Allocation Patterns and Temporal Dynamics of Chinese Fir Biomass in Hunan Province, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Plot Setting and Investigation
2.3. Sample Tree Selection and Sample Collection
2.4. Data Statistics and Analysis
3. Results
3.1. Allocation Pattern of Biomass in Different Organs
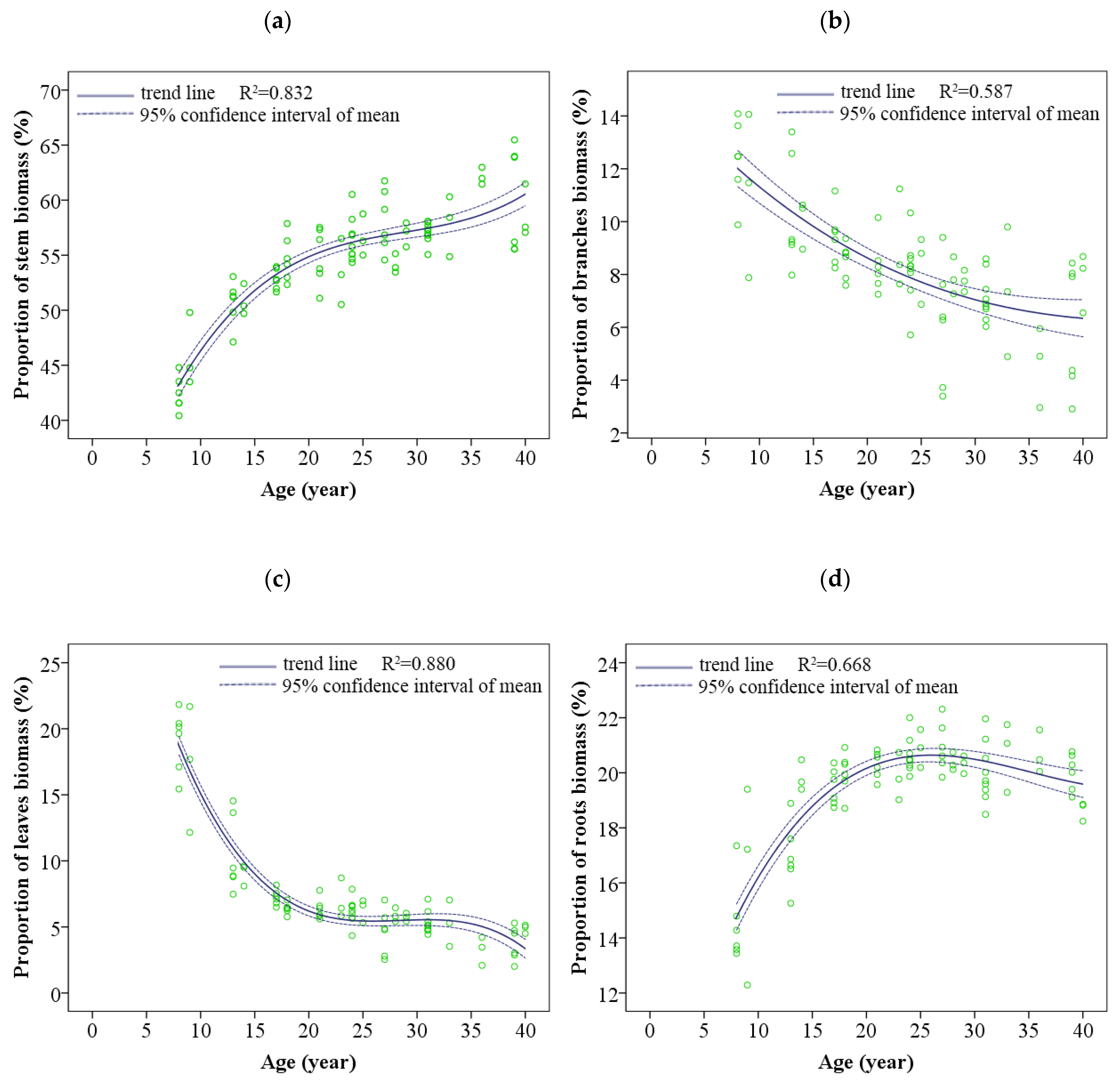
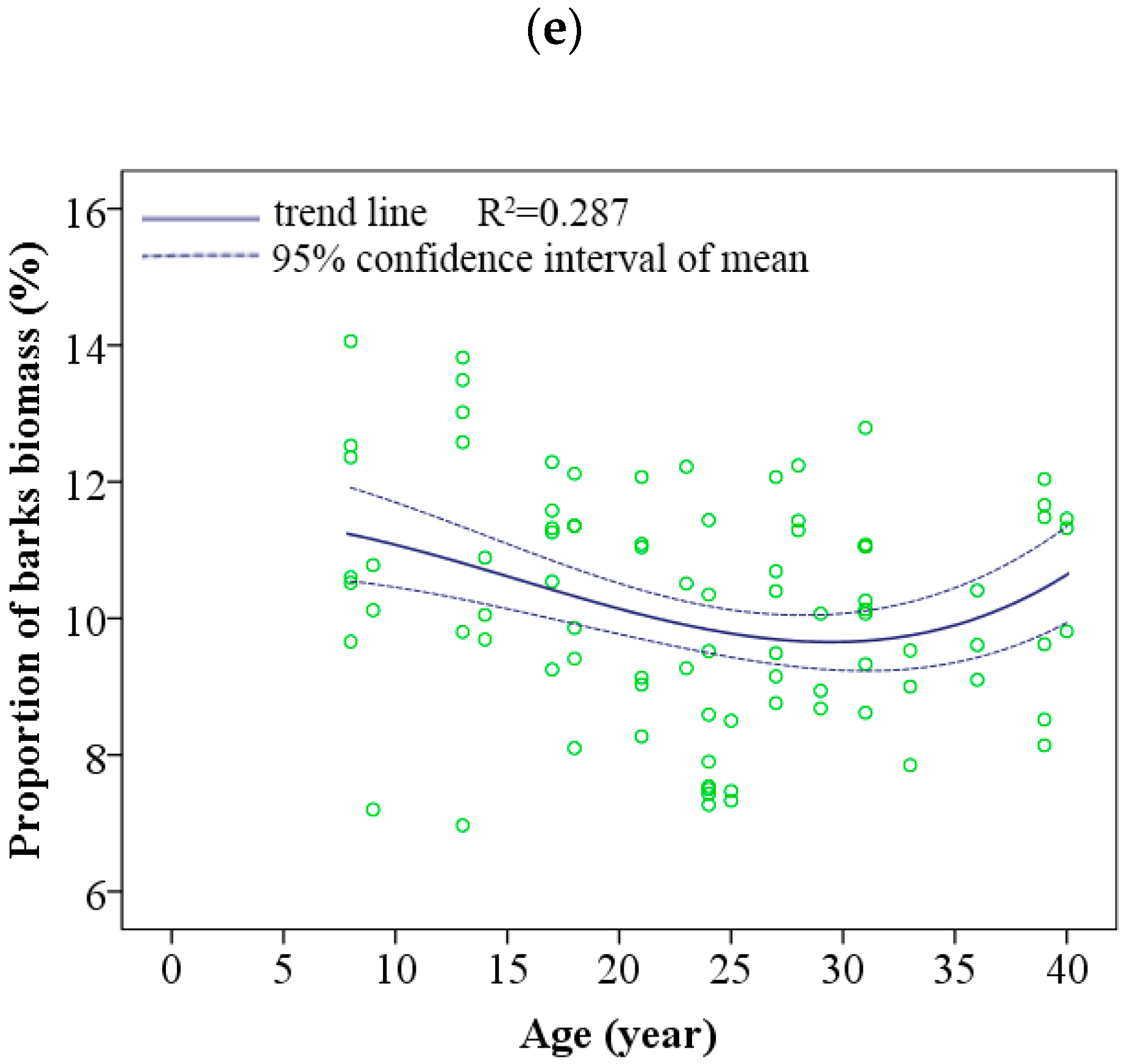
3.2. Dynamic Changes in Biomass Allocation Patterns
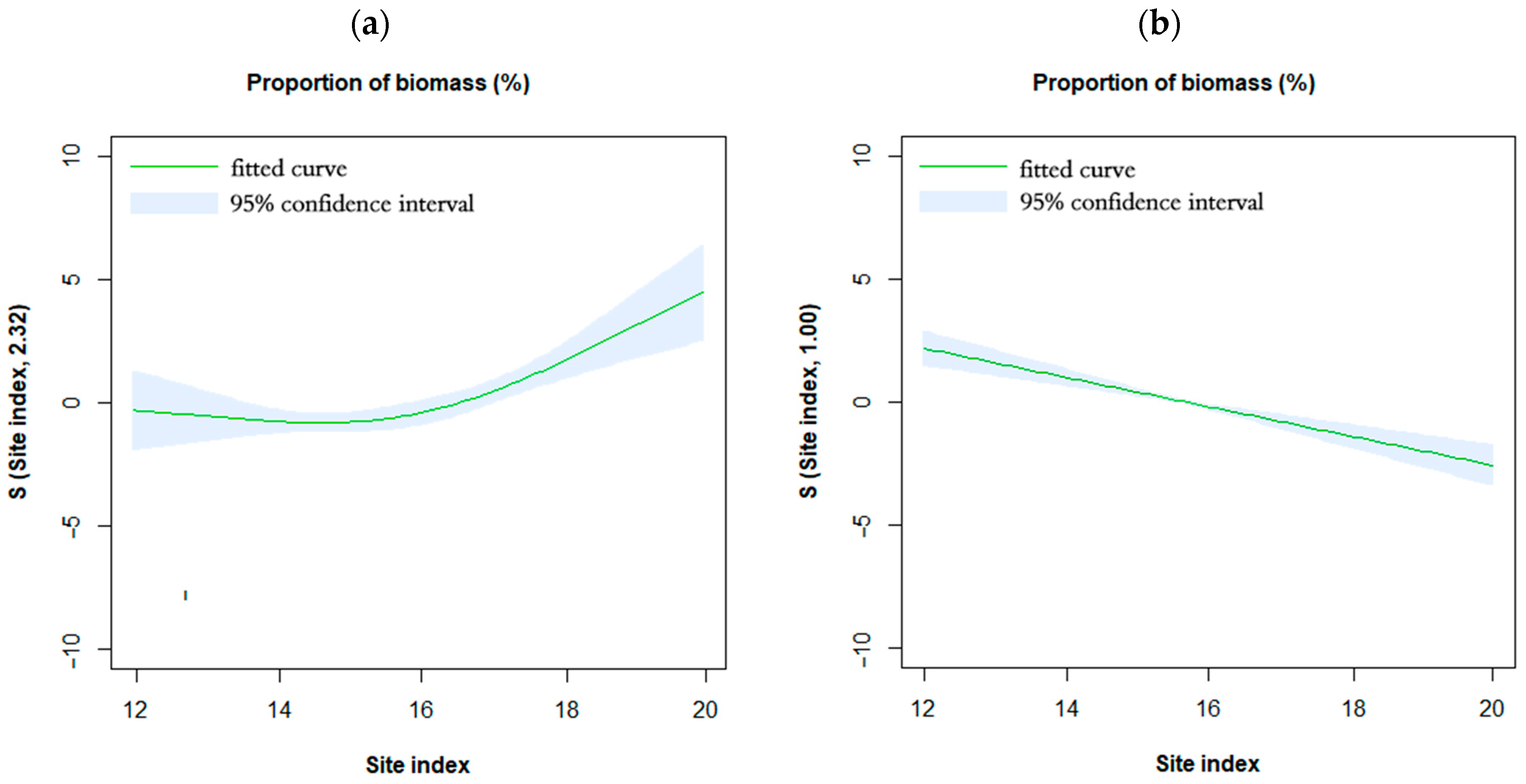
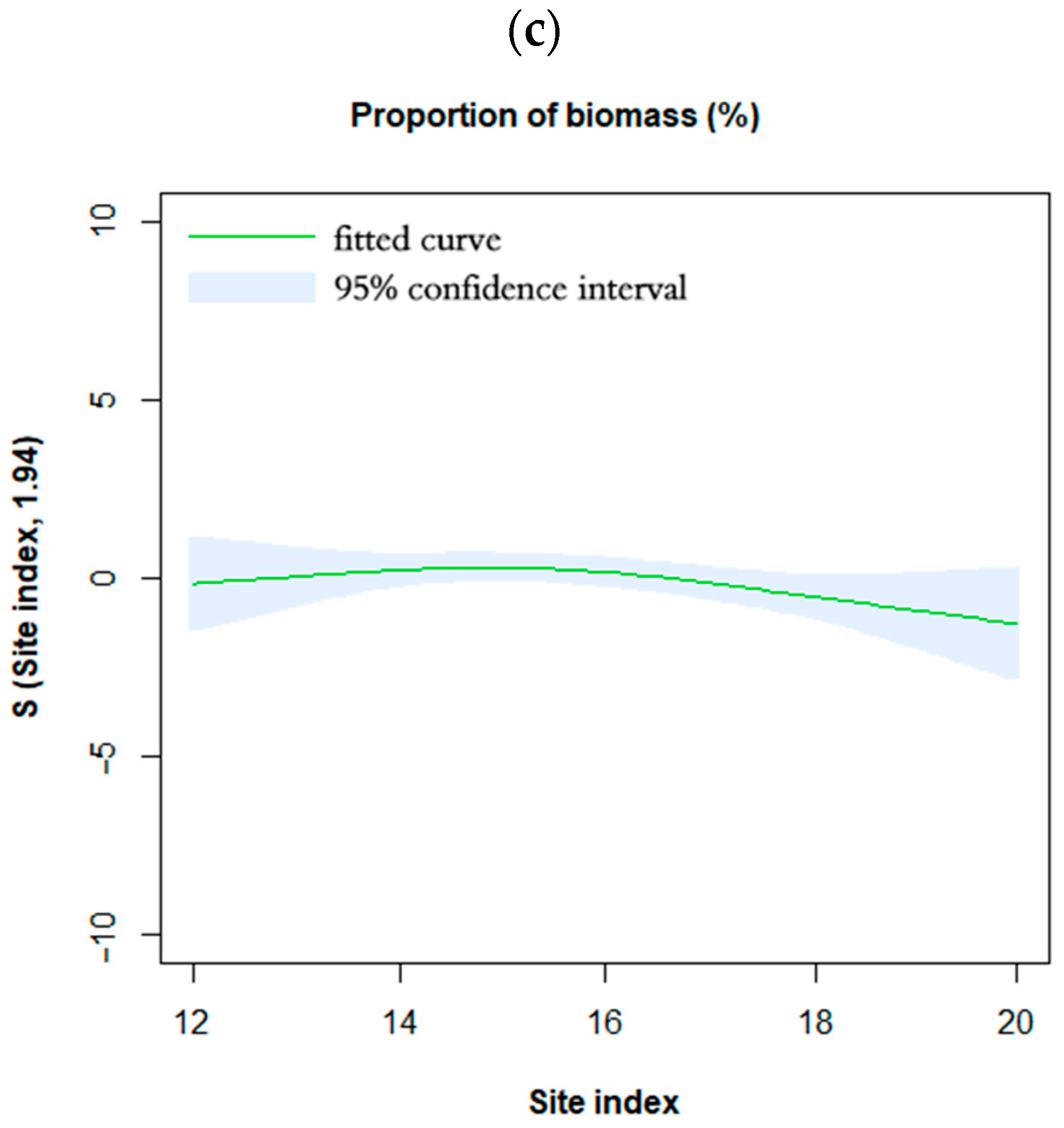
3.3. Effects of Site Quality on Biomass Allocation Pattern
3.4. Interaction of Site Quality and Tree Age on Biomass Allocation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miner, R.; Abt, R.; Bowyer, J.; Buford, M.; Malmsheimer, R.; O’Laughlin, J.; Oneil, E.; Sedjo, R.; Skog, K. Forest carbon accounting considerations in US bioenergy policy. J. For. 2014, 112, 591–606. [Google Scholar]
- Birdsey, R.; Pregitzer, K.; Lucier, A. Forest carbon management in the United States: 1600–2100. J. Environ. Qual. 2006, 35, 1461–1469. [Google Scholar] [CrossRef]
- Fang, J.; Chen, A.; Peng, C.; Zhao, S.; Ci, L. Changes in forest biomass carbon storage in China between 1949 and 1998. Science 2001, 292, 2320–2322. [Google Scholar] [CrossRef] [PubMed]
- McKinley, D.; Ryan, M.; Birdsey, R.; Giardina, C.; Harmon, M.; Heath, L.; Houghton, R.; Jackson, R.; Morrison, J.; Murray, B.; et al. A synthesis of current knowledge on forests and carbon storage in the United States. Ecol. Appl. 2011, 21, 1902–1924. [Google Scholar] [CrossRef] [PubMed]
- Lu, D. The potential and challenge of remote sensing-based biomass estimation. Int. J. Remote Sens. 2006, 27, 1297–1328. [Google Scholar] [CrossRef]
- Weiskittel, A.; MacFarlane, D.; Radtke, P.; Affleck, D.; Temesgen, H.; Woodall, C.; Westfall, J.; Coulston, J. A call to improve methods for estimating tree biomass for regional and national assessments. J. For. 2015, 113, 414–424. [Google Scholar] [CrossRef]
