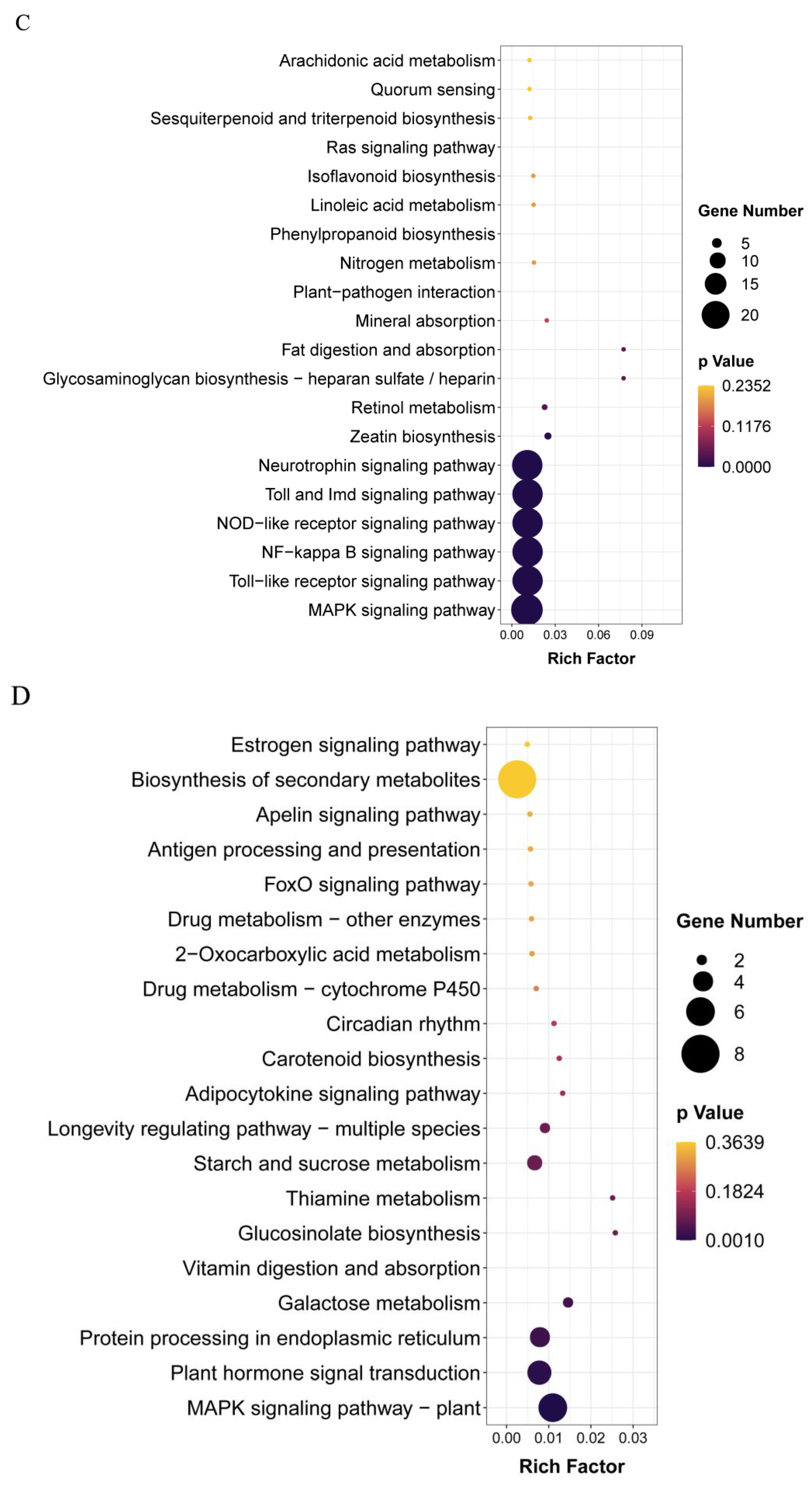
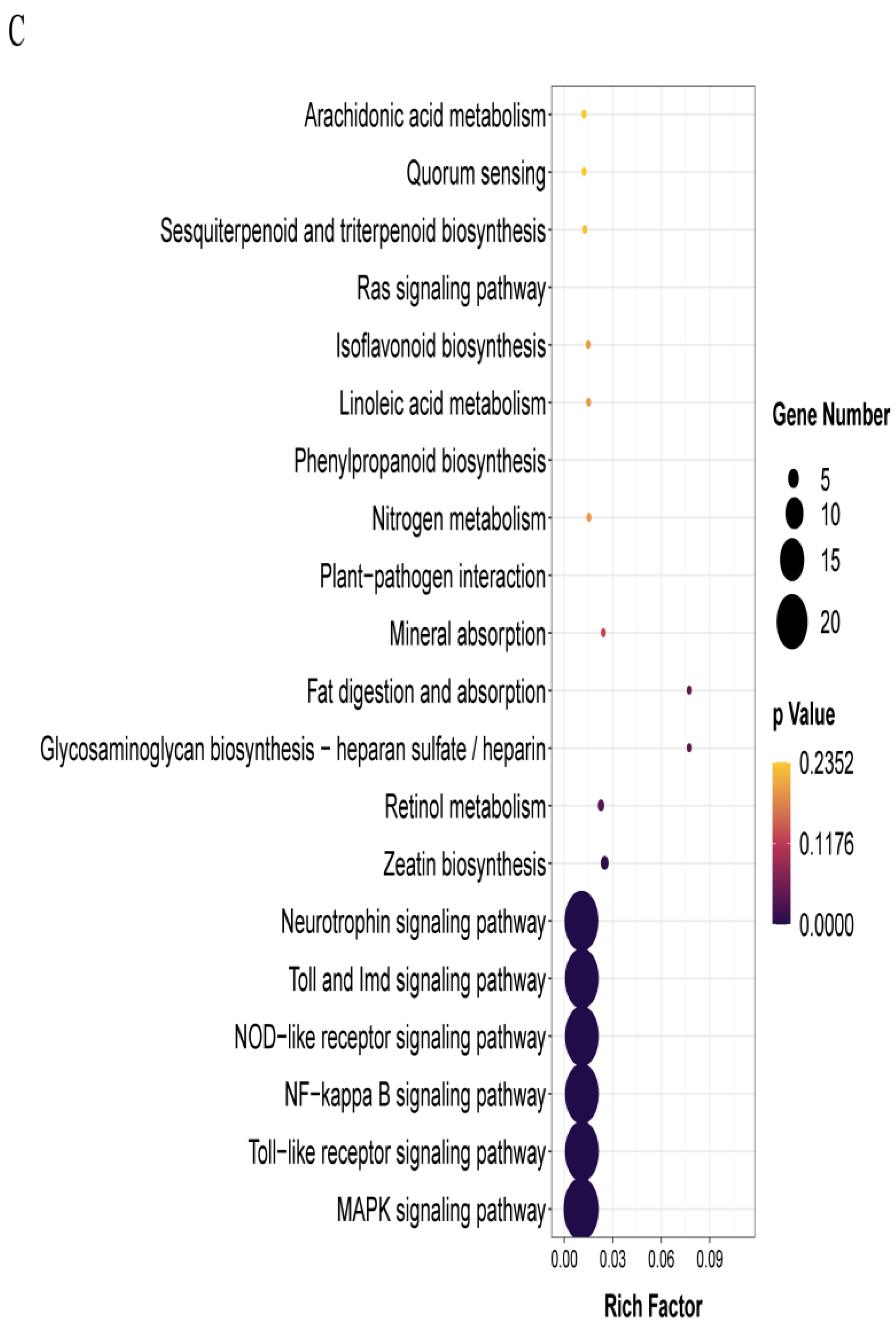
3.3. T-4 Treatment Mainly Altered Transcription Patterns of Genes Relevant to Membrane Signaling Pathway and Secondary Metabolites
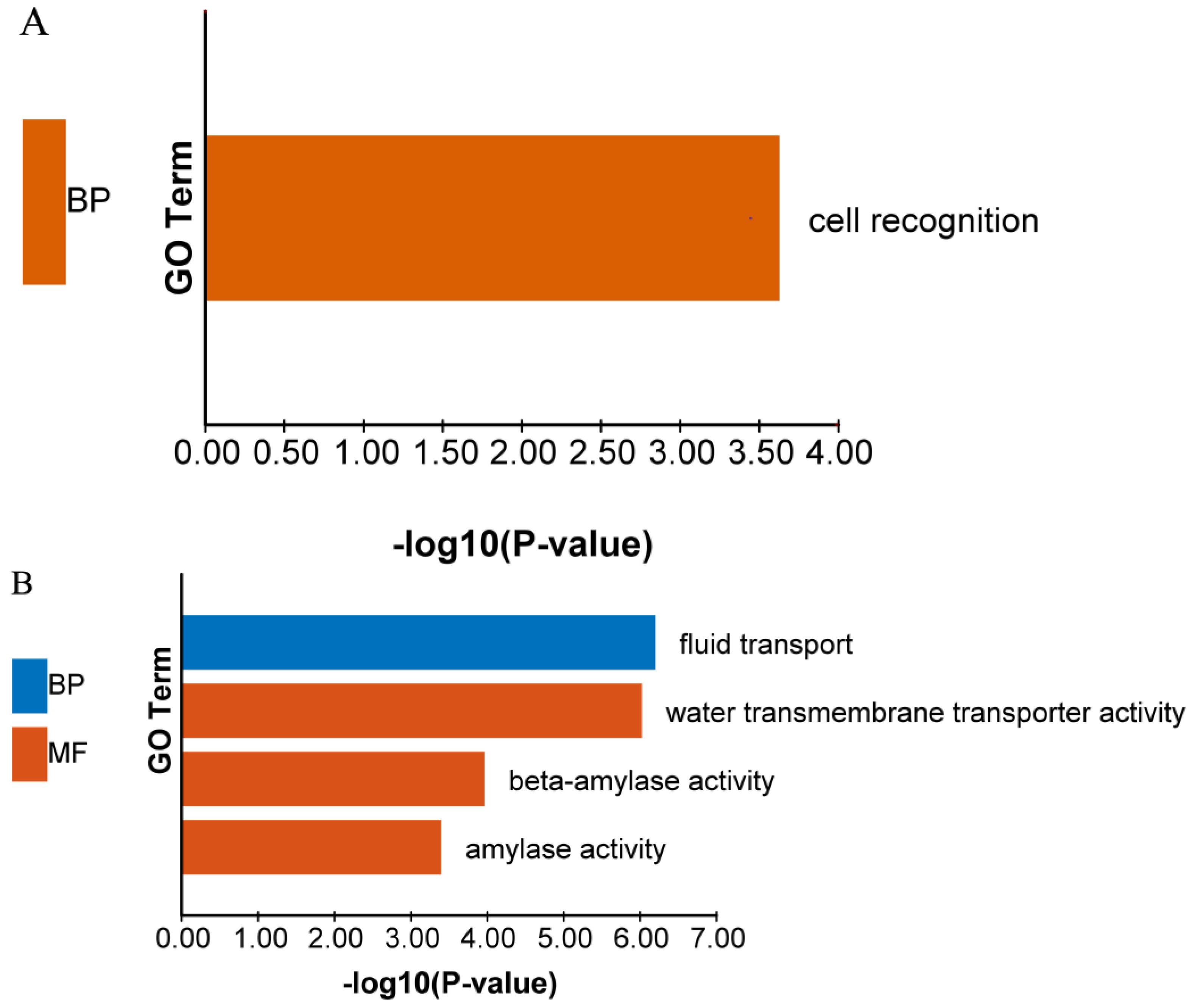
3.4. Exogenous ALA Spraying Triggered Abundant Transcription Fluctuation Related to MAPK Signaling Pathway and Membrane Transport
3.5. ALA Pretreatment Can Induce Up-Regulated Expression Genes of Diterpenoid Biosynthesis and Down-Regulated Expression Genes of Glutathione Metabolism
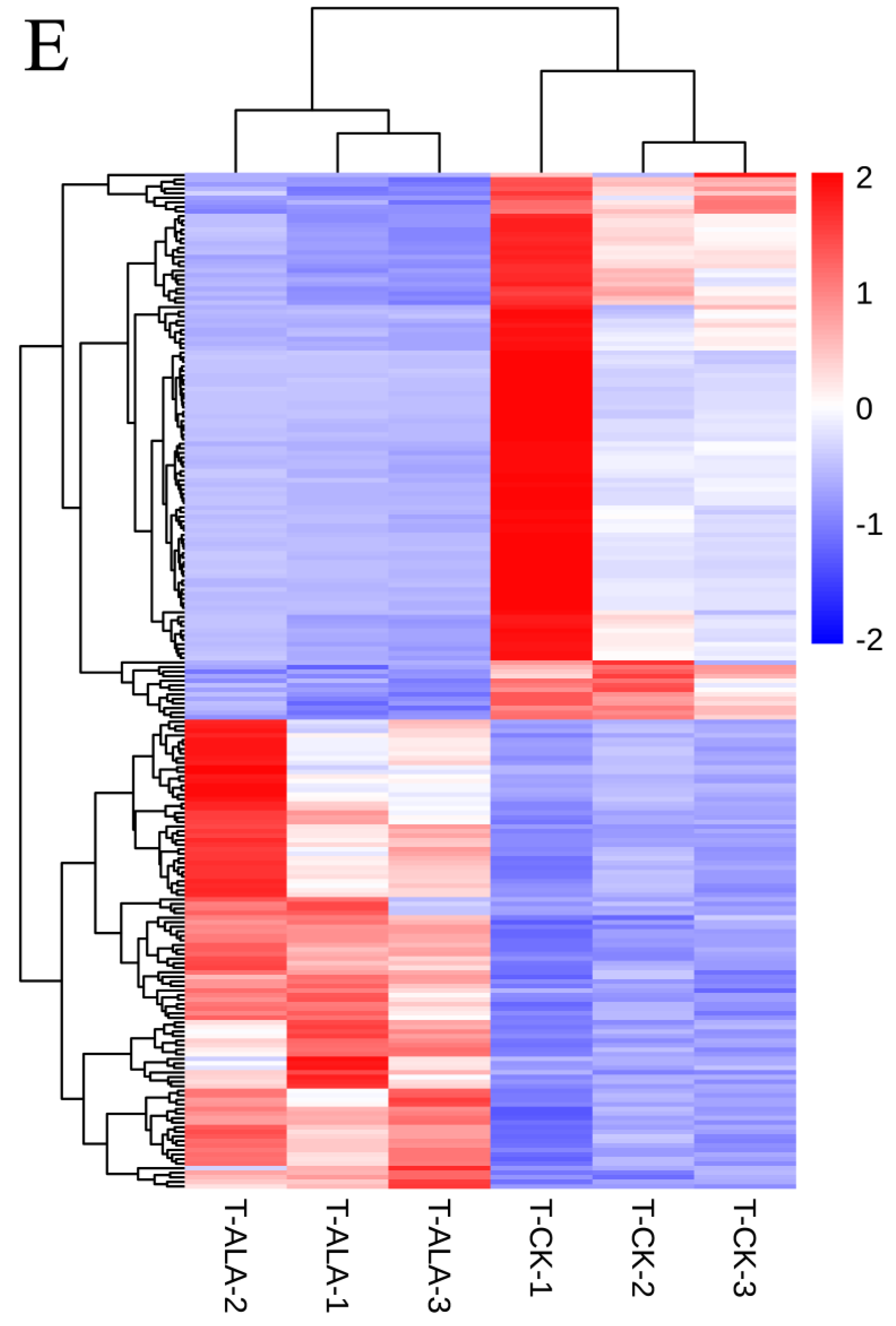
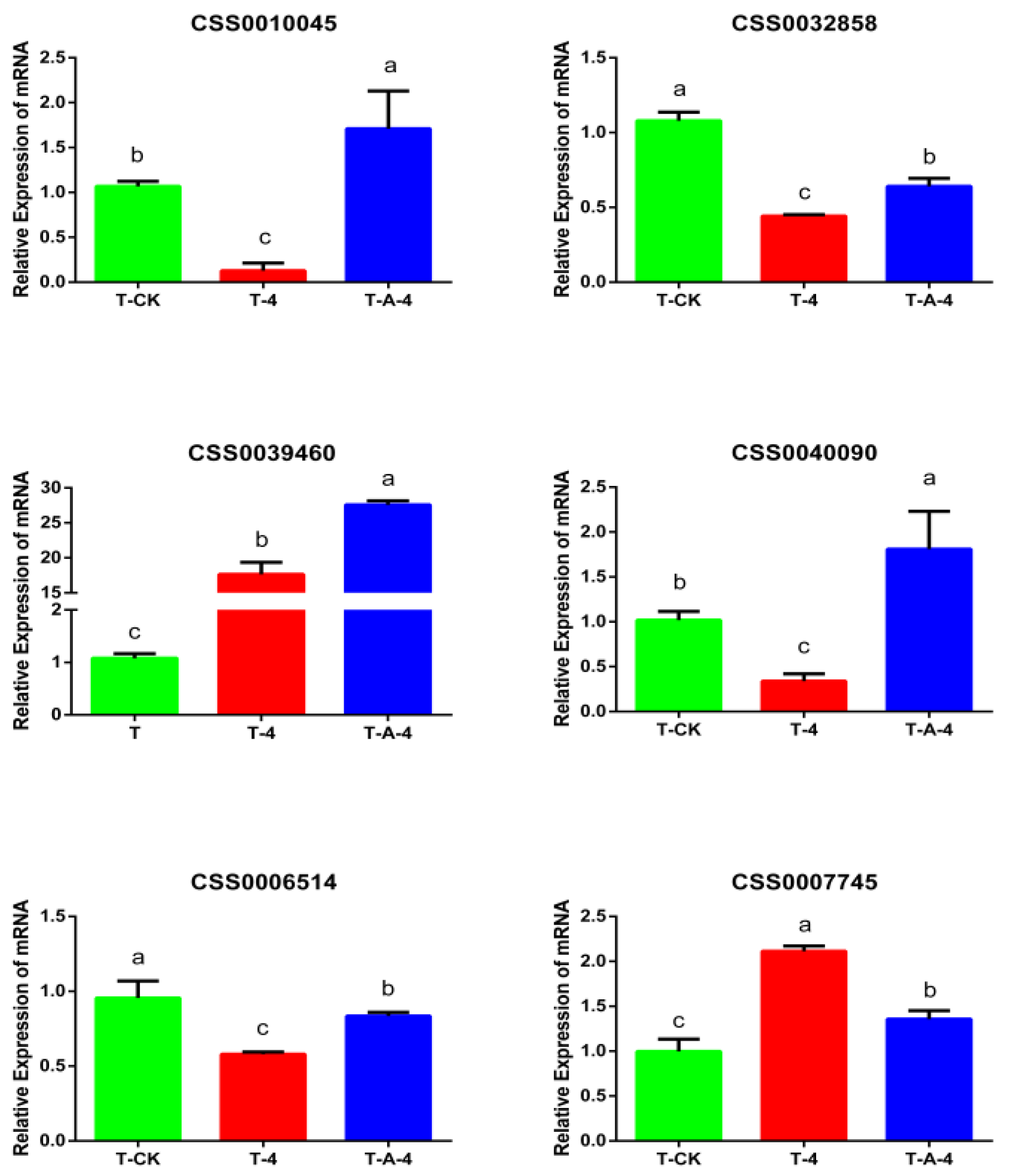
3.6. Comparisons of Gene Expression Patterns of Tea Leaves in T-CK, T-4 and T-A-4 Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kanwar, J.; Taskeen, M.; Mohammad, I.; Huo, C.; Chan, T.H.; Dou, Q.P. Recent advances on tea polyphenols. Front. Biosci. (Elite Ed.) 2012, 4, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Xia, E.H.; Tong, W.; Wu, Q.; Wei, S.; Zhao, J.; Zhang, Z.Z.; Wei, C.L.; Wan, X.C. Tea plant genomics: Achievements, challenges and perspectives. Hortic. Res. 2020, 7, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeed, M.; Naveed, M.; Arif, M.; Akar, M.U.; Manzoor, R.; Abd El-Hack, M.E.; Alagawany, M.; Tiwari, R.; Khandia, R.; Munjal, A.; et al. Green tea (Camellia sinensis) and l-theanine: Medicinal values and beneficial applications in humans—A comprehensive review. Biomed. Pharmacother. 2017, 95, 1260–1275. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Mukhtar, H. Tea Polyphenols in Promotion of Human Health. Nutrients 2018, 11, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancini, E.; Beglinger, C.; Drewe, J.; Zanchi, D.; Lang, U.E.; Borgwardt, S. Green tea effects on cognition, mood and human brain function: A systematic review. Phytomedicine 2017, 34, 26–37. [Google Scholar] [CrossRef] [Green Version]
- Kochman, J.; Jakubczyk, K.; Antoniewicz, J.; Mruk, H.; Janda, K. Health Benefits and Chemical Composition of Matcha Green Tea: A Review. Molecules 2020, 26, 85. [Google Scholar] [CrossRef]
- Hayat, K.; Iqbal, H.; Malik, U.; Bilal, U.; Mushtaq, S. Tea and its consumption: Benefits and risks. Crit. Rev. Food Sci. Nutr. 2015, 55, 939–954. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, H.; Zhang, D.; Li, J.; Xiong, H.; Yu, J.; Li, J.; Rashid, M.A.; Li, G.; Ma, X.; et al. Genetic analysis of cold tolerance at the germination and booting stages in rice by association mapping. PLoS ONE 2015, 10, e0120590. [Google Scholar] [CrossRef] [Green Version]
- Sato, Y.; Masuta, Y.; Saito, K.; Murayama, S.; Ozawa, K. Enhanced chilling tolerance at the booting stage in rice by transgenic overexpression of the ascorbate peroxidase gene, OsAPXa. Plant Cell Rep. 2011, 30, 399–406. [Google Scholar] [CrossRef]
- Venema, J.H.; Linger, P.; van Heusden, A.W.; van Hasselt, P.R.; Brüggemann, W. The inheritance of chilling tolerance in tomato (Lycopersicon spp.). Plant Biol. (Stuttg.) 2005, 7, 118–130. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [Green Version]
