Predicting Spruce Taiga Distribution in Northeast Asia Using Species Distribution Models: Glacial Refugia, Mid-Holocene Expansion and Future Predictions for Global Warming
Abstract
:1. Introduction
2. Materials and Methods
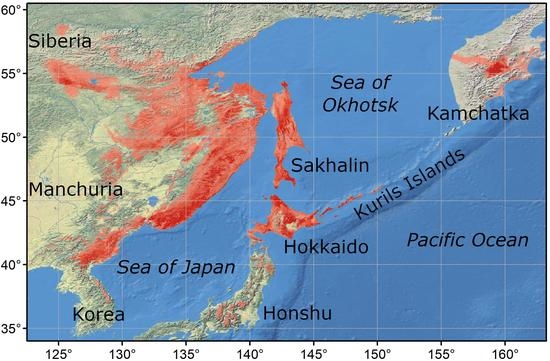
2.1. Study Area
2.2. Presence Points
2.3. Climatic Data
2.4. Model Building
3. Results
4. Discussion
4.1. Model of Current Distribution
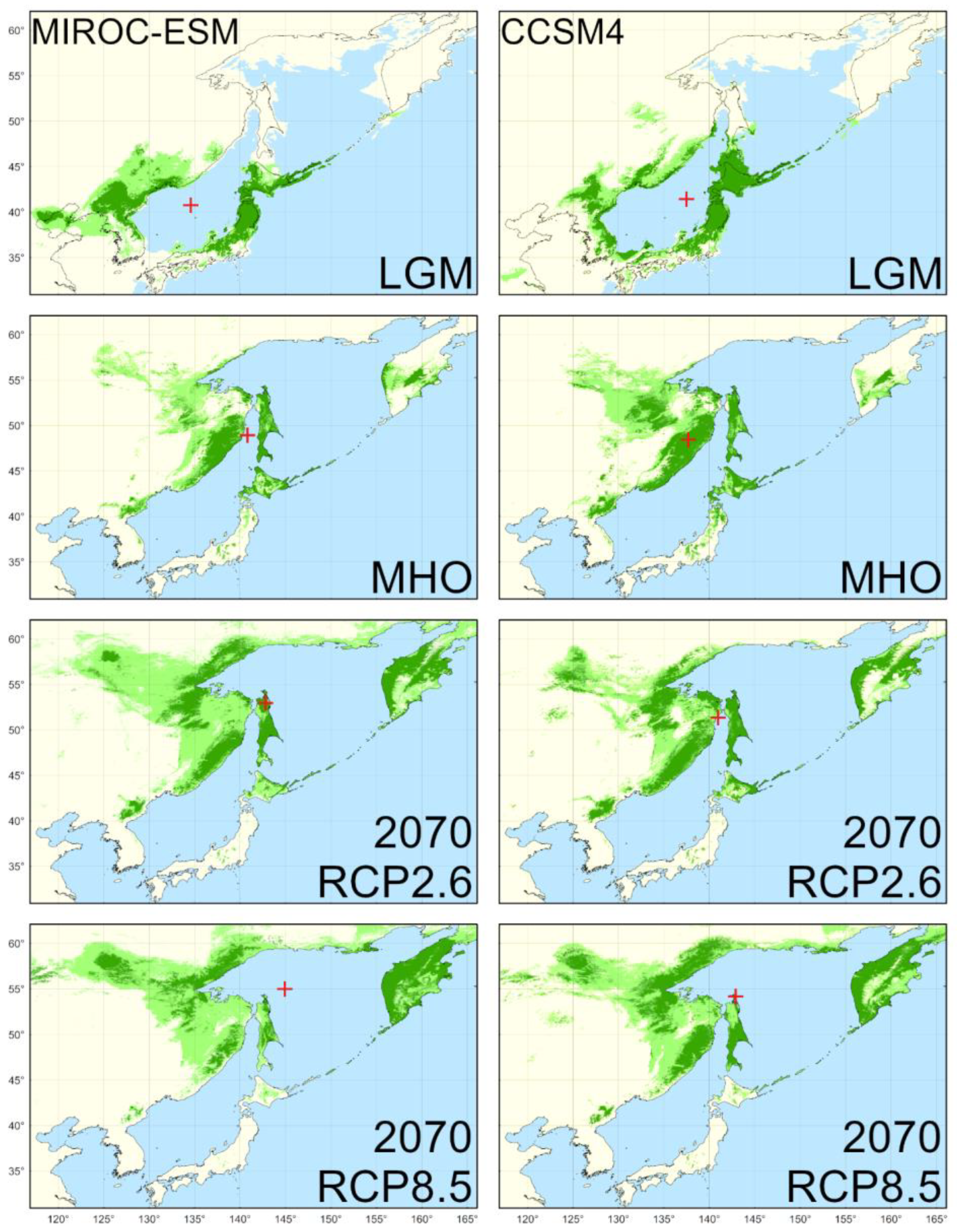
4.2. Reconstructed Distribution in the LGM
4.3. Reconstructed Distribution in the MHO
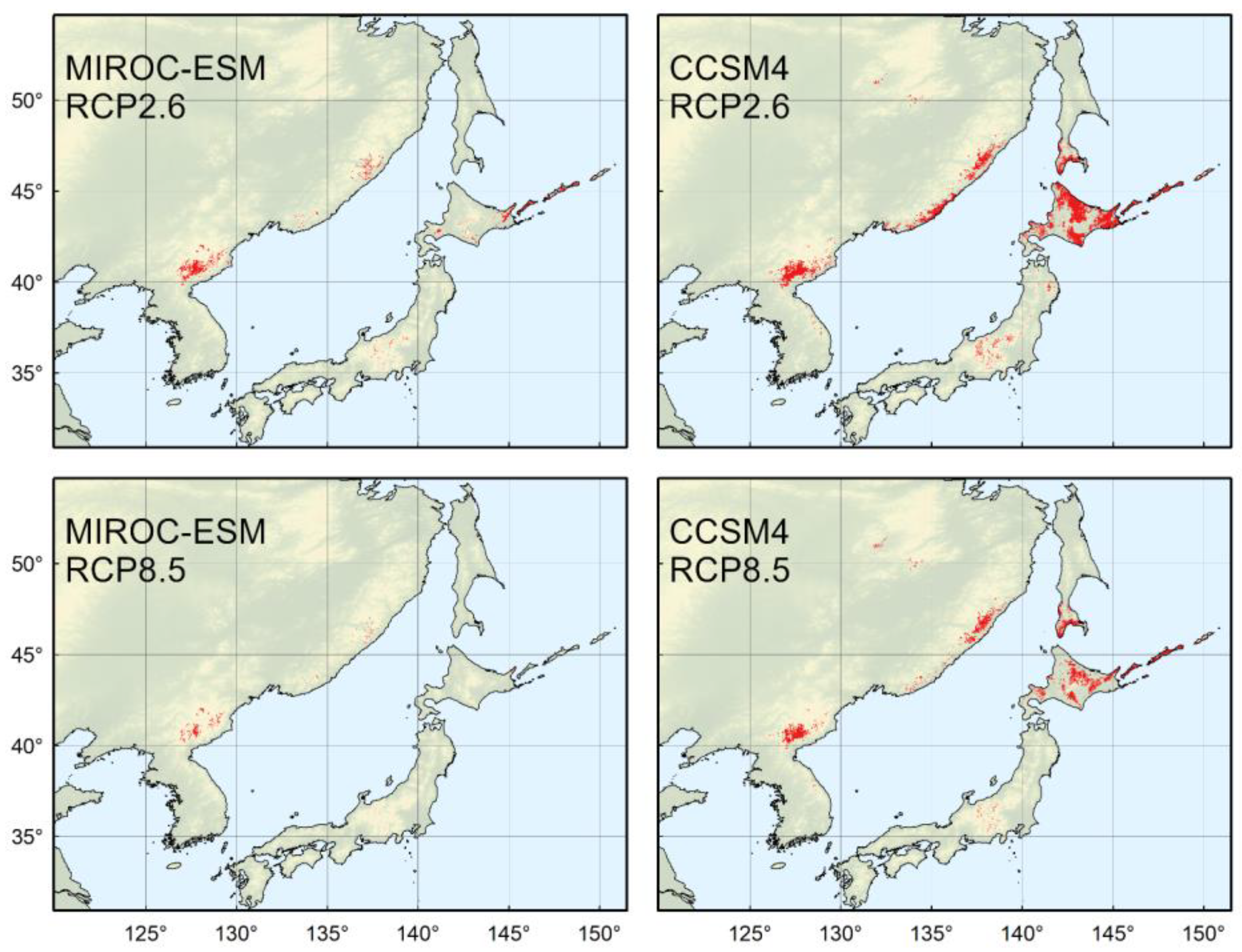
4.4. Predicted Distribution in the Year 2070
4.5. Implications for Conservation and Management
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, I.-C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid Range Shifts of Species Associated with High Levels of Climate Warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- Becknell, J.M.; Desai, A.R.; Dietze, M.C.; Schultz, C.A.; Starr, G.; Duffy, P.A.; Franklin, J.F.; Pourmokhtarian, A.; Hall, J.; Stoy, P.C.; et al. Assessing Interactions Among Changing Climate, Management, and Disturbance in Forests: A Macrosystems Approach. Bioscience 2015, 65, 263–274. [Google Scholar] [CrossRef] [Green Version]
- Seidl, R.; Thom, D.; Kautz, M.; Martin-Benito, D.; Peltoniemi, M.; Vacchiano, G.; Wild, J.; Ascoli, D.; Petr, M.; Honkaniemi, J.; et al. Forest disturbances under climate change. Nat. Clim. Chang. 2017, 7, 395–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guisan, A.; Zimmermann, N.E. Predictive habitat distribution models in ecology. Ecol. Model. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Elith, J.H.; Graham, C.P.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef] [Green Version]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Kearney, M.; Porter, W. Mechanistic niche modelling: Combining physiological and spatial data to predict species’ ranges. Ecol. Lett. 2009, 12, 334–350. [Google Scholar] [CrossRef]