- Planck, N.; MacFarlane, D. A vertically integrated whole-tree biomass model. Trees-Struct. Funct. 2015, 29, 449–460. [Google Scholar] [CrossRef]
- Domke, G.; Woodall, C.; Smith, J.; Westfall, J.; McRoberts, R. Consequences of alternative tree-level biomass estimation procedures on U.S. forest carbon stock estimates. For. Ecol. Manag. 2012, 270, 108–116. [Google Scholar] [CrossRef]
- Le Toan, T.; Quegan, S.; Davidson, M.; Balzter, H.; Paillou, P.; Papathanassiou, K.; Plummer, S.; Rocca, F.; Saatchi, S.; Shugart, H.; et al. The biomass mission: Mapping global forest biomass to better understand the terrestrial carbon cycle. Remote Sens. Environ. 2011, 115, 2850–2860. [Google Scholar] [CrossRef]
- Fang, J.; Guo, Z.; Hu, H.; Kato, T.; Muraoka, H.; Son, Y. Forest biomass carbon sinks in East Asia, with special reference to the relative contributions of forest expansion and forest growth. Glob. Chang. Biol. 2014, 20, 2019–2030. [Google Scholar] [CrossRef]
- Kurz, W.A.; Beukema, S.J.; Apps, M.J. Estimation of root biomass and dynamics for the carbon budget model of the Canadian forest sector. Can. J. For. Res. 1996, 26, 1973–1979. [Google Scholar] [CrossRef]
- van Breugel, M.; Ransijn, J.; Craven, D.; Bongers, F.; Hall, J. Estimating carbon stock in secondary forests: Decisions and uncertainties associated with allometric biomass models. For. Ecol. Manag. 2011, 262, 1648–1657. [Google Scholar] [CrossRef]
- Rutishauser, E.; Noor’an, F.; Laumonier, Y.; Halperin, J.; Hergoualc’h, K.; Verchot, L. Generic allometric models including height best estimate forest biomass and carbon stocks in Indonesia. For. Ecol. Manag. 2013, 307, 219–225. [Google Scholar] [CrossRef]
- Ketterings, Q.M.; Coe, R.; van Noordwijk, M.; Ambagau’, Y.; Palm, C.A. Reducing uncertainty in the use of allometric biomass equations for predicting above-ground tree biomass in mixed secondary forests. For. Ecol. Manag. 2001, 146, 199–209. [Google Scholar] [CrossRef]
