Abstract
The present study evaluated the potentials of phytoremediation and the biomonitoring of potentially toxic metals (PTMs) (Zn, Ni, Fe, Pb, and Cu) in the mangrove leaves of Rhizophora apiculata from the tropical mangrove ecosystem in the Sepang Besar River and Lukut River, Peninsular Malaysia. Overall, the present studies concluded that (a) the levels of essential Fe, Cu, and Zn in lamina are significantly (p < 0.05) higher than in MP, (b) the levels of Pb and Ni in MP are significantly (p < 0.05) higher than in lamina, (c) the lamina has better potential as a phytoremediator of Cu, Zn, and Fe, while MP is a better potential phytoremediator of Pb and Ni, (d) lamina is a potential biomonitoring agent of potentially toxic metals based on better correlation coefficients with the surface sedimentary geochemical fractions, and (e) metal uptake in the mangrove leaves and comparative levels of metals is low with reported studies. Specifically, based on bioconcentration factors (BCF), their most obtained values were considered low (<1), suggesting that R. apiculata can be considered as a low-efficiency plant for the bioaccumulation of PTM. However, the present findings also suggested that R. apiculata may be classified as a potential phytoremediator for Zn, Cu, Pb, and Ni in the leaves, as indicated by higher metal accumulation in the MP, with BCFEFLE values > 1.0; BCFAR > 1.0 for Cu, Pb, and Ni. The mangrove leaves are potential biomonitors of PTMs since positive correlations of PTMs were found between the leaves and their habitat surface sediments. Having been identified as a potential phytoremediator and biomonitor of PTMs, the present study emphasized the possibility of establishing a framework for managing the coastal aquatic ecosystems along the mangrove ecosystems of Sepang and Lukut.
1. Introduction
Mangrove ecosystems are significant intertidal estuarine wetlands along tropical coasts [1]. Mangroves are woody plant communities and are regarded as distinct halophytes, an uncommon variety of evergreen trees [2]. The mangrove plants are extremely valuable to marine species as habitats, food sources, and refuges [3]. Mangrove environments may suffer due to anthropogenic pressure and potentially toxic metals (PTMs) brought on by population expansion. The PTMs are major inorganic pollutants with potentially detrimental effects on the ecological quality of the mangrove ecosystems [4]. The PTM pollution in the ecosystem of mangroves has drawn more attention in recent years [2,5] since increased PTM concentrations in surface sediments from mangrove ecosystems have been widely reported in the literature [2]. This is based on the many reported studies on the levels of status of toxic metals or pollutants on the mangrove trees [6,7,8] with their phytoremediation potentials [9,10,11,12,13,14,15].
Typically, industrial effluents, agrochemical-based industries, agricultural runoff, sewage treatment facilities, leaching from residential garbage dumps, urbanisation, and chemical and oil spills are linked to mangrove pollution by PTMs [16,17,18,19,20,21]. This mangrove ecosystem contributes to the sequestration of carbon and the prevention of coastal erosion, and it provides a habitat for local and migratory animals. Due to these distinguishing characteristics, those concerned with conservation issues have recently begun to pay more attention to the contamination of the mangrove ecosystems by PTMs [22,23]. In tropical and subtropical marine ecosystems, mangroves are crucial components of the metal accumulator system [7]. In studies on PTMs in the mangrove leaves and their habitat sediments, many researchers have reported the same from Saudi Arabia [3,16,17,19,20,24] and Australia [22,25].
There have been prior reports of the genus Rhizophora (family: Rhizophoraceae) from the mangrove habitats in Peninsular Malaysia [26,27,28,29]. According to these reports, R. apiculata is a primary mangal vegetation in the Sepang mangrove habitat [30]. R. apiculata Blume, 1827, has a ‘Least Concern’ rating on the International Union for Conservation of Nature (IUCN) Red List [31]. This species can be found in the mid-intertidal intermediate estuary zone [32], and it may grow best in salinities between 8 and 15 ppt, with a maximum salinity of 65 ppt [33]. Na+ concentrations in the xylem of non-salt-secreting species such as Rhizophora spp. are typically less than one-hundredth in seawater, and absorption is mostly regulated at the root endodermis [34]. In addition to the processes that control Na influx/transport, it is likely that mangroves achieve metal influx and transport regulation by many other routes.
The studies of mangrove plants for ecotoxicological monitoring [19,20,24,35,36,37,38,39,40,41,42] and phytoremediation [19,43] studies have been reported in the literature. In general, the mangrove Rhizophora species have been specifically focused upon [44,45,46,47,48,49]. In particular, R. apiculata has been investigated for Zn removal from the study site [50] and elemental distribution in the different parts of the plants [51], Setiu mangrove forest, Terengganu [52] and Balok mangrove forest, Pahang [53].
The mangrove is endangered by the disappearance of its habitat across its range, which is mostly due to resource exploitation and coastal development. Studies on the presence of PTMs in the leaves of the R. apiculata have been reported from several locations, including the Setiu Mangrove Forest in Malaysia [54], the Matang Mangrove Forest Reserve in Malaysia [52], the Balok mangrove forest in Malaysia [53], the Can Gio Mangrove Forest in Southern Vietnam [55], and Belawan in North Sumatera of Indonesia [56]. However, very limited studies on the west coast of Peninsular Malaysia are found. Furthermore, its potential to act as a biomonitor and its phytoremediator potentials are limitedly documented in the literature.
Therefore, the objectives of the present study were to (a) determine the potential of mangrove R. apiculata to act as a biomonitor of PTMs and (b) evaluate its potential to act as a phytoremediator of PTMs in the mangrove ecosystem at Sepang estuary and Lukut estuary.
2. Materials and Methods
2.1. Sampling Site Descriptions
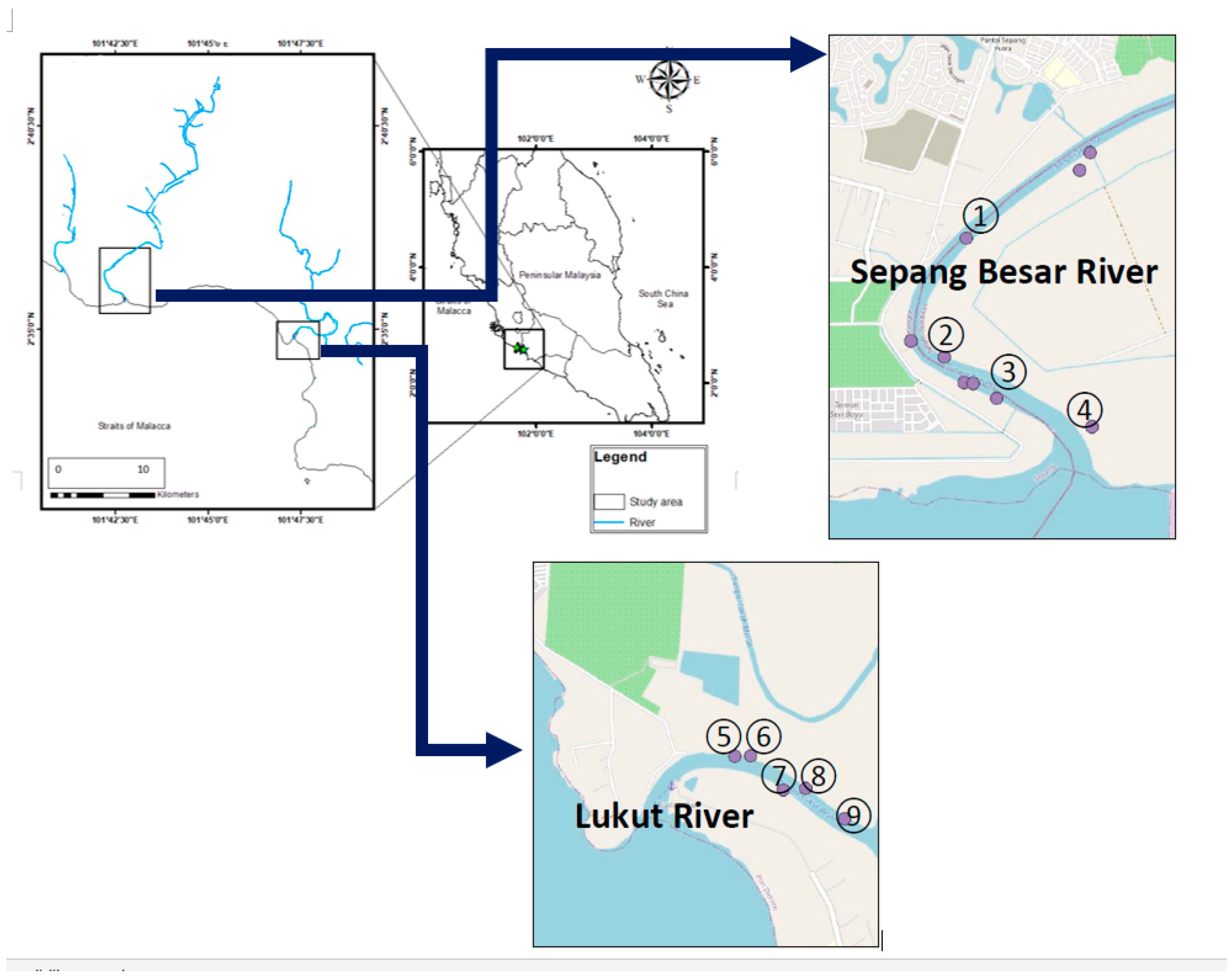
The mangrove leaves of R. apiculata and their surface sediments (0–10 cm) were collected from four sampling sites (1–4) in the Sepang Besar River and all five sampling sites (5–9) in the Lukut River (Figure 1; Table S1) on 1 December 2007. The sampling sites were selected based on sample availability and safety considerations. Only one sampling site was at Sepang Besar River S3 where the mangrove roots were collected. All the samples were transferred to the lab in zipped-lock polyethylene bags.
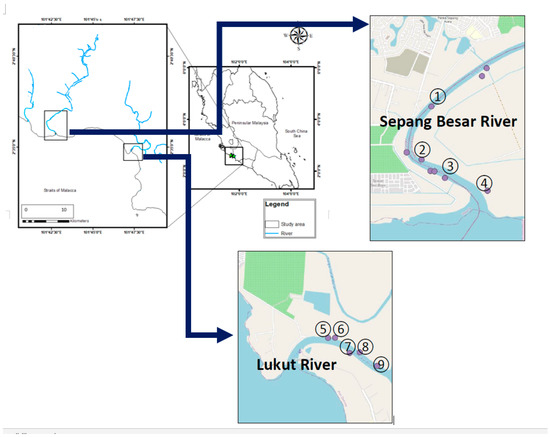
Figure 1.
Sampling sites in the Sepang Besar River (1–4), and Lukut River (5–9) from the present study.
All the samples were brought to the laboratory and oven-dried at 80 °C for 72 h to a constant dry weight. All dried sediments were passed through 63 µm sieves. The leaves samples were separated into the lamina and the midrib plus petiole (MP) (Figure S1). The roots were separated into barks and vascular bundles for further analysis. The plant samples were ground to homogenize the samples using a pestle and mortar.
2.2. Metal Analysis
The sediment and leave samples were digested using a wet digestion technique. The aqua-regia technique was applied to extract metals in the plant samples. Then, 5 mL of concentrated nitric acid (HNO3, AnalaR grade, BDH 69%) was added to the dried tissues. Each dried sediment sample (1.0 g) was digested using a combination of concentrated nitric acid (HNO3, AnalaR grade, BDH 69%) and perchloric acid (HClO4, AnalaR grade, BDH 60%) in the ratio 4:1 (10 mL) [57,58,59,60,61,62].
Samples were then placed in a digestion block at 40 °C for 1 h, and the samples were then fully digested at 140 °C for 3 h [61,62]. They were then diluted to 40 mL with double de-ionised water. Later, the diluted samples were filtered through Whatman No. 1 (filter speed: medium) filter paper into acid-washed pill boxes and stored at 4 °C until metal determination.
The sediment samples were fractionated into four fractions based on Badri and Aston [63]. The four fractions employed in this study were (i) ‘Easily, freely, leachable, or exchangeable’ (EFLE), (ii) ‘Acid-reducible’ (AR), (iii) ‘Oxidisable-organic’ (OO), and (iv) ‘Resistant’ (RES). The total concentrations are the summation (SUM) of all four geochemical fractions.
2.3. Metal Analysis
All the samples were analysed for Zn, Ni, Fe, Pb, and Cu by using an air-acetylene flame atomic absorption spectrophotometer (FAAS, Perkin Elmer Model AAnalyst 800; Perkin Elmer LLC, Waltham, CT, USA). Standard solutions were prepared from the stock solution provided by MERCK Titrisol for the six metals, and the data were presented in mg/kg dry weight basis.
2.4. Quality Control and Quality Assurance
All glassware and non-metal apparatuses used in this study were soaked in an acid bath (5% HNO3) for 72 h, after being washed with laboratory-grade detergent (Decon 90), to avoid possible contamination. The metal-made apparatuses were washed and soaked in laboratory-grade detergent (Decon 90) for at least 3 h before the analysis. Procedural blanks were employed, and quality control samples were made by diluting the standard solutions of the metals to be tested. These standard solutions were analysed after every 5–10 samples to check for the accuracy of the analysed samples.
There were four types of Certified Reference Materials (CRMs) checked with the samples to ensure the accuracy of the FAAS measurements. These CRMs included Lagarosiphon major (NR.60), Dogfish Liver-DOLT-3 (National Research Council Canada), marine sediments-(MESS-3, National Research Council Canada, Beaufort Sea), and NSC DC 73,319 (soil). Their recoveries were mostly acceptable (between 70%–120%) (Table S2). The detection limits for Fe, Cu, Ni, Pb, and Zn were 0.010, 0.010, 0.010, 0.009, and 0.007 mg/L, respectively.