- Pearson, R.G.; Dawson, T.P. Predicting the impacts of climate change on the distribution of species: Are bioclimate envelope models useful? Glob. Ecol. Biogeogr. 2003, 12, 361–371. [Google Scholar] [CrossRef] [Green Version]
- Hijmans, R.J.; Graham, C.H. The ability of climate envelope models to predict the effect of climate change on species distributions. Glob. Chang. Biol. 2006, 12, 2272–2281. [Google Scholar] [CrossRef]
- Booth, T.H. Species distribution modelling tools and databases to assist managing forests under climate change. For. Ecol. Manag. 2018, 430, 196–203. [Google Scholar] [CrossRef]
- Santini, L.; Benítez-López, A.; Maiorano, L.; Čengić, M.; Huijbregts, M.A.J. Assessing the reliability of species distribution projections in climate change research. Divers. Distrib. 2021, 27, 1035–1050. [Google Scholar] [CrossRef]
- Tang, C.Q.; Matsui, T.; Ohashi, H.; Dong, Y.-F.; Momohara, A.; Herrando-Moraira, S.; Qian, S.; Yang, Y.; Ohsawa, M.; Luu, H.T.; et al. Identifying long-term stable refugia for relict plant species in East Asia. Nat. Commun. 2018, 9, 4488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janowiak, M.K.; Swanston, C.; Nagel, L.M.; Brandt, L.A.; Butler, P.R.; Handler, S.D.; Shannon, P.D.; Iverson, L.R.; Matthews, S.; Prasad, A.; et al. A Practical Approach for Translating Climate Change Adaptation Principles into Forest Management Actions. J. For. 2014, 112, 424–433. [Google Scholar] [CrossRef] [Green Version]
- Schelhaas, M.-J.; Nabuurs, G.-J.; Hengeveld, G.; Reyer, C.; Hanewinkel, M.; Zimmermann, N.E.; Cullmann, D. Alternative forest management strategies to account for climate change-induced productivity and species suitability changes in Europe. Reg. Environ. Chang. 2015, 15, 1581–1594. [Google Scholar] [CrossRef] [Green Version]
- Krestov, P.V. Forest Vegetation of Easternmost Russia (Russian Far East). In Forest Vegetation of Northeast Asia. Geobotany; Kolbek, J., Šrůtek, M., Box, E.O., Eds.; Springer: Dordrecht, The Netherlands, 2003; Volume 28, pp. 93–180. [Google Scholar]
- Manko, Y.I. El’ Ajanskaya (Picea Ajanensis); Nauka: Leningrad, Russia, 1987. (In Russian) [Google Scholar]