- Goodman, R.; Phillips, O.; Baker, T. The importance of crown dimensions to improve tropical tree biomass estimates. Ecol. Appl. 2014, 24, 680–698. [Google Scholar] [CrossRef] [PubMed]
- McTague, J.; Weiskittel, A. Evolution, history, and use of stem taper equations: A review of their development, application, and implementation. Can. J. For. Res. 2021, 51, 210–235. [Google Scholar] [CrossRef]
- Hame, T.; Salli, A.; Andersson, K.; Lohi, A. A new methodology for the estimation of biomass of coniferdominated boreal forest using NOAA AVHRR data. Int. J. Remote Sens. 1997, 18, 3211–3243. [Google Scholar] [CrossRef]
- Powell, S.; Cohen, W.; Kennedy, R.; Healey, S.; Huang, C. Observation of trends in biomass loss as a result of disturbance in the conterminous US: 1986–2004. Ecosystems 2014, 17, 142–157. [Google Scholar] [CrossRef]
- Liao, R.Y.; Wu, X.Q.; Jin, C.; Huang, L.; Qian, S.H.; Yang, Y.C. Effect of canopy condition on Machilus nanmu seedling configuration and biomass allocation. Chin. J. Appl. Ecol. 2021, 32, 2061–2069. (In Chinese) [Google Scholar]
- Potkay, A.; Trugman, A.; Wang, Y.; Venturas, M.; Anderegg, W.; Mattos, C.; Fan, Y. Coupled whole-tree optimality and xylem hydraulics explain dynamic biomass partitioning. New Phytol. 2021, 230, 2226–2245. [Google Scholar] [CrossRef]
- Agathokleous, E.; Belz, R.; Kitao, M.; Koike, T.; Calabrese, E. Does the root to shoot ratio show a hormetic response to stress? An ecological and environmental perspective. J. For. Res. 2019, 30, 1569–1580. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.; Reich, P.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.L.; Schroeder, P.; Kern, J.S. Spatial distribution of biomass in forests of the eastern USA. For. Ecol. Manag. 1999, 123, 81–90. [Google Scholar] [CrossRef]