2.5. Calculation of Bioconcentration Factor
The plant’s capacity to absorb and tolerate PTMs was calculated using the bioconcentration factor (BCF). This indicator is frequently used to assess whether plants might make effective phytoremediators [22,64]. The plant’s capacity to bioaccumulate metals from sediments is assessed using BCF. In the present study, five BCF values were used, as defined in the following Equations (1)–(5):
2.6. Statistical Analysis
The present data’s overall statistics and graphical histograms were achieved using Kaleida Graphs, version 5.0 (1986–2022 by Synergy Software, Eden Prairie, MN, USA). The Shapiro–Wilk test was chosen because it is the most extensively employed method to detect non-normality when the sample size is small (N < 50) [65,66,67].
Before doing correlation analysis, multiple linear (forward) stepwise regression analysis (MLSRA), and cluster analysis, those data that considerably deviated from a normal distribution (based on the Shapiro–Wilk normality test; <0.05) were converted using the log10 [value + 1] formula. This log10-transformation was used to stabilise the variance and the lack of normality to produce a frequency distribution that was more akin to a normal distribution and to satisfy the need for normality for the three statistical analytical models [68,69]. The correlation analysis, MLSRA, and cluster analysis were performed using STATISTICA (Version 10; StatSoft. Inc., Tulsa, OK, USA, 1984–2011). This has been shown by many studies on relationships between a dependent variable and independent variables [70,71,72,73,74,75].
The MLSRA was based on lamina and MP as dependent variables and the values of BCF (BCFEFLE, BCFAR, BCFOO, BCFRES, BCFSUM), while the independent variables included sedimentary geochemical fractions (EFLE, AR, OO, RES, and SUM) and surface water parameters (temperature, conductivity, and salinity).
For cluster analysis, the clustering patterns of the nine sampling sites were based on the metal concentrations (Cu, Fe, Ni, Pb, and Zn) in the leaves (lamina, as well as midrib plus petiole), the geochemical fractions of the sediments (EFLE, AR, OO, RES, and SUM), the values of BCFEFLE, BCFAR, BCFOO, BCFRES, BCFSUM, and surface water parameters (temperature, conductivity, and salinity).
3. Results
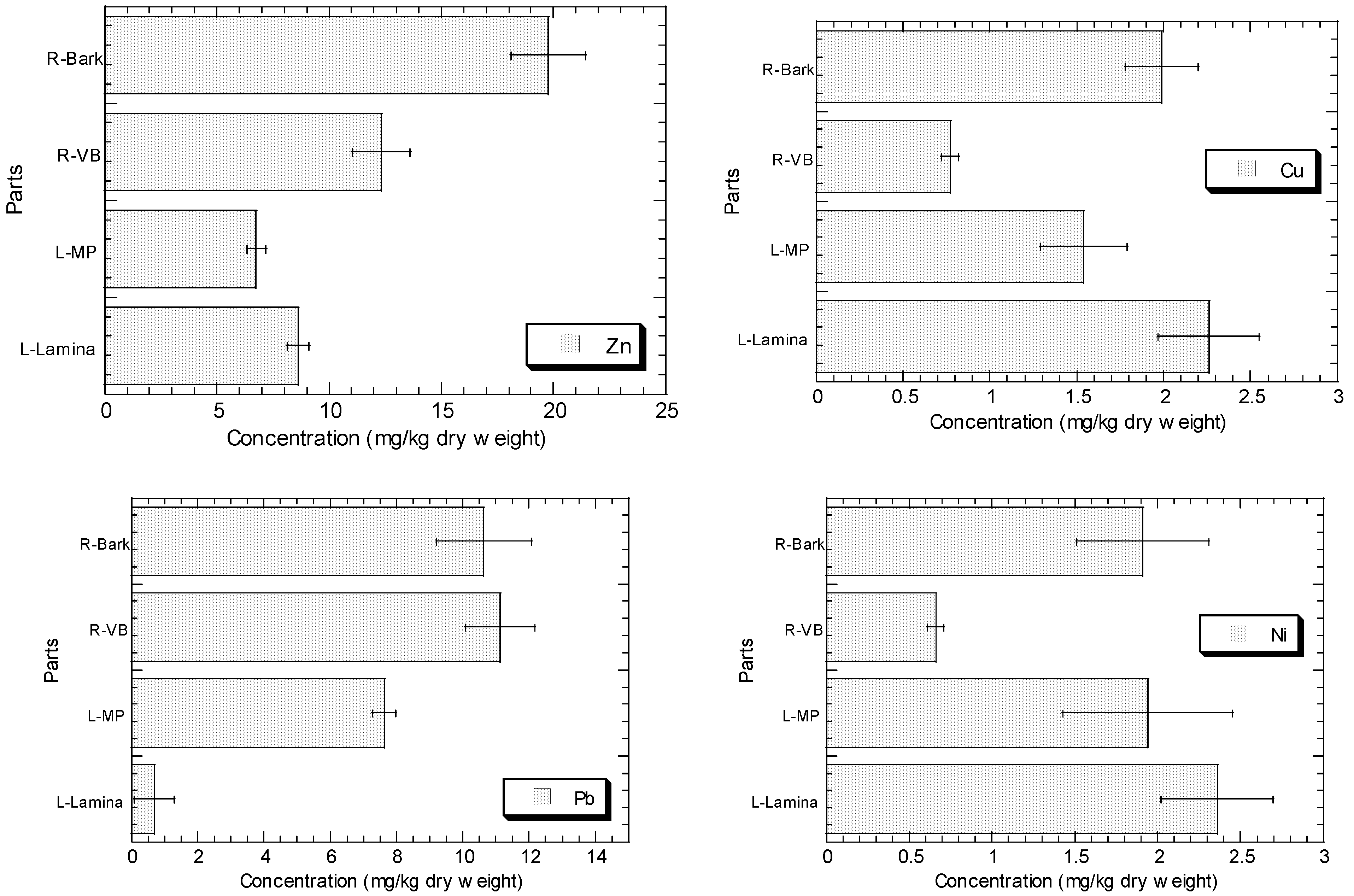
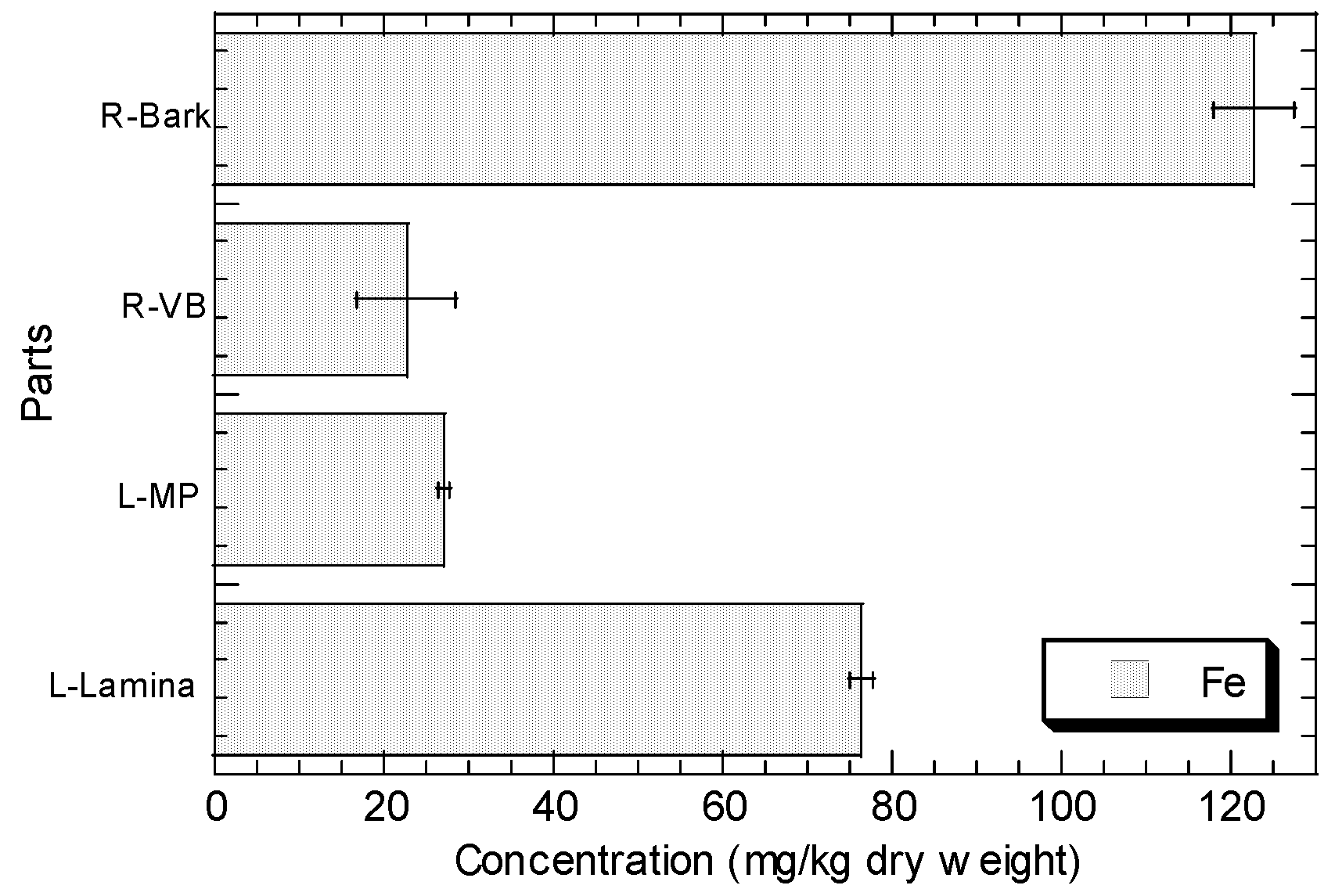
The concentrations of Cu, Fe, Ni, Pb, and Zn in the root barks, root vascular bundles, MP, and lamina of R. apiculata collected from Sepang (S3) are presented in Figure 2. In general, the levels of Zn, Cu, Fe, and Ni in the lamina are higher than those in MP, while Pb shows the contrast. The levels of Zn, Cu, Fe, and Ni in the root bark are higher than those in root vascular bundle, but Pb shows the contrast.
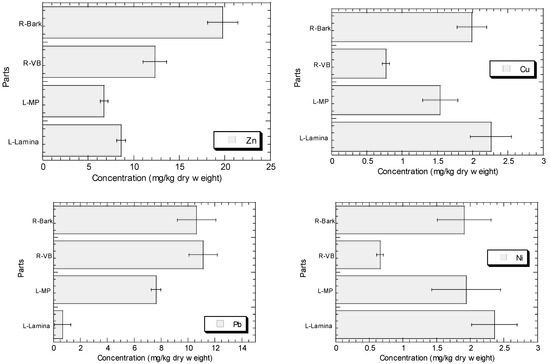
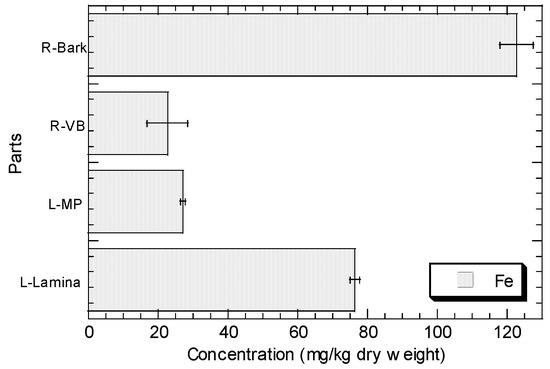
Figure 2.
Concentrations (mean ± standard error, mg/kg dry weight) of Cu, Fe, Ni, Pb, and Zn in the root barks (R-Bark), root vascular bundles (R-VB), leaf midrib plus petiole (L-MP), and leaf lamina (L-Lamina) of Rhizophora apiculata collected from Sepang (S3).