- Pareek, A.; Khurana, A.; Sharma, A.K.; Kumar, R. An overview of signaling regulons during cold stress tolerance in plants. Curr. Genomics. 2017, 18, 498–511. [Google Scholar] [CrossRef]
- Fotinos, N.; Campo, M.A.; Popowycz, F.; Gurny, R.; Lange, N. 5-Aminolevulinic acid derivatives in photomedicine: Characteristics, application and perspectives. Photochem. Photobiol. 2006, 82, 994–1015. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, N.; Hu, L.; Liao, W.; Tang, Z.; Xiao, X.; Lyu, J.; Xie, J.; Calderón-Urrea, A.; Yu, J. 5-Aminolevulinic acid improves morphogenesis and Na+ subcellular distribution in the apical cells of Cucumis sativus L. under salinity stress. Front. Plant Sci. 2021, 12, 636121. [Google Scholar] [CrossRef] [PubMed]
- Akram, N.A.; Iqbal, M.; Muhammad, A.; Ashraf, M.; Al-Qurainy, F.; Shafiq, S. Aminolevulinic acid and nitric oxide regulate oxidative defense and secondary metabolisms in canola (Brassica napus L.) under drought stress. Protoplasma 2018, 255, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Jiang, W.B.; Huang, B.J. Promotion of 5-aminolevulinic acid on photosynthesis of melon (Cucumis melo) seedlings under low light and chilling stress conditions. Physiol. Plant. 2004, 121, 258–264. [Google Scholar] [CrossRef]
- Farid, M.; Ali, S.; Saeed, R.; Rizwan, M.; Bukhari, S.A.H.; Abbasi, G.H.; Hussain, A.; Ali, B.; Zamir, M.S.I.; Ahmad, I. Combined application of citric acid and 5-aminolevulinic acid improved biomass, photosynthesis and gas exchange attributes of sunflower (Helianthus annuus L.) grown on chromium contaminated soil. Int. J. Phytoremediation 2019, 21, 760–767. [Google Scholar] [CrossRef]