- Rodriguez-Veiga, P.; Saatchi, S.; Tansey, K.; Balzter, H. Magnitude, spatial distribution and uncertainty of forest biomass stocks in Mexico. Remote Sens. Environ. 2016, 183, 265–281. [Google Scholar] [CrossRef]
- Mitchard, E.T.; Saatchi, S.S.; Baccini, A.; Asner, G.P.; Goetz, S.J.; Harris, N.L.; Brown, S. Uncertainty in the spatial distribution of tropical forest biomass: A comparison of pan-tropical maps. Carbon Balanc. Manag. 2013, 8, 10. [Google Scholar] [CrossRef]
- Verkerk, P.; Fitzgerald, J.; Datta, P.; Dees, M.; Hengeveld, G.; Lindner, M.; Zudin, S. Spatial distribution of the potential forest biomass availability in Europe. For. Ecosyst. 2019, 6, 5. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.; Phillips, O.; Jackson, R. The structure, distribution, and biomass of the world’s forests. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 593–622. [Google Scholar] [CrossRef]
- Poorter, H.; Jagodzinski, A.; Ruiz-Peinado, R.; Kuyah, S.; Luo, Y.; Oleksyn, J.; Usoltsev, V.; Buckley, T.; Reich, P.; Sack, L. How does biomass distribution change with size and differ among species? An analysis for 1200 plant species from five continents. New Phytol. 2015, 208, 736–749. [Google Scholar] [CrossRef]
- Hu, M.; Lehtonen, A.; Minunno, F.; Makela, A. Age effect on tree structure and biomass allocation in Scots pine (Pinus sylvestris L.) and Norway spruce (Picea abies [L.] Karst.). Ann. For. Sci. 2020, 77, 90. [Google Scholar] [CrossRef]
- Xiang, W.; Li, L.; Ouyang, S.; Xiao, W.; Zeng, L.; Chen, L.; Lei, P.; Deng, X.; Zeng, Y.; Fang, J.; et al. Effects of stand age on tree biomass partitioning and allometric equations in Chinese fir (Cunninghamia lanceolata) plantations. Eur. J. For. Res. 2021, 140, 317–332. [Google Scholar] [CrossRef]
- Wertz, B.; Bembenek, M.; Karaszewski, Z.; Ochal, W.; Skorupski, M.; Strzelinski, P.; Wegiel, A.; Mederski, P. Impact of stand density and tree social status on aboveground biomass allocation of Scots pine Pinus sylvestris L. Forests 2020, 11, 765. [Google Scholar] [CrossRef]
