A Functional Trait-Based Approach to Evaluate the Resilience of Key Ecosystem Functions of Tropical Savannas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area

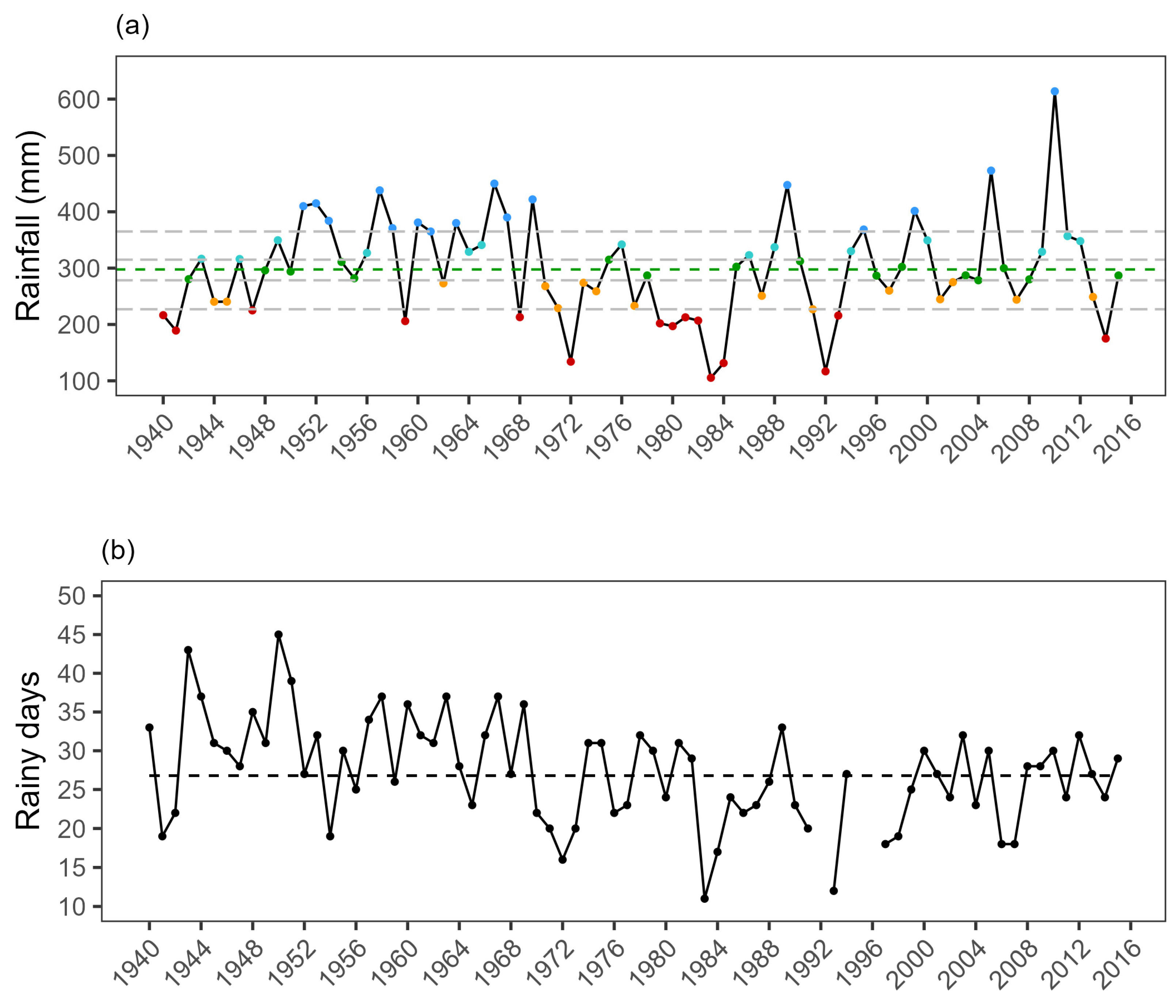
2.2. Determining Species’ Abundances from 1970 to 2015
2.3. Trait-Based Approach
2.3.1. Selecting Response and Effect Traits
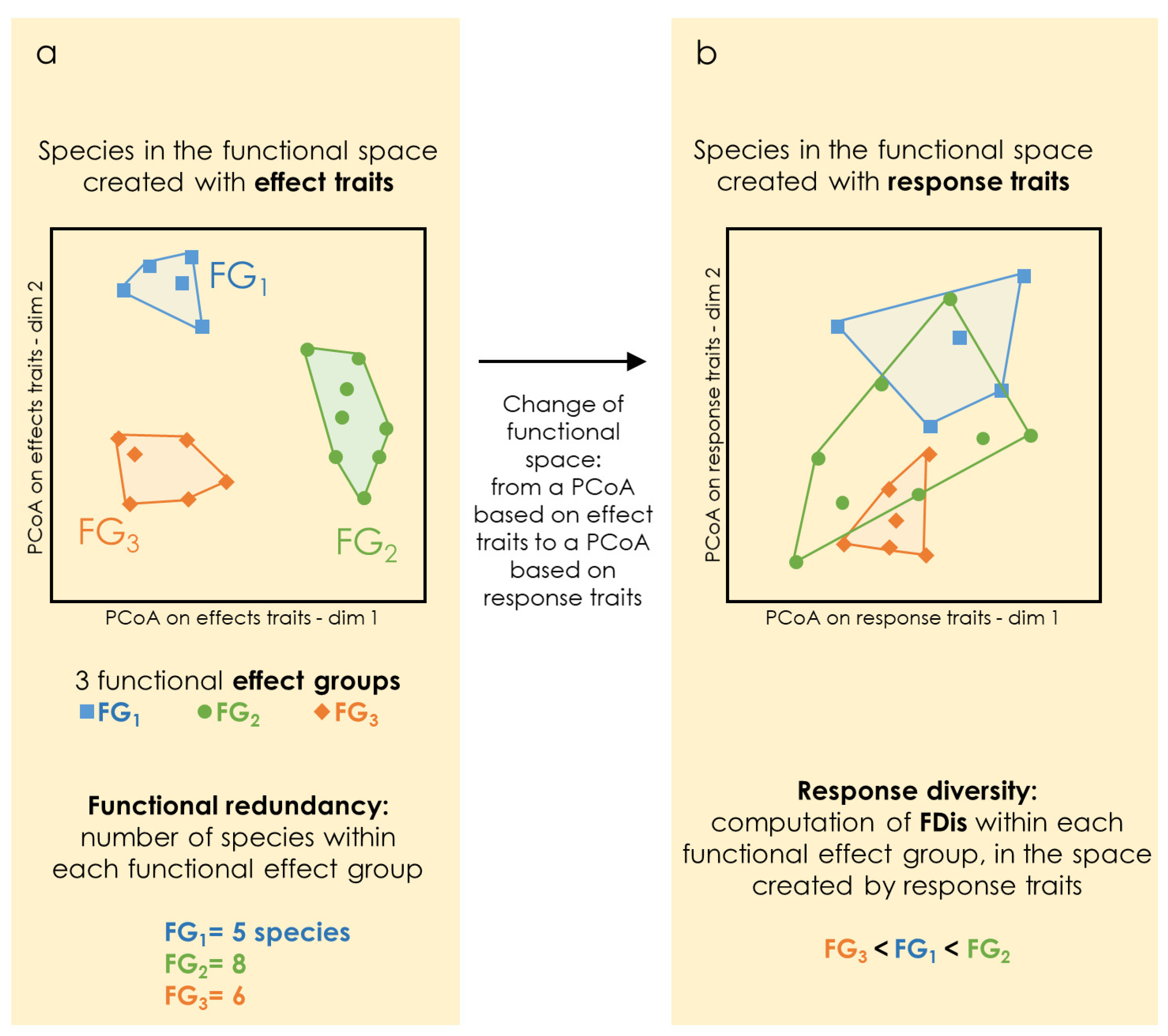
2.3.2. Functional Groups (FGs), Functional Redundancy (FRe), and Response Diversity (RDiv)
2.4. Persistence of the Studied Functions
3. Results
3.1. Functional Groups
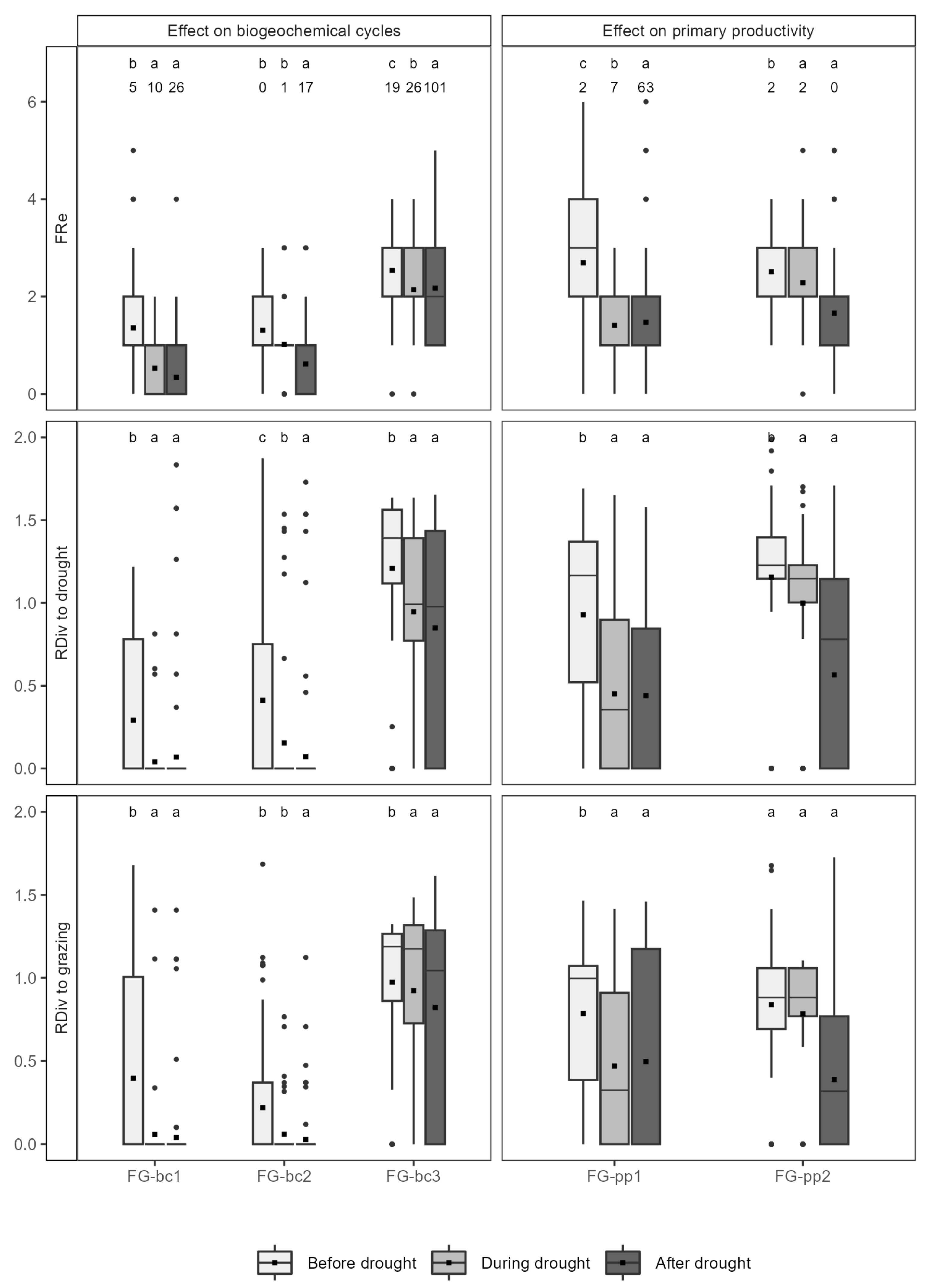
3.2. Functional Redundancy and Response Diversity from 1970 to 2015
3.3. Influence of Topography on Functional Redundancy and Response Diversity in 2015
3.4. Persistence through Time of the Studied Functions
4. Discussion
4.1. Evolution of Functional Redundancy and Response Diversity from 1970 to 2015
4.2. Persistence of the Ecosystem Functions
4.3. Strengths and Limitations of the Methodological Approach
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reynolds, J.F.; Maestre, F.T.; Kemp, P.R.; Stafford-Smith, D.M.; Lambin, E. Natural and Human Dimensions of Land Degradation in Drylands: Causes and Consequences. In Terrestrial Ecosystems in a Changing World; Canadell, J.G., Pataki, D.E., Pitelka, L.F., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 247–257. [Google Scholar]
- Pennington, R.T.; Lehmann, C.E.R.; Rowland, L.M. Tropical savannas and dry forests. Curr. Biol. 2018, 28, R541–R545. [Google Scholar] [CrossRef] [PubMed]
- Osborne, C.P.; Charles-Dominique, T.; Stevens, N.; Bond, W.J.; Midgley, G.; Lehmann, C.E.R. Human impacts in African savannas are mediated by plant functional traits. New Phytol. 2018, 220, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Mirzabaev, A.; Wu, J.; Evans, J.; Garcia-Oliva, F.; Hussein, I.A.G.; Iqbal, M.H.; Kimutai, J.; Knowles, T.; Meza, F.; Nedjraoui, D.; et al. Desertification. In Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; Shukla, P., Buendia, E.C., Masson-Delmotte, V., Pörtner, H.-O., Roberts, D., Slade, R., Connors, S., van Diemen, R., Ferrat, M., et al., Eds.; IPCC: Geneva, Switzerland, 2019; pp. 249–343. [Google Scholar] [CrossRef]
- FAO. Trees, Forests and Land Use in Drylands: The First Global Assessment; Full Report; FAO Forestry Paper 184: Rome, Italy, 2019; p. 193. [Google Scholar]
- Le Houérou, H.N. The rangelands of the Sahel. J. Range Manag. 1980, 33, 41–46. [Google Scholar] [CrossRef]
- Nicholson, S.E. Sahel, West Africa. In Encyclopedia of Environmental Biology; Nierenberg, W.A., Ed.; Academic Press: London, UK, 1995; Volume 3, pp. 261–275. [Google Scholar]
- Hiernaux, P.; Le Houérou, H.N. Les parcours du Sahel. Sécheresse 2006, 17, 51–71. [Google Scholar]
- Cornet, A. Relations entre la structure spatiale des peuplements végétaux et le bilan hydrique des sols de quelques phytocénoses en zone aride. In L’Aridité: Une Contrainte Au Développement: Caractérisation, Réponses Biologiques, Stratégies des Sociétés; Le Floc’h, E., Grouzis, M., Cornet, A., Bille, J.-C., Eds.; Didactiques; ORSTOM: Paris, France, 1992; pp. 245–265. [Google Scholar]
- Coughenour, M.B.; Ellis, J.E. Landscape and climatic control of woody vegetation in a dry tropical ecosystem: Turkana district, Kenya. J. Biogeogr. 1993, 20, 383–398. [Google Scholar] [CrossRef]
- Dendoncker, M.; Vincke, C. Low topographic positions enhance woody vegetation stability in the Ferlo (Senegalese Sahel). J. Arid Environ. 2020, 175, 104087. [Google Scholar] [CrossRef]
- Nicholson, S.E. Climatic and environmental change in Africa during the last two centuries. Clim. Res. 2001, 17, 123–144. [Google Scholar] [CrossRef]