- Cai, C.; He, S.; An, Y.; Wang, L. Exogenous 5-aminolevulinic acid improves strawberry tolerance to osmotic stress and its possible mechanisms. Physiol. Plant. 2020, 168, 948–962. [Google Scholar] [CrossRef] [Green Version]
- Ali, B.; Tao, Q.; Zhou, Y.; Gill, R.A.; Ali, S.; Rafiq, M.T.; Xu, L.; Zhou, W. 5-Aminolevolinic acid mitigates the cadmium-induced changes in Brassica napus as revealed by the biochemical and ultra-structural evaluation of roots. Ecotoxicol. Environ. Saf. 2013, 92, 271–280. [Google Scholar] [CrossRef]
- Luksiene, Z.; Danilcenko, H.; Taraseviciene, Z.; Anusevicius, Z.; Maroziene, A.; Nivinskas, H. New approach to the fungal decontamination of wheat used for wheat sprouts: Effects of aminolevulinic acid. Int. J. Food Microbiol. 2007, 116, 153–158. [Google Scholar] [CrossRef]
- Niu, K.; Ma, X.; Liang, G.; Ma, H.; Jia, Z.; Liu, W.; Yu, Q. 5-Aminolevulinic acid modulates antioxidant defense systems and mitigates drought-induced damage in Kentucky bluegrass seedlings. Protoplasma 2017, 254, 2083–2094. [Google Scholar] [CrossRef]
- Rhaman, M.S.; Imran, S.; Karim, M.M.; Chakrobortty, J.; Mahamud, M.A.; Sarker, P.; Tahjib-Ul-Arif, M.; Robin, A.H.K.; Ye, W.; Murata, Y.; et al. 5-aminolevulinic acid-mediated plant adaptive responses to abiotic stress. Plant Cell Rep. 2021, 40, 1451–1469. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.F. Experimental Guidance for Plant Physiology; Higher Education Press of China: Beijing, China, 2006; pp. 140–144. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant-environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef] [PubMed]
- Xia, E.H.; Zhang, H.B.; Sheng, J.; Li, K.; Zhang, Q.J.; Kim, C.; Zhang, Y.; Liu, Y.; Zhu, T.; Li, W.; et al. The Tea Tree Genome Provides Insights into Tea Flavor and Independent Evolution of Caffeine Biosynthesis. Mol. Plant 2017, 10, 866–877. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Liu, Z.; Luo, S.; Li, J.; Zhang, J.; Li, L.; Xie, J. 5-Aminolevulinic acid and hydrogen sulphide alleviate chilling stress in pepper (Capsicum annuum L.) seedlings by enhancing chlorophyll synthesis pathway. Plant Physiol. Biochem. 2021, 167, 567–576. [Google Scholar] [CrossRef]
- An, Y.Y.; Feng, X.X.; Ding, H.Y.; Liu, L.B.; Wang, L.J. Effects of 5-aminolevulinic acid (5-ALA) on growth and quality components of spring tea. Jiangsu Agric. Sci. 2016, 44, 224–228. [Google Scholar]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial properties of green tea catechins. Int. J. Mol. Sci. 2020, 4, 1744. [Google Scholar] [CrossRef] [Green Version]
- Barton, D.A.; Cantrill, L.C.; Law, A.M.; Phillips, C.G.; Sutton, B.G.; Overall, R.L. Chilling to zero degrees disrupts pollen formation but not meiotic microtubule arrays in Triticum aestivum L. Plant Cell Environ. 2014, 37, 2781–2794. [Google Scholar] [CrossRef]