- Lu, D.; Wang, G.; Yan, Q.; Gao, T.; Zhu, J. Effects of gap size and within-gap position on seedling growth and biomass allocation: Is the gap partitioning hypothesis applicable to the temperate secondary forest ecosystems in Northeast China? For. Ecol. Manag. 2018, 429, 351–362. [Google Scholar] [CrossRef]
- Lie, Z.; Xue, L.; Jacobs, D. Allocation of forest biomass across broad precipitation gradients in China’s forests. Sci. Rep. 2018, 8, 10536. [Google Scholar] [CrossRef]
- Wang, X.; Huang, X.; Wang, Y.; Yu, P.; Guo, J. Impacts of site conditions and stand structure on the biomass allocation of single trees in Larch Plantations of Liupan Mountains of Northwest China. Forests 2022, 13, 177. [Google Scholar] [CrossRef]
- Jevon, F.; Lang, A. Tree biomass allocation differs by mycorrhizal association. Ecology 2022, 103, 6. [Google Scholar] [CrossRef] [PubMed]
- Zianis, D.; Muukkonen, P.; Mäkipää, R.; Mencuccini, M. Biomass and Stem Volume Equations for Tree Species in Europe; Tammer-Paino Oy: Tampere, Finland, 2005. [Google Scholar]
- Deng, C.; Zhang, S.; Lu, Y.; Li, Q. Determining the ecological compensation standard based on forest multifunction evaluation and financial net present value analysis: A case study in Southwestern Guangxi, China. J. Sustain. For. 2020, 39, 730–749. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, C.; Chen, X.; Jia, L.; Guo, X.; Chen, R.; Zhang, M.; Chen, Z.; Wang, H. Carbon peak and carbon neutrality in China: Goals, implementation path and prospects. China Geol. 2021, 4, 720–746. [Google Scholar] [CrossRef]
- National Forestry and Grassland Administration. China Forest Resources Report; China Forestry Publishing House: Beijing, China, 2019. [Google Scholar]
- Wood, S.; Scheipl, F. gamm4: Generalized Additive Mixed Models Using “mgcv” and “lme4”. R Package Version 0.2-6. Available online: https://cran.r-project.org/web/packages/gamm4/index.html (accessed on 31 December 2022).