- Epule, E.; Peng, C.; Lepage, L.; Chen, Z. The causes, effects and challenges of Sahelian droughts: A critical review. Reg. Environ. Change 2014, 14, 145–156. [Google Scholar] [CrossRef]
- Nicholson, S.E.; Tucker, C.J.; Ba, M.B. Desertification, drought, and surface vegetation: An example from the West African Sahel. Bull. Am. Meteorol. Soc. 1998, 79, 815–829. [Google Scholar] [CrossRef]
- Hiernaux, P.; Diarra, L.; Trichon, V.; Mougin, E.; Soumaguel, N.; Baup, F. Woody plant population dynamics in response to climate changes from 1984 to 2006 in Sahel (Gourma, Mali). J. Hydrol. 2009, 375, 103–113. [Google Scholar] [CrossRef] [Green Version]
- Le Houérou, H.N. The Grazing Land Ecosystems of the African Sahel; Springer: Berlin/Heidelberg, Germany, 1989; p. 282. [Google Scholar]
- Poupon, H. Structure et Dynamique de la Strate Ligneuse D’une Steppe Sahélienne au Nord du Sénégal; OS: Ouvrages Scientifiques; ORSTOM: Paris, France, 1980. [Google Scholar]
- Brandt, M.; Romankiewicz, C.; Spiekermann, R.; Samimi, C. Environmental change in time series—An interdisciplinary study in the Sahel of Mali and Senegal. J. Arid Environ. 2014, 105, 52–63. [Google Scholar] [CrossRef]
- Dendoncker, M.; Brandt, M.; Rasmussen, K.; Taugourdeau, S.; Fensholt, R.; Tucker, C.J.; Vincke, C. 50 years of woody vegetation changes in the Ferlo (Senegal) assessed by high-resolution imagery and field surveys. Reg. Environ. Change 2020, 20, 137. [Google Scholar] [CrossRef]
- Lykke, A.M.; Fog, B.; Madsen, J.E. Woody vegetation changes in the Sahel of Burkina Faso assessed by means of local knowledge, aerial photos, and botanical investigations. Dan. J. Geogr. 1999, 2, 57–68. [Google Scholar]
- Vincke, C.; Diédhiou, I.; Grouzis, M. Long term dynamics and structure of woody vegetation in the Ferlo (Senegal). J. Arid Environ. 2010, 74, 268–276. [Google Scholar] [CrossRef]
- Wezel, A.; Lykke, A.M. Woody vegetation change in Sahelian West Africa: Evidence from local knowledge. Environ. Dev. Sustain. 2006, 8, 553–567. [Google Scholar] [CrossRef]
- Touré, O. Where Herders Don’t Herd Anymore: Experience from the Ferlo, Northern Senegal; IIED, International Institute for Environment and Development: London, UK, 1990; p. 22. [Google Scholar]
- Sinclair, A.R.E.; Fryxell, J.M. The Sahel of Africa: Ecology of a disaster. Can. J. Zool. 1985, 63, 987–994. [Google Scholar] [CrossRef]
- Chillo, V.; Ojeda, R.A.; Capmourteres, V.; Anand, M. Functional diversity loss with increasing livestock grazing intensity in drylands: The mechanisms and their consequences depend on the taxa. J. Appl. Ecol. 2017, 54, 986–996. [Google Scholar] [CrossRef]
- Rasmussen, K.; Brandt, M.; Tong, X.; Hiernaux, P.; Diouf, A.A.; Assouma, M.H.; Tucker, C.J.; Fensholt, R. Does grazing cause land degradation? Evidence from the sandy Ferlo in Northern Senegal. Land Degrad. Dev. 2018, 29, 4337–4347. [Google Scholar] [CrossRef]
- Hanan, N.P.; Prevost, Y.; Diouf, A.; Diallo, O. Assessment of desertification around deep wells in the Sahel using satellite imagery. J. Appl. Ecol. 1991, 28, 173–186. [Google Scholar] [CrossRef]
- Miehe, S.; Kluge, J.; von Wehrden, H.; Retzer, V. Long-term degradation of Sahelian rangeland detected by 27 years of field study in Senegal. J. Appl. Ecol. 2010, 47, 692–700. [Google Scholar] [CrossRef]
- Miehe, S. Inventaire et Suivi de la Végétation dans le Périmètre Expérimental de Widou Thiengoly dans le Cadre du Projet Sénégalo-Allemand D’autopromotion Pastorale dans le Ferlo (PAPF); Rapport Final; Deutsche Gesellschaft für Technische Zusammenarbeit: Eschborn, Germany, 2002; p. 57. [Google Scholar]
- Brandt, M.; Tappan, G.; Diouf, A.A.; Beye, G.; Mbow, C.; Fensholt, R. Woody vegetation die off and regeneration in response to rainfall variability in the West African Sahel. Remote Sens. 2017, 9, 39. [Google Scholar] [CrossRef] [Green Version]