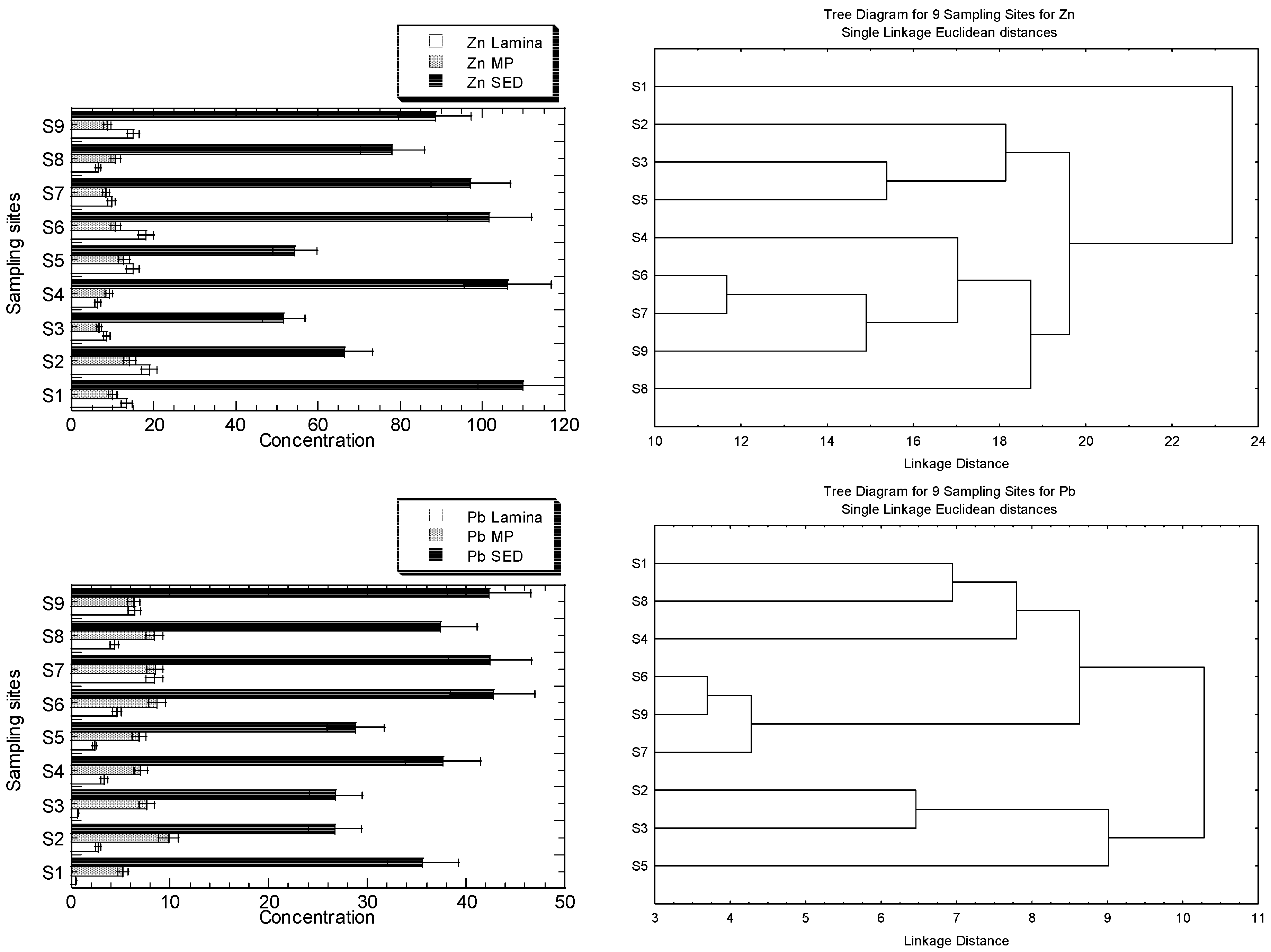
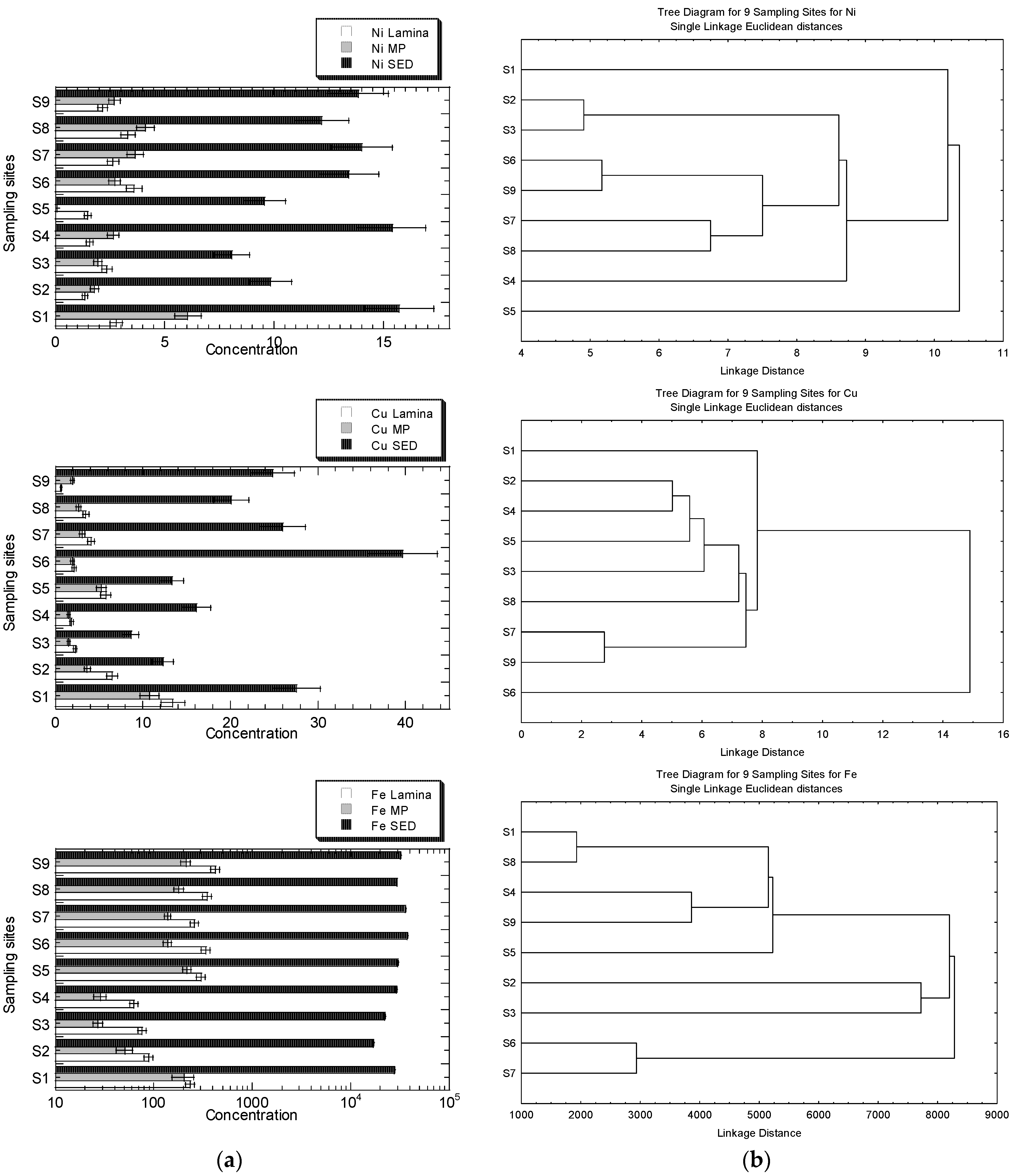
The concentrations of Cu, Fe, Ni, Pb, and Zn in the MP), lamina of R. apiculata, and their habitat surface of four geochemical fractions in the sediments, are presented in Figure 3a. The clustering patterns of the nine sampling sites for Cu, Fe, Ni, Pb, and Zn, based on the metal concentrations in the leaves (lamina and midrib plus petiole) and the geochemical fractions of the sediments, are presented in Figure 3b.
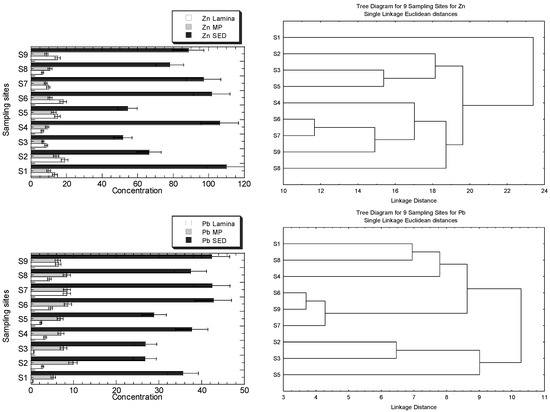
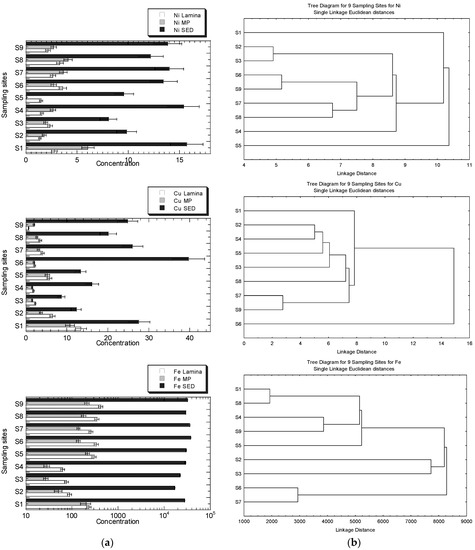
Figure 3.
(a) Concentrations (mean ± standard error, mg/kg dry weight) of Cu, Fe, Ni, Pb, and Zn in the leave midrib plus petiole (MP), leaf lamina (Lamina) of Rhizophora apiculata, and their habitat surface sediments (SED = summation of four geochemical fractions) collected from Sepang River (S1–S4) and Lukut River (S5–S9). Note: Fe X-axis is drawn in logarithmic scale. (b) Clustering patterns of the nine sampling sites for Cu, Fe, Ni, Pb, and Zn, based on the metal concentrations in the leaves (lamina, and midrib plus petiole), and the geochemical fractions of the sediments (EFLE, AR, OO, RES, and SUM), as well as the values of BCFEFLE, BCFAR, BCFOO, BCFRES, and BCFSUM surface water parameters (temperature, conductivity, and salinity). Only the parameters with p < 0.05, based on the Shapiro–Wilk normality test, were log10 (value + 1) transformed before cluster analysis; sampling sites’ information followed those in Table S1.
The highest level of Zn in the sediment is found in S1, but it is not visibly found in the mangrove leaves. Overall, the levels of Zn and Ni in S1 and S4 are higher than in other sites, but the clustering patterns for both metals are not well indicated. The higher levels of Zn in S2 and S6 are also not visibly indicated in the clustering patterns. Only the highest levels of Ni in S1, shown by MP and sediments, are indicated by the clustering pattern where S1 is clustered as a single entity. There have been no clear clustering patterns for Zn for the highest levels of Zn in MP at S2 and sediment at S6. The highest level of Cu in the sediments at S6 is well indicated as a single entity, while the highest Cu in lamina, with comparatively higher Cu levels in sediments at S1, is single-subclustered. The higher levels of Pb, at S6, S7, and S9 in the sediments, are well-indicated as subclusters, while the highest Pb level in MP at S2 is not indicated in the clustering pattern. There has been no visibly clear pattern of clustering patterns for Fe, but the levels of Fe in the lamina and MP at S2, S3, and S4 are comparatively lower than at other sites. However, only S2 and S3 are subclustered as a single entity for Fe.
3.1. Zinc
The Zn levels (mg/kg dry weight) in MP and lamina are 6.08–14.2, and 7.28–18.9, respectively (Table S3; Table 1). The mean Zn values indicate that the Zn lamina (13.6) is significantly (p < 0.05) higher than that (9.43) in MP.

Table 1.
Overall statistics of Zn concentrations (mg/kg dry weight) in the midrib plus petiole (MP) and lamina of Rhizophora apiculata, with their habitat geochemical fractions of sediments and their bioconcentration factors (BCF) values, collected from nine sampling sites in the mangrove areas of Sepang and Lukut. N = 9.
The total Zn concentrations in the surface sediments ranged from 45.5 to 88.2 mg/kg dry weight. When comparing to the sediment quality guideline values (Table 1), all Zn levels were below those by Chapman et al. [76], Macdonald et al. [77], and Long et al. [78]. When compared to the reference background values (Table S4), the maximum range of Zn levels of the Sepang and Lukut mangroves were higher than the background Zn levels proposed by Taylor and McLennan [79], Rudnick and Gao [80], Wedepohl [81], and the background level of Peninsular Malaysia [60], while they are within the mangrove sediments reported by Cheng and Yap [61].
The overall mean values in the MP for BCFEFLE, BCFAR, BCFOO, BCFRES, and BCFSUM are 5.22, 0.53, 0.37, 0.33, and 0.12, respectively. These BCF values are lower than those in the lamina, in which their values of BCFEFLE, BCFAR, BCFOO, BCFRES, and BCFSUM are 7.59, 0.75, 0.50, 0.46, and 0.17, respectively. This indicates that lamina has a better potential as a phytoremediator of Zn.
The relationships of Zn concentrations between mangrove leaves (lamina and MP) and geochemical fractions in the surface mangrove sediments are shown in Table 2: It is observed that the Zn lamina shows non-significant (p > 0.05) correlations with all geochemical fractions but positive correlations with all five BCF values (R = 0.53 to 0.81; p < 0.05). The Zn MP levels also correlate positively (R = 0.38 to 0.79; p < 0.05) with EFLE and four BCF values (BCFAR, BCFOO, BCFRES, and BCFSUM). The overall R values between Zn lamina–BCF pairwises are higher than those between Zn MP–BCF pairwises.

Table 2.
Zinc correlation coefficients between variable A (Zn levels in the lamina and Fe bioconcentration factors (BCF)) and variable B (geochemical fractions in the sediments and surface water parameters). Values in red are significant at p < 0.05.
3.2. Iron
The Fe levels (mg/kg dry weight) in MP and lamina are 27.1–217, and 63.2–424, respectively (Table S5; Table 3). The mean Fe values indicated the Fe lamina (237) is significantly (p < 0.05) higher than that (133) in MP. The total Fe concentrations in the surface sediments ranged from 13,564 to 32,489 mg/kg dry weight, which were lower than the Fe UCC concentration by Wedepohl [81].

Table 3.
Overall statistics of Fe concentrations (mg/kg dry weight) in the midrib plus petiole (MP) and lamina of Rhizophora apiculata, with their habitat geochemical fractions of sediments and their bioconcentration factor (BCF) values, collected from nine sampling sites in the mangrove areas of Sepang and Lukut. N = 9.