- Shao, C.-C.; Shen, T.-T.; Jin, W.-T.; Mao, H.-J.; Ran, J.-H.; Wang, X.-Q. Phylotranscriptomics resolves interspecific relationships and indicates multiple historical out-of-North America dispersals through the Bering Land Bridge for the genus Picea (Pinaceae). Mol. Phylogenet. Evol. 2019, 141, 106610. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Krestov, P.V. Coniferous forests of the temperate zone of Asia. Coniferous forests. Ser. Ecosyst. World 2005, 6, 163–220. [Google Scholar]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef] [Green Version]
- Gauthier, S.; Bernier, P.; Kuuluvainen, T.; Shvidenko, A.Z.; Schepaschenko, D.G. Boreal forest health and global change. Science 2015, 349, 819–822. [Google Scholar] [CrossRef]
- Kuuluvainen, T.; Gauthier, S. Young and old forest in the boreal: Critical stages of ecosystem dynamics and management under global change. For. Ecosyst. 2018, 5, 26. [Google Scholar] [CrossRef]
- Svenning, J.-C.; Eiserhardt, W.L.; Normand, S.; Ordonez, A.; Sandel, B. The Influence of Paleoclimate on Present-Day Patterns in Biodiversity and Ecosystems. Annu. Rev. Ecol. Evol. Syst. 2015, 46, 551–572. [Google Scholar] [CrossRef] [Green Version]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- WorldClim. 1.4 Downscaled Paleo Climate. Available online: https://www.worldclim.org/data/v1.4/paleo1.4.html (accessed on 17 January 2023).
- Watanabe, S.; Hajima, T.; Sudo, K.; Nagashima, T.; Takemura, T.; Okajima, H.; Nozawa, T.; Kawase, H.; Abe, M.; Yokohata, T.; et al. MIROC-ESM 2010: Model description and basic results of CMIP5-20c3m experiments. Geosci. Model Dev. 2011, 4, 845–872. [Google Scholar] [CrossRef] [Green Version]
- Kawamiya, M.; Hajima, T.; Tachiiri, K.; Watanabe, S.; Yokohata, T. Two decades of Earth system modeling with an emphasis on Model for Interdisciplinary Research on Climate (MIROC). Prog. Earth Planet. Sci. 2020, 7, 64. [Google Scholar] [CrossRef]
- Gent, P.R.; Danabasoglu, G.; Donner, L.J.; Holland, M.M.; Hunke, E.C.; Jayne, S.R.; Lawrence, D.M.; Neale, R.B.; Rasch, P.J.; Vertenstein, M.; et al. The Community Climate System Model Version 4. J. Clim. 2011, 24, 4973–4991. [Google Scholar] [CrossRef] [Green Version]