- Olsson, L.; Eklundh, L.; Ardö, J. A recent greening of the Sahel-trends, patterns and potential causes. J. Arid Environ. 2005, 63, 556–566. [Google Scholar] [CrossRef]
- Lykke, A.M.; Kristensen, M.K.; Ganaba, S. Valuation of local use and dynamics of 56 woody species in the Sahel. Biodivers. Conserv. 2004, 13, 1961–1990. [Google Scholar] [CrossRef]
- Herrmann, S.M.; Tappan, G.G. Vegetation impoverishment despite greening: A case study from central Senegal. J. Arid Environ. 2013, 90, 55–66. [Google Scholar] [CrossRef]
- Anyamba, A.; Tucker, C.J. Analysis of Sahelian vegetation dynamics using NOAA-AVHRR NDVI data from 1981–2003. J. Arid Environ. 2005, 63, 596–614. [Google Scholar] [CrossRef]
- Dardel, C.; Kergoat, L.; Hiernaux, P.; Mougin, E.; Grippa, M.; Tucker, C.J. Re-greening Sahel: 30 years of remote sensing data and field observations (Mali, Niger). Remote Sens. Environ. 2014, 140, 350–364. [Google Scholar] [CrossRef]
- Brandt, M.; Mbow, C.; Diouf, A.A.; Verger, A.; Samimi, C.; Fensholt, R. Ground- and satellite-based evidence of the biophysical mechanisms behind the greening Sahel. Glob. Chang. Biol. 2015, 21, 1610–1620. [Google Scholar] [CrossRef] [PubMed]
- MEA. Ecosystems and Human Well-Being: Synthesis; Island Press: Washington, DC, USA, 2005; p. 137. [Google Scholar]
- Sankaran, M.; Hanan, N.P.; Scholes, R.J.; Ratnam, J.; Augustine, D.J.; Cade, B.S.; Gignoux, J.; Higgins, S.I.; Le Roux, X.; Ludwig, F.; et al. Determinants of woody cover in African savannas. Nature 2005, 438, 846–849. [Google Scholar] [CrossRef] [PubMed]
- Berdugo, M.; Delgado-Baquerizo, M.; Soliveres, S.; Hernández-Clemente, R.; Zhao, Y.; Gaitán, J.J.; Gross, N.; Saiz, H.; Maire, V.; Lehmann, A.; et al. Global ecosystem thresholds driven by aridity. Science 2020, 367, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Sinare, H.; Gordon, L.J. Ecosystem services from woody vegetation on agricultural lands in Sudano-Sahelian West Africa. Agric. Ecosyst. Environ. 2015, 200, 186–199. [Google Scholar] [CrossRef]
- Chillo, V.; Anand, M.; Ojeda, R. Assessing the Use of Functional Diversity as a Measure of Ecological Resilience in Arid Rangelands. Ecosystems 2011, 14, 1168–1177. [Google Scholar] [CrossRef]
- De Bello, F.; Lavorel, S.; Díaz, S.; Harrington, R.; Cornelissen, J.H.C.; Bardgett, R.D.; Berg, M.P.; Cipriotti, P.; Feld, C.K.; Hering, D.; et al. Towards an assessment of multiple ecosystem processes and services via functional traits. Biodivers. Conserv. 2010, 19, 2873–2893. [Google Scholar] [CrossRef]
- Díaz, S.; Cabido, M. Vive la différence: Plant functional diversity matters to ecosystem processes. Trends Ecol. Evol. 2001, 16, 646–655. [Google Scholar] [CrossRef]
- Díaz, S.; Lavorel, S.; de Bello, F.; Quétier, F.; Grigulis, K.; Robson, T.M. Incorporating plant functional diversity effects in ecosystem service assessments. Proc. Natl. Acad. Sci. USA 2007, 104, 20684–20689. [Google Scholar] [CrossRef]
- Elmqvist, T.; Folke, C.; Nyström, M.; Peterson, G.; Bengtsson, J.; Walker, B.; Norberg, J. Response diversity, ecosystem change, and resilience. Front. Ecol. Environ. 2003, 1, 488–494. [Google Scholar] [CrossRef]
- Harrison, P.A.; Berry, P.M.; Simpson, G.; Haslett, J.R.; Blicharska, M.; Bucur, M.; Dunford, R.; Egoh, B.; Garcia-Llorente, M.; Geamănă, N.; et al. Linkages between biodiversity attributes and ecosystem services: A systematic review. Ecosyst. Serv. 2014, 9, 191–203. [Google Scholar] [CrossRef]