- Kaniuga, Z. Chilling response of plants: Importance of galactolipase, free fatty acids and free radicals. Plant Biol. (Stuttg.) 2008, 10, 171–184. [Google Scholar] [CrossRef]
- Sun, Y.P.; Zhang, Z.P.; Xu, C.X.; Shen, C.M.; Gao, C.; Wang, L.J. Effect of ALA on Fast Chlorophyll Fluorescence Induction Dynamics of Watermelon Leaves under Chilling Stress. Acta Hortic. Sin. 2009, 5, 671–678. [Google Scholar]
- Chen, H.; Xu, L.; Li, X.; Wang, D.Y.; An, Y.Y.; Wang, L.J. Promotive effect of 5-aminolevulinic acid improved cold tolerance of Rhododendron simissi and Cinnamomum camphora leaves. Plant Physiol. J. 2017, 53, 2103–2113. [Google Scholar]
- Wu, Y.; Liao, W.B.; Dawuda, M.M.; Hu, L.L.; Yu, J.H. 5-Aminolevulinic acid (ALA) biosynthetic and metabolic pathways and its role in higher plants: A review. Plant Growth Regul. 2019, 87, 357–374. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, K.; Cheng, D.; Zhang, C.; Li, R.; Liu, F.; Wen, H.; Tao, L.; Zhang, Y.; Li, C.; et al. Characterization of Cuticular Wax in Tea Plant and Its Modification in Response to Low Temperature. J. Agric. Food Chem. 2022, 2, 13849–13861. [Google Scholar] [CrossRef] [PubMed]
- Saucedo-García, M.; González-Córdova, C.D.; Ponce-Pineda, I.G.; Cano-Ramírez, D.; Romero-Colín, F.M.; Arroyo-Pérez, E.E.; King-Díaz, B.; Zavafer, A.; Gavilanes-Ruíz, M. Effects of MPK3 and MPK6 kinases on the chloroplast architecture and function induced by cold acclimation in Arabidopsis. Photosynth. Res. 2021, 149, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, M.; Gu, C.; Jiang, H.; Sun, J.; Li, J. Genome-wide