- Van Vuuren, D.P.; Stehfest, E.; den Elzen, M.G.J.; Kram, T.; Van Vliet, J.; Deetman, S.; Isaac, M.; Goldewijk, K.K.; Hof, A.; Beltran, A.M.; et al. RCP2.6: Exploring the possibility to keep global mean temperature increase below 2 °C. Clim. Chang. 2011, 109, 95–116. [Google Scholar] [CrossRef]
- Riahi, K.; Rao, S.; Krey, V.; Cho, C.; Chirkov, V.; Fischer, G.; Kindermann, G.E.; Nakicenovic, N.; Rafaj, P. RCP 8.5—A scenario of comparatively high greenhouse gas emissions. Clim. Chang. 2011, 109, 33–57. [Google Scholar] [CrossRef] [Green Version]
- Rokach, L.; Maimon, M.O. Classification Trees. In Data Mining and Knowledge DisCovery Handbook; Maimon, O., Rokach, L., Eds.; Springer: Boston, MA, USA, 2009; pp. 149–174. [Google Scholar]
- Krestov, P.V.; Nakamura, Y. Phytosociological study of thePicea jezoensis forests of the far east. Folia Geobot. 2002, 37, 441–473. [Google Scholar] [CrossRef]
- Potenko, V.V.; Knysh, Y.D. Genetic variation of Yeddo spruce populations in Russia. For. Genet. 2003, 10, 55–64. [Google Scholar]
- Potenko, V.V. Allozyme Variation and Phylogenetic Relationships in Picea jezoensis (Pinaceae) Populations of the Russian Far East. Biochem. Genet. 2007, 45, 291–304. [Google Scholar] [CrossRef]
- Aizawa, M.; Yoshimaru, H.; Saito, H.; Katsuki, T.; Kawahara, T.; Kitamura, K.; Shi, F.; Kaji, M. Phylogeography of a northeast Asian spruce, Picea jezoensis, inferred from genetic variation observed in organelle DNA markers. Mol. Ecol. 2007, 16, 3393–3405. [Google Scholar] [CrossRef]
- Aizawa, M.; Yoshimaru, H.; Saito, H.; Katsuki, T.; Kawahara, T.; Kitamura, K.; Shi, F.; Sabirov, R.; Kaji, M. Range-wide genetic structure in a north-east Asian spruce (Picea jezoensis) determined using nuclear microsatellite markers. J. Biogeogr. 2009, 36, 996–1007. [Google Scholar] [CrossRef]
- GBIF. Global Biodiversity Information Facility. Available online: https://www.gbif.org/ (accessed on 9 October 2022).