The overall mean values in the MP for BCFEFLE, BCFAR, BCFOO, BCFRES, and BCFSUM are 0.71, 0.104, 0.03, 0.006, and 0.004, respectively. These BCF values are lower than those in lamina, in which their values of BCFEFLE, BCFAR, BCFOO, BCFRES, and BCFSUM are 1.24, 0.184, 0.05, 0.011, and 0.008, respectively. This indicates that lamina has a better potential as a phytoremediator of Fe.
The relationships of Fe concentrations between mangrove leaves (lamina and MP) and their geochemical fractions in the surface mangrove sediments are shown in Table 4: It is observed that the Fe lamina shows positive correlations with all the geochemical fractions (R = 0.48 to 0.68; p < 0.05), except for EFLE and all five BCF values (R = 0.53 to 0.96; p < 0.05). The Zn MP levels also correlate positively with F4 (R = 0.46; p < 0.05) and all five BCF values (R = 0.60 to 0.93; p < 0.05). The overall R values between Fe lamina–BCF pairwises are higher than those between Fe MP–BCF pairwises. Interestingly, the number of significant and positive correlations between Fe lamina–geochemical fractions is higher than those between Fe MP–geochemical fractions.

Table 4.
Iron correlation coefficients between variable A (Fe levels in the lamina (L), midrib plus petiole (MP), and Fe bioconcentration factors (BCF)) and variable B (geochemical fractions in the sediments and surface water parameters). Values in red are significant at p < 0.05.
3.3. Lead
The Pb levels (mg/kg dry weight) in MP and lamina are 5.25–9.88 and 0.41–8.43, respectively (Table S6; Table 5). The mean Pb values indicated that the Pb lamina (3.70) is significantly (p < 0.05) lower than that (7.39) in MP.

Table 5.
Overall statistics of Pb concentrations (mg/kg dry weight) in the midrib plus petiole (MP) and lamina of Rhizophora apiculata, with their habitat geochemical fractions of sediments and their bioconcentration factors (BCF) values, collected from nine sampling sites in the mangrove areas of Sepang and Lukut. N = 9.
The total Pb concentrations in the surface sediments ranged from 25.6 to 47.4 mg/kg dry weight. When comparing them to the sediment quality guideline values (Table 1), the present maximum Pb levels were below those by Chapman et al. [76], Macdonald et al. [77] (except for TEL), and Long et al. [78] (except for ERL). When compared to the reference background values (Table 1), the maximum range of Pb levels of the Sepang and Lukut mangrove was higher than the background Zn levels proposed by Taylor and McLennan [79], Rudnick and Gao [80], Wedepohl [81], and the Pb background level of Peninsular Malaysia [59], while it is within the mangrove sediments reported by Cheng and Yap [61].
The overall mean values in the MP for BCFEFLE, BCFAR, BCFOO, BCFRES, and BCFSUM are 5.88, 17.97, 1.04, 0.31 and 0.22, respectively. These BCF values are higher than those in lamina, in which their values of BCFEFLE, BCFAR, BCFOO, BCFRES, and BCFSUM are 2.89, 10.5, 0.48, 0.14, and 0.10, respectively. This indicates that MP has better potential as a phytoremediator of Ni.
The relationships of Pb concentrations between mangrove leaves (lamina and MP), and their geochemical fractions in the surface mangrove sediments are shown in Table 6: It is observed that the Pb lamina shows positive correlations with all the geochemical fractions (R = 0.46 to 0.72; p < 0.05), except for EFLE and AR fractions and all five BCF values (R = 0.79 to 0.98; p < 0.05). The Pb MP levels also correlate positively with EFLE (R = 0.76; p < 0.05) and three BCF values (R = 0.60 to 0.76; p < 0.05). The overall R values between Pb lamina–BCF pairwises are higher than those between Pb MP–BCF pairwises.

Table 6.
Lead correlation coefficients between variable A (Pb levels in the lamina (L), midrib plus petiole (MP), and Pb bioconcentration factors (BCF)) and variable B (geochemical fractions in the sediments and surface water parameters). Values in red are significant at p < 0.05.
3.4. Copper
The Cu levels (mg/kg dry weight) in MP and lamina are 1.53–10.8, and 0.69–13.4, respectively (Table S7; Table 7). The mean Cu values indicate the Cu lamina (4.46) is significantly (p < 0.05) higher than that (3.60) in MP.

Table 7.
Overall statistics of Cu concentrations (mg/kg dry weight) in the midrib plus petiole (MP) and lamina of Rhizophora apiculata, with their habitat geochemical fractions of sediments and their bioconcentration factors (BCF) values, collected from nine sampling sites in the mangrove areas of Sepang and Lukut. N = 9.
The total Cu concentrations in the surface sediments ranged from 9.00 to 22.7 mg/kg dry weight. When comparing them the sediment quality guideline values (Table 1), all Cu levels were below those by Chapman et al. [76], Macdonald et al. [77] (except for TEL), and Long et al. [78]. When compared to the reference background values (Table 1), the maximum range of Cu levels of the Sepang and Lukut mangroves were higher than the background Cu levels proposed by Wedepohl [81] and the Cu background level of Peninsular Malaysia [57], while they are within the mangrove sediments reported by Cheng and Yap [61].
The overall mean values in the MP for BCFEFLE, BCFAR, BCFOO, BCFRES, and BCFSUM are 51.1, 39.3, 0.63, 0.28 and 0.19, respectively. These BCF values are lower than those in lamina, in which their values of BCFEFLE, BCFAR, BCFOO, BCFRES, and BCFSUM are 72.4, 44.2, 0.82, 0.37, and 0.25, respectively. This indicated that lamina has a better potential as a phytoremediator of Cu.
The relationships of Cu concentrations between mangrove leaves (lamina and MP), and their geochemical fractions in the surface mangrove sediments are shown in Table 8: It is observed that the Cu lamina shows a positive correlation with AR (R = 0.70; p < 0.05), and all five BCF values (R = 0.53 to 0.87; p < 0.05). The Cu MP levels also correlate positively with AR (R = 0.63; p < 0.05) and four BCF values (R = 0.45 to 0.84; p < 0.05). The overall Cu R values between lamina–BCF pairwises are higher than those between Cu MP–BCF pairwises.

Table 8.
Copper correlation coefficients between variable A (Cu levels in the lamina (L), midrib plus petiole (MP), and Cu bioconcentration factors (BCF)) and variable B (geochemical fractions in the sediments and surface water parameters). Values in red are significant at p < 0.05.
3.5. Nickel
The Ni levels (mg/kg dry weight) in MP and lamina are 1.79–6.05 and 1.35–3.60, respectively (Table S8; Table 9). The mean Ni values indicate that the Ni lamina (2.36) is significantly (p < 0.05) lower than that (3.14) in MP.

Table 9.
Overall statistics of Ni concentrations (mg/kg dry weight) in the midrib plus petiole (MP), and lamina of Rhizophora apiculata, with their habitat geochemical fractions of sediments and their bioconcentration factors (BCF) values, collected from nine sampling sites in the mangrove areas of Sepang and Lukut. N = 9.
The total Ni concentrations in the surface sediments ranged from 8.49 to 17.1 mg/kg dry weight. When comparing the sediment quality guideline values (Table 1), all Ni levels were below those by Chapman et al. [76], Macdonald et al. [77] (except for TEL), and Long et al. [78]. When compared to the reference background values (Table 1), the maximum Ni range of the Sepang and Lukut mangroves was lower than all the values and only higher than the Ni background level of Peninsular Malaysia [58], while it is within the mangrove sediments reported by Cheng and Yap [61].
The overall mean values in the MP for BCFEFLE, BCFAR, BCFOO, BCFRES, and BCFSUM are 21.2, 10.5, 0.81, 0.40 and 0.25, respectively. These BCF values are higher than those in lamina, in which their values of BCFEFLE, BCFAR, BCFOO, BCFRES, and BCFSUM are 15.3, 7.75, 0.63, 0.31, and 0.19, respectively. This indicates that MP has better potential as a phytoremediator of Ni.
The relationships of Ni concentrations between mangrove leaves (lamina, and MP), and their geochemical fractions in the surface mangrove sediments are shown in Table 10: It is observed that the Ni lamina shows a positive correlation with RES fraction (R = 0.49; p < 0.05) and all four BCF values (R = 0.40 to 0.78; p < 0.05). The Ni MP levels also correlate positively with all geochemical fractions (R = 0.54 to 0.74; p < 0.05) and five BCF values (R = 0.67 to 0.93; p < 0.05). The overall R values between Ni MP–BCF pairwises are higher than those between Ni lamina–BCF pairwises. Interestingly, the number of significant and positive correlations between Ni MP–geochemical fractions is higher than those between Ni lamina–geochemical fractions.

Table 10.
Nickel correlation coefficients between variable A (Ni levels in the lamina (L), midrib plus petiole (MP), and Ni bioconcentration factors (BCF)) and variable B (geochemical fractions in the sediments and surface water parameters). Values in red are significant at p < 0.05.
3.6. Influential Parameters for the Metal Accumulation by the Mangrove
The MLSRA outputs are based on lamina and MP as dependent variables, and the independent variables, including the BCF values, sedimentary geochemical fractions, and surface water parameters, are presented in Table 11.

Table 11.
Multiple linear stepwise regression analytical outputs based on Rhizophora leaves (lamina (L); midrib plus petiole (M)), as dependent variables and independent variables, are the bioconcentration factors, sedimentary geochemical fractions, and surface water parameters.
For Zn, six similar variables (BCFAR, AR, Temp, BCFOO, BCFSUM, and BCFRES) are similarly selected as influential factors in Zn lamina’s and Zn MP’s accumulations. However, only Sal and OO are selected for Zn lamina, while additional three variables (SUM, EFLE, and RES) are selected for Zn MP.
For Fe, three similar variables (BCFSUM, Cond, and Sal) are similarly selected as influential factors in the accumulations of Fe lamina and Fe MP. However, only SUM is selected for Fe lamina, while five additional variables (SUM, AR, BCFRES, BCFEFLE, and BCFAR) are selected for Fe MP.
For Pb, four similar variables (BCFSUM, RES, Temp, and Sal) are similarly selected as influential factors in Pb lamina and Pb MP. However, six additional variables (SUM, BCFRES, EFLE, BCFAR, AR, and BCFEFLE) are selected for Pb MP.
For Cu, seven similar variables (BCFRES, RES, SUM, BCFSUM, BCFOO, OO, and Cond) are similarly selected as influential factors in the accumulations of Cu lamina and Cu MP. However, only Temp and BCFAR are selected for Cu lamina, while additional AR and EFLE are selected for Cu MP.
For Ni, four similar variables (BCFSUM, OO, Cond, and Temp) are similarly selected as influential factors in the accumulations of Ni lamina and Ni MP. However, only OO is selected for Ni lamina, while six additional variables (SUM, BCFRES, BCFOO, BCFEFLE, BCFAR, and AR) are selected for Ni MP.
4. Discussion
4.1. The Levels of Essential Fe, Cu and Zn in Lamina Are Significantly (p < 0.05) Higher Than in Midrib plus Petiole
The higher number of significant and positive correlations between the essential metal lamina–geochemical fractions than those between Fe MP–geochemical fractions could support the higher levels of Fe, Cu, and Zn in the lamina than MP. This phenomenon was also indicated by the differences in influential variables selected for the accumulation of Fe, Cu, and Zn between lamina and MP.
This demonstrated that the lamina had better potential to accumulate the necessary essential Fe, Cu, and Zn than the MP. The higher levels of essential metals in the lamina could be because these metals are needed for the metabolism during the photosynthetic activities of the lamina, and these metals are required to produce carbohydrates for the plant.
The lamina is in charge of the plant’s photosynthesis. Managing the leaf lamina function by the leaf lamina area is crucial for maintaining the viability of plant life, depending on the ecology. It contains chloroplast, which gives leaves their green colour, so a leaf is referred to as the kitchen of the plant. The petiole, which connects the leaf’s flat surface to the plant, is referred to as the lamina. The main portion of the leaf, or lamina, has a network of stomata that allow carbon dioxide and oxygen to be taken in and exhaled. These stomata, which are connected by primary and secondary veins, open and close in response to the plant’s needs for processing food. The lamina receives its structure and shape from the midrib. Chlorophyll is found in the lamina, which serves as a trap for the energy from sunshine. The mixture of water and carbon dioxide then uses the energy trapped to produce glucose. The plant’s food, glucose, is created and transported to different sections of the plant by the phloem, or vascular tissue, which is also found in the lamina. The primary purpose of green leaves is a process known as photosynthesis [82].
Essential micronutrients, Cu, Fe, and Zn, and their uptake and allocation to plant organs, such as photosynthetic leaf tissue, are high and active [51]. The highest value of the essential metals compared to non-essential metals of R. apiculata is closely related to the role of this element in growth and plant cell maintenance. Due to the significant roles that vital metals play in the organelle mechanisms of the leaf structure, it is possible that the greater amounts of Fe, Cu, and Zn in the lamina are the result. The Fe substance is required to provide them with the chlorophyll that gives them their distinctive green colour and absorbs light energy. Cu and Zn are required to construct a protective leaf epidermis at the lamina levels.
Fe is a necessary transition metal, with a redox-active character, under biological conditions [83] since it is a crucial micronutrient [84]. Redox enzymes use several Fe compounds as cofactors. They involve metabolic activities such as DNA synthesis, respiration, and photosynthesis [84]. Despite being common in most well-aerated soils, Fe has a low biological activity because, at neutral pH levels, it largely forms very insoluble ferric compounds. Iron plays a crucial role in numerous physiological and metabolic processes in plants. It is needed for various biological processes, as it is a component of numerous essential enzymes, including cytochromes of the electron transport chain. Fe is required to maintain chloroplast structure and function in plants, and it is involved in synthesising chlorophyll. However, little is known about how Fe status is regulated in leaves.