- Yang, Y.; Huang, S.; Vassov, R.; Pinno, B.; Chhin, S. Climate-sensitive height-age models for top height trees in natural and reclaimed oil sands stands in Alberta, Canada. Can. J. For. Res. 2020, 50, 297–307. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, D. Inference in generalized additive mixed modelsby using smoothing splines. J. R. Stat. Soc. Ser. B-Stat. Methodol. 1999, 61, 381–400. [Google Scholar] [CrossRef]
- Groll, A.; Tutz, G. Regularization for generalized additive mixed models by likelihood-based boosting. Methods Inf. Med. 2012, 51, 168–177. [Google Scholar]
- Mensah, S.; Kakai, R.; Seifert, T. Patterns of biomass allocation between foliage and woody structure: The effects of tree size and specific functional traits. Ann. For. Res. 2016, 59, 49–60. [Google Scholar] [CrossRef]
- Tian, D.; Xiang, W.; Chen, X.; Yan, W.; Fang, X.; Kang, W.; Dan, X.; Peng, C.; Peng, Y. A long-term evaluation of biomass production in first and second rotations of Chinese fir plantations at the same site. Forests 2011, 84, 411–418. [Google Scholar] [CrossRef]
- Zhou, L.; Shalom, A.; Wu, P.; He, Z.; Liu, C.; Ma, X. Biomass production, nutrient cycling and distribution in age-sequence Chinese fir (Cunninghamia lanceolate) plantations in subtropical China. J. For. Res. 2016, 27, 357–368. [Google Scholar] [CrossRef]
- Meng, S.; Jia, Q.; Liu, Q.; Zhou, G.; Wang, H.; Yu, J. Aboveground biomass allocation and additive allometric models for natural Larix gmelinii in the western Daxing’anling Mountains, Northeastern China. Forests 2019, 10, 150. [Google Scholar] [CrossRef]
- Yang, B.; Xue, W.; Yu, S.; Zhou, J.; Zhang, W. Effects of stand age on biomass allocation and allometry of Quercus acutissima in the Central Loess Plateau of China. Forests 2019, 10, 41. [Google Scholar] [CrossRef]
- Peng, C.; Ai, W.; Qi, L.; Tu, J.; Meng, Y.; Yang, M.; Li, M. Cunninghamia lanceolata sprout population structure, dynamics and biomass allocation changes during Phyllostachys edulis expansion in middleo-southern China. Trees-Struct. Funct. 2022, 36, 1207–1218. [Google Scholar] [CrossRef]
- Xiang, W.; Liu, S.; Deng, X.; Shen, A.; Lei, X.; Tian, D.; Zhao, M.; Peng, C. General allometric equations and biomass allocation of Pinus massoniana trees on a regional scale in southern China. Ecol. Res. 2011, 26, 697–711. [Google Scholar] [CrossRef]
- Larson, P.R. Stem form development of forest trees. For. Sci. 1963, 9, 1–42. [Google Scholar] [CrossRef]
- Kohler, S.; Koehler, H.; Figueiredo, A.; Arce, J.; Machado, S. Evolution of tree stem taper in Pinus taeda stands. Cienc. Rural 2016, 46, 1185–1191. [Google Scholar] [CrossRef]
- Rather, S.A.; Qaisar, K.; Nabi, S.; Banyal, R.; Khan, P.; Islam, M. Biomass allocation and carbon stock in Elm (Ulmus Wallichiana Planch) plantation. Curr. World Environ. 2017, 12, 339–344. [Google Scholar] [CrossRef]
- Xu, Z.; Du, W.; Zhou, G.; Qin, L.; Meng, S.; Yu, J.; Sun, Z.; SiQing, B.; Liu, Q. Aboveground biomass allocation and additive allometric models of fifteen tree species in northeast China based on improved investigation methods. For. Ecol. Manag. 2022, 505, 119918. [Google Scholar] [CrossRef]
- Pajtik, J.; Konopka, B.; Lukac, M. Individual biomass factors for beech, oak and pine in Slovakia: A comparative study in young naturally regenerated stands. Trees-Struct. Funct. 2011, 25, 277–288. [Google Scholar] [CrossRef]