- GeoPy’s Documentation. Available online: https://geopy.readthedocs.io/en/stable/ (accessed on 9 October 2022).
- Average Nearest Neighbor, ArcMap 10.8. Available online: https://desktop.arcgis.com/en/arcmap/latest/tools/spatial-statistics-toolbox/average-nearest-neighbor.htm (accessed on 9 October 2022).
- Nakamura, Y.; Krestov, P.V.; Omelko, A.M. Bioclimate and zonal vegetation in Northeast Asia: First approximation to an integrated study. Phytocoenologia 2007, 37, 443–470. [Google Scholar] [CrossRef]
- Noce, S.; Caporaso, L.; Santini, M. A new global dataset of bioclimatic indicators. Sci. Data 2020, 7, 398. [Google Scholar] [CrossRef] [PubMed]
- NumPy. The Fundamental Package for Scientific Computing with Python. Available online: https://numpy.org/ (accessed on 9 October 2022).
- Imdadullah, M.; Aslam, M.; Altaf, S. mctest: An R Package for Detection of Collinearity among Regressors. R J. 2016, 8, 495–505. [Google Scholar] [CrossRef]
- GDAL Documentation. Available online: https://gdal.org/ (accessed on 9 October 2022).
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar] [CrossRef]
- Čengić, M.; Rost, J.; Remenska, D.; Janse, J.H.; Huijbregts, M.A.J.; Schipper, A.M. On the importance of predictor choice, modelling technique, and number of pseudo-absences for bioclimatic envelope model performance. Ecol. Evol. 2020, 10, 12307–12317. [Google Scholar] [CrossRef]
- Zhao, Z.; Xiao, N.; Shen, M.; Li, J. Comparison between optimized MaxEnt and random forest modeling in predicting potential distribution: A case study with Quasipaa boulengeri in China. Sci. Total Environ. 2022, 842, 156867. [Google Scholar] [CrossRef] [PubMed]
- Petrenko, T.Y.; Korznikov, K.A.; Kislov, D.E.; Belyaeva, N.G.; Krestov, P.V. Modeling of cold-temperate tree Pinus koraiensis (Pinaceae) distribution in the Asia-Pacific region: Climate change impact. For. Ecosyst. 2022, 9, 100015. [Google Scholar] [CrossRef]
- Boyce, M.S.; Vernier, P.R.; E. Nielsen, S.; Schmiegelow, F.K. Evaluating resource selection functions. Ecol. Model. 2002, 157, 281–300. [Google Scholar] [CrossRef] [Green Version]
- Lobo, J.M.; Jiménez-Valverde, A.; Real, R. AUC: A misleading measure of the performance of predictive distribution models. Glob. Ecol. Biogeogr. 2007, 17, 145–151. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; White, M.; Newell, G. Selecting thresholds for the prediction of species occurrence with presence-only data. J. Biogeogr. 2013, 40, 778–789. [Google Scholar] [CrossRef]
- Konowalik, K.; Nosol, A. Evaluation metrics and validation of presence-only species distribution models based on distributional maps with varying coverage. Sci. Rep. 2021, 11, 1482. [Google Scholar] [CrossRef]
- USGS EROS Archive—Digital Elevation—Shuttle Radar Topography Mission (SRTM) 1 Arc-Second Global. Available online: https://www.usgs.gov/centers/eros/science/usgs-eros-archive-digital-elevation-shuttle-radar-topography-mission-srtm-1 (accessed on 9 October 2022).