Cu is a vital cofactor for many metalloproteins, and it is involved in some physiological processes. However, elevated bioaccumulation of Cu in the lamina could stunt the growth and compromises crucial cellular functions, such as photosynthetic electron transport [85]. Different mechanisms have developed in plants to properly manage Cu homeostasis as a function of the environmental Cu level because Cu is both a vital cofactor and a harmful element, including a complex network of metal trafficking routes. Plastocyanin (PC) is an abundant and crucial Cu protein needed for photosynthesis in higher plants, according to Shahbaz et al. [86]. Due to a lack of PC, severe Cu shortage may result in a flaw in photosynthetic electron transport.
Besides playing a crucial part in the growth, Zn also plays a role in plants’ ability to protect themselves, with a focus on superoxide dismutases [87,88,89]. The amount of available Zn and the type of plant impact Zn distribution and transport in plants. Zn concentrations are often higher in developing tissue than in mature tissue when plants have low to adequate Zn supplies [88]. Accumulation has been seen in plants’ root cortices and leaves that can tolerate harmful Zn levels. Zn was accumulated in the cell walls or stored in vacuoles in the lamina [89].
4.2. The Levels of Non-Essential Pb and Ni in Midrib plus Petiole Is Significantly (p < 0.05) Higher Than in Lamina
The higher number of significant and positive correlations between Ni MP–geochemical fractions are higher than those between Ni lamina–geochemical fractions, supporting that the non-essential metal in MP is higher than in lamina.
The non-essentiality of Pb and Ni to plant growth, with low solubility and their passive uptake, caused their translocation from roots to other plant organs to be generally low [51]. The lower mean values of Pb and Ni also indicated that these non-essential elements are not significantly involved in the major process of R. apiculata. This phenomenon was also indicated by the differences in influential variables selected for accumulating Pb and Ni between lamina and MP.
Although Pb is not necessary for plants, it is easily absorbed and accumulates in many plant tissues [90]. Particle size, cation exchange capability, pH, root exudation, and other physico-chemical factors affect how much Pb plants can absorb. Mechanisms of Pb detoxification include binding to glutathione and amino acids, sequestering Pb in the vacuole, phytochelatin production, and other processes. Pb tolerance is linked to a plant’s ability to confine Pb to its cell walls, producing osmolytes and activating its antioxidant defence system. Using phytoremediation and rhizofiltration technologies to clean up Pb-contaminated soils appears to have many potential metabolisms, while growth and photosynthetic activity of the lamina were found to be harmed by the high intake of Pb. However, excessive Pb bioaccumulation in plant leaves might result in a 42% reduction in root growth [91].
Although generally considered a non-essential metal, Ni is a component of urease and hydrogenase. It has been acknowledged as a necessary element for plants and was the most recent element to be added to the list of essential nutrients for plants [92]. According to van der Ent et al. [93], particular metal sequestering and transport processes cause a high level of Ni accumulation in hyperaccumulator plants. Understanding these processes is essential for understanding how these plants regulate the metabolism of transition elements. The chemical speciation of Ni2+ is unmistakably linked to citrate and does not significantly vary between species, plant tissues, or transport fluids.
4.3. The Lamina Has a Better Potential as a Phytoremediator of Cu, Zn and Fe, while Midrib plus Petiole Has a Better Potential as a Phytoremediator of Pb and Ni
The overall R values between the essential metal (Fe, Cu, and Zn) lamina–BCF pairwises, which are higher than those between MP–BCF pairwises, could support that lamina is a better phytoremediator of Cu, Zn, and Fe than MP. The relatively similar numbers of influential variables selected for the accumulation of Zn lamina (8 variables) compared to Zn MP (9 variables), as well as similar numbers of influential variables for the accumulation of Cu lamina (9 variables) and Cu MP (9 variables), could be used to understand why lamina could be a good phytoremediator for Zn and Cu for lamina.
The overall R values between Ni MP–BCF pairwises are higher than those between Ni lamina–BCF pairwises, supporting that MP has a better potential as a phytoremediator of Ni. The clear higher numbers of influential variables selected for the accumulation of Pb MP (10 variables) compared to Pb lamina (4 variables), as well as for the accumulation of Ni MP (10 variables) compared to Ni lamina (5 variables), indicated one point. The more variables with higher R values for Pb and Ni in MP supported the correlation pairwises mentioned previously. Hence, this could be a proxy of MP, which is a better phytoremediator for Pb and Ni than lamina.
According to Kamaruzzaman et al. [52], BCF values showed that R. apiculata contains substantially less lead than silt, at just 0.96 times the levels. It is believed that the mechanism behind the increased accumulation of metals in plant tissues is the translocation of air absorbed by lenticels in pneumatophores to subterranean roots. In the anaerobic soil environment, this leads to the production of oxidised rhizospheres, a decline in complexing sulphides, a reduction in the stability of Fe plaques, and an increase in the concentration of exchangeable trace metals [94].
MacFarlane and Burchett [95] reported that A. marina revealed that Pb showed little absorption and minimal movement, while Cu and Zn had the largest accumulation. Sazon and Veronica [18] reported that Zn (0.7) in Avicennia sp. had the highest BCF values, whereas metals with high mean levels were in sediments (4244 mg/kg Ni and 137,049 mg/kg Fe) had lower BCF values. They concluded that R. apiculata could be useful in the remediation of Ni-Fe-laden sediments, particularly for phytostabilization, based on the limited translocation of Ni and Fe in these plants.
From the present study, all values of BCFOO, BCFRES, and BCFSUM were found below 1.0, indicating a low PTM uptake. The BCFSUM value is conventionally used, which is based on total metal concentration. However, the present BCF values were higher than 1.0 for BCFEFLE and BCFAR, indicating the substantial update of PTM at the EFLE and AR fractions of the mangrove habitat sediments. Even though BCF calculates species’ potential for phytoremediation [1], the effectiveness that was based on the total metal concentration of the sediments is debatable. This debate is somewhat masked by the many reported studies concluding that hyperaccumulators are interpreted by BCF > 1, which are also tolerant to high concentrations of PTMs [96].
Usman et al. [3] claimed that the BCF, which measures the ratio of metal concentration in tissue compared to that in sediment, can be used to quantify how well plants and aquatic creatures can absorb contaminants from sediments. Qiu et al. [1] reported that the leaf BCF values for Cu, Ni, and Zn are, respectively, 1.25–9.08, 0–1.02, and 0.20–4.71. They found that most of these BCF values were deemed excessive (>1), indicating that A. marina may be a highly effective plant for bioaccumulating PTMs.
Low bioavailability of PTMs in sediments and/or preventing metal uptake by mangroves can account for the lowest BCF values found in highly metal-contaminated sediments. It was speculated that, by complexing with organic matter and/or precipitating sulphides under decreasing conditions, mangrove sediments could immobilise PTMs in inaccessible forms [94].
Due to greater metal concentrations in the leaves (284–496 mg/kg) and BCF values >1, Usman and Mohamed [97] concluded that A. marina might be categorised as a possible accumulator for Cu (1.25–9.08). However, they cannot be regarded as prospective hyperaccumulators since they lack the necessary quantities of Cu in the above-ground tissues, which should be >1000 mg/kg [69]. In R. apiculata from Setiu Mangrove Forest, Terengganu, Malaysia, Kamaruzzaman et al. [52] reported Cu concentrations of 2.73 and 9.42 mg/kg dry weight in the leaves and sediments, respectively, and Zn concentrations of 1.43 and 11.7 mg/kg dry weight in the leaves and sediments, respectively. According to Khan et al. [54], essential Fe and Cu contents in tissue samples from R. apiculata were higher at the 80-year-old site than at the 15-year-old location. Due to the 80-year-old compartment’s proximity to an urban area, this may be a side effect.
Interestingly, mangroves thriving in less contaminated environments had the highest BCF values [98]. In contrast, plants from the most contaminated area had the highest translocation factors, showing that Avicennia schaueriana could adapt to unfavourable circumstances [97]. Nath et al. [25] concluded that A. marina could be used as phytostabilizers in this heavily altered estuary, shielding the aquatic ecosystem from direct or indirect sources of PTM contamination. Usman et al. [3] also investigated the capacity of mangroves (A. marina) from Farasan Island, the coast of the Red Sea, to store and translocate PTMs inside their numerous compartments. Based on BCF values, their most often obtained values were found to be too high (>1), indicating that A. marina can be regarded as a highly effective plant for the bioaccumulation of PTMs.
4.4. Lamina Is a Potential Biomonitoring Agent of Potentially Toxic Metals
The MP generally exhibits negative connections with all geochemical fractions, but the Zn lamina exhibits positive relationships with all geochemical fractions. Except for EFLE, all geochemical fractions and total summation fractions indicate positive associations between Fe levels in the two leaf portions (lamina and MP), with lamina exhibiting higher R values for SUM, AR, OO, and RES. The Pb lamina exhibits favourable correlations with each geochemical fraction (except for AR). The MP, however, exhibits adverse associations with each geochemical fraction (except for EFLE, AR and OO). Except for AR, all geochemical fractions had poor associations with the Cu levels in the lamina and MP of leaves. Except for EFLE, the Ni concentrations in both the lamina and the MP of leaves demonstrate positive associations with all geochemical fractions and total summation fractions, with the MP displaying higher R values for SUM, OO, and RES.
Some authors have reported the positive correlation of metals between mangrove leaves and their habitat sediments. Except for Cu and Cd, which don’t correlate with other metal concentrations, Aljahdali and Alhassan [19] found a substantial link between the PTM concentrations in sediment and A. marina leaves. Bakshi et al. [24] found that the substantial association between the metal concentration in A. officinalis leaves collected from Sundarban mangrove wetland and the sediment metals suggested that there has been extensive bioaccumulation. According to Usman et al. [3], the highest levels of Cu in the leaves of A. marina are correlated with the highest Cu levels in the nearby sediments.
According to Kamaruzzaman et al. [52], Pb and Cu are observed to have greater concentrations in the sediments in Setiu mangrove habitats. Their concentrations are on the rise, especially close to the estuary, which is consistent with their predominately lithogenous origin. Furthermore, compared to the coarse-grained sediments of the inner portion of the rivers, the fine-grained sediments in the mangrove and the surrounding area are characterised by higher quantities of these trace elements. The contents of Pb and Cu are positively correlated with grain size, as documented by several scientists [99,100], indicating the role of the fine fraction in their assimilation into the sediments. Lower Pb and Cu ratios and larger mean particle sizes indicate sediments located further from the estuary. Cu content was higher than Pb, according to Yunasfi et al. [39], who used R. apiculata samples from Belawan (North Sumatra).
Ganeshkumar et al. [49] suggested that R. mucronata could be used as a biomonitor for monitoring Pb pollution in the Muthupet mangrove ecosystem, based on the high accumulation and translocation rate of Pb. Based on the values of the biota–sediment accumulation factors (BSAF) of metals in mangrove tissues, Qiu and Qiu [48] concluded that Rhizophora stylosa from Dongzhai Harbor and Sanya Bay of Hainan Island can be used as a biomonitor of Cu and Zn pollution with temporal monitoring. Being a good biomonitor of metal pollutants is attributed to the mangrove’s characteristics, including tolerance to metal pollution in the mangrove ecosystem. For instance, Shams et al. [42] confirmed that the antioxidative defence system plays a critical role in R. mucronata to tolerate the multiple PTMs stress. Nualla-ong et al. [41] also suggested that the concentration of glutathione and phytochelatin synthase play an important role in PTM tolerance in R. mucronata seedlings. Based on the study of the efficiency of R. apiculata mangrove plants in Zn metal removal made from Zn artificial solution using a laboratory scale reactor, Razif and Farhan [40] reported the positive efficiency of Zn removal of R. apiculata.
4.5. Low Metal Uptake in the Mangrove Leaves
According to data from Avicennia marina, gathered from eight stations at the Rabigh lagoon (Saudi Arabia), Aljahdali and Alhassan [19] found that there was a positive connection between antioxidants and Pb, which may be due to A. marina’s capacity to exclude or detoxify this metal through its mechanism of exclusion or detoxification. Therefore, low levels of PTM bioaccumulation in the mangrove leaves are expected.
According to Arumugam et al. [16], samples of A. marina have higher concentrations of non-essential metals such as Cd and Pb, in which the metal accumulation was influenced by the seasons, with the summer and the post-monsoon being the most polluted. When compared to non-essential metals, they found that essential elements such as Cu and Zn were more abundant in the sediment of the rhizosphere of A. marina. As a result, the plant was using Cu and Zn for their active metabolism quickly. This phenomenon could explain the low bioaccumulation of Cu and Zn in the leaves of R. apiculata from the present study.
Using both species-level analyses and a phylogenetic approach, MacFarlane et al. [22] conducted a comparative analysis analysing patterns of accumulation and partitioning of Cu, Pb, and Zn in mangroves from accessible field-based studies to date. Avicennia mangroves have several adaptive mechanisms for overcoming the difficulty of saline and extremely anoxic conditions. Although they are different species of mangrove, metal accumulation and partitioning for Cu, Pb, and Zn were discovered to be comparable across Avicennia genera and vast geographical ecosystems. With root BCF values of 1, metals were collected in roots to concentrations similar to those of nearby sediments. For all metals, the root BCFs were consistent during the exposure range. The metal levels in leaves were either half or less than those in roots. Cu and Zn, two important elements, saw a decline in leaf BCF when environmental concentrations rose. Regardless of ambient quantities, the non-essential metal Pb was not found in leaf tissue. As a result, mangroves, as a whole, often act as species that exclude non-essential metals and regulate vital metals.