- Konopka, B.; Pajtik, J.; Seben, V.; Surovy, P.; Merganicova, K. Biomass allocation into woody parts and foliage in young common aspen (Populus tremula L.)-trees and a stand-level study in the Western Carpathians. Forests 2020, 11, 464. [Google Scholar] [CrossRef]
- Dimobe, K.; Mensah, S.; Goetze, D.; Ouedraogo, A.; Kuyahf, S.; Porembski, S.; Thiombiano, A. Aboveground biomass partitioning and additive models for Combretum glutinosum and Terminalia laxiflora in West Africa. Biomass Bioenerg. 2018, 115, 151–159. [Google Scholar] [CrossRef]
- Veronica, G.; Luis, P.; Gerardo, R. Allometric relations for biomass partitioning of Nothofagus antarctica trees of different crown classes over a site quality gradient. For. Ecol. Manag. 2010, 259, 1118–1126. [Google Scholar]
- Cairns, M.A.; Brown, S.; Helmer, E.H.; Baumgardner, G.A. Root biomass allocation in the world’s upland forests. Oecologia 1997, 111, 1–11. [Google Scholar] [CrossRef]
- Peichl, M.; Arain, M. Allometry and partitioning of above- and belowground tree biomass in an age-sequence of white pine forests. For. Ecol. Manag. 2007, 253, 68–80. [Google Scholar] [CrossRef]
- Billard, A.; Bauer, R.; Mothe, F.; Jonard, M.; Colin, F.; Longuetaud, F. Improving aboveground biomass estimates by taking into account density variations between tree components. Ann. For. Sci. 2020, 77, 103. [Google Scholar] [CrossRef]
- Baishya, R.; Barik, S.; Upadhaya, K. Distribution pattern of aboveground biomass in natural and plantation forests of humid tropics in northeast India. Trop. Ecol. 2009, 50, 295–304. [Google Scholar]
- Dicus, C.; Dean, T.J. Stand density effects on biomass allocation patterns and subsequent soil nitrogen demand. In Proceedings of the Ninth Biennial Southern Silvilcultural Research Conference, Clemson, SC, USA, 25–27 February 1997; p. 564. [Google Scholar]
- He, Y.; Xi, B.; Bloomberg, M.; Jia, L.; Zhao, D. Effects of drip irrigation and nitrogen fertigation on stand growth and biomass allocation in young triploid Populus tomentosa plantations. For. Ecol. Manag. 2020, 461, 1–8. [Google Scholar] [CrossRef]
- Thornley, J.H.M. A balanced quantitative model for root: Shoot ratios in vegetative plants. Ann. Bot. 1972, 36, 431–441. [Google Scholar] [CrossRef]
- Bloom, A.J.; Chapin, F.S.; Mooney, H.A. Resource limitation in plants—An economic analogy. Annu. Rev. Ecol. Syst. 1985, 16, 363–392. [Google Scholar] [CrossRef]
- Morote, F.; Serrano, F.; Andres, M.; Rubio, E.; Jimenez, J.; de las Heras, J. Allometries, biomass stocks and biomass allocation in the thermophilic Spanish juniper woodlands of Southern Spain. For. Ecol. Manag. 2012, 270, 85–93. [Google Scholar] [CrossRef]
- Thomas, S.C.; Martin, A.R. Carbon Content of Tree Tissues: A Synthesis. Forests 2012, 3, 332–352. [Google Scholar] [CrossRef]
- Ma, S.; He, F.; Tian, D.; Zou, D.; Yan, Z.; Yang, Y.; Zhou, T.; Huang, K.; Shen, H.; Fang, J. Variations and determinants of carbon content in plants: A global synthesis. Biogeosciences 2018, 15, 693–702. [Google Scholar] [CrossRef]
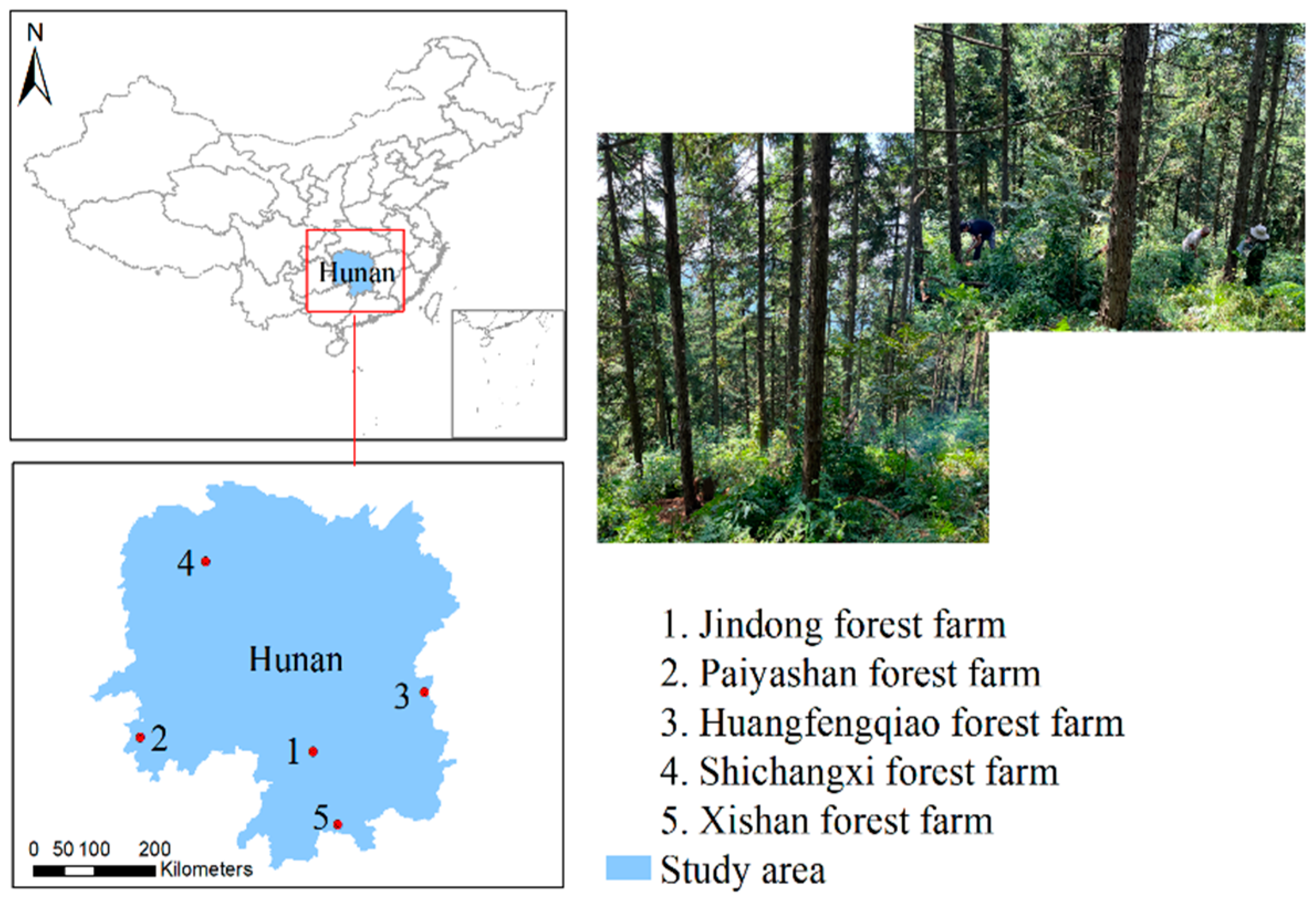


| Sampling Forest Farm | Plot No. | Stand Age (a) | Age Group | Site Index | Mean Diameter at Breast Height (cm) | Average Total Tree Height (m) | Stand Density (Stems/ha) |
|---|---|---|---|---|---|---|---|
| Jingdong | JD-1 | 8 | I | 14 | 8.3 | 6.4 | 3250 |
| JD-2 | 17 | II | 14 | 12.1 | 10.8 | 2550 | |
| JD-3 | 25 | III | 16 | 19 | 15 | 1200 | |
| JD-4 | 27 | IV | 18 | 20.4 | 17.4 | 1175 | |
| JD-5 | 31 | IV | 12 | 14.9 | 12.2 | 1675 | |