- Černý, T.; Kopecký, M.; Petřík, P.; Song, J.-S.; Šrůtek, M.; Valachovič, M.; Altman, J.; Doležal, J. Classification of Korean forests: Patterns along geographic and environmental gradients. Appl. Veg. Sci. 2014, 18, 5–22. [Google Scholar] [CrossRef]
- Herzschuh, U.; Birks, H.J.B.; Laepple, T.; Andreev, A.; Melles, M.; Brigham-Grette, J. Glacial legacies on interglacial vegetation at the Pliocene-Pleistocene transition in NE Asia. Nat. Commun. 2016, 7, 11967. [Google Scholar] [CrossRef] [Green Version]
- Tsukada, M. Vegetation in prehistoric Japan: The last 20,000 years, In Windows on the Japanese Past: Studies in Archeology and Prehistory; Center for Japanese Studies, University of Michigan: Ann Arbor, MI, USA, 1986; pp. 11–56. [Google Scholar]
- Sakaguchi, S.; Sakurai, S.; Yamasaki, M.; Isagi, Y. How did the exposed seafloor function in postglacial northward range expansion of Kalopanax septemlobus? Evidence from ecological niche modelling. Ecol. Res. 2010, 25, 1183–1195. [Google Scholar] [CrossRef]
- Vereshchagin, N.K.; Baryshnikov, G.F. The ecological structure of the “Mammoth Fauna” in Eurasia. Ann. Zool. Fenn. 1991, 28, 253–259. Available online: https://www.jstor.org/stable/23735450 (accessed on 9 October 2022).
- Markova, A.K.; Smirnov, N.G.; Kozharinov, A.V.; Kazantseva, N.E.; Simakova, A.N.; Kitaev, L.M. Late Pleistocene distribution and diversity of mammals in Northern Eurasia (PALEOFAUNA database). Paleontol. Evol. 1995, 28–29, 5–66. [Google Scholar]
- Igarashi, Y.; Zharov, A.E. Climate and vegetation change during the late Pleistocene and early Holocene in Sakhalin and Hokkaido, northeast Asia. Quat. Int. 2011, 237, 24–31. [Google Scholar] [CrossRef]
- Igarashi, Y. Vegetation and climate during the LGM and the last deglaciation on Hokkaido and Sakhalin Islands in the northwest Pacific. Quat. Int. 2016, 425, 28–37. [Google Scholar] [CrossRef]
- Belyanin, P.S.; Belyanina, N.I. On the Prikhanka depression vegetation cover evolution and its mountain framing in the Late Neopleistocene-Holocene (from palynological data). Russ. J. Pac. Geol. 2012, 31, 96–108. (In Russian) [Google Scholar]
- Li, X.; Zhao, C.; Zhou, X. Vegetation pattern of Northeast China during the special periods since the Last Glacial Maximum. Sci. China Earth Sci. 2019, 62, 1224–1240. [Google Scholar] [CrossRef]
- Herzschuh, U. Legacy of the Last Glacial on the present-day distribution of deciduous versus evergreen boreal forests. Glob. Ecol. Biogeogr. 2019, 29, 198–206. [Google Scholar] [CrossRef] [Green Version]
- Zimov, S.; Zimov, N.; Tikhonov, A.; Chapin, F. Mammoth steppe: A high-productivity phenomenon. Quat. Sci. Rev. 2012, 57, 26–45. [Google Scholar] [CrossRef]
- Ooi, N. Vegetation history of Japan since the last glacial based on palynological data. Jpn. J. Hist. Bot. 2016, 25, 1–101. [Google Scholar] [CrossRef]
- Cao, X.; Herzschuh, U.; Ni, J.; Zhao, Y.; Böhmer, T. Spatial and temporal distributions of major tree taxa in eastern continental Asia during the last 22,000 years. Holocene 2014, 25, 79–91. [Google Scholar] [CrossRef] [Green Version]
- Binney, H.; Edwards, M.; Macias-Fauria, M.; Lozhkin, A.; Anderson, P.; Kaplan, J.O.; Andreev, A.; Bezrukova, E.; Blyakharchuk, T.; Jankovska, V.; et al. Vegetation of Eurasia from the last glacial maximum to present: Key biogeographic patterns. Quat. Sci. Rev. 2017, 157, 80–97. [Google Scholar] [CrossRef]