Greger [101] hypothesised that a high degree of soil salinity, which leads to the development of metal-chloride complexes, may be to blame for plants’ inadequate absorption of heavy metals. The PTMs are less readily absorbed and accumulated by plants due to these complexes. Kamaruzzaman et al. [52] reported, in contrast to the current study, that the concentration of Cu was highest in sediments, followed by roots, barks, and leaves. Roots had larger quantities of Fe and Cu than shoots, indicating effective absorption and accumulation that may be related to improved oxidation in the rhizosphere [102].
Collected from the Alinsaog River (Philippines), Sazon and Veronica [18] reported the concentrations (mg/kg dry weight) of Zn, Ni, and Fe in leaves of R. apiculata, and the sediments surrounding it were 13.9, 95.1, 351.2, and 137,049 mg/kg dry weight, respectively. According to Ariyanto et al. [21], the mean concentrations of Pb in the leaves and habitat sediments of R. apiculata mangrove were 0.00035 mg/kg dry weight and sediment (9.06·0.05 mg/kg), respectively, in Asahan East Coast, Asahan Regency, North Sumatra.
Additionally, Usman et al. [3] demonstrated that the metal contents of A. marina’s leaf, branch, and root varied. The leaves of A. marina were the component of the plant that accumulated the greatest Cd and Cu in the majority of the research sites. However, there is no discernible pattern among the examined sites for Zn and Ni. According to Usman et al. [3], the metal levels in the mangrove tissues were high compared to mangrove concentrations worldwide for most of the studied metals [1,94,95,103]. Qiu et al. [1] reported that nine species of mangroves from Hainan Island, China have average amounts of Cu, Zn, Cd, and Pb of 2.8, 8.7, 0.03, and 1.1 mg/kg dry weight, respectively. Defew et al. [4] measured leaf concentrations of Cu and Zn at 3.73 and 35.8 mg/kg dry weight, respectively, in a study on the assessment of metal contamination in mangrove sediments and leaves from Punta Mala Bay, Pacific Panama.
Based on R. apiculata collected from Balok mangrove forest, Pahang, Kamaruzzaman et al. [53] reported that the mean Pb concentrations (mg/kg dry weight) in the leaf and root of R. apiculata and their habitat surface sediment were 4.30, 22.5, and 31.2, respectively. The average Cu concentrations recorded were 2.93, 4.81, and 15.5, respectively. Based on R. apiculata from the Setiu mangrove forest (Terengganu), Kamaruzzaman et al. [52] also reported the mean concentrations (mg/kg dry weight) of Cu in the mangrove’s leaf, root, and their habitat sediment as: 2.73, 5.21, and 9.42, respectively. Meanwhile, the mean concentrations (mg/kg dry weight) of Pb in leaf, root, and sediment were 1.43, 2.05, and 11.66, respectively. Abdullah et al. [51] reported ratios of 48.5:9.23 between roots and leaves for Fe, 3.60:1.14 between roots and leaves for Zn, and 0.22:0.08 between roots and leaves for Pb in R. apiculata collected from Kuala Sepetang. Based on R. stylosa collected from Belawan Stream Estuary (Medan, Northern Sumatra), Yunasfi et al. [39] reported that the levels of Cu and Pb were higher in the roots and stems than in the leaves of the mangrove. Based on R. mucronata in the Kelantan Delta, Baruddin et al. [47] found that the roots accumulated higher levels of Pb than the leaves.
The above three findings confirmed that the levels of Cu, Pb, and Zn were lowest in leaves of R. apiculata, followed by roots and habitat sediments. This also indicated that metal accumulation generally occurs at the root levels of R. apiculata, with restricted transport to leafy or aerial portions of the mangrove plants. Based on Rhizophora spp. collected from Tanjung Piai, Halim et al. [40] concluded that the Rhizophora did not meet the criteria for hyperaccumulators due to low metal accumulation, necessitating further research to identify species suited for phytoextraction of Cd, Pb, and Zn. Pahalawattaarachchi et al. [44] concluded that the trend of the phytostabilization capacity of R. mucronata in the Alibag mangrove ecosystem is confined to only the highly abundant metals in nature, and the phytoremediation capacity of R. mucronata varies from metal to metal.
These indicated that plants actively avoid the uptake of PTMs. Qiu and Qiu [48] proposed that total organic carbon in the mangrove sediment could be one of the important factors for regulating metals in mangrove tissues. Hence, the present findings indicated that the leaves of R. apiculata played important roles as biofilters and natural pollution treatment centres because the root system could manage to trap the PTMs into the sediments, as well as particulates, which are transported by the current into the oceans from the estuaries [52,53,99,100].
The low level of metal uptake by R. apiculata from the present study suggested that A. apiculata from Sepang and Lukut may not be stressed due to the unpolluted condition of the study sites. According to Kabata–Pendias and Pendias [104], the gene plants’ general heavy metal concentration depends on the amounts of metals that were measured in the plant tissues. At Yanbu Red Sea, Saudi Arabia, Alharbi et al. [20] evaluated the geographical distribution and concentrations (mg/kg dry weight) of Cu, Ni, Pb, and Zn in the mangrove Avicennia marina) leaves (16.1–56.3, 18.1–40.2, 2.3–9.90, and 36.8–84.9, respectively) and their habitat sediments (27.3–242, 17.2–217, 11.5–111, and 48.8–512, respectively). These results showed that the mangrove leaves accumulated far lower levels of PTMs than their habitat sediments.
4.6. Implications for Conservation
In PTM-contaminated sites, R. apiculata is a viable choice for phytoremediation. As the salinity around roots rises due to thin roots excluding seawater’s salts, certain metals, which could have adverse consequences, are either not eliminated or are weak. R. apiculata stands could, thus, be re-established in degraded mangrove habitats, serving as a valuable phytoremediator for some PTMs in contaminated areas and for ecosystem design. Overall, it is advised to preserve and increase the planting of R. apiculata for remediation purposes, in places with PTM contamination, in accordance with the ecosystem design concept. Mangrove habitats are arguably best used as phytostabilizers in phytoremediation efforts, with the ability to help with the retention of PTMs and, hence, reduce transmission to nearby estuarine and marine systems.
Mangroves can help reduce PTM pollution; however, models and data analyses have indicated that, if the environment is overly contaminated, the quality and vitality of this ecosystem would be severely compromised. Additionally, a data-driven and model-driven management tool must be developed to sustain mangrove environmental resources [55]. Future research should investigate improving mangroves’ abilities to absorb metals by raising PTM bioavailability. The emphasis of the current study was on the opportunity to develop a framework for managing the coastal aquatic ecosystems in the mangrove areas of Sepang and Lukut. This strained estuarine habitat is currently contaminated, and the study can provide decision-makers with a clear picture of this situation to support coastal management and environmental protection initiatives.
5. Conclusions
In this work, Cu, Ni, Fe, Pb, and Zn levels in tropical mangrove ecosystems in the Sepang Besar River and Lukut River, Peninsular Malaysia, were assessed for their potential for phytoremediation and biomonitoring in mangrove leaves of R. apiculata. Overall, the results of the present studies showed that the levels of essential Fe, Cu, and Zn in lamina are significantly (p < 0.05) higher than in MP; the levels of Pb and Ni are significantly (p < 0.05) higher than in lamina. Therefore, the lamina has better potential as a phytoremediator of Cu, Zn, and Fe, while MP has better potential as a phytoremediator of Pb and Ni.
The findings provided insightful information on R. apiculata’s ability to function as a biomonitoring agent and phytoremediator of PTMs in the mangrove ecosystem. The findings showed that the levels of Cu, Fe, Ni, Pb, and Zn in R. apiculata leaves (MP, and lamina) were lower than those in the surface sediments of their environment. The findings provided insightful information on R. apiculata’s ability to function as a biomonitoring agent and phytoremediator of PTMs in the mangrove ecosystem. The current study highlighted the potential for creating a framework for managing the coastal aquatic ecosystems in the mangrove area of Sepang and Lukut after identifying R. apiculata as a potential phytoremediator and biomonitor of PTMs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f14020237/s1, Figure S1: Parts of leaves (midrib plus petiole, and lamina) of mangrove (Rhizophora apiculata) investigated in the present study; Table S1: Sampling information in the mangrove of Sepang Besar River (1–4), and Lukut River (4–9) from the present study; Table S2: Heavy metals analysis recovery percentages of the certified reference materials (CRM); Table S3: Mean concentrations (mg/kg dry weight) of Zn in the leaf parts (lamina (L); midrib plus petiole (MP)) of Rhizophora apiculata and their habitat geochemical fraction sediments, with their bioconcentration factor (BCF) values in the mangrove of Sepang Besar River (1–4), and Lukut River (4–9) from the present study; Table S4: Comparisons of concentrations (mg/kg dry weight) of Zn, Cu, Fe, Ni and Pb between surface sediments from this study with those cited from sediment quality guidelines, and reference values; Table S5: Mean concentrations (mg/kg dry weight) of Fe in the leaf parts (lamina (L); midrib plus petiole (MP)) of Rhizophora apiculata and their habitat geochemical fraction sediments, with their bioconcentration factor (BCF) values in the mangrove of Sepang Besar River (1–4), and Lukut River (5–9) from the present study; Table S6: Mean concentrations (mg/kg dry weight) of Pb in the leaf parts (lamina (L); midrib plus petiole (MP)) of Rhizophora apiculata and their habitat geochemical fraction sediments, with their bioconcentration factor (BCF) values in the mangrove of Sepang Besar River (1–4), and Lukut River (5–9) from the present study; Table S7: Mean concentrations (mg/kg dry weight) of Cu in the leaf parts (lamina (L); midrib plus petiole (MP)) of Rhizophora apiculata and their habitat geochemical fraction sediments, with their bioconcentration factor (BCF) values in the mangrove of Sepang Besar River (1–4), and Lukut River (5–9) from the present study; Table S8: Mean concentrations (mg/kg dry weight) of Ni in the leaf parts (lamina (L); midrib plus petiole (MP)) of Rhizophora apiculata and their habitat geochemical fraction sediments, with their bioconcentration factor (BCF) values in the mangrove of Sepang Besar River (1–4), and Lukut River (5–9) from the present study. References [57,58,59,61,62,74,76,77,78,79,80,81,85,105] are cited in the supplementary materials.