identification of MAPK family genes and their response to abiot-ic stresses in tea plant (Camellia sinensis). Open Life Sci. 2022, 8, 1064–1074. [Google Scholar] [CrossRef]



| Treatment | Soluble Sugar/mg·g−1 FW | Soluble Protein/mg·g−1 FW | Catechin/% |
|---|---|---|---|
| T-4 | 2.00 ± 0.28 b | 6.5 ± 0.03 b | 21.2953 ± 0.18 b |
| T-A-4 | 2.51 ± 0.37 a | 12.5 ± 0.01 a | 25.1329 ± 0.78 a |
| Treatment | Pn/μmol·m−2·s−1 | Gs/mmol·m−2·s−1 | Ci/μmol·m−2·s−1 | Tr/mmol·m−2·s−1 |
|---|---|---|---|---|
| T-4 | 7.84 ± 1.27 b | 0.15 ± 0.02 b | 333.93 ± 7.18 b | 2.32 ± 0.28 b |
| T-A-4 | 9.71 ± 0.95 a | 0.18 ± 0.03 a | 344.83 ± 6.82 a | 2.75 ± 0.37 a |
| Sample | Raw_ Reads | Clean_ Reads | Clean_ Bases | Total Mapped | Q30 (%) | GC (%) |
|---|---|---|---|---|---|---|
| T-4-1 | 46,131,834 | 43,884,132 | 6.06 G | 36,372,232 (82.88%) | 92.66 | 45.6 |
| T-4-2 | 53,488,410 | 51,423,374 | 7 G | 43,689,622 (84.96%) | 93.01 | 46.92 |
| T-4-3 | 67,969,468 | 65,244,804 | 8.9 G | 56,146,303 (86.05%) | 92.98 | 46.79 |
| T-A-4-1 | 48,710,748 | 46,941,144 | 6.28 G | 40,265,286 (85.78%) | 93.38 | 46.71 |
| T-A-4-2 | 48,831,254 | 47,023,426 | 6.32 G | 40,779,946 (86.72%) | 93.23 | 46.96 |
| T-A-4-3 | 66,347,650 | 64,169,130 | 8.29 G | 56,095,572 (87.42%) | 93.3 | 46.67 |
| T-A-1 | 45,848,706 | 44,118,740 | 5.85 G | 38,279,165 (86.76%) | 92.52 | 46.67 |
| T-A-2 | 45,982,254 | 44,344,884 | 5.86 G | 37,938,206 (85.55%) | 93.17 | 46.64 |