- Mori, A.S.; Furukawa, T.; Sasaki, T. Response diversity determines the resilience of ecosystems to environmental change. Biol. Rev. 2013, 88, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Sterk, M.; Gort, G.; Klimkowska, A.; van Ruijven, J.; van Teeffelen, A.J.A.; Wamelink, G.W.W. Assess ecosystem resilience: Linking response and effect traits to environmental variability. Ecol. Indic. 2013, 30, 21–27. [Google Scholar] [CrossRef]
- Funk, J.L.; Larson, J.E.; Ames, G.M.; Butterfield, B.J.; Cavender-Bares, J.; Firn, J.; Laughlin, D.C.; Sutton-Grier, A.E.; Williams, L.; Wright, J. Revisiting the Holy Grail: Using plant functional traits to understand ecological processes. Biol. Rev. 2017, 92, 1156–1173. [Google Scholar] [CrossRef]
- Garnier, E.; Navas, M.-L. A trait-based approach to comparative functional plant ecology: Concepts, methods and applications for agroecology. A review. Agron. Sustain. Dev. 2012, 32, 365–399. [Google Scholar] [CrossRef]
- Violle, C.; Navas, M.-L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Nock, C.A.; Vogt, R.J.; Beisner, B.E. Functional Traits. In eLS; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2016. [Google Scholar]
- Tilman, D. Functional diversity. In Encyclopedia of Biodiversity; Levin, S.A., Ed.; Academic Press: Cambridge, MA, USA, 2001; pp. 109–120. [Google Scholar]
- Holling, C.S. Resilience and Stability of Ecological Systems. Annu. Rev. Ecol. Syst. 1973, 4, 1–23. [Google Scholar] [CrossRef]
- Laliberté, E.; Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Naeem, S. Species Redundancy and Ecosystem Reliability. Conserv. Biol. 1998, 12, 39–45. [Google Scholar] [CrossRef]
- Yachi, S.; Loreau, M. Biodiversity and ecosystem productivity in a fluctuating environment: The insurance hypothesis. Proc. Natl. Acad. Sci. USA 1999, 96, 1463–1468. [Google Scholar] [CrossRef] [PubMed]
- Gladstone-Gallagher, R.V.; Pilditch, C.A.; Stephenson, F.; Thrush, S.F. Linking Traits across Ecological Scales Determines Functional Resilience. Trends Ecol. Evol. 2019, 34, 1080–1091. [Google Scholar] [CrossRef]
- Lavorel, S.; Grigulis, K.; Lamarque, P.; Colace, M.-P.; Garden, D.; Girel, J.; Pellet, G.; Douzet, R. Using plant functional traits to understand the landscape distribution of multiple ecosystem services. J. Ecol. 2011, 99, 135–147. [Google Scholar] [CrossRef]
- Mouillot, D.; Villéger, S.; Scherer-Lorenzen, M.; Mason, N.W.H. Functional Structure of Biological Communities Predicts Ecosystem Multifunctionality. PLoS ONE 2011, 6, e17476. [Google Scholar] [CrossRef]
- Tappan, G.G.; Sall, M.; Wood, E.C.; Cushing, M. Ecoregions and land cover trends in Senegal. J. Arid Environ. 2004, 59, 427–462. [Google Scholar] [CrossRef]
- Akpo, L.-E. Dynamique des Systèmes Ecologiques Sahéliens: Structure Spécifique, Productivité et Qualité des Herbages: Le Forage de Widdu Thiengoly. Master’s Thesis, Mémoire de fin d’étude, Université Cheikh Anta Diop de Dakar, Dakar, Senegal, 1990. [Google Scholar]
- Thébaud, B. Politiques d’hydraulique pastorale et gestion de l’espace au Sahel. Cah. Des Sci. Hum. 1990, 26, 13–31. [Google Scholar]
- Barral, H. Le Ferlo des Forages: Gestion Ancienne et Actuelle de L’espace Pastoral: Étude de Géographie Humaine; ORSTOM: Dakar, Senegal, 1982; p. 85. [Google Scholar]
- Touré, I.; Ickowicz, A.; Wane, A.; Garba, I.; Gerber, P. Atlas des Evolutions des Systèmes Pastoraux au Sahel; FAO et CIRAD: Rome, Italy, 2012; p. 32. [Google Scholar]
- Taugourdeau, S.; Daget, P.; Chatelain, C.; Mathieu, D.; Juanes, X.; Huguenin, J.; Ickowicz, A. FLOTROP, a massive contribution to plant diversity data for open ecosystems in northern tropical Africa. Sci. Data 2019, 6, 118. [Google Scholar] [CrossRef]
- Dendoncker, M.; Vincke, C. Inventory data of woody plants surveyed and measured in North Senegal (Ferlo) in 2015–2017. Zenodo. 2022. Available online: https://zenodo.org/record/7041354 (accessed on 29 January 2023).