Author Contributions
Conceptualisation, C.K.Y. and K.A.A.-M.; methodology and validation, C.K.Y. and K.A.A.-M.; formal analysis, C.K.Y.; investigation, C.K.Y.; resources, K.A.A.-M.; data curation, C.K.Y.; writing—original draft preparation, C.K.Y.; writing—review and editing, C.K.Y. and K.A.A.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank undergraduate student Dzul Khairool Mohamad for laboratory analysis of the heavy metal data presented in this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Qiu, Y.-W.; Yu, K.-F.; Zhang, G.; Wang, W.-X. Accumulation and partitioning of seven trace metals in mangroves and sediment cores from three estuarine wetlands of Hainan Island, China. J. Hazard. Mater. 2011, 190, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.; Nayak, G.N.; Ilangovan, D. Geochemical assessment of metal concentrations in mangrove sediments along Mumbai Coast, India. Eng. Technol. 2012, 61, 258–263. [Google Scholar]
- Usman, A.R.A.; Alkredaa, R.S.; Al-Wabel, M.I. Heavy metal contamination in sediments and mangroves from the coast of Red Sea: Avicennia marina as potential metal bioaccumulator. Ecotox. Environ. Saf. 2013, 97, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Defew, L.H.; Mair, J.M.; Guzman, H.M. An assessment of metal contamination in mangrove sediments and leaves from Punta Mala Bay, Pacific Panama. Mar. Pollut. Bull. 2005, 50, 547–552. [Google Scholar] [CrossRef]
- Bodin, N.; N′Gom-Kâ, R.; Kâ, S.; Thiaw, O.T.; Tito de Morais, L.; Le Loc′h, F.; Rozuel-Chartier, E.; Auger, D.; Chiffoleau, J.F. Assessment of trace metal contamination in mangrove ecosystems from Senegal West Africa. Chemosphere 2013, 90, 150–157. [Google Scholar] [CrossRef]
- Al-Hasawi, Z. Determination of Potentially Toxic Metals in Mangrove Trees and Associated Sediments Along Saudi Red Sea Coast. Egypt. J. Aquat. Biol. Fish. 2022, 26, 595–617. [Google Scholar] [CrossRef]
- Besley, C.H.; Birch, G.F. Comparison of mangrove (Avicennia marina) metal tissue concentrations to ambient sediment with an extensive range of contaminant levels in a highly-modified estuary (Sydney estuary, Australia). Mar. Pollut. Bull. 2021, 171, 112680. [Google Scholar] [CrossRef]
- Chai, M.W.; Li, R.L.; Ding, H.; Zan, Q.J. Occurrence and contamination of heavy metals in urban mangroves: A case study in Shenzhen, China. Chemosphere 2019, 219, 165–173. [Google Scholar] [CrossRef]
- Al-Solaimani, S.G.; Abohassan, R.A.; Alamri, D.A.; Yang, X.; Rinklebe, J.; Shaheen, S.M. Assessing the risk of toxic metals contamination and phytoremediation potential of mangrove in three coastal sites along the Red Sea. Mar. Pollut. Bull. 2022, 176, 113412. [Google Scholar] [CrossRef]
- Hossain, M.B.; Masum, Z.; Rahman, M.S.; Yu, J.; Noman, M.A.; Jolly, Y.N.; Begum, B.A.; Paray, B.A.; Arai, T. Heavy Metal Accumulation and Phytoremediation Potentiality of Some Selected Mangrove Species from the World’s Largest Mangrove Forest. Biology 2022, 11, 1144. [Google Scholar] [CrossRef]
- Mosa, A.; Selim, E.M.; El-Kadi, S.M.; Khedr, A.K.; Elnaggar, A.A.; Hefny, W.A.; Abdelhamid, A.S.; El Kenawy, A.M.; El-Naggar, A.; Wang, H.; et al. Ecotoxicological assessment of toxic elements contamination in mangrove ecosystem along the Red Sea coast, Egypt. Mar. Pollut. Bull. 2022, 176, 113446. [Google Scholar] [CrossRef]
- Nguyen, K.L.; Nguyen, H.A.; Richter, O.; Pham, M.T.; Nguyen, V.P. Ecophysiological responses of young mangrove species Rhizophora apiculata (Blume) to different chromium contaminated environments. Sci. Total Environ. 2017, 574, 369–380. [Google Scholar] [CrossRef]
- Su, R.; Ou, Q.; Wang, H.; Luo, Y.; Dai, X.; Wang, Y.; Chen, Y.; Shi, L. Comparison of phytoremediation potential of Nerium indicum with inorganic modifier calcium carbonate and organic modifier mushroom residue to lead-zinc tailings. Int. J. Environ. Res. Public Health 2022, 19, 10353. [Google Scholar] [CrossRef]
- Richter, O.; Nguyen, A.; Nguyen, K.L.; Nguyen, V.P.; Biester, H.; Schmidt, P. Phytoremediation by mangrove trees: Experimental studies and model development. Chem. Eng. J. 2016, 294, 389–399. [Google Scholar] [CrossRef]
- Sarkar, S.K. Phytoremediation of Trace Metals by Mangrove Plants of Sundarban Wetland. In Trace Metals in a Tropical Mangrove Wetland; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Arumugam, G.; Rajendran, R.; Ganesan, A.; Sethu, R. Bioaccumulation and translocation of heavy metals in mangrove rhizosphere sediments to tissues of Avicenia marina—A field study from tropical mangrove forest. Environ. Nanotech. Monitor. Manag. 2018, 10, 272–279. [Google Scholar] [CrossRef]
- Alzahrani, D.A.; El-Metwally, M.S.; El-Sherbiny, M.M. Ecological assessment of heavy metals in the grey mangrove (Avicennia marina) and associated sediments along the Red Sea coast of Saudi Arabia. Oceanologia 2018, 60, 513–526. [Google Scholar] [CrossRef]
- Sazon, R.; Veronica, P.M. Accumulation of Heavy Metals in Sediments and Tissues of Rhizopora apiculata, Sonneratia alba and Avicennia sp. in Alinsaog River, Zambales, Central Luzon, Philippines. 2019. Available online: https://ssrn.com/abstract=3489099 (accessed on 5 January 2023). [CrossRef]
- Aljahdali, M.O.; Alhassan, A.B. Ecological risk assessment of heavy metal contamination in mangrove habitats, using biochemical markers and pollution indices: A case study of Avicennia marina L. in the Rabigh lagoon, Red Sea. Saudi J. Biol. Sci. 2020, 27, 1174–1184. [Google Scholar] [CrossRef]
- Alharbi, O.M.L.; Khattab, R.A.; Ali, I.; Binnaser, Y.S.; Aqeel, A. Assessment of heavy metals contamination in the sediments and mangroves (Avicennia marina) at Yanbu coast, Red Sea, Saudi Arabia. Mar. Poll. Bull. 2019, 149, 110669. [Google Scholar] [CrossRef]
- Ariyanto, D.; Gunawan, H.; Purba, D.W. Heavy Metal (Pb) in the Rhizophora apiculata Mangrove in Asahan, North Sumatera, Indonesia. Adv. Biol. Sci. Res. 2020, 13, 373–378. [Google Scholar]
- MacFarlane, G.F.; Claudia, E.K.; Blomberg, S.P. Accumulation and partitioning of heavy metals in mangroves: A synthesis of field-based studies. Chemosphere 2007, 69, 1454–1464. [Google Scholar] [CrossRef]
- Liu, J.; Ma, K.; Qu, L. Ecological risk assessments and context-dependence analysis of heavy metal contamination in the sediments of mangrove swamp in Leizhou Peninsula, China. Mar. Pollut. Bull. 2015, 100, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, M.; Ghosh, S.; Chakraborty, D.; Hazra, S.; Chaudhuri, P. Assessment of potentially toxic metal (PTM) pollution in mangrove habitats using biochemical markers: A case study on Avicennia officinalis L. in and around Sundarban, India. Mar. Pollut. Bull. 2018, 133, 157–172. [Google Scholar] [CrossRef]
- Nath, B.; Birch, G.; Chaudhuri, P. Assessment of sediment quality in Avicennia marina-dominated embayments of Sydney Estuary: The potential use of pneumatophores (aerial roots) as a bio-indicator of trace metal contamination. Sci. Total Environ. 2014, 472, 1010–1022. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Eong, O.J. Effects of the Heavy Metals Zn and Pb on R. mucronata and A. alba Seedlings. In Proceedings of the Asian Symposium on Mangroves and Environment: Research and Management, Kuala Lumpur, Malaysia, 25–29 August 1980; Soepadmo, E., Rao, A.M., MacIntosh, M.D., Eds.; ISME: Kuala Lumpur, Malaysia, 1984; pp. 568–574. [Google Scholar]
- Chan, H.T. A note on the discovery of Rhizophora lamarckii in Peninsular Malaysia. J. Trop. Forest Sci. 1996, 9, 128–130. [Google Scholar]
- Mohd Nasir, H.; Safiah Yusmah, M.Y. Distribution of Rhizophora stylosa in Peninsular Malaysia. J. Trop. For. Sci. 2007, 19, 57–60. [Google Scholar]
- Hamdan, O.; Khali Aziz, H.; Shamsudin, I.; Raja Barizan, R.S. Status of Mangroves in Peninsular Malaysia; Forest Research Institute: Kuala Lumpur, Malaysia; Gemilang Press Sdn Bhd: Sungai Buloh, Malaysia, 2012; 169p. [Google Scholar]
- Hossain, M.; Othman, S.; Bujang, J.S.; Lim, M.T. Distribution of copper in the Sepang mangrove reserve forest environment, Malaysia. J. Trop. For. Sci. 2002, 13, 130–139. [Google Scholar]
- Duke, N.; Kathiresan, K.; Salmo, S.G., III; Fernando, E.S.; Peras, J.R.; Sukardjo, S.; Miyagi, T. Rhizophora apiculata—The IUCN Red List of Threatened Species; Version 2014.3. 2010. Available online: https://www.iucnredlist.org/species/31382/9623321 (accessed on 24 November 2022).
- Waycott, M.; McKenzie, L.J.; Mellors, J.E.; Ellison, J.; Sheaves, M.T.; Coller, C.; Schwarz, A.-M.; Webb, A.; Johnson, J.E.; Payri, C.E. Vulnerability of Mangroves, Seagrasses and Intertidal Flats in the Tropical Pacific to Climate Change. 2011. Available online: https://horizon.documentation.ird.fr/exl-doc/pleins_textes/divers13-07/010058147.pdf (accessed on 30 December 2022).
- Robertson, A.I.; Alongi, D.M. Tropical Mangrove Ecosystems; American Geophysical Union: Washington, DC, USA, 1992; Volume 41, pp. 1–330. [Google Scholar]
- Tomlinson, P.B. The Botany of Mangroves; Cambridge University Press: Cambridge, UK, 1986; 413p. [Google Scholar]
- Ghosh, S.; Bakshi, M.; Mahanty, S.; Chaudhuri, P. Understanding potentially toxic metal (PTM) induced biotic response in two riparian mangrove species Sonneratia caseolaris and Avicennia officinalis along river Hooghly, India: Implications for sustainable sediment quality management. Mar. Environ. Res. 2021, 172, 105486. [Google Scholar] [CrossRef]
- Ghosh, S.; Bakshi, M.; Mahanty, S.; Chaudhuri, P. Assessment of role of rhizosphere process in bioaccumulation of heavy metals in fine nutritive roots of riparian mangrove species in river Hooghly: Implications to global anthropogenic environmental changes. Mar. Poll. Bull. 2022, 174, 113157. [Google Scholar] [CrossRef]
- Kholoud, A.S.; Mohammad, S.A.; Ahmed, A.S.; Asma, A. Assessing Heavy Metals Accumulation in the Leaves and Sediments of Urban Mangroves (Avicennia marina (Forsk.) Vierh.) in Bahrain. Int. J. Ecol. 2017, 2017, 3978216. [Google Scholar]
- Yunasfi, L.; Singh, K.P. The heavy metal of cuprum (Cu) and lead(Pb) content in Avicennia marina and Rhizophora mucranata. IOP Conf. Ser. Earth Environ. Sci. 2019, 374, 012064. [Google Scholar] [CrossRef]
- Yunasfi, L.; Dalimunthe, R.A.; Siregar, A.S. Rhizophora stylosa as a protector against overwhelming copper (Cu) and lead (Pb) metal substances and how they affect on Belawan’s water quality. IOP Conf. Ser. Earth Environ. Sci. 2021, 912, 012060. [Google Scholar] [CrossRef]
- Halim, N.H.A.; Abdullah, R.; Kadir, W.R.; Ajeng, A.A.; Zawawi, N.Z.B. Heavy metals distribution and fractionation in mangrove sediments linked to organic deposits Vis-à-vis accumulation in Rhizophora spp. At Tanjung Piai, Johor, Malaysia. Appl. Ecol. Environ. Res. 2022, 20, 4011–4030. [Google Scholar] [CrossRef]
- Nualla-ong, A.; Phongdara, A.; Buapet, P. Copper and zinc differentially affect root glutathione accumulation and phytochelatin synthase gene expression of Rhizophora mucronata seedlings: Implications for mechanisms underlying trace metal tolerance. Ecotox. Environ. Saf. 2020, 205, 111175. [Google Scholar] [CrossRef]
- Shams, S.; Ismail, S.; Siddiqui, M.F.; Azeem, M.; Saifullah, M.; Rasool, F. Impact of heavy metal stress on antioxidant mechanisms of Avicennia marina (Forsk.) and Rhizophora mucronata Lamk. Pak. J. Sci. Ind. Res. Ser. B Biol. Sci. 2021, 64, 126–135. [Google Scholar] [CrossRef]
- Ghasemi, S.; Siavash, M.S.; Rahimi, A.; Damalas, C.A.; Naji, A. Phytomanagement of trace metals in mangrove sediments of Hormozgan, Iran, using gray mangrove (Avicennia marina). Environ. Sci. Pollut. Res. 2018, 25, 28195–28205. [Google Scholar] [CrossRef]
- Pahalawattaarachchi, V.; Purushothaman, C.S.; Vennila, A. Metal phytoremediation potential of Rhizophora mucronata (Lam.). Indian J. Mar. Sci. 2009, 38, 178–183. [Google Scholar]
- Mullai, P.; Yogeswari, M.K.; Saravanakumar, K.; Kathiresan, K. Phytoremediation of heavy metals using Avicennia marina and rhizophora mucronata in the uppanar river. Int. J. Chem. Tech. Res. 2014, 6, 4984–4990. [Google Scholar]
- Isroni, W.; Suryanto, A.M.; Yanuhar, U. The content of lead (Pb) heavy metal in mangrove (Rhizophora mucronata) at Mlaten Village, Indonesia. Int. J. Chem. Tech. Res. 2016, 9, 259–262. [Google Scholar]
- Baruddin, N.A.; Shazili, N.A.M.; Pradit, S. Sequential extraction analysis of heavy metals in relation to bioaccumulation in mangrove, Rhizophora mucronata from Kelantan Delta, Malaysia. AACL Bioflux 2017, 10, 172–181. [Google Scholar]
- Qiu, Y.W.; Qiu, H.L. Comparison of metals levels in two mangrove species (Rhizophora stylosa and Sonneratia hainanensis) from Hainan Island, South China. IOP Conf. Ser. Earth Environ. Sci. 2017, 52, 012050. [Google Scholar] [CrossRef]