| JD-6 | 40 | V | 14 | 19.9 | 16.5 | 950 | |
| Paiyashan | PYS-1 | 18 | II | 20 | 18.4 | 17.2 | 1875 |
| PYS-2 | 21 | III | 12 | 11.9 | 9.9 | 2025 | |
| PYS-3 | 24 | III | 12 | 17 | 11.8 | 1350 | |
| PYS-4 | 28 | IV | 14 | 15.8 | 12.9 | 1475 | |
| PYS-5 | 29 | IV | 20 | 27.7 | 20.4 | 800 | |
| PYS-6 | 36 | V | 16 | 24 | 19 | 1150 | |
| PYS-7 | 39 | V | 20 | 25.2 | 23 | 975 | |
| Huangfengqiao | HFQ-1 | 14 | II | 14 | 11.4 | 9 | 2650 |
| HFQ-2 | 18 | II | 16 | 16.2 | 12.5 | 2125 | |
| HFQ-3 | 24 | III | 18 | 22.5 | 17.9 | 1200 | |
| HFQ-4 | 24 | III | 18 | 22.2 | 16 | 1325 | |
| HFQ-5 | 33 | IV | 16 | 22.3 | 18.7 | 900 | |
| Shichangxi | SCX-1 | 8 | I | 12 | 7.9 | 6.1 | 3350 |
| SCX-2 | 13 | II | 16 | 14.8 | 10.3 | 2700 | |
| SCX-3 | 17 | II | 16 | 17.2 | 11.8 | 2175 | |
| SCX-4 | 23 | III | 16 | 19.8 | 13.3 | 1325 | |
| SCX-5 | 27 | IV | 18 | 25 | 16.7 | 925 | |
| SCX-6 | 31 | IV | 18 | 27.2 | 17.9 | 1075 | |
| Xishan | XS-1 | 9 | I | 12 | 8.5 | 6.4 | 2850 |
| XS-2 | 13 | II | 12 | 11 | 8 | 2750 | |
| XS-3 | 21 | III | 18 | 22 | 14.7 | 1150 | |
| XS-4 | 31 | IV | 14 | 21.8 | 14.6 | 950 | |
| XS-5 | 39 | V | 14 | 22 | 14.7 | 825 |
| Age Group | Number of Sample Trees | Diameter Classes Range (cm) | Quadratic Mean Diameter (cm) | Average Total Tree Height (m) | Average Biomass (kg) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total Tree | Stem | Branch | Leaf | Bark | Root | |||||
| I | 9 | 8–12 | 8.1 | 6.1 | 11.88 | 5.10 | 1.46 | 2.29 | 1.27 | 1.77 |
| II | 21 | 8–20 | 14.4 | 11.6 | 55.45 | 29.25 | 5.26 | 4.42 | 5.81 | 10.71 |
| III | 21 | 12–26 | 19.2 | 14.2 | 107.27 | 60.30 | 8.87 | 6.74 | 9.38 | 21.97 |
| IV | 24 | 14–32 | 21.9 | 16.8 | 180.90 | 103.37 | 13.24 | 9.80 | 17.62 | 36.87 |
| V | 12 | 18–26 | 22.7 | 17.6 | 171.29 | 104.11 | 9.88 | 6.47 | 16.37 | 34.46 |
| Total | 87 | 6–32 | 18.4 | 13.9 | 114.04 | 65.02 | 8.58 | 6.53 | 10.92 | 23.00 |
| Age Group | Total Tree | Stem | Lower Part of Stem | Middle Part of Stem | Upper Part of Stem | Branch | Leaf | Bark | Root |
|---|---|---|---|---|---|---|---|---|---|
| I | 100.00 | 43.61 | 28.77 | 11.88 | 2.96 | 11.95 | 18.45 | 10.87 | 15.12 |
| II | 100.00 | 52.47 | 31.77 | 16.47 | 4.24 | 9.50 | 8.18 | 10.89 | 18.96 |
| III | 100.00 | 55.59 | 32.59 | 18.33 | 4.67 | 8.37 | 6.37 | 9.21 | 20.46 |
| IV | 100.00 | 57.08 | 33.37 | 18.93 | 4.77 | 7.09 | 5.26 | 10.17 | 20.41 |
| V | 100.00 | 59.94 | 34.30 | 20.24 | 5.40 | 6.06 | 3.88 | 10.26 | 19.85 |
| Total | 100.00 | 54.61 | 32.44 | 17.65 | 4.52 | 8.34 | 7.41 | 10.20 | 19.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, C.; Ma, F.; Xu, X.; Zhu, B.; Tao, J.; Li, Q. Allocation Patterns and Temporal Dynamics of Chinese Fir Biomass in Hunan Province, China. Forests 2023, 14, 286. https://doi.org/10.3390/f14020286
Deng C, Ma F, Xu X, Zhu B, Tao J, Li Q. Allocation Patterns and Temporal Dynamics of Chinese Fir Biomass in Hunan Province, China. Forests. 2023; 14(2):286. https://doi.org/10.3390/f14020286
Chicago/Turabian StyleDeng, Cheng, Fengfeng Ma, Xiaojun Xu, Baoqi Zhu, Ji Tao, and Qingfen Li. 2023. "Allocation Patterns and Temporal Dynamics of Chinese Fir Biomass in Hunan Province, China" Forests 14, no. 2: 286. https://doi.org/10.3390/f14020286
APA StyleDeng, C., Ma, F., Xu, X., Zhu, B., Tao, J., & Li, Q. (2023). Allocation Patterns and Temporal Dynamics of Chinese Fir Biomass in Hunan Province, China. Forests, 14(2), 286. https://doi.org/10.3390/f14020286