- Wikum, D.A.; Shanholtzer, G.F. Application of the Braun-Blanquet cover-abundance scale for vegetation analysis in land development studies. Environ. Manag. 1978, 2, 323–329. [Google Scholar] [CrossRef]
- Diop, A.T.; Sy, O.; Ickowicz, A.; Touré, I. Politique D’hydraulique et Gestion de L’espace et des Ressources Dans la Région Sylvopastorale du Sénégal, Ferlo. In Proceedings of the Actes du Colloque International Umr Sagert, Montpellier, France, 25–27 February 2003; p. 9. [Google Scholar]
- Assouma, H. Approche écosystémique du Bilan des Gaz à Effet de Serre d’un Territoire Sylvo-Pastoral Sahélien: Contribution de L’élevage. Ph.D. Thesis, AgroParisTech, Montpellier, France, 2016. [Google Scholar]
- Cornet, A. Observations sur les précipitations dans la partie centrale du secteur sahélien sénégalais. Notes Afr. 1978, 34–42. [Google Scholar]
- Gross, N.; Bagousse-Pinguet, Y.L.; Liancourt, P.; Berdugo, M.; Gotelli, N.J.; Maestre, F.T. Functional trait diversity maximizes ecosystem multifunctionality. Nat. Ecol. Evol. 2017, 1, 0132. [Google Scholar] [CrossRef] [PubMed]
- Laliberté, E.; Wells, J.A.; DeClerck, F.; Metcalfe, D.J.; Catterall, C.P.; Queiroz, C.; Aubin, I.; Bonser, S.P.; Ding, Y.; Fraterrigo, J.M.; et al. Land-use intensification reduces functional redundancy and response diversity in plant communities. Ecol. Lett. 2010, 13, 76–86. [Google Scholar] [CrossRef]
- Lavorel, S.; Garnier, E. Predicting Changes in Community Composition and Ecosystem Functioning from Plant Traits: Revisiting the Holy Grail. Funct. Ecol. 2002, 16, 545–556. [Google Scholar] [CrossRef]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Díazaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.B.; Steege, H.t.; Morgan, H.D.; Heijden, M.G.A.v.d.; et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef]
- Kattge, J.; Bönisch, G.; Díaz, S.; Lavorel, S.; Prentice, I.C.; Leadley, P.; Tautenhahn, S.; Werner, G.D.A.; Aakala, T.; Abedi, M.; et al. TRY plant trait database—Enhanced coverage and open access. Glob. Chang. Biol. 2020, 26, 119–188. [Google Scholar] [CrossRef]
- Burke, A. Savanna trees in Namibia—Factors controlling their distribution at the arid end of the spectrum. Flora Morphol. Distrib. Funct. Ecol. Plants 2006, 201, 189–201. [Google Scholar] [CrossRef]
- Schmidt, M.; Traoré, S.; Ouédraogo, A.; Mbayngone, E.; Ouédraogo, O.; Zizka, A.; Kirchmair, I.; Kaboré, E.; Tindano, E.; Thiombiano, A.; et al. Geographical Patterns of Woody Plants’ Functional Traits in Burkina Faso. Candollea 2013, 68, 197–207. [Google Scholar] [CrossRef]
- Walker, B.; Kinzig, A.; Langridge, J. Plant Attribute Diversity, Resilience, and Ecosystem Function: The Nature and Significance of Dominant and Minor Species. Ecosystems 1999, 2, 95–113. [Google Scholar] [CrossRef]
- Diaz, S.; Hodgson, J.G.; Thompson, K.; Cabido, M.; Cornelissen, J.H.C.; Jalili, A.; Montserrat-Martí, G.; Grime, J.P.; Zarrinkamar, F.; Asri, Y.; et al. The plant traits that drive ecosystems: Evidence from three continents. J. Veg. Sci. 2004, 15, 295–304. [Google Scholar] [CrossRef]
- Gower, J.C. A General Coefficient of Similarity and Some of Its Properties. Biometrics 1971, 27, 857–871. [Google Scholar] [CrossRef]
- Ward, J.H. Hierarchical Grouping to Optimize an Objective Function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Curtis, J.T. The Vegetation of Wisconsin: An Ordination of Plant Communities; The University of Wisconsin Press: Madison, WI, USA, 1959; p. 663. [Google Scholar]
- Diémé, J.S.; Diouf, M.; Armas, C.; Rusch, G.; Pugnaire, F. Functional groups of Sahelian trees in a semiarid agroforestry system of Senegal. J. Plant Ecol. 2017, 11, 375–384. [Google Scholar] [CrossRef]
- Nunes, A.; Köbel, M.; Pinho, P.; Matos, P.; Bello, F.d.; Correia, O.; Branquinho, C. Which plant traits respond to aridity? A critical step to assess functional diversity in Mediterranean drylands. Agric. For. Meteorol. 2017, 239, 176–184. [Google Scholar] [CrossRef]
- Aguirre-Gutiérrez, J.; Malhi, Y.; Lewis, S.L.; Fauset, S.; Adu-Bredu, S.; Affum-Baffoe, K.; Baker, T.R.; Gvozdevaite, A.; Hubau, W.; Moore, S.; et al. Long-term droughts may drive drier tropical forests towards increased functional, taxonomic and phylogenetic homogeneity. Nat. Commun. 2020, 11, 3346. [Google Scholar] [CrossRef]
- Oliveira, A.C.P.d.; Nunes, A.; Oliveira, M.A.; Rodrigues, R.G.; Branquinho, C. How Do Taxonomic and Functional Diversity Metrics Change Along an Aridity Gradient in a Tropical Dry Forest? Front. Plant Sci. 2022, 13. [Google Scholar] [CrossRef]
- Gonzalez, P. Desertification and a shift of forest species in the West African Sahel. Clim. Res. 2001, 17, 217–228. [Google Scholar] [CrossRef]
- Maestre, F.T.; Le Bagousse-Pinguet, Y.; Delgado-Baquerizo, M.; Eldridge, D.J.; Saiz, H.; Berdugo, M.; Gozalo, B.; Ochoa, V.; Guirado, E.; García-Gómez, M.; et al. Grazing and ecosystem service delivery in global drylands. Science 2022, 378, 915–920. [Google Scholar] [CrossRef]
- Dendoncker, M.; Vincke, C.; Bazan, S.; Madingou, M.P.N.; Taugourdeau, S. The size of topographic depressions in a Sahelian savanna is a driver of woody vegetation diversity. J. Arid Environ. 2023, 210, 104923. [Google Scholar] [CrossRef]
- Le Bagousse-Pinguet, Y.; Soliveres, S.; Gross, N.; Torices, R.; Berdugo, M.; Maestre, F.T. Phylogenetic, functional, and taxonomic richness have both positive and negative effects on ecosystem multifunctionality. Proc. Natl. Acad. Sci. USA 2019, 116, 8419. [Google Scholar] [CrossRef] [PubMed]
- Grime, J.P. Benefits of Plant Diversity to Ecosystems: Immediate, Filter and Founder Effects. J. Ecol. 1998, 86, 902–910. [Google Scholar] [CrossRef]