- Ganeshkumar, A.; Arun, G.; Vinothkumar, S.; Rajaram, R. Bioaccumulation and translocation efficacy of heavy metals by Rhizophora mucronata from tropical mangrove ecosystem, Southeast coast of India. Ecohydr. Hydrobiol. 2019, 19, 66–74. [Google Scholar] [CrossRef]
- Razif, M.; Farhan, I. A removal of Zn metal concentrate using Rhizophora apiculata mangrove plants. Asian J. Agric. Biol. 2017, 5, 328–336. [Google Scholar]
- Abdullah, F.; Shaari, H.; Satyanarayana, B.; Khalik, W.M.A.W.M.; Jaafar, M.Z.M. Macro, micro, and non-essential elements in different parts of Rhizophora apiculata. Malays. J. Anal. Sci. 2018, 22, 570–578. [Google Scholar]
- Kamaruzzaman, B.Y.; Ong, M.C.; Jalal, K.C.A.; Shahbudin, S.; Mohd, N.O. Accumulation of lead and copper in Rhizophora apiculata from Setiu Mangrove Forest, Terengganu, Malaysia. J. Environ. Biol. 2009, 30 (Suppl. S5), 821–824. [Google Scholar] [PubMed]
- Kamaruzzaman, B.Y.; Rina Sharlinda, M.Z.; John, B.A.; Waznah, A.S. Accumulation and distribution of lead and copper in Avicennia marina and Rhizophora apiculata from Balok mangrove forest, Pahang, Malaysia. Sains Malay. 2011, 40, 555–560. [Google Scholar]
- Khan, W.R.; Rasheed, F.; Zulkifli, S.Z.; Kasim, M.R.; Zimmer, M.; Pazi, A.M.; Kamrudin, N.A.; Zafar, Z.; Faridah-Hanum, I.; Nazre, M. Phytoextraction potential of Rhizophora apiculata: A case study in Matang Mangrove Forest Reserve, Malaysia. Trop. Conser. Sci. 2020, 13, 1940082920947344. [Google Scholar] [CrossRef]
- Nguyen, A.; Richter, O.; Le, B.V.Q.; Phuong, N.T.K.; Dinh, K.C. Long-term heavy metal retention by mangroves and effect on its growth: A field inventory and scenario simulation. Int. J. Environ. Res. Public Health 2020, 17, 9131. [Google Scholar] [CrossRef]
- Yunasfi, R.; Dalimunthe, L.; Rakesya, N.Z. Rhizophora apiculata on copper and lead heavy metal substances and their effect on water quality in Belawan. IOP Conf. Ser. Earth Environ. Sci. 2022, 995, 012043. [Google Scholar] [CrossRef]
- Yap, C.K.; Arifin, N.; Tan, S.G. Relationships of copper concentrations between the different soft tissues of Telescopium telescopium and the surface sediments collected from tropical intertidal areas. Int. J. Chem. 2013, 5, 8–19. [Google Scholar] [CrossRef]
- Yap, C.K.; Noorhaidah, A.; Tan, S.G. Digestive cecum and tissue redistribution in gills of Telescopium telescopium as indicators of Ni bioavailabilities and contamination in tropical intertidal areas. Water Air Soil Pollut. 2012, 223, 2891–2905. [Google Scholar] [CrossRef]
- Yap, C.K.; Noorhaidah, A. Gill and digestive caecum of Telescopium telescopium as biomonitors of Pb bioavailability and contamination by Pb in the tropical intertidal area. Sains Malays. 2011, 40, 1075–1085. [Google Scholar]
- Noorhaidah, A.; Yap, C.K. Correlations between speciation of Zn in sediment and their concentrations in different soft tissue of Telescopium telescopium from the intertidal area of Peninsular Malaysia. Pertanika J. Trop. Agric. Sci. 2010, 33, 79–90. [Google Scholar]
- Cheng, W.H.; Yap, C.K.; Ismail, A.; Abdul Rahim, I. Distribution and concentrations of Ni in tissues of the gastropod Nerita lineata collected from intertidal areas of Peninsular Malaysia. Pertanika J. Trop. Agric. Sci. 2012, 35, 723–736. [Google Scholar]
- Cheng, W.H.; Yap, C.K. Potential human health risks from toxic metals via mangrove snail consumption and their ecological risk assessments in the habitat sediment from Peninsular Malaysia. Chemosphere 2015, 135, 156–165. [Google Scholar] [CrossRef]
- Badri, M.A.; Aston, S.R. Observation on heavy metal geochemical associations in polluted and non-polluted estuarine sediments. Environ. Pollut. Ser. B Chem. Phys. 1983, 6, 181–193. [Google Scholar] [CrossRef]
- Mukherjee, P.; Pramanick, P.; Zaman, S.; Mitra, A. Phytoremediation of heavy metals by the dominant mangrove associate species of Indian Sundarbans. J. Environ. Eng. Landsc. Manag. 2021, 29, 391–402. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Ghasemi, A.; Zahedias, L.S. Normality tests for statistical analysis: A guide for non-statisticians. Int. J. Endocrinol. Metab. 2012, 10, 486–489. [Google Scholar] [CrossRef]
- Mishra, P.; Pandey, C.M.; Singh, U.; Gupta, A.; Sahu, C.; Keshri, A. Descriptive statistics and normality tests for statistical data. Ann. Card. Anaesth. 2019, 22, 67–72. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 3rd ed.; Prentice-Hall International: Hoboken, NJ, USA, 1996. [Google Scholar]
- Manly, B.F.J. Multivariate Statistical Methods: A Primer, 2nd ed.; Chapman and Hall: London, UK, 1997. [Google Scholar]
- Yap, C.K.; Rahim Ismail, A.; Ismail, A.; Tan, S.G. Studies on heavy metal accumulations in green-lipped mussel Perna viridis by using multiple linear stepwise regression analysis. Pertanika J. Sci. Technol. 2003, 11, 43–55. [Google Scholar]
- Yap, C.K.; Rahim Ismail, A. Relationships of distribution of macrobenthic invertebrates and the physico-chemical parameters from Semenyih River by using correlation and multiple linear stepwise regression analyses. Pertanika J. Trop. Agric. Sci. 2011, 34, 229–245. [Google Scholar]
- Yap, C.K.; Rahim Ismail, A.; Ismail, A.; Tan, S.G. Analysis of heavy metal level data (Cd, Cu, Pb and Zn) in different geochemical fractions of the surface sediments in the Straits of Malacca by the use of correlation and multiple linear stepwise regression analyses. Malays. Appl. Biol. 2005, 34, 51–59. [Google Scholar]
- Yap, C.K.; Edward, F.B.; Tan, S.G. Similarities and differences of metal distributions in the tissues of molluscs by using multivariate analyses. Environ. Monitor. Assess. 2010, 165, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Yap, C.K.; Noorhaidah, A.; Tan, S.G. Zn Concentrations in the Different Soft Tissues of Telescopium telescopium and Their Relationships with Zn Speciation by Sequential Extraction in Surface Sediments: A Statistical Multiple Linear Stepwise Regression Analysis. In Gastropods: Diversity, Habitat and Genetics; Bianchi, A.M., Fields, J.N., Eds.; Nova Science Publishers: New York, NY, USA, 2011; pp. 127–148. [Google Scholar]
- Peng, S.H.T.; Chee, K.H.; Saud, H.M.; Yusop, M.R.; Tan, G.H. A Cost-Effective Novel Biochemical Fertilizer for Better Managing Nutrient Levels and Vegetative Growth in the Immature Oil Palm (Elaeis guineensis Jacq.). Horticulturae 2022, 8, 758. [Google Scholar] [CrossRef]
- Chapman, P.M.; Allard, P.J.; Vigers, G.A. Development of sediment quality values for Hong Kong special administrative region: A possible model for other jurisdictions. Mar. Pollut. Bull. 1999, 38, 161–169. [Google Scholar] [CrossRef]
- Macdonald, D.D.; Carr, R.S.; Calder, F.D.; Long, E.R.; Ingersoll, C.G. Development and evaluation of sediment quality guidelines for Florida coastal waters. Ecotoxicology 1996, 5, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Long, E.R.; Macdonald, D.D.; Smith, S.L.; Calder, F.D. Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ. Manag. 1995, 19, 81–97. [Google Scholar] [CrossRef]
- Taylor, S.R.; McLennan, S.M. The Geochemical Evolution of the Continental Crust. Rev. Geophys. 1995, 33, 241–265. [Google Scholar] [CrossRef]
- Rudnick, R.L.; Gao, S. 3.01—Composition of the Continental Crust. In Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Eds.; Pergamon: Oxford, UK, 2003; pp. 1–64. ISBN 978-0-08-043751-4. [Google Scholar]
- Wedepohl, K.H. The Composition of the Continental Crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Sarath, N.G.; Manzil, S.A.; Ali, S.; Alsahli, A.A.; Puthur, J.T. Physio-anatomical modifications and elemental allocation pattern in Acanthus ilicifolius L. subjected to zinc stress. PLoS ONE 2022, 17, e0263753. [Google Scholar] [CrossRef]
- Sagi-Kazar, M.; Solymosi, K.; Solti, A. Iron in leaves: Chemical forms, signalling, and in-cell distribution. J. Exp. Bot. 2022, 73, 1717–1734. [Google Scholar] [CrossRef]
- Rout, G.R.; Sahoo, S. Role of iron in plant growth and metabolism. Rev. Agric. Sci. 2015, 3, 1–24. [Google Scholar] [CrossRef]
- Yruela, I. Copper in plants. Braz. J. Plant Physiol. 2005, 17, 145–156. [Google Scholar] [CrossRef]
- Shahbaz, M.; Ravet, K.; Peers, G.; Pilon, M. Prioritization of copper for the use in photosynthetic electron transport in developing leaves of hybrid poplar. Front. Plant Sci. 2015, 6, 407. [Google Scholar] [CrossRef]
- Cabot, C.; Martos, S.; Llugany, M.; Gallego, B.; Tolrà, R.; Poschenrieder, C. A role for zinc in plant defense against pathogens and herbivores. Front. Plant Sci. 2019, 10, 1171. [Google Scholar] [CrossRef]
- Longnecker, N.E.; Robson, A.D. Distribution and Transport of Zinc in Plants. In Zinc in Soils and Plants; Developments in Plant and Soil Sciences Book Series; Robson, A.D., Ed.; Springer: Dordrecht, The Netherlands, 1993; Volume 55. [Google Scholar] [CrossRef]
- Hacisalihoglu, G. Zinc (Zn): The last nutrient in the alphabet and shedding light on Zn efficiency for the future of crop production under suboptimal Zn. Plants 2020, 9, 1471. [Google Scholar] [CrossRef]
- Sharma, P.; Dubey, R.S. Lead toxicity in plants. Brazil. J. Plant Physiol. 2005, 17, 35–52. [Google Scholar]
- Geevarghese, D.M.; Syed Ali, M.N.V.; Bahubali, P.; Choudhary, R.; Lvov, V.; Tovar, G.I.; Senatov, F.; Koppala, S.; Swamiappan, S. Bioaccumulation of lead (Pb) and its effects in plants: A review Samuel Collin a, Amritha Baskar. J. Hazard. Mat. Lett. 2022, 3, 100064. [Google Scholar] [CrossRef]
- Siqueira Freitas, D.; Wurr Rodak, B.; Rodrigues dos Reis, A.; de Barros Reis, F.; Soares de Carvalho, T.; Schulze, J.; Carbone Carneiro, M.A.; Guimarães Guilherme, L.R. Hidden nickel deficiency? Nickel fertilization via soil improves nitrogen metabolism and grain yield in soybean genotypes. Front. Plant Sci. 2018, 9, 614. [Google Scholar] [CrossRef]
- Van der Ent, A.; Callahan, D.L.; Noller, B.N.; Mesjasz-Przybylowicz, J.; Przybylowicz, W.J.; Barnabas, A.; Harris, H.H. Nickel biopathways in tropical nickel hyperaccumulating trees from Sabah (Malaysia). Sci. Rep. 2017, 7, 41861. [Google Scholar] [CrossRef]
- MacFarlane, G.R.; Pulkownik, A.; Burchett, M.D. Accumulation and distribution of heavy metals in the grey mangrove, Avicennia marina (Forsk.) Vierh: Biological indication potential. Environ. Pollut. 2003, 123, 139–151. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, G.R.; Burchett, M.D. Toxicity, growth and accumulation relationships of copper, lead and zinc in the Grey Mangrove Avicennia marina (Forsk.) Veirh. Mar. Environ. Res. 2002, 54, 65–84. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.J.M.; Brooks, R.R. Terrestrial higher plants which hyperaccumulate metallic elements-a review of their distribution, ecology and phytochemistry. Biorecovery 1989, 1, 81–126. [Google Scholar]
- Usman, A.R.A.; Mohamed, H.M. Effect of microbial inoculation and EDTA on the uptake and translocation of heavy metal by corn and sunflower. Chemosphere 2009, 76, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Souza, I.C.; Rocha, L.D.; Morozesk, M.; Bonomo, M.M.; Arrivabene, H.P.; Duarte, I.D.; Furlan, L.M.; Monferrán, M.V.; Mazik, K.; Elliott, M.; et al. Changes in bioaccumulation and translocation patterns between root and leafs of Avicennia schaueriana as adaptive response to different levels of metals in mangrove system. Mar. Pollut. Bull. 2015, 94, 176–184. [Google Scholar] [CrossRef]
- Kamaruzzaman, B.Y.; Ong, M.C.; Noor Azhar, M.S.; Shahbudin, S.; Jalal, K.C.A. Geochemistry of sediments in the major estuarine mangrove forest of Terengganu Region, Malaysia. Am. J. Appl. Sci. 2008, 5, 1707–1712. [Google Scholar]
- Kamaruzzaman, B.Y.; Ong, M.C. The distribution of some geochemical elements in the surface sediment of Kerteh mangrove forest, Terengganu, Malaysia. Sains Malays. 2008, 37, 337–340. [Google Scholar]
- Greger, M. Metal Availability, Uptake, Transport and Accumulation in Plants. In Heavy Metal Stress in Plants; Prasad, M.N.V., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 1–27. [Google Scholar]
- Marchand, C.; Allenbach, M.; Lallier-Verges, E. Relationships between heavy metals distribution and organic matter cycling in mangrove sediments (conception Bay), New Caledonia. Geoderma 2011, 160, 444–456. [Google Scholar] [CrossRef]
- Peng, L.; Wenjian, Z.; Zhenji, L. Distribution and accumulation of heavy metals in Avicennia marina community in Shenzhen, China. J. Environ. Sci. 1997, 9, 472–479. [Google Scholar]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants, 2nd ed.; CRC Press Inc.: Boca Raton, FL, USA, 1992. [Google Scholar]
- Hakanson, L. An Ecological Risk Index for Aquatic Pollution Control. A Sedimentological Approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).