| T-A-3 | 49,396,794 | 47,419,010 | 6.45 G | 38,364,744 (80.91%) | 93.02 | 46.66 |
| T-CK-1 | 49,388,822 | 47,395,516 | 6.47 G | 40,176,843 (84.77%) | 93.02 | 47.95 |
| T-CK-2 | 49,277,524 | 47,575,956 | 6.25 G | 39,837,399 (83.73%) | 93.24 | 46.95 |
| T-CK-3 | 43,145,130 | 41,371,492 | 5.62 G | 34,751,359 (84.00%) | 93.04 | 46.91 |
| DEGs Set | Up-Regulated | Down-Regulated | Total DEGs |
|---|---|---|---|
| T-CK vs. T-4 | 2375 | 2615 | 4900 |
| T-CK vs. T-A | 103 | 120 | 223 |
| T-4 vs. T-A-4 | 13 | 79 | 92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, F.; Qu, D.; Chen, X.; Yang, J.; Zeng, H.; Li, X. Transcriptome Analysis of 5-Aminolevulinic Acid Contributing to Cold Tolerance in Tea Leaves (Camellia sinensis L.). Forests 2023, 14, 198. https://doi.org/10.3390/f14020198
Yan F, Qu D, Chen X, Yang J, Zeng H, Li X. Transcriptome Analysis of 5-Aminolevulinic Acid Contributing to Cold Tolerance in Tea Leaves (Camellia sinensis L.). Forests. 2023; 14(2):198. https://doi.org/10.3390/f14020198
Chicago/Turabian StyleYan, Fei, Dong Qu, Xiaohua Chen, Jianjun Yang, Haitao Zeng, and Xinsheng Li. 2023. "Transcriptome Analysis of 5-Aminolevulinic Acid Contributing to Cold Tolerance in Tea Leaves (Camellia sinensis L.)" Forests 14, no. 2: 198. https://doi.org/10.3390/f14020198
APA StyleYan, F., Qu, D., Chen, X., Yang, J., Zeng, H., & Li, X. (2023). Transcriptome Analysis of 5-Aminolevulinic Acid Contributing to Cold Tolerance in Tea Leaves (Camellia sinensis L.). Forests, 14(2), 198. https://doi.org/10.3390/f14020198