- Aubin, I.; Munson, A.D.; Cardou, F.; Burton, P.J.; Isabel, N.; Pedlar, J.H.; Paquette, A.; Taylor, A.R.; Delagrange, S.; Kebli, H.; et al. Traits to stay, traits to move: A review of functional traits to assess sensitivity and adaptive capacity of temperate and boreal trees to climate change. Environ. Rev. 2016, 24, 164–186. [Google Scholar] [CrossRef]
- Charles-Dominique, T.; Davies, T.J.; Hempson, G.P.; Bezeng, B.S.; Daru, B.H.; Kabongo, R.M.; Maurin, O.; Muasya, A.M.; van der Bank, M.; Bond, W.J. Spiny plants, mammal browsers, and the origin of African savannas. Proc. Natl. Acad. Sci. USA 2016, 113, E5572. [Google Scholar] [CrossRef]
- Midgley, J.; Sawe, T.; Abanyam, P.; Hintsa, K.; Gacheru, P. Spinescent East African savannah acacias also have thick bark, suggesting they evolved under both an intense fire and herbivory regime. Afr. J. Ecol. 2016, 54, 118–120. [Google Scholar] [CrossRef]
- O’Brien, M.J.; Engelbrecht, B.M.J.; Joswig, J.; Pereyra, G.; Schuldt, B.; Jansen, S.; Kattge, J.; Landhäusser, S.M.; Levick, S.R.; Preisler, Y.; et al. A synthesis of tree functional traits related to drought-induced mortality in forests across climatic zones. J. Appl. Ecol. 2017, 54, 1669–1686. [Google Scholar] [CrossRef]
- Santiago, L.S.; Bonal, D.; De Guzman, M.E.; Ávila-Lovera, E. Drought Survival Strategies of Tropical Trees. In Tropical Tree Physiology: Adaptations and Responses in a Changing Environment; Goldstein, G., Santiago, L.S., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 243–258. [Google Scholar]
- Poorter, L.; Markesteijn, L. Seedling Traits Determine Drought Tolerance of Tropical Tree Species. Biotropica 2008, 40, 321–331. [Google Scholar] [CrossRef]
- Mouillot, D.; Graham, N.A.J.; Villéger, S.; Mason, N.W.H.; Bellwood, D.R. A functional approach reveals community responses to disturbances. Trends Ecol. Evol. 2013, 28, 167–177. [Google Scholar] [CrossRef]
- Craven, D.; Filotas, E.; Angers, V.A.; Messier, C. Evaluating resilience of tree communities in fragmented landscapes: Linking functional response diversity with landscape connectivity. Divers. Distrib. 2016, 22, 505–518. [Google Scholar] [CrossRef]
- Mensah, S.; Veldtman, R.; Assogbadjo, A.E.; Glèlè Kakaï, R.; Seifert, T. Tree species diversity promotes aboveground carbon storage through functional diversity and functional dominance. Ecol. Evol. 2016, 6, 7546–7557. [Google Scholar] [CrossRef] [PubMed]
- Arbonnier, M. Arbres, Arbustes et Lianes des Zones Sèches d’Afrique de l’Ouest (3e Edition); CIRAD, MHNM: Montpellier, France, 2009; p. 573. [Google Scholar]
- Chave, J.; Coomes, D.; Jansen, S.; Lewis, S.L.; Swenson, N.G.; Zanne, A.E. Towards a Worldwide Wood Economics Spectrum. Ecol. Lett. 2009, 12, 351–366. [Google Scholar] [CrossRef]
- Zanne, A.E.; Westoby, M.; Falster, D.S.; Ackerly, D.D.; Loarie, S.R.; Arnold, S.E.J.; Coomes, D.A. Angiosperm Wood Structure: Global Patterns in Vessel Anatomy and Their Relation to Wood Density and Potential Conductivity. Am. J. Bot. 2010, 97, 207–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wheeler, E. Insidewood—A web resource for hardwood anatomy. IAWA J. 2011, 32, 199–211. [Google Scholar] [CrossRef]
- Kindt, R.; Ordonez, J.; Smith, E.; Orwa, C.; Kehlenbeck, K.; Asmara, D.H.; Luedeling, E.; Munjuga, M.; Mwanzia, L.; Sinclair, F. ICRAF Species Switchboard; Version 1.0; World Agroforestry Centre: Nairobi, Kenya, 2013. [Google Scholar]
- Royal Botanic Gardens. Survey of Economic Plants for Arid and Semi-Arid Lands (SEPASAL) Database. Royal Botanic Gardens, Kew, UK. 1999. Available online: http://www.rbgkew.org.uk/ceb/sepasal/internet/ (accessed on 29 January 2023).
- Van den Bilcke, N.; De Smedt, S.; Simbo, D.J.; Samson, R. Sap flow and water use in African baobab (Adansonia digitata L.) seedlings in response to drought stress. South Afr. J. Bot. 2013, 88, 438–446. [Google Scholar] [CrossRef]
- Robert, N.; Björn, E. Stem basic density and bark proportion of 45 woody species in young savanna coppice forests in Burkina Faso. Ann. For. Sci. 2000, 57, 143–153. [Google Scholar] [CrossRef]
- Chiveu, C.J.; Dangasuk, O.G.; Omunyin, M.E.; Wachira, F.N. Quantitative variation among Kenyan populations of Acacia senegal (L.) Willd. for gum production, seed and growth traits. New For. 2009, 38, 1. [Google Scholar] [CrossRef]
- Hiernaux, P.; Cissé, M.I.; Diarra, L.; de Leeuw, P.N. Fluctuations saisonnières de la feuillaison des arbres et des buissons sahéliens. Conséquences pour la quantification des ressources fourragères. Rev. D’élevage Médecine Vétérinaire Pays Trop. 1994, 47, 117–125. [Google Scholar] [CrossRef]
- De Bie, S.; Ketner, P.; Paasse, M.; Geerling, C. Woody plant phenology in the West Africa savanna. J. Biogeogr. 1998, 25, 883–900. [Google Scholar] [CrossRef]
- Seghieri, J.; Do, F.; Devineau, J.-L.; Fournier, A. Phenology of woody species along the climatic gradient in West Tropical Africa. In Phenology and Climate Change; Zhang, X., Ed.; InTechOpen: Rijeka, Croatia, 2012; p. 320. [Google Scholar]
- Poupon, H.; Bille, J.C. Recherches écologiques sur une savane sahélienne du Ferlo septentrional, Sénégal: Influence de la sécheresse de l’année 1972–1973 sur la strate ligneuse. La Terre Vie Revue d’Ecologie Appliquée 1974, 1, 49–75. [Google Scholar]
- Poupon, H. Etude de la phénologie de la strate ligneuse à Fété-Olé (Sénégal septentrional) de 1971 à 1977. Bull. l’IFAN Série A Sci. Nat. 1979, 1, 44–91. [Google Scholar]
- Brandt, M.; Hiernaux, P.; Tagesson, T.; Verger, A.; Rasmussen, K.; Diouf, A.A.; Mbow, C.; Mougin, E.; Fensholt, R. Woody plant cover estimation in drylands from Earth Observation based seasonal metrics. Remote Sens. Environ. 2016, 172, 28–38. [Google Scholar] [CrossRef]
- Grouzis, M.; Le Floc’h, E. Un Arbre au Désert: Acacia Raddiana; IRD: Paris, France, 2003; p. 313.