- Zhao, C.; Li, X.; Zhou, X.; Zhao, K.; Yang, Q. Holocene vegetation succession and responses to climate change in the northern sector of Northeast China. Sci. China Earth Sci. 2016, 59, 1390–1400. [Google Scholar] [CrossRef]
- Razjigaeva, N.; Ganzey, L.; Grebennikova, T.; Mokhova, L.; Kudryavtseva, E.; Arslanov, K.; Maksimov, F.; Starikova, A. Landscape and environmental changes along the Eastern Primorye coast during the middle to late Holocene and human effects. J. Asian Earth Sci. 2018, 158, 160–172. [Google Scholar] [CrossRef]
- Razjigaeva, N.; Ganzey, L.; Lyaschevskaya, M.; Makarova, T.; Kudryavtseva, E.; Grebennikova, T.; Panichev, A.; Arslanov, K.; Maksimov, F.; Petrov, A.Y.; et al. Climatic and human impacts on landscape development of the Murav’ev Amursky Peninsula (Russian South Far East) in the Middle/Late Holocene and historical time. Quat. Int. 2019, 516, 127–140. [Google Scholar] [CrossRef]
- Jang, W.; Park, P.S. Stand Structure and Maintenance of Picea jezoensis in a Northern Temperate Forest, South Korea. J. Plant Biol. 2010, 53, 180–189. [Google Scholar] [CrossRef]
- Jang, W.; Keyes, C.R.; Running, S.W.; Lim, J.-H.; Park, P.S. Climate–growth relationships of relict Picea jezoensis at Mt. Gyebang, South Korea. For. Sci. Technol. 2014, 11, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.; Wang, Q.; Wang, Y.; Zhou, W.; Ding, H.; Fang, X.; Jiang, S.; Dai, L. Climatic effects on radial growth of major tree species on Changbai Mountain. Ann. For. Sci. 2011, 68, 921–933. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Shao, X.-M.; Jiang, Y.; Fang, X.-Q.; Wu, S.-H. The impacts of climate change on the radial growth of Pinus koraiensis along elevations of Changbai Mountain in northeastern China. For. Ecol. Manag. 2013, 289, 333–340. [Google Scholar] [CrossRef]
- Gai, X.; Wang, S.; Zhou, L.; Wu, J.; Zhou, W.; Bi, J.; Cao, L.; Dai, L.; Yu, D. Spatiotemporal evidence of tree-growth resilience to climate variations for Yezo spruce (Picea jezoensis var. komarovii) on Changbai Mountain, Northeast China. J. For. Res. 2018, 31, 927–936. [Google Scholar] [CrossRef]
- Hiura, T.; Go, S.; Iijima, H. Long-term forest dynamics in response to climate change in northern mixed forests in Japan: A 38-year individual-based approach. For. Ecol. Manag. 2019, 449, 117469. [Google Scholar] [CrossRef]
- Kim, E.S.; Lee, J.S.; Park, G.E.; Lim, J.-H. Change of subalpine coniferous forest area over the last 20 years. J. Korean Soc. For. Sci. 2019, 108, 10–20. [Google Scholar] [CrossRef]
- Zhu, L.; Cooper, D.J.; Yang, J.; Zhang, X.; Wang, X. Rapid warming induces the contrasting growth of Yezo spruce (Picea jezoensis var. microsperma) at two elevation gradient sites of northeast China. Dendrochronologia 2018, 50, 52–63. [Google Scholar] [CrossRef]
- Pulliam, H. On the relationship between niche and distribution. Ecol. Lett. 2000, 3, 349–361. [Google Scholar] [CrossRef]
- Michalet, R.; Maalouf, J.-P.; Choler, P.; Clément, B.; Rosebery, D.; Royer, J.-M.; Schöb, C.; Lortie, C. Competition, facilitation and environmental severity shape the relationship between local and regional species richness in plant communities. Ecography 2014, 38, 335–345. [Google Scholar] [CrossRef]