- Bille, J.C. Etude de la Production Primaire Nette d’un Ecosystème Sahélien; ORSTOM: Paris, France, 1977; Volume 65, p. 86. [Google Scholar]
- Ali, M.; Saadou, M.; Jean, L. Phénologie de quelques espèces ligneuses du parc national du « W » (Niger). Sci. Changements Planétaires/Sécheresse 2007, 18, 354–358. [Google Scholar] [CrossRef]
- Breman, H.; De Ridder, N. Manuel sur les Pâturages des Pays Sahéliens; ACCT-CTA-Karthala: Wageningen, Pays-Bas, 1991; p. 488. [Google Scholar]
- Breman, H.; Kessler, J.J. Woody Plants in Agro-Ecosystems of Semi-Arid Regions; Springer: Berlin/Heidelberg, Germany, 1995; Volume 23, p. 340. [Google Scholar]
- Fall, S.T. Digestibilité in vitro et dégradabilité in situ dans le rumen de ligneux fourragers disponibles sur pâturages naturels au Sénégal: Premiers résultats. Rev. D’élevage Médecine Vétérinaire Pays Trop. 1991, 44, 345–354. [Google Scholar] [CrossRef]
- Le Houérou, H.N. Composition chimique et valeur nutritive des fourrages ligneux en Afrique tropicale occidentale. In Les Fourrages Ligneux en Afrique: État Actuel des Connaissances; Le Houerou, H.N., Ed.; CIPEA: Addis Abeba, Ethiopie, 1980; pp. 259–284. [Google Scholar]
- Kgope, B.S.; Musil, C.F. Differential photosynthetic responses of broad- and fine-leafed savanna trees to elevated temperatures. South Afr. J. Bot. 2004, 70, 760–766. [Google Scholar] [CrossRef] [Green Version]
- Tezara, W.; Colombo, R.; Coronel, I.; Marin, O. Water relations and photosynthetic capacity of two species of Calotropis in a tropical semi-arid ecosystem. Ann. Bot. 2011, 107, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Farahat, A.E.; Galal, M.T.; El-Midany, M.M.; Hassan, M.L. Phenology, biomass and reproductive characteristics of Calotropis procera (Aiton) W.T. Aiton in South Cairo, Egypt. Rend. Lincei 2016, 27, 197–204. [Google Scholar] [CrossRef]
- Baumer, M. Notes on Trees and Shrubs in Arid and Semi-Arid Regions; Food and Agriculture Organization of the United Nations: Rome, Italy, 1983; p. 270. [Google Scholar]
- Mayus, M.; Van Keulen, H.; Stroosnijder, L. A model of tree-crop competition for windbreak systems in the Sahel: Description and evaluation. Agrofor. Syst. 1998, 43, 183–201. [Google Scholar] [CrossRef]
- Kizito, F.; Dragila, M.; Sène, M.; Lufafa, A.; Diedhiou, I.; Dick, R.P.; Selker, J.S.; Dossa, E.; Khouma, M.; Badiane, A.; et al. Seasonal soil water variation and root patterns between two semi-arid shrubs co-existing with Pearl millet in Senegal, West Africa. J. Arid Environ. 2006, 67, 436–455. [Google Scholar] [CrossRef]
- Breman, H.; Sissoko, F. L’Intensification Agricole au Sahel; Broché; Karthala Editions: Paris, France, 1998; p. 996. ISBN 978-2-86537-809-8. [Google Scholar]
- Clanet, J.-C.; Gillet, H. Le Commiphora africana, véritable arbre fourrager sahélien. In Les Fourrages Ligneux en Afrique: État des Connaissances Actuelles; Le Houerou, H.N., Ed.; CIPEA: Addis Abeba, Ethiopie, 1980; pp. 431–433. [Google Scholar]
- Gebrekirstos, A.; Teketay, D.; Fetene, M.; Mitlöhner, R. Adaptation of five co-occurring tree and shrub species to water stress and its implication in restoration of degraded lands. For. Ecol. Manag. 2006, 229, 259–267. [Google Scholar] [CrossRef]
- Diouf, J.C.; Akpo, L.E.; Ickowicz, A.; Lesueur, D.; Chotte, J.-L. Dynamique des peuplements ligneux et pratiques pastorales au Sahel (Ferlo, Sénégal). In Conférence Internationale: Biodiversité Science et Gouvernance; Barbault, R., Le Duc, J.P., Eds.; MNHN: Paris, France, 2005; p. 9. [Google Scholar]
- Tybirk, K.; Univ, A. Regeneration of Woody Legumes in Sahel; Aarhus (Denmark) Botanical Institute, Aarhus University: Aarhus, Denmark, 1991; p. 81. [Google Scholar]
- Guissou, T.G. La Symbiose Mycorhizienne à Arbuscules Chez des Espèces D’arbres: Diversité des Glomales, Dépendance Mycorhizienne, Utilisation des Phosphates Naturels et Tolérance à un Stress Hydrique. Ph.D. Thesis, Univeristé de Ouagadougou, Burkina Faso, Ouagadougou, 2001. [Google Scholar]
- Royal Botanic Gardens Kew. Seed Information Database (SID) Version 7.1; Royal Botanic Gardens Kew: Richmond, UK, 2008. [Google Scholar]
- Joker, D. Acacia senegal (L.) Willd. Seed Leafl. 2000, 5, 2. [Google Scholar]
- Joker, D. Acacia seyal Del. Seed Leafl. 2000, 34, 2. [Google Scholar]
- Joker, D. Acacia tortilis (Forssk.) Hayne. Seed Leafl. 2000, 19, 2. [Google Scholar]
- Joker, D. Faidherbia albida (Del.) A. Chev. Seed Leafl. 2000, 28, 2. [Google Scholar]
- Joker, D. Ziziphus mauritiana. Seed Leafl. 2003, 85, 2. [Google Scholar]
- Joker, D.; Erdey, D. Sclerocarya birrea (A. Rich.) Hochst. Seed Leafl. 2003, 72, 2. [Google Scholar]
- Sacande, M.; Ronne, C.; Sanon, M.; Joker, D. Adansonia digitata L. Seed Leafl. 2006, 109, 2. [Google Scholar]
- Sacande, M.; Sanogo, S. Anogeissus leiocarpus (DC.) Guill. & Perr. Seed Leafl. 2007, 119, 2. [Google Scholar]
- Sacande, M.; Sanon, M.; Schmidt, L.H. Sterculia setigera Delile. Seed Leafl. 2007, 134, 2. [Google Scholar]
- Sacande, M.; Vautier, H.; Sanon, M.; Schmidt, L.H. Dalbergia melanoxylon Guill. & Perr. Seed Leafl. 2007, 135, 2. [Google Scholar]
- Sanon, M.; Sacandé, M.; Schmidt, L.H. Combretum aculeatum Vent. Seed Leafl. 2007, 127, 2. [Google Scholar]
- Schmidt, L.H.; Joker, D. Balanites aegyptiaca (L.) Del. Seed Leafl. 2000, 21, 2. [Google Scholar]
- Schmidt, L.H.; Mbora, A. Acacia nilotica (L.) Del. Seed Leafl. 2008, 137, 2. [Google Scholar]
- Schmidt, L.H.; Mbora, A. Commiphora africana (A. Rich.) Engel. Seed Leafl. 2008, 138, 2. [Google Scholar]
- Vautier, H.; Sanon, M.; Sacandé, M.; Schmidt, L.H. Combretum glutinosum Perrot. ex DC. Seed Leafl. 2007, 128, 2. [Google Scholar]
- Kirchmair, I.; Schmidt, M.; Zizka, G.; Erpenbach, A.; Hahn, K. Biodiversity Islands in the savanna: Analysis of the phytodiversity on termite mounds in Northern Benin. Flora Veg. Sudano-Sambesica 2012, 15, 3–14. [Google Scholar] [CrossRef]
- Colombo, R.C.; Favetta, V.; Yamamoto, L.Y.; Alves, G.A.C.; Abati, J.; Takahashi, L.S.A.; Faria, R.T.d. Biometric description of fruits and seeds, germination and imbibition pattern of desert rose [Adenium obesum (Forssk.), Roem. & Schult.]. J. Seed Sci. 2015, 37, 206–213. [Google Scholar]
- Steentoft, M. Flowering plants in West Africa; Cambridge University Press: New York, NY, USA, 1988; p. 350. [Google Scholar]
- Tréca, B.; Tamba, S. Rôle des oiseaux sur la régénération du ligneux Boscia senegalensis (pers.) Lam. en savane sahélienne au Nord Sénégal. Revue D‘Ecol. La Terre La Vie 1997, 52, 239–260. [Google Scholar]
- Sen, D.N. Ecology of desert plants and observations on their seedlings. II. Germination behaviour of seeds in Asclepiadaceae. Osterr. Bot. Z. 1968, 115, 18–27. [Google Scholar] [CrossRef]
- Midgley, J.J. Dispersibility, cost and allometry of tumblers (Combretum, Combretaceae) and parachutes (Leucadendron, Proteaceae) of different size. Plant Syst. Evol. 1998, 211, 141–147. [Google Scholar] [CrossRef]
- Dukku, U.H. Plants for bees: Guiera senegalensis, an important nectar plant in the savanna. Bee World 2010, 87, 77. [Google Scholar] [CrossRef]
- Helm, C.V.; Scott, S.L.; Witkowski, E.T.F. Reproductive potential and seed fate of Sclerocarya birrea subsp. caffra (marula) in the low altitude savannas of South Africa. South Afr. J. Bot. 2011, 77, 650–664. [Google Scholar] [CrossRef] [Green Version]
- Abdourhamane, H.; Rabiou, H.; Diouf, A.; Morou, B.; Mahamane, A.; Bellefontaine, R. Structure démographique et répartition spatiale des populations de Sclerocarya birrea (A.Rich.) Hochst. du secteur sahélien du Niger. Bois Des Trop. 2017, 333, 55–66. [Google Scholar]
- Danthu, P.; Ickowicz, A.; Friot, D.; Manga, D.; Sarr, A. Effet du passage par le tractus digestif des ruminants domestiques sur la germination des graines de légumineuses ligneuses des zones tropicales sèches. Revue Elev. Méd. Vét. Pays Trop. 1996, 49, 235–241. [Google Scholar] [CrossRef]
- Wilson, T.B.; Witkowski, E.T.F. Water requirements for germination and early seedling establishment in four African savanna woody plant species. J. Arid Environ. 1998, 38, 541–550. [Google Scholar] [CrossRef]
- Garner, R.D.; Witkowski, E.T.F. Variations in seed size and shape in relation to depth of burial in the soil and pre-dispersal predation in Acacia nilotica, Acacia tortilis and Dichrostachys cinerea. S. Afr. J. Bot. 1997, 63, 371–377. [Google Scholar] [CrossRef]
- Lahoreau, G.; Barot, S.; Gignoux, J.; Hoffmann, W.A.; Setterfield, S.A.; Williams, P.R. Positive effect of seed size on seedling survival in fire-prone savannas of Australia, Brazil and West Africa. J. Trop. Ecol. 2006, 22, 719–722. [Google Scholar] [CrossRef]
- Bellefontaine, R. Synthèse des espèces des domaines sahélien et soudanien qui se multiplient naturellement par voie végétative. In Fonctionnement et Gestion des Ecosystèmes Contractés Sahéliens; d’Herbès, J.M., Ambouta, J.-M.K., Régis Peltier, R., Eds.; John Libbey Eurotext: Paris, France, 1997; pp. 95–104. [Google Scholar]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Sankaran, M. Droughts and the ecological future of tropical savanna vegetation. J. Ecol. 2019, 107, 1531–1549. [Google Scholar] [CrossRef] [Green Version]


| Traits | Type | Units or Attributes | Completeness (%) | Set of Traits |
|---|---|---|---|---|
| Whole-plant traits | ||||
| Growth form | categorical | tree, bush, and shrub | 100 | Dr, Pp, and Bc |
| Maximum height | continuous | meters (m) | 100 | Gr, Pp, and Bc |
| Spinescence | ordinal | from 1 (no spine) to 4 (numerous, hard, and long spines) | 100 | Gr |
| Leaf traits | ||||
| Digestible protein | continuous | % of dry matter | 74 | Gr |
| Leaf area | continuous | mm2 | 59 | Gr, Dr, Pp, and Bc |
| Leaf area category | categorical | nanophyllous (20–200 mm2), microphyllous (2–6 cm2), submicrophyllous (6–20 cm2), and mesophyllous (20–100 cm2). | 72 | Gr, Dr, Pp, and Bc |
| Leaf N content | continuous | mg.g−1 | 90 | Pp and Bc |
| Leaf P content | continuous | mg.g−1 | 82 | Bc |
| Leaf persistence | categorical | deciduous, evergreen, and semi-evergreen | 100 | Dr and Bc |
| Leaf phenology (duration of leafing period) | continuous | months | 87 | Gr, Dr, and Bc |
| Specific leaf area | continuous | mm2.mg1 | 41 | Dr, Pp, and Bc |
| Stem traits | ||||
| Bark thickness | ordinal | from 1 (thin) to 3 (thick) | 100 | Dr |
| Stem specific density | continuous | g.cm−3 | 92 | Dr, Pp, and Bc |
| Vessel area | continuous | mm2 | 59 | Dr |
| Below-ground traits | ||||
| N fixing capacity | categorical | yes or no | 87 | Bc |
| Presence of a taproot | categorical | yes or no | 46 | Dr |
| Regenerative traits | ||||
| Dispersal mode | categorical | Anemochorous and zoochorous | 97 | Gr |
| Resprouting capacity (coppice) | categorical | yes or no | 87 | Gr, Dr, and Bc |
| Root suckering capacity | categorical | yes or no | 72 | Dr |
| Seed weight | continuous | mg | 97 | Dr |
| Species | Species Acronym | IVI (%) | FG-pp | FG-bc | Regeneration | Dynamic Status |
|---|---|---|---|---|---|---|
| Hilltops | ||||||
| Boscia senegalensis (Pers.) Lam. ex Poir | BOS | 30.7 | 1 | 3 | Numerous | Stable |
| Balanites aegyptiaca (L.) Del. | BAA | 23.3 | 2 | 3 | Numerous | Stable |
| Sclerocarya birrea (A. Rich.) Hochst. | SCB | 19.2 | 2 | 2 | Absent | Decrease |
| Acacia tortilis subsp. raddiana (Savi) Brenan | ACT | 7.5 | 2 | 3 | Numerous | Increase |
| Calotropis procera (Aiton) W.T. Aiton | CAP | 6.5 | 1 | 3 | Numerous | Stable |
| Combretum glutinosum Perr. ex DC. | COG | 3.4 | 2 | 3 | Absent | Decrease |
| Acacia senegal (L.) Willd. | ASN | 2.6 | 1 | 1 | Moderate | Decrease |
| Adansonia digitata L. | ADD | 2.3 | 2 | 2 | Absent | Stable |
| Grewia bicolor Juss. | GRB | 1.4 | 1 | 1 | Absent | Decrease |
| Leptadenia pyrotechnica (Forssk.) Decne. | LEP | 1.1 | 1 | 3 | Moderate | Stable |
| Guiera senegalensis J. F. Gmel. | GUS | 1.1 | 1 | 3 | Low | Decrease |
| Depressions | ||||||
| Balanites aegyptiaca (L.) Del. | BAA | 29.6 | 2 | 3 | Numerous | Stable |
| Boscia senegalensis (Pers.) Lam. ex Poir | BOS | 22.7 | 1 | 3 | Numerous | Stable |
| Calotropis procera (Aiton) W.T. Aiton | CAP | 8.9 | 1 | 3 | Numerous | Stable |
| Sclerocarya birrea (A. Rich.) Hochst. | SCB | 8.8 | 2 | 2 | Absent | Decrease |
| Acacia tortilis subsp. raddiana (Savi) Brenan | ACT | 6.2 | 2 | 3 | Numerous | Increase |
| Grewia bicolor Juss. | GRB | 5.6 | 1 | 1 | Low | Decrease |
| Acacia seyal Del. | ASY | 5.0 | 2 | 2 | Moderate | Decrease |
| Guiera senegalensis J. F. Gmel. | GUS | 2.1 | 1 | 3 | Low | Decrease |
| Ziziphus mauritiana Lam. | ZIM | 2.0 | 2 | 3 | Low | Stable |
| Acacia nilotica (L.) Willd. ex Del. | ACN | 1.5 | 2 | 2 | Absent | Rare |
| Combretum glutinosum Perr. ex DC. | COG | 1.5 | 2 | 3 | Absent | Decrease |
| Acacia senegal (L.) Willd. | ASN | 1.5 | 1 | 1 | Moderate | Decrease |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dendoncker, M.; Taugourdeau, S.; Messier, C.; Vincke, C. A Functional Trait-Based Approach to Evaluate the Resilience of Key Ecosystem Functions of Tropical Savannas. Forests 2023, 14, 291. https://doi.org/10.3390/f14020291
Dendoncker M, Taugourdeau S, Messier C, Vincke C. A Functional Trait-Based Approach to Evaluate the Resilience of Key Ecosystem Functions of Tropical Savannas. Forests. 2023; 14(2):291. https://doi.org/10.3390/f14020291
Chicago/Turabian StyleDendoncker, Morgane, Simon Taugourdeau, Christian Messier, and Caroline Vincke. 2023. "A Functional Trait-Based Approach to Evaluate the Resilience of Key Ecosystem Functions of Tropical Savannas" Forests 14, no. 2: 291. https://doi.org/10.3390/f14020291