- Antúnez, P. Main environmental variables influencing the abundance of plant species under risk category. J. For. Res. 2021, 33, 1209–1217. [Google Scholar] [CrossRef]
- Kolström, M.; Lindner, M.; Vilén, T.; Maroschek, M.; Seidl, R.; Lexer, M.J.; Netherer, S.; Kremer, A.; Delzon, S.; Barbati, A.; et al. Reviewing the Science and Implementation of Climate Change Adaptation Measures in European Forestry. Forests 2011, 2, 961–982. [Google Scholar] [CrossRef] [Green Version]
- Jandl, R.; Spathelf, P.; Bolte, A.; Prescott, C.E. Forest adaptation to climate change—Is non-management an option? Ann. For. Sci. 2019, 76, 48. [Google Scholar] [CrossRef] [Green Version]
- Vozmishcheva, A.S.; Bondarchuk, S.N.; Gromyko, M.N.; Kislov, D.E.; Pimenova, E.A.; Salo, M.A.; Korznikov, K.A. Strong Disturbance Impact of Tropical Cyclone Lionrock (2016) on Korean Pine-Broadleaved Forest in the Middle Sikhote-Alin Mountain Range, Russian Far East. Forests 2019, 10, 1017. [Google Scholar] [CrossRef] [Green Version]
- Kislov, D.E.; Korznikov, K.A.; Altman, J.; Vozmishcheva, A.S.; Krestov, P.V. Extending deep learning approaches for forest disturbance segmentation on very high-resolution satellite images. Remote Sens. Ecol. Conserv. 2021, 7, 355–368. [Google Scholar] [CrossRef]
- Korznikov, K.; Kislov, D.; Doležal, J.; Petrenko, T.; Altman, J. Tropical cyclones moving into boreal forests: Relationships between disturbance areas and environmental drivers. Sci. Total Environ. 2022, 844, 156931. [Google Scholar] [CrossRef]

| Model Predictor | Importance (Mean ± SE, n = 100) |
|---|---|
| Pp | 0.234 ± 0.010 |
| Pn | 0.234 ± 0.009 |
| WKI | 0.210 ± 0.005 |
| CKI | 0.164 ± 0.003 |
| IC | 0.158 ± 0.001 |
| Scenario | CCSM4 | MIROC-ESM |
|---|---|---|
| LGM | 546,250 * | 456,471 |
| MHO | 494,278 | 322,155 |
| RCP2.6 | 614,347 * | 581,760 * |
| RCP8.5 | 625,076 * | 483,805 |
| Climate Model | Scenario | MIROC-ESM |
|---|---|---|
| MIROC-ESM | RCP2.6 | 18,293 |
| RCP8.5 | 4480 | |
| CCSM4 | RCP2.6 | 54,725 |
| RCP8.5 | 20,416 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korznikov, K.; Petrenko, T.; Kislov, D.; Krestov, P.; Doležal, J. Predicting Spruce Taiga Distribution in Northeast Asia Using Species Distribution Models: Glacial Refugia, Mid-Holocene Expansion and Future Predictions for Global Warming. Forests 2023, 14, 219. https://doi.org/10.3390/f14020219
Korznikov K, Petrenko T, Kislov D, Krestov P, Doležal J. Predicting Spruce Taiga Distribution in Northeast Asia Using Species Distribution Models: Glacial Refugia, Mid-Holocene Expansion and Future Predictions for Global Warming. Forests. 2023; 14(2):219. https://doi.org/10.3390/f14020219
Chicago/Turabian StyleKorznikov, Kirill, Tatyana Petrenko, Dmitry Kislov, Pavel Krestov, and Jiří Doležal. 2023. "Predicting Spruce Taiga Distribution in Northeast Asia Using Species Distribution Models: Glacial Refugia, Mid-Holocene Expansion and Future Predictions for Global Warming" Forests 14, no. 2: 219. https://doi.org/10.3390/f14020219
APA StyleKorznikov, K., Petrenko, T., Kislov, D., Krestov, P., & Doležal, J. (2023). Predicting Spruce Taiga Distribution in Northeast Asia Using Species Distribution Models: Glacial Refugia, Mid-Holocene Expansion and Future Predictions for Global Warming. Forests, 14(2), 219. https://doi.org/10.3390/f14